Abstract
The protein product of the Multiple Endocrine Neoplasia Type I (MEN1) gene is thought to be involved in predominantly nuclear functions; however, immunohistochemistry (IHC) data on cellular localization are conflicting. To further investigate menin expression, we analyzed human pancreas (an MEN1 target organ) using IHC and six antibodies raised against full-length menin or its peptides. In 10 normal pancreas specimens, two independently raised antibodies showed unexpected cytoplasmic immunoreactivity in peripheral cells in each islet examined (over 100 total across all 10 patients). The staining exhibited a distinct punctate pattern and subsequent immunoelectron microscopy indicated the target antigen was in secretory granules. Exocrine pancreas and pancreatic stroma were not immunoreactive. In MEN1 patients, unaffected islets stained similar to those in normal samples but with a more peripheral location of positive cells, whereas hyperplastic islets and tumorlets showed increased and diffuse cytoplasmic staining, respectively. Endocrine tumors from MEN1 patients were negative for menin, consistent with a two-hit loss of a tumor suppressor gene. Secretory granule localization of menin in a subset of islet cells suggests a function of the protein unique to a target organ of familial endocrine neoplasia, although the IHC data must be interpreted with some caution due to the possibility of antibody cross reaction. The identity, cellular trafficking, and role of this putative secretory granule-form of menin warrant additional investigation.
Keywords: Endocrine tumor, menin localization, human tissue, immunohistochemistry
Introduction
Multiple endocrine neoplasia type I (MEN1) is an inherited, autosomal dominant syndrome in which patients develop parathyroid, pancreatic, gastroduodenal, and pituitary gland tumors (1, 2). Germline MEN1 gene mutations are highly penetrant and affect the majority of those with a DNA alteration, generally starting in the third decade. Clinical management includes gene mutation testing, symptomatic treatment, and surveillance (3, 4). Although MEN1 tumors are predominantly benign, a subset of duodeno-pancreatic neoplasms will metastasize and produce significant morbidity and mortality.
MEN1 was first described as a distinct syndrome by Wermer in the 1950s and later became the subject of study by research groups worldwide (5). In 1988, Larsson and colleagues in Sweden identified chromosome 11 as the location of the responsible gene, which was subsequently cloned and characterized in 1997 (6, 7). Studies since then show the gene is mutated in germline in approximately 80% of affected MEN1 kindreds, as well as in a significant fraction of counterpart neuroendocrine tumors with somatic mutation (8-19).
Biochemical and functional studies indicate menin resides primarily in the nucleus and interacts with transcription factors, although there are also published reports that implicate the protein in other cellular processes and the most central partners in its actions remain to be confirmed (20-32). The goal of the present study was to utilize IHC to investigate menin expression in normal and pathological human pancreas specimens.
Material and Methods
Antibodies
Anti-peptide antibodies were generated by injecting rabbits with peptides corresponding to amino acid sequence in human menin (Table). Full-length menin (FLM) antibody was generated in rabbits using recombinant human menin produced in E. coli. All antibodies were affinity purified on columns with a peptide or recombinant FLM, respectively.
Table.
Antibodies used in the study, location within menin, and IHC staining results.
| Antibody | Menin Sequence for Immunization | Pancreatic Islet Staining | MEN1 Tumor Staining |
|---|---|---|---|
| Full-Length Menin (FLM) | Whole Protein | Peripheral Islet Cells; Cytoplasmic | None |
| 498 | 584-610 | Peripheral Islet Cells; Cytoplasmic | None |
| SQV | 583-610 | None | None |
| GEE | 386-410 | None | None |
| GPN | 187-211 | None | None |
| 620 | 596-610 | None | None |
Tissue Specimens and Histopathology
Human pancreatic tissue samples after informed consent and from surgical resections, or from autopsy, were from IRB-approved studies in the intramural program of the National Institute of Diabetes, Digestive, and Kidney Disease at NIH. Ten cases of histologically normal pancreatic tissue (containing over 100 total islets for evaluation) were studied. Also, six pancreas samples and four non-pancreatic endocrine tumors (three parathyroid adenomas and one pulmonary carcinoid) from patients with MEN1 were examined, including five tumors from kindreds with known germline MEN1 mutations and allelic deletion encompassing the MEN1 locus on chromosome 11q13 (12, 13, 33). Tumor tissue and its MEN1 gene mutation were: 357del4, parathyroid tumor; 357del4, parathyroid tumor; K119del, pancreatic endocrine tumor (glucagonoma); 512delC, parathyroid tumor; and 512delC, pulmonary carcinoid.
The histological and IHC sections were evaluated by a consensus of two pathologists (LVD and MRE-B) who used a definition of hyperplastic islet as an enlarged (250 μ and more) islet of normal or somewhat irregular shape. Tumorlets/microadenomas differed from hyperplastic islets based on their monotonous cellular content, trabecular/serpentine architecture, increased amount of collagen stroma, and were <0.5 cm in size. Lesions >0.5 cm were classified as islet cell tumors (34).
Immunohistochemistry
IHC staining for menin in formalin-fixed, paraffin-embedded (FFPE) sections was performed with six antibodies using an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) according to the company's protocols, with slight modifications. Briefly, 5 μm thick histological sections were mounted on Fisherbrand/Plus Superfrost Precleaned slides (Fisher Scientific, Pittsburgh, PA) and attached by overnight heating at 58°C. After deparaffinization and rehydration the slides were placed in a microwave pressure cooker in 0.01 mol/L citrate buffer (pH 6.0) containing 0.1% Tween 20 and heated in a microwave oven at maximum power (800 W) for 20 minutes and then cooled in Tris-buffered saline. Thereafter, all sections were incubated in Tris-buffered saline (pH 7.6) containing 5% normal goat serum (Cell Signaling Technology) for 40 minutes. The primary antibodies (1:2000 dilution for 498 and 1:300 dilution for FLM) were incubated overnight at room temperature. The remainder of the procedure (secondary antibody, avidin-biotin complex, color development, and counterstain) was performed on a Ventana immunostainer. The sections were lightly counterstained in Mayer's hematoxylin and then mounted. Negative controls were established by replacing the primary antibody with buffer or with rabbit polyclonal IgG (Abcam, Cambridge, MA). Peptide competition experiments were performed for the FLM and 498 antibodies using the same protocol except the primary antibodies were pre-incubated with either FLM, 498 peptide, a menin C-terminal peptide (NHGRI 498), or a non-C-terminal peptide (NHGRI KM28) at a 50X molar concentration.
Immunoblots
Protein lysates were obtained from cell lines or a homogenized frozen pancreas using radioimmunoprecipitation assay buffer (Cell Signaling, Danvers, MA) containing a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO). Concentrations were measured using a protein assay dye reagent (BioRad, Hercules, CA). Following denaturation with β-mercaptoethanol, the lysates were run on a 10% BIS-TRIS polyacrylamide gel (Life Technologies, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes (Amersham Parmacia Biotech, Piscataway, NJ). The membranes were blