Abstract
KLF5 possesses both tumor suppressing and tumor promoting activities, though the mechanism controlling these opposing functions is unknown. In cultured non-cancerous epithelial cells, KLF5 converts from pro-proliferative to anti-proliferative activity upon TGFβ-induced acetylation, which sequentially alters the KLF5 transcriptional complex and the expression of genes such as p15 and MYC. In this study, we tested whether the acetylation status of KLF5 also determines its opposing functions in tumorigenesis using the PC-3 and DU 145 prostate cancer cell lines, whose proliferation is inhibited by TGFβ. KLF5 inhibited the proliferation of these cancer cells, and the inhibition was dependent on KLF5 acetylation. MYC and p15 showed the same patterns of expression change found in non-cancerous cells. In nude mice, KLF5 also suppressed tumor growth in an acetylation-dependent manner. Furthermore, deacetylation switched KLF5 to tumor promoting activity, and blocking TGFβ signaling attenuated the tumor suppressor activity of KLF5. RNA-Seq and comprehensive data analysis suggest that multiple molecules, including RELA, p53, CREB1, MYC, JUN, ER, AR and SP1, mediate the opposing functions of AcKLF5 and unAcKLF5. These results provide novel insights into the mechanism by which KLF5 switches from anti-tumorigenic to pro-tumorigenic function, and also suggest the roles of AcKLF5 and unAcKLF5, respectively, in the tumor suppressing and tumor promoting functions of TGFβ.
Keywords: KLF5, TGFβ, acetylation, tumorigenesis, prostate cancer
INTRODUCTION
KLF5 is a basic transcriptional factor that is highly expressed in epithelial cells 1. By regulating a large number of genes, KLF5 functions in multiple cellular processes including cell proliferation, differentiation, and apoptosis 1–7. In tumorigenesis, KLF5 has opposing functions, being tumor suppressing in some studies 8 and tumor promoting in other studies 9, 10. In the intestine, KLF5 appears to be a mediator of tumorigenesis caused by combined ApcMin and KRASV12 mutations 11, 12. It is unusual for a molecule to be both pro- and anti-tumorigenic, and the mechanisms controlling KLF5’s functional switching remain unknown.
Similar to its opposing functions in tumorigenesis, KLF5 can be both anti- and pro-proliferative in cultured epithelial cells in the context of TGFβ 1. Detailed biochemical analyses indicate that KLF5 is necessary for cell proliferation without TGFβ, but becomes anti-proliferative when TGFβ is present, and TGFβ-induced acetylation of KLF5 is the key to this functional reverse 13–15. Biochemically, the pro-proliferative transcriptional complex formed with unacetylated KLF5 (unAcKLF5) is altered upon the acetylation of KLF5, leading to a change in the complex and subsequent reversal in the expression of genes such as MYC and p15 13–15. It seems possible that acetylated KLF5 (AcKLF5) would be anti-tumorigenic and unAcKLF5 pro-tumorigenic, though this remains unexplored.
In this study, we determined whether and how the acetylation status of KLF5 determines its function in tumorigenesis in the context of TGFβ. We used the PC-3 and DU 145 prostate cancer cell lines, both of which secrete a certain amount of TGFβ 16, express KLF5 at lower levels 4, and are suppressed in cell proliferation by TGFβ 17. We found that KLF5 not only inhibited the proliferation of these cancer cells in vitro but also suppressed their tumorigenesis in nude mice in an acetylation-dependent manner. In fact, deacetylation converted KLF5 from a tumor-suppressive to tumor-promoting function. Blocking TGFβ signaling attenuated the tumor suppressor activity of KLF5. Molecularly, MYC and p15 showed the same patterns of KLF5 acetylation-responsive expression changes found in non-cancerous epithelial cells, and RNA-Seq and comprehensive data analysis suggest that multiple molecules, including RELA, p53, CREB1, MYC, JUN, ER, AR and SP1, mediate the opposing functions of AcKLF5 and unAcKLF5.
MATERIALS AND METHODS
Cell lines and other materials
The two prostate cancer cell lines, PC-3 and DU 145, were purchased from the American Type Culture Collection (ATCC, Manassas, VA), and propagated according to the ATCC’s instructions.
The TGFβ used in this study was TGFβ1, purchased from R&D Systems (Minneapolis, MN). SB431542, an antagonist against TGFβ type I receptor that blocks TGFβ1 signaling, was purchased from Sigma-Aldrich (Beijing, China). Matrigel was from BD Biosciences (Beijing, China).
Retroviral expression of KLF5 and K369R
PCR was performed to amplify the coding regions of wildtype KLF5 and the acetylation-deficient K369R mutant from plasmids as described in a previous study 15, with primers 5′-CCCAAGCTTATGGCTACAAGGGTGCTGA-3′ (forward) and 5′-CCATCGATTCAGTTCTGGTGCCTCTTC -3′ (reverse). PCR products were digested with HindIII and ClaI restriction enzymes, purified and subsequently cloned into the pLHCX vector (Clontech, Mountain View, CA) to construct vectors pLHCX-KLF5 and pLHCX-K369R. Plasmids containing KLF5, K369R or the empty vector were cotransfected with the envelope vector VSV-G and the gal/pol expression vector Ecopac (Clontech) into HEK293T cells (ATCC) using the Lipofectamine 2000 reagent (Invitrogen, Beijing, China). Viruses were harvested 48 and 72 hours after transfection and filtered with 0.45 μm filters (Millipore, Beijing, China). Cells infected with viruses were selected in media containing Hygromycin B at 800 μg/ml (Roche, Beijing, China) for 14 days before use.
Cell proliferation assay
The Cell Counting Kit-8 (Dojindo, Beijing, China) was used to measure cell proliferation rates. Cells were seeded at 30% confluence onto 24-well plates, grown for 48 hours with the indicated treatments (TGFβ or SB431542), and then 60 μl of the CCK-8 solution was added. After 1.5 hours of incubation, optical density (OD) was measured at the 450 nm wavelength.
Western blotting
Western blotting and the anti-KLF5 and anti-AcKLF5 polyclonal antibodies have been described in our previous studies 15, 18. The KLF5 antibody was developed using purified partial KLF5 protein (residues 88-374) to detect total KLF5 protein, while the AcKLF5 antibody was developed using a short synthesized peptide (residues 362-375) with acetylated lysine 369 to detect only acetylated KLF5 15, 18. Other antibodies used in this study included: MYC (rabbit polyclonal, 1:500 dilution, catalogue #9402, Cell Signaling Technology, Beverly, MA, USA), p15 (mouse monoclonal, 1:500 dilution, catalogue #sc-271791, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (catalogue #A1978, Sigma-Aldrich).
Tumorigenesis assay
Male BALB/c nude mice 3–4 weeks old were used in this assay. Cancer cells were resuspended in a mixture of PBS and Matrigel (equal volumes) at 5 × 107 cells/ml for PC-3 and 2 × 107 cells/ml for DU 145, and 100 μl of cells were injected subcutaneously into both flanks. Six mice were used in each group. Tumor volumes were measured twice a week after palpable tumors appeared. At 5–7 weeks post injection, mice were sacrificed, and tumors were surgically isolated, weighed and photographed. To block TGFβ signaling, SB431542 in DMSO was intraperitoneally injected into mice at 10 mg/kg body weight one day before cell injection and twice a week after cell injection to maintain TGFβ inhibition. Mice in the control group were injected with DMSO, the solvent for SB431542.
RNA sequencing (RNA-Seq), qPCR validation, and identification of differentially expressed genes
Tumors of DU 145 cells expressing KLF5, K369R or the PLHCX vector control were used for RNA isolation and RNA-Seq analysis at the Shanghai Biotechnology Corporation (Shanghai, China). Tumors of PC-3 cells were not used because those expressing KLF5 were too small (see Results for details). Three tumors were used for each group (KLF5, K369R or PLHCX). Briefly, total RNA was extracted using the TRIZOL reagent (Invitrogen), and 4 μg of total RNA from each of the three tumors were pooled and purified using the RNeasy Micro kit (Qiagen) for each group. The quality and concentration of RNA samples were determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Ten μg of pooled total RNA were used to construct non-directional RNA-Seq libraries (300 bp average insert size) using the Truseq RNA-Seq Library Prep Kit v2 (Illumina, San Diego, CA, USA) following the manufacturer’s instruction. Libraries were quantified by using the Qubit 2.0 Fluorometer (Invitrogen) and validated by using the Agilent 2100 Bioanalyzer. Clusters were generated by using the cBot with libraries diluted to 20 pM and sequenced on a HiSeq2500 platform (Illumina). The sequencing was single-end with 50 nt read length.
Data analysis for identifying differentially expressed genes was also performed by Shanghai Biotechnology. Briefly, clean raw reads in the fastq format were mapped to the human transcriptome sequences downloaded from the UCSC Genome Bioinformatics Site (http://hgdownload.cse.ucsc.edu/goldenPath/hg19/bigZips/chromFa.tar.gz) using the TopHat computer program (Version 2.0.9) 19. Each sample had 30–40 × 106 reads, and approximately 85% of these reads were mapped to the human genome, indicating a high quality of RNA-Seq. Expression level for a gene was established by the number of fragments per kilobase of exon per million fragments mapped (FPKM) reads using the Cufflink software (Version 2.1.1) 20. Differentially expressed genes were identified using the DEGseq software 21.
We then performed quantitative real-time PCR (qPCR) to validate RNA-Seq data. We evaluated the expression of 9 genes (5 for KLF5 vs. PLHCX and 4 for K369R vs. PLHCX comparisons) with >20 fold change, 2 (1 for KLF5 vs. PLHCX and 1 for K369R vs. PLHCX) with 1.5–2 fold change, and 25 (4 for KLF5 vs. PLHCX and 21 for K369R vs. PLHCX) with 2–20 fold change. Briefly, cDNA was generated from total RNA using the M-MLV Reverse Transcriptase (Invitrogen) with oligo(dT) primers, and real-time quantitative PCR (qPCR) was performed using the SYBR Green MasterMix (TaKaRa, Dalian, China) on a Mastercycler ep realplex thermal cycler (Eppendorf, Shanghai, China), with GAPDH as an internal control. The assay was performed in duplicate or triplicate for each gene. Gene names and primer sequences for qPCR are listed in Table S1. KLF5 expression at the mRNA level was also detected by regular RT-PCR as previously described 3.
Considering that none of the 9 genes with >20 fold change were validated by qPCR, 2 of 2 with 1.5–2 fold change and 22 of 25 with 2–20 fold change were successfully validated, and 11 of 13 genes with a p value <0.05 but FDR >0.05 were validated (Table S2), we defined differentially expressed genes as those having a fold change of 1.5–20 and a p value <0.05, regardless of FDR. Twenty-four of the 27 (88%) genes that met this criterion were validated by qPCR for differential expression (Table S2). The RNA-Seq data have been deposited in NCBI’s Gene Expression Omnibus database 22, 23 and are accessible through GEO Series accession number GSE56343 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE5643).
Identification of the largest possible number of molecular pathways/networks and key molecules that mediate the opposing function of unAcKLF5
Differentially expressed genes between two groups (i.e., xenografts carrying KLF5 vs. PLHCX vector control, K369R vs. PLHCX, and K369R vs. KLF5) were uploaded to the web-based MetaCore program (http://thomsonreuters.com/metacore), and different algorithms were run with MetaCore’s database to identify functional processes and molecular pathways and networks that are differentially regulated by K369R and KLF5. Details of the MetaCore platform and its application have been described previously 24. Top-ranked networks were reviewed to identify all the possible molecules that mediate the opposing function of unAcKLF5 in tumorigenesis.
Statistical analysis
The unpaired t-test was used to determine the significance of differences between the two groups.
RESULTS
Acetylation is necessary for KLF5 to inhibit the proliferation of prostate cancer cells
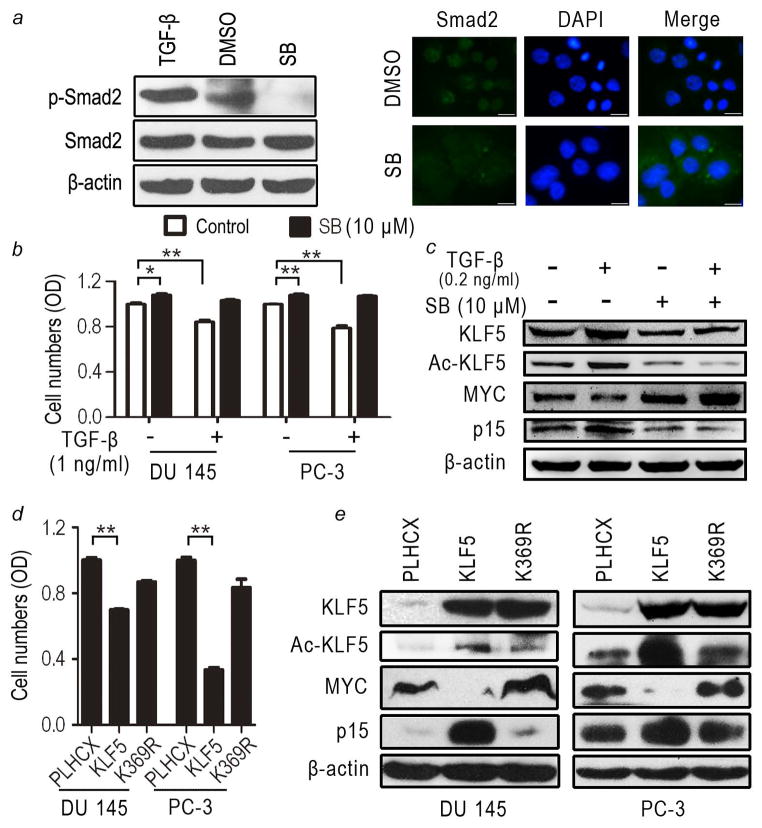
We determined whether KLF5 inhibits cancer cell proliferation in an acetylation-dependent manner as described previously in non-cancerous cells 13–15. Using the PC-3 and DU 145 prostate cancer cell lines, we first confirmed that TGFβ signaling was active in these cells. The phosphorylation of Smad2 and the nuclear translocation of p-Smad2, both of which are indicators of TGFβ signaling, were increased by the addition of TGFβ to the medium, and reduced by blocking TGFβ signaling using the SB431542 TGFβ type I receptor antagonist 25 (Fig. 1a). PC-3 and DU 145 cells secrete TGFβ 16, and this endogenous TGFβ was sufficient to induce the phosphorylation of Smad2 (Fig. 1a).
Figure 1. Acetylation of KLF5 is necessary for KLF5 to inhibit the proliferation of prostate cancer cells.
(a) SB431542 (SB) effectively inhibited TGFβ signaling in DU 145 cells, as indicated by reduced phosphorylation of Smad2 (p-Smad2) and interrupted nuclear translocation of p-Smad2, as determined by western blotting and immunofluorescence imaging, respectively. DMSO is the solvent control for SB431542. DAPI shows nuclei. Scale bar, 50 μm. (b, c) Addition of TGFβ slowed, while inhibition of TGFβ signaling by SB431542 enhanced, the proliferation of PC-3 and DU 145 cells (b), with accompanied alterations in KLF5 acetylation and the expression of MYC and p15 (shown only for DU 145 cells) (c). + and − indicate the presence and absence, respectively, of a treatment. Gene expression was determined by western blotting. (d, e) Interruption of acetylation abolished the anti-proliferative function of KLF5 in PC-3 and DU 145 prostate cancer cells (d), accompanied with changes in the expression of MYC and p15 (e). Stable cell populations were used, and expression of KLF5 and K369R was mediated by retroviral infection. Cell proliferation was determined by CCK-8 assay (d) and gene expression was evaluated by western blotting (e). Each bar in panels b and d is the mean of 4 samples, with the standard error indicated at the top. β-actin in panels a, c and e served as the loading control. Each experiment was repeated at least twice and consistent results were obtained. *, P<0.05; **, P<0.01.
KLF5 is expressed in PC-3 and DU 145 cells, although at lower levels compared to that in non-cancerous cells 4, so we next tested the effect of TGFβ on cell proliferation without ectopically expressed KLF5. In both cell lines, the addition of TGFβ decreased, while blocking TGFβ by SB431542 increased, cell proliferation (Fig. 1b) as previously reported 17. Consistent with findings from non-cancerous epithelial cells 15, TGFβ increased and SB431542 decreased KLF5 acetylation, and MYC and p15 showed the opposite expression changes in response to KLF5 acetylation, with p15 upregulated and MYC downregulated by increased AcKLF5 expression (Fig. 1c).
Since the endogenous TGFβ in both PC-3 and DU 145 cell lines 16 was sufficient to cause the phosphorylation of Smad2 (Fig. 1a), we then tested the effect of KLF5 acetylation on cell proliferation. Whereas the expression of wildtype KLF5, as mediated by retroviral infection, significantly inhibited cell proliferation as expected, the K369R mutant lost this inhibitory function (Fig. 1d). At the molecular level, as evaluated by western blotting, KLF5 downregulated MYC but upregulated p15 as expected, while the K369R mutant upregulated MYC and had no effect on p15 expression (Fig. 1e).
Deficiency in acetylation converts KLF5 from tumor suppressor to tumor promoter
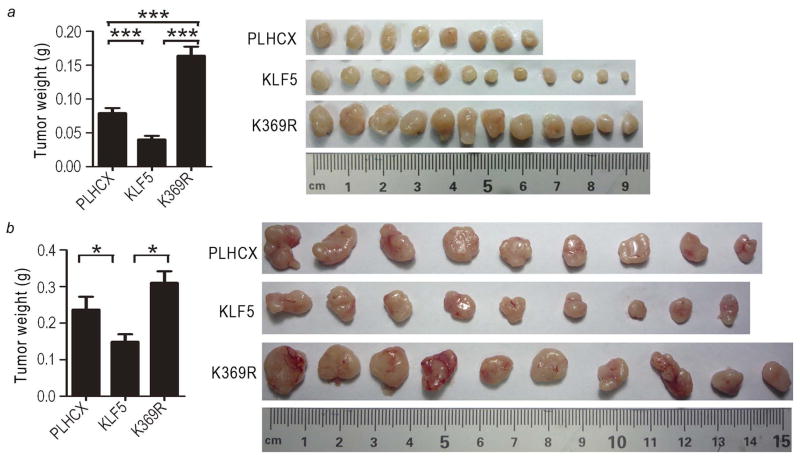
To test the effect of acetylation on the function of KLF5 in tumorigenesis, we subcutaneously injected cancer cells stably expressing KLF5 or K369R, along with the vector control, into nude mice. Tumors were allowed to grow for about 5 weeks. Compared to the vector control (PLHCX), KLF5 expression significantly reduced PC-3 and DU 145 tumor weight by 37–49%. Interestingly, K369R expression not only abolished the tumor suppressor activity of KLF5, it actually significantly increased tumor weight by 31–107% (Fig. 2, panels at left). Differences in tumor sizes among different groups were also visually apparent (Fig. 2, panels at right). A tumor volume-based growth curve analysis also confirmed the differential effects of KLF5 and K369R on tumorigenesis (data not shown).
Figure 2. Interruption of KLF5 acetylation reverses its activity from tumor suppressing to tumor promoting in prostate cancer cells.
Cancer cells expressing KLF5, acetylation-deficient K369R, or the vector control (PLHCX) were subcutaneously injected into nude mice, and tumors were surgically isolated at day 35 for PC-3 cells (a) and day 38 for DU 145 cells (b) post injection. The average tumor weight (grams, g) is shown in the bar figures at left, with the standard error indicated at the top of each bar. Images of all tumors are shown at right. *, P<0.05; ***, P<0.0001.
Blocking TGFβ signaling attenuates the tumor suppressor activity of KLF5
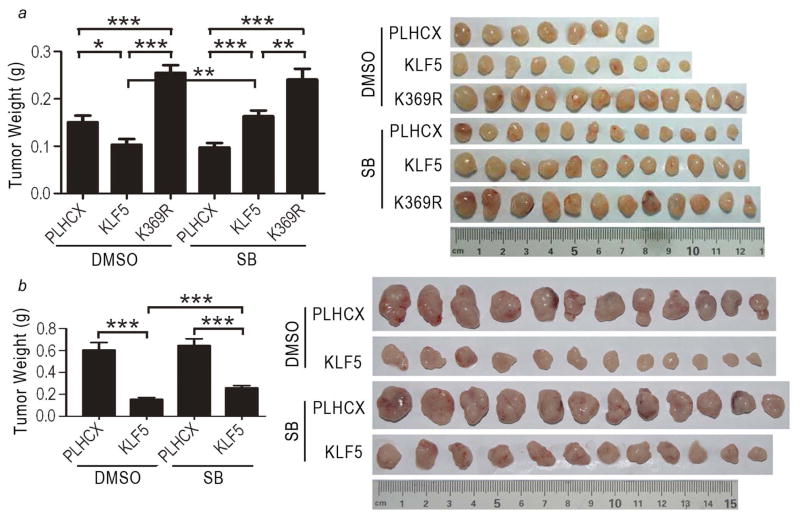
To evaluate whether the tumor suppressor activity of KLF5 also depends on active TGFβ signaling as in the proliferation of cultured cells (Fig. 1) 15, we administered the TGFβ type I receptor inhibitor SB431542 to mice via intraperitoneal injection, and evaluated tumor growth by measuring tumor volumes and weights and photographing surgically isolated tumors (data for tumor volumes are not shown). In PC-3 cells, which developed smaller tumors than DU 145 cells, blocking TGFβ signaling abolished the tumor suppressor activity of KLF5 (Fig. 3a, KLF5 group with SB431542 versus the group without) but did not affect the tumor promoting activity of K369R (Fig. 3a, K369R group with SB431542 versus the group without). The PLHCX control group with SB431542 treatment formed smaller tumors than the group without (Fig. 3a), which could have been caused by the effect of SB431542 on cells with lower levels of KLF5 expression. In DU 145 cells, whose tumors grew significantly faster than PC-3 tumors, blocking TGFβ signaling also attenuated the tumor suppressor activity of KLF5 (Fig. 3b, KLF5 group with SB431542 versus the group without), although the effect was milder than in PC-3 cells (Fig. 3b, the two bars at right).
Figure 3. Blocking TGFβ signaling attenuates the tumor suppressor activity of KLF5 in prostate cancer cells.
Tumor cells expressing KLF5, acetylation-deficient K369R or the vector control (PLHCX) were subcutaneously injected into nude mice, and tumors were surgically isolated at day 42 for PC-3 cells (a) and day 34 for DU 145 cells (b) post injection. The TGFβ receptor inhibitor SB431542 (SB) was administered to mice via intraperitoneal injection. Average tumor weights (grams, g) are indicated by bar figures at left, with the standard errors indicated at the top of bars. Images of all tumors are shown at right. *, P<0.05; **, P<0.01; ***, P<0.0001.
Interruption of KLF5 acetylation alters global gene expression
To understand how interruption of KLF5 acetylation converts its function to tumor promoting, we performed RNA-Seq with tumors of DU 145 cells expressing KLF5, K369R or the PLHCX vector control to identify differentially expressed genes between KLF5 and K369R. Tumors from PC-3 cells carrying KLF5 were too small to be used in this analysis (Fig. 2). We confirmed that ectopic KLF5 and K369R were still highly expressed after tumor growth by qPCR (Table S2).
Compared to the PLHCX vector control, ectopic expression of KLF5 altered the expression of 65 genes (39 upregulated and 26 downregulated) (Table S3), while K369R expression dysregulated 540 genes (275 upregulated and 265 downregulated) (Table S4). Between K369R and KLF5 xenografts, 501 genes were differentially expressed (275 upregulated and 226 downregulated by K369R) (Table S5). We noticed that 372 of the 540 (69%) differentially expressed genes between K369R and PLHCX xenografts were also differentially expressed between K369R and KLF5, indicating a similarity between KLF5 and PLHCX (Tables S4–S5). Consistent with its tumor promoting function, K369R downregulated the BCOR tumor suppressor gene by 5.3-fold and upregulated the MET oncogene by 5.7-fold, both of which are driver genes in tumorigenesis 26. K369R also upregulated CD44, an established marker for cancer-like stem cells that plays a role in tumorigenesis.
Multiple signaling pathways, molecular networks and key molecules appear to be responsible for the opposing function of unAcKLF5 in tumorigenesis
To further explore the molecular mechanisms underlying different functions of KLF5 and K369R in tumorigenesis, we uploaded the three lists of differentially expressed genes to the MetaCore platform to identify significant gen ontology (GO) processes, signaling pathways and molecular networks that are dysregulated upon the interruption of KLF5 acetylation.
While KLF5 and K369R were seen to affect several common processes and pathways such as inflammation, cell proliferation and cell cycle (Fig. S1–S2 and Table S6–S11), the effect of K369R was much different than that of KLF5 compared to the PLHCX vector control. In fact, affected processes and pathways between K369R and KLF5, as shown by the p value, were quite similar to those between K369R and PLHCX (Fig. S1–S2). Compared to the PLHCX vector control, while genes affected by K369R mostly related to the processes of muscle cell movement, cell adhesion, cytoskeleton remodeling, TGFβ signaling, tissue-factor induced signaling and Wnt signaling in cancer (Fig. S1–S2 and Table S7, S10), those affected by KLF5 were mainly related to protein folding processes, leptin signaling, and AP-1 and ZNF202 signaling in transcriptional regulation of genes (Fig. S1–S2, Table S6, S9).
Directly related to the tumor promoting function of K369R, it upregulated 7 of 13 proliferative genes by 2–14 fold, including JUN, basomuclin, FGFR1, HGFR (MET), while KLF5 only upregulated 2, β-arrestin1 and MDM2 (both < 2 fold) and downregulated one, VEGF-A (Table S3–S4 and S6–S7). In addition, K369R upregulated 19 of 20 genes that promote cell movement and contraction, including actin (ACT) and myosin heavy chain (MYH) (Table S7); and 17 of 20 genes that promote cell adhesion, including LAMC, ITGB4, and CD44 (Table S7). These findings indicate that K369R and KLF5 clearly have different effects on cellular processes and signaling pathways.
We also used the network analysis algorithm to reveal connections and interactions among K369R-regulated genes. The algorithm limited each network to 50 nodes with canonical pathways, and networks with at least 4 seed nodes (genes differentially expressed among our groups) were considered instructive and meaningful.
In the KLF5 vs. PLHCX comparison, only 4 networks were identified, and they centered around HSP70, MYC and PKC (Fig. S3 and Table S12). In the K369R vs. PLHCX comparison, 29 networks were built, and the top two networks with the smallest p values were centered around the RELA (p65) NF-κB subunit and p53, respectively (Fig. S4 and Table S13). Interestingly, 23 of the 29 networks shared the same key knot – the CREB1 transcription factor, and 3 of them centered around MYC (Fig. S4 and Table S13), suggesting the relevance of CREB1 and MYC in the function of K369R in tumorigenesis. The TGFβ pathway, which uses KLF5 as a cofactor 13–15, ranked the 5th with 9 genes involved, 7 of which appeared to be TGFβ target genes (Table S10).
For the K369R vs. KLF5 comparison, CREB1, MYC and p53 were also identified as key knots, as in the K369R vs. PLHCX comparison, centering on 17, 2 and 1, respectively, of the 28 networks (Fig. S5 and Table S14). Other networks also appeared, including those centered around SP1 (3/28) and α6β4 complex, STAT3, ubiquitin, tubulin alpha and FAK1 (one each). Interestingly, the top two networks identified as differentially regulated between K369R and KLF5 were those centered around the estrogen receptor (ER) and the androgen receptor (AR), linking 28 and 26 seed nodes, respectively (Fig. S5 and Table S14). We noticed that 21 of 24 processes from the K369R vs. KLF5 comparison were also identified in the K369R vs. PLHCX comparison, further suggesting that KLF5 and PLHCX were similar while K369R acted very differently.
We further determined which transcription factors most likely interacted with K369R or KLF5 target genes by building transcription factor-centered networks. Most of the identified transcription factors were shared by all three comparisons (K369R vs. PLHCX, KLF5 vs. PLHCX, and K369R vs. KLF5), including CREB1, HIF1A, GCR-alpha, MYC, SP1, p53, Oct-3/4, STAT3, C/EBPbeta, JUN, E2F1, RELA (p65 subunit of NF-κB), Nanog, AR, ER and p63 (Table S15–17). However, these transcription factors were implicated by different genes among different comparisons, as 38 of the 62 genes from the KLF5 vs. PLHCX comparison did not appear in the K369R vs. PLHCX comparison, and 4 of the 62 genes even showed opposite trends of expression change between the two comparisons (Tables S15–16). On the other hand, AP-1, Smad3, PPARγ and C-Fos are unique to KLF5 vs. PLHCX. There were also some transcription factors that were unique to one comparison, including KLF4 and STAT1 for KLF5 vs. K369R, ERG1 for KLF5 vs. PLHCX and K369R vs. PLHCX but not for K369R vs. KLF5 (Table S15–17). Among the transcription factors common to all three comparisons, CREB1, p53, SP1, MYC, AR, ER, STAT3 and NF-κB were also identified as key knots by the network analysis, further indicating their relevance to K369R and KLF5 functions. We noticed that MYC was the only key knot that was identified by both network and transcription factor analyses in all three groups. In addition, JUN was not only identified as a key transcription factor that affected 81 of 540 genes’ expression in the K369R vs. PLHCX comparison, it was also upregulated by K369R when compared to PLHCX or KLF5, and showed up in most of the processes and signaling pathways identified above. In total, 8 molecules, including RELA, p53, CREB1, MYC, JUN, ER, AR and SP1, were more likely to mediate the opposing functions of AcKLF5 and unAcKLF5 in tumorigenesis (Table 1).
Table 1.
Key molecules potentially responsible for K369R’s tumor promoting function and K369R-regulated genes that identified these key molecules in MetaCore analysis.
| Key molecules | Genes regulated by K369R in DU 145 tumor |
|---|---|
| From the comparison between K369R and the PLHCX vector control | |
| RELA (p65) | Upregulated: JUN, C3, CD44, CAV1, CFB, GNAI2, LGALS1, KLF6, LAMB3, OPTN, PKM, PRKCDBP, RIPK2, PSAP, SMS, TGM2, TNFAIP2, CASP4, ERAP2, MET, SERPINE1, TNC; Downregulated: STAMBPL1, EPAS1, EHF, FBLN1, GLRX, IGFBP2, IGFBP3, ICAM1, NFKBIA, PAG1, GDF15, TFPI2, VEGFA, ZFP36 |
| p53 | Upregulated: ACTA2, SERPINA1, CRYAB, DDIT3, TNFSF9, COL4A1, CAV1, CCDC85B, FHL2, GADD45A, GPX1, RNF128, HSPB1, ITGB4, CKM, LAMC2, PMAIP1, P4HA2, PERP, PHLDA3, PLK2, PRNP, RRAD, RHOC, S100A2, TNC, THBS1, AXL, VIM; Downregulated: ANXA4, CPSF6, CHEK2, DAPK1, DKK1, EDIL3, EPCAM, IGFBP2, IGFBP3, LIF, DUSP4, NDRG1, GDF15, PTP4A1, RGCC, SLC6A6, STMN1, TFPI2, TOP2A, TXNIP, TMEM205, VEGFA, ZFP36, PLAGL1 |
| CREB1 | Upregulated: VGF, IL-32, RRAD, ETS2, NOXA, Cyr61, SMRT, PAI-1; Downregulated: ASS1, BTG1, CEBPD, CGA, CHMP1B, GJA1, ICA1, LAPTM4B, LIF, LTBP1, NR4A3, NR4A1, PDE4D, GDF15, PER1, PTP4A1, STMN1, TOB1, VEGFA. |
| MYC | Upregulated: JUN, BMI1, CD44, CLIC4, S100A11, DSG2, ETS2, ETV6, FGFR1, FSTL3, GNAI2, GADD45A, GALNT2, LGALS1, HERPUD1, KLF6, MYO1B, MYO1C, PMAIP1, OPTN, CDH3, PEA15, ATP2B4, PMEPA1, RALB, THBS1; Downregulated: ABCA2, ASS1, ADM, BASP1, BTG1, CEBPD, CEACAM5, CHEK2, DKK1, DNAJC12, ICAM1, PRPS2, NME4, NDRG1, NOL6, NUB1, NR4A1, PTP4A1, PEG10, JUP, VEGFA, WIF1, ZFP36 |
| JUN | Upregulated: CD44, CLIC4, CYR61, GADD45A, MET, IL18, IL32, SPARC, SERPINE1, TSPO, PSAP, ST5, TNC, VIM; Downregulated: CEBPD, DKK1, NFKBIA, BPIFA1, SLC6A6, VEGFA, CYBA, ALDH2, CGA, NR4A1, SFTPB, F3 |
| From the comparison between K369R and KLF5 | |
| ER | Upregulated: JUN, ANXA3, TGFBI, CD44, CAV1, HES1, HSPB1, PMAIP1, SAT1, TNS4, DDIT3, C6orf141, CASP4, DCBLD2, GAS6, KRT19, KRT7, CLIC1, CDH3, PLAUR, PLOD2, PITX1, TGM2, TMEM2; Downregulated: APOE, ASS1, ATP11A, AQP3, CGA, SLC9A3R1, AGR2, IGFBP2, IGFBP3, MALL, ERRFI1, MUC1, NELL2, NR4A1, PDK4, SLC26A2, SLC6A6, F3, XBP1, FOS |
| AR | Upregulated: KRT7, ADAM9, AMIGO2, JUN, CD44, CAV1, CYR61, GAS6, FBXO32, PMAIP1, CDH3, PMEPA1, TNS4; Downregulated: SLPI, CITED2, AGR2, IGFBP3, ITGB5, MUC1, NDRG1, PER1, PEG10, SLC26A2, STOM, TM4SF1, F3, XBP1, ZNF189 |
| SP1 | Upregulated: JUN, SERPINA1, BMI1, BNC1, DDIT3, COL4A1, COL4A2, S100A6, CAV1, F13A1, FGFR1, FHL2, GFPT2, MET, HSPB1, HSPA1A, KRT19, KRT7, LAMC2, MICB, MYH9, SPARC, SERPINE1, PKM, PLAU, PLAUR, SAT1, TGM2, TIMP2, TNC, TNNT3, AXL, VIM, XAGE1B; Downregulated: AKAP12, AKR1C2, AKR1C1, APOE, ASS1, CEBPD, CEACAM6, CITED2, CRABP2, CEA, CGA, CCND3, EPAS1, EPCAM, GSTA4, IGFBP2, IGFBP3, ICAM1, IDH2, ITPR1, ITGB5, LAMA1, MDM2, KITLG, MUC1, NR4A1, GDF15, PDK4, PGD, SMARCA1, SFTPB, TFPI2, F3, UGDH, FOS |
| CREB1 | Upregulated: CD68, Cyr61, VGF, RRAD, PAI1, IL32, ETS2, NOXA, HES1, HSPA1A; Downregulated: ASS1, BTG1, CEBPD, CGA, PFKFB3, LAPTM4B, LTBP1, NR4A3, NR4A1, PDE4D, GDF15, PER1, SMARCA1, FOS |
| JUN | Upregulated: JUN, CD44, CLIC4, CYR61, GADD45A, MET, DNAJB1, IL18, IL32, SPARC, SERPINE1, PLAU, PLAUR, TNC, VIM; Downregulated: ALDH1A1, CEBPD, CGA, DKK1, MDM2, NR4A1, SLC6A6, SFTPB, F3, FOS, CYBA |
DISCUSSION
In this study, we investigated whether the switch in KLF5 function from tumor-suppressive to tumor-promoting activity is determined by its acetylation status in the context of TGFβ, as suggested by previous studies of non-cancerous epithelial cells where KLF5 is pro-proliferative without TGFβ but becomes anti-proliferative upon TGFβ-induced acetylation 13–15. We used two prostate cancer cell lines, PC-3 and DU 145, both of which produce endogenous TGFβ 16, express lower levels of KLF5 4, and are suppressed in cell proliferation by TGFβ 17. We first confirmed that TGFβ plays an anti-proliferative role in these cells as previously described 17, showing that TGFβ treatment decreased, while blocking TGFβ enhanced, cell proliferation (Fig. 1b). In this context of TGFβ function, we demonstrated that, as in non-cancerous cells 15, KLF5 expression inhibited cell proliferation while interruption of KLF5 acetylation abolished this inhibitory effect (Fig. 1d). The expected molecular alterations also found in non-cancerous epithelial cells were detectable, including TGFβ-associated KLF5 acetylation and alterations in the expression of MYC and p15 in response to AcKLF5 or unAcKLF5 (Fig. 1c, e). These findings indicate that, in cancer cells in which TGFβ suppresses cell proliferation, KLF5 also inhibits cell proliferation, and the inhibition is dependent on the acetylation of KLF5.
Our tumorigenesis experiments demonstrated for the first time that KLF5 can function in both a pro- and anti-tumorigenic capacity in the same cells, and that acetylation of KLF5 is the key to controlling its function in tumorigenesis. KLF5 has been shown to suppress tumor growth of PC-3 and DU 145 prostate cancer cells 8, which was confirmed in this study (Fig. 2). Interestingly, when the acetylation of KLF5 was interrupted by mutation, unAcKLF5 no longer suppressed tumorigenesis; rather, it promoted tumor growth (Fig. 2). These results suggest that the acetylation status of KLF5 determines whether KLF5 suppresses or promotes tumor growth, with AcKLF5 suppressing and unAcKLF5 promoting tumor growth. This point of view is consistent with a previous study in KLF5-expressing TSU-Pr1 bladder cancer cells, in which ectopically expressed KLF5 promoted tumor growth 9 but no acetylation of KLF5 was detectable (unpublished data). In normal mouse prostates, AcKLF5 is expressed in most luminal cells while unAcKLF5 is expressed exclusively in basal cells 27. Luminal cells are differentiated and mostly non-proliferative, while basal cells are undifferentiated and proliferative. It is thus possible that the tumor suppressor activity of AcKLF5 is related to its pro-differentiating function, while the tumor promoting activity of unAcKLF5 is related to its pro-proliferative function. We have begun testing the expression of both AcKLF5 and unAcKLF5 in human prostate cancers to determine whether KLF5 deacetylation occurs in human cancer, and whether it affects prostate cancer behavior.
Tumor suppression by AcKLF5 and tumor promotion by unAcKLF5 could share the same molecular mechanisms as the control of proliferation in cultured cells, i.e., via the transcriptional regulation of genes such as MYC and p15 14, 15. For example, MYC and p15 showed the same patterns of AcKLF5-responsive expression in cancer cell lines (Fig. 1) as in non-cancerous epithelial cells 14, 15. It is possible that additional genes mediate the different functions of AcKLF5 and unAcKLF5, but no comprehensive studies have been reported for the identification and validation of such genes, to the best of our knowledge.
To better understand how KLF5’s tumor suppressor function is reversed by deacetylation, we performed gene expression profiling and pathway and network analyses using tumors expressing K369R, KLF5 or the PLHCX vector control. There appear to be multiple mechanisms involved. Among the differentially expressed genes, two previously established tumorigenesis driver genes may play a role: one is the BCOR tumor suppressor gene 26, which was downregulated by K369R by 5.3-fold, and the other is the MET oncogene 26, which was upregulated by K369R by 5.7-fold when compared to PLHCX (Table S4). Another molecule of note is CD44, which was upregulated by K369R by about 2-fold compared to either PLHCX or KLF5 (Table S4, S5). CD44 has been established as a key marker for cancer-like stem cells, and some miRNAs inhibit prostate cancer growth and metastasis by directly downregulating CD44 expression 28, 29.
Comprehensive MetaCore analysis further suggests the involvement of additional pathways and molecular networks, particularly those centering around the following 8 molecules: RELA (the p65 subunit of NF-κB), p53, CREB1, MYC, JUN, ER, AR and SP1, as identified by the network reconstruction analysis (Table 1). Among the 8 molecules, RELA, p53 and MYC were identified by comparing differentially expressed genes between K369R and PLHCX; ER, AR and SP1 by comparing K369R and KLF5; and CREB1 and JUN in both comparisons. All these 8 molecules have been well implicated in tumorigenesis, and they all may mediate KLF5’s functional reverse upon acetylation interruption. CREB1, a transcription factor involved in many signaling pathways and cellular processes, is more likely to mediate KLF5’s functional reverse than other molecules for the following reasons. First, CREB1 was identified as the most commonly occurring core molecule in the network reconstruction analysis from K369R-regulated genes (Fig. S4, S5). Second, a previous study has demonstrated that KLF5 and ERβ interact to recruit CREB1 to a transcriptional complex on the promoter of the tumor suppressor gene FOXO1, leading to transcriptional activation of FOXO1 and subsequent suppression of tumor growth in both DU 145 and PC-3 prostate cancer cells 8. One possibility is that deacetylation of KLF5 alters or prevents its interaction with ERβ and CREB1, leading to the downregulation of FOXO1 and enhanced tumor growth. We have begun to test this possibility.
The other 7 of the 8 molecules have also been shown to associate with KLF5 in different ways. In intestinal epithelial cells, NF-κB mediates lipopolysaccharide (LPS)-induced proinflammatory response, and KLF5 has been shown to upregulate both the p50 and p65 (RELA) subunits of NF-κB during the response 30. KLF5 and NF-κB also directly interact to regulate gene transcription, as shown for RELA in keratinocytes 7 and for p50 in other types of cells 31. p53 directly interacts with KLF5 to regulate the transcription of genes such as survivin 32 and HIF-1α 33; and the interaction also plays a role in tumor suppression, as loss of KLF5 in the context of p53 deletion drives tumor progression 34, and the effect of KLF5 on keratinocyte proliferation is reversed upon p53 mutation 35. For MYC, its gene transcription is directly regulated by KLF5, and interestingly, KLF5 and K369R suppress and enhance MYC expression, respectively 13, 14 (Fig. 1), suggesting that MYC may also mediate KLF5’s functional reverse. In addition, Klf5 can substitute for Klf4 in reprogramming a differentiated cell to a stem cell with MYC and two other transcription factors (Oct4 and Sox2) 36. KLF5 also interacts with the JUN transcription factor to regulate gene expression 37, 38; and KLF5 expression in esophageal cancer cells can activate JNK (JUN N-terminal kinases), which activates JUN to induce cell transformation and tumorigenesis 39. It is worth noting that the JUN gene itself was upregulated by K369R (Table S4, S5).
ERα also interacts with KLF5, and the interaction suppresses ERα’s function in gene regulation and cell proliferation in ERα-positive breast cancer cells 40. For AR, although little is known about whether it interacts with KLF5 as a transcription factor, some studies suggest a functional association between the two. For example, androgen and AR can induce KLF5 transcription in prostate cancer cells 41, and in mouse prostates without androgen due to castration, knockout of Klf5 causes more severe shrinkage of the organ 27. For SP1, even less is known about its interaction with KLF5, except that SP1 plays a role in the transcription of KLF5 42.
EMT was the second most relevant process affected by K369R when compared to PLHCX, as indicated by 15 differentially expressed genes (Table S7). K369R-induced expression changes for 12 of the 15 genes, including upregulation of actin, JUN, desmin, PAI1, tropomyosin-1, vimentin and downregulation of claudin-2, have been shown in the literature to promote EMT. Taken together with our recent finding that KLF5 itself inhibits EMT 43 and K369R and KLF5 have opposing functions in cell proliferation 15 and tumorigenesis (Fig. 2), we predict that K369R promotes EMT via multiple molecules. Furthermore, considering that K369R upregulated genes involved in cell motility and adhesion (Table S7), it is possible that K369R promotes tumor metastasis, a process related to increased EMT and cell motility. We are currently testing whether K369R promotes EMT and tumor metastasis.
Whereas K369R was very different from the PLHCX control in terms of gene regulation, KLF5 was more similar to the PLHCX control. First, the number of genes differentially expressed between K369R and PLHCX or KLF5 was much greater than that between KLF5 and PLHCX (Tables S3–S5). Second, 372 of the 540 (69%) differentially expressed genes between K369R and PLHCX were also differentially expressed between K369R and KLF5. Third, cellular processes and molecular pathways affected by K369R were much different from that of KLF5 when compared to PLHCX control. Finally, many of the individual network key knots and transcription factors also showed a similar pattern. It is known that DU 145 cells not only express a certain level of endogenous KLF5 4 but also secrete TGFβ 16, so additional expression of KLF5 in the KLF5 group was more quantitative than qualitative in gene regulation. K369R on the other hand, showed opposing effects on gene regulation and cell proliferation 15.
KLF5 could participate in the dual functions of TGFβ in tumorigenesis. It is well established that TGFβ possesses dual functions during carcinogenesis, being suppressive at early stages but tumor-promoting at advanced stages 44, 45. Although different mechanisms have been suggested by different studies for how TGFβ executes these opposing functions 45, the question remains unresolved. In our xenograft tumorigenesis assay, TGFβ was available from both the surrounding stroma and cancer cells themselves. Our previous studies established KLF5 as a cofactor for TGFβ in the formation of the TGFβ-induced transcriptional complex, and loss of KLF5 acetylation alters this complex and thus compromises TGFβ function in the regulation of gene expression and cell proliferation 13–15. Taken together with our findings that blocking TGFβ signaling attenuated the tumor suppressor activity of KLF5 (Fig. 3) and that deacetylation of KLF5 promoted tumor growth (Fig. 2), we hypothesize that AcKLF5 mediates the tumor suppressor activity of TGFβ, while unAcKLF5 mediates the tumor promoter activity. For example, activated oncogenic signals could interfere with TGFβ-induced acetylation of KLF5, thus converting the tumor suppressor functions of TGFβ and KLF5 to tumor promoting. While remaining to be tested, this hypothesis is consistent with previous findings from TSU-Pr1 bladder cancer cells, in which HRAS is activated due to an activating mutation 46, both TGFβ and KLF5 promote its tumor growth in xenograft models 9, 47, blockage of Ras-MAPK oncogenic signaling restores the tumor suppressor activity of TGFβ 46, and the acetylation of KLF5 is not detectable (unpublished data). Furthermore, TGFβ signaling was affected by K369R in the pathway analysis (Fig. S2), and MYC, an oncogene downregulated by TGFβ and KLF5 14, 15, was identified as one of the key molecules mediating K369R’s pro-tumorigenesis function. Currently we are developing transgenic mice in which TGFβ signaling and KLF5 acetylation can be modulated in a tissue-specific manner, and we will thus be able to determine whether TGFβ and KLF5 form a signal axis to govern the dual functions of TGFβ in tumorigenesis.
In summary, we examined whether acetylation status determines the opposing functions of KLF5 in tumorigenesis in the context of TGFβ. As in non-cancerous cells, KLF5 inhibited the proliferation of prostate cancer cells in an acetylation-dependent manner. In addition, KLF5 suppressed tumor growth, and its deacetylation not only abolished the tumor suppressor activity of KLF5 but also converted its activity to that of tumor promotion. Furthermore, blocking TGFβ signaling attenuated the tumor suppressor activity of KLF5, and known target genes of KLF5 and TGFβ showed the same patterns of KLF5 acetylation-responsive expression as did non-cancerous epithelial cells. RNA-Seq and detailed data analysis suggest that multiple molecules, including RELA, p53, CREB1, MYC, JUN, ER, AR and Sp1, likely mediate the opposing functions of AcKLF5 and unAcKLF5. These results provide a novel mechanism that controls how KLF5 can be both pro- and anti-tumorigenic. They also suggest that AcKLF5 and unAcKLF5 can respectively mediate the tumor suppressing and tumor promoting functions of TGFβ.
Supplementary Material
Novelty & Impact Statements.
KLF5 is both tumor suppressing and tumor promoting. Here the authors demonstrated that acetylation was essential for KLF5’s tumor suppressor activity, and deacetylation switched KLF5 to tumor promoting activity. Furthermore, blocking TGFβ signaling attenuated KLF5’s tumor suppressor activity. Target genes of KLF5 and TGFβ showed KLF5 acetylation-responsive patterns of expression. RNA-Seq and molecular network analysis identified 8 molecules that could mediate KLF5’s functional reverse. The study provides a novel mechanism by which KLF5 switches function in tumorigenesis and implicates unacetylated KLF5 in the tumor promoting activity of TGFβ.
Acknowledgments
Grant sponsor: National Natural Science Foundation of China, 81130044; National Cancer Institute, NIH, R01CA171189
We thank Dr. Peng Guo of Xi’an Jiaotong University School of Medicine for advice; Dr. Anthea Hammond for editing the manuscript; and Dr. Changsheng Xing, Dr. Xiao Wu, Leilei Qi, Dr. Mei Li, Ang Gao, Lili Huang and Na Li for their help during the study.
Abbreviations
- AcKLF5
acetylated KLF5
- ATCC
American Type Culture Collection
- CCK-8
the Cell Counting Kit-8
- DAPI
4,6-diamino-2-phenyl indole
- DMSO
dimethyl sulfoxide
- K369R
acetylation-deficient KLF5 mutant
- KLF5
Krüppel-like factor
- OD
optical density
- PBS
phosphate buffer solution
- PCR
polymerase chain reaction
- p-Smad2
phosphorylated Smad2
- SB
SB431542
- TGFβ
tumor growth factor beta 1
- unAcKLF5
unacetylated KLF5
- RNA-Seq
RNA sequencing
References
- 1.Dong JT, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci. 2009;66:2691–706. doi: 10.1007/s00018-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman NW, Tan D, Pestell RG, Black JD, Black AR. Intestinal tumor progression is associated with altered function of KLF5. J Biol Chem. 2004;279:12093–101. doi: 10.1074/jbc.M311532200. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Bhalala HV, Qiao H, Dong JT. A possible tumor suppressor role of the KLF5 transcription factor in human breast cancer. Oncogene. 2002;21:6567–72. doi: 10.1038/sj.onc.1205817. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Bhalala HV, Vessella RL, Dong JT. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate. 2003;55:81–8. doi: 10.1002/pros.10205. [DOI] [PubMed] [Google Scholar]
- 5.Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, Babbin BA, Robine S, Yang VW. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–30. doi: 10.1053/j.gastro.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sur I, Rozell B, Jaks V, Bergstrom A, Toftgard R. Epidermal and craniofacial defects in mice overexpressing Klf5 in the basal layer of the epidermis. J Cell Sci. 2006;119:3593–601. doi: 10.1242/jcs.03070. [DOI] [PubMed] [Google Scholar]
- 7.Sur I, Unden AB, Toftgard R. Human Kruppel-like factor5/KLF5: synergy with NF-kappaB/Rel factors and expression in human skin and hair follicles. Eur J Cell Biol. 2002;81:323–34. doi: 10.1078/0171-9335-00257. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima Y, Akaogi K, Suzuki T, Osakabe A, Yamaguchi C, Sunahara N, Ishida J, Kako K, Ogawa S, Fujimura T, Homma Y, Fukamizu A, et al. Estrogen regulates tumor growth through a nonclassical pathway that includes the transcription factors ERbeta and KLF5. Sci Signal. 2011;4:ra22. doi: 10.1126/scisignal.2001551. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong XY, Bao Y, Zhou Z, Cheng X, Simons JW, Dong JT. KLF5 promotes cell proliferation and tumorigenesis through gene regulation in the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006;118:1346–55. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 10.Zheng HQ, Zhou Z, Huang J, Chaudhury L, Dong JT, Chen C. Kruppel-like factor 5 promotes breast cell proliferation partially through upregulating the transcription of fibroblast growth factor binding protein 1. Oncogene. 2009;28:3702–13. doi: 10.1038/onc.2009.235. [DOI] [PubMed] [Google Scholar]
- 11.McConnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW. Haploinsufficiency of Kruppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. 2009;69:4125–33. doi: 10.1158/0008-5472.CAN-08-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandan MO, Ghaleb AM, McConnell BB, Patel NV, Robine S, Yang VW. Kruppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations. Mol Cancer. 2010;9:63. doi: 10.1186/1476-4598-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo P, Zhao KW, Dong XY, Sun X, Dong JT. Acetylation of KLF5 alters the assembly of p15 transcription factors in transforming growth factor-beta-mediated induction in epithelial cells. J Biol Chem. 2009;284:18184–93. doi: 10.1074/jbc.M109.007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo P, Dong XY, Zhao KW, Sun X, Li Q, Dong JT. Opposing effects of KLF5 on the transcription of MYC in epithelial proliferation in the context of transforming growth factor beta. J Biol Chem. 2009;284:28243–52. doi: 10.1074/jbc.M109.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo P, Dong XY, Zhang X, Zhao KW, Sun X, Li Q, Dong JT. Pro-proliferative factor KLF5 becomes anti-proliferative in epithelial homeostasis upon signaling-mediated modification. J Biol Chem. 2009;284:6071–8. doi: 10.1074/jbc.M806270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu N, Kozlowski JM, Park, Chen L, Zhang Q, Xu D, Doll JA, Crawford SE, Brendler CB, Lee C. Overexpression of transforming growth factor beta1 in malignant prostate cells is partly caused by a runaway of TGF-beta1 auto-induction mediated through a defective recruitment of protein phosphatase 2A by TGF-beta type I receptor. Urology. 2010;76:1519, e8–e13. doi: 10.1016/j.urology.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein D, O’Leary M, Mitchen J, Borden EC, Wilding G. Effects of interferon beta ser and transforming growth factor beta on prostatic cell lines. J Urol. 1991;146:1173–7. doi: 10.1016/s0022-5347(17)38034-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Sun X, Ran Q, Wilkinson KD, Murphy TJ, Simons JW, Dong JT. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene. 2005;24:3319–27. doi: 10.1038/sj.onc.1208497. [DOI] [PubMed] [Google Scholar]
- 19.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991–5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekins S, Nikolsky Y, Bugrim A, Kirillov E, Nikolskaya T. Pathway mapping tools for analysis of high content data. Methods Mol Biol. 2007;356:319–50. doi: 10.1385/1-59745-217-3:319. [DOI] [PubMed] [Google Scholar]
- 25.Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab. 2005;16:73–8. doi: 10.1016/j.tem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing C, Fu X, Sun X, Guo P, Li M, Dong JT. Different expression patterns and functions of acetylated and unacetylated Klf5 in the proliferation and differentiation of prostatic epithelial cells. PLoS ONE. 2013;8:e65538. doi: 10.1371/journal.pone.0065538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saini S, Majid S, Shahryari V, Arora S, Yamamura S, Chang I, Zaman MS, Deng G, Tanaka Y, Dahiya R. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer Res. 2012;72:3618–30. doi: 10.1158/0008-5472.CAN-12-0540. [DOI] [PubMed] [Google Scholar]
- 30.Chanchevalap S, Nandan MO, McConnell BB, Charrier L, Merlin D, Katz JP, Yang VW. Kruppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006;34:1216–23. doi: 10.1093/nar/gkl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aizawa K, Suzuki T, Kada N, Ishihara A, Kawai-Kowase K, Matsumura T, Sasaki K, Munemasa Y, Manabe I, Kurabayashi M, Collins T, Nagai R. Regulation of platelet-derived growth factor-A chain by Kruppel-like factor 5: new pathway of cooperative activation with nuclear factor-kappaB. J Biol Chem. 2004;279:70–6. doi: 10.1074/jbc.M306621200. [DOI] [PubMed] [Google Scholar]
- 32.Zhu N, Gu L, Findley HW, Chen C, Dong JT, Yang L, Zhou M. KLF5 interacts with P53 in regulating survivin expression in acute lymphoblastic leukemia. J Biol Chem. 2006;281:14711–8. doi: 10.1074/jbc.M513810200. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, Yun CC. Regulation of hypoxia-iinducible factor 1alpha (HIF-1alpha) by lysophosphatidic acid is dependent on interplay between p53 and Kruppel-like factor 5. J Biol Chem. 2013 doi: 10.1074/jbc.M113.489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Nakagawa H, Tetreault MP, Billig J, Victor N, Goyal A, Sepulveda AR, Katz JP. Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Tarapore RS, Jarmel MH, Tetreault MP, Katz JP. p53 mutation alters the effect of the esophageal tumor suppressor KLF5 on keratinocyte proliferation. Cell Cycle. 2012;11:4033–9. doi: 10.4161/cc.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–60. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wen JK, Dong LH, Zheng B, Han M. Kruppel-like factor (KLF) 5 mediates cyclin D1 expression and cell proliferation via interaction with c-Jun in Ang II-induced VSMCs. Acta Pharmacol Sin. 2010;31:10–8. doi: 10.1038/aps.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He M, Han M, Zheng B, Shu YN, Wen JK. Angiotensin II stimulates KLF5 phosphorylation and its interaction with c-Jun leading to suppression of p21 expression in vascular smooth muscle cells. J Biochem. 2009;146:683–91. doi: 10.1093/jb/mvp115. [DOI] [PubMed] [Google Scholar]
- 39.Vogt PK. Jun, the oncoprotein. Oncogene. 2001;20:2365–77. doi: 10.1038/sj.onc.1204443. [DOI] [PubMed] [Google Scholar]
- 40.Guo P, Dong XY, Zhao KW, Sun X, Li Q, Dong JT. Estrogen-induced interaction between KLF5 and estrogen receptor (ER) suppresses the function of ER in ER-positive breast cancer cells. Int J Cancer. 2010;126:81–9. doi: 10.1002/ijc.24696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frigo DE, Sherk AB, Wittmann BM, Norris JD, Wang Q, Joseph JD, Toner AP, Brown M, McDonnell DP. Induction of Kruppel-like factor 5 expression by androgens results in increased CXCR4-dependent migration of prostate cancer cells in vitro. Mol Endocrinol. 2009;23:1385–96. doi: 10.1210/me.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C, Zhou Y, Zhou Z, Sun X, Otto KB, Uht RM, Dong JT. Regulation of KLF5 involves the Sp1 transcription factor in human epithelial cells. Gene. 2004;330:133–42. doi: 10.1016/j.gene.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, Zhang Z, Xia S, Xing C, Ci X, Li X, Zhao R, Tian S, Ma G, Zhu Z, Fu L, Dong JT. KLF5 activates microRNA 200 transcription to maintain epithelial characteristics and prevent induced epithelial-mesenchymal transition in epithelial cells. Mol Cell Biol. 2013;33:4919–35. doi: 10.1128/MCB.00787-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhurst RJ, Derynck R. TGF-beta signaling in cancer-a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 45.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 46.Park BJ, Park JI, Byun DS, Park JH, Chi SG. Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-Ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 2000;60:3031–8. [PubMed] [Google Scholar]
- 47.Lamm ML, Sintich SM, Lee C. A proliferative effect of transforming growth factor-beta1 on a human prostate cancer cell line, TSU-Pr1. Endocrinology. 1998;139:787–90. doi: 10.1210/endo.139.2.5907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.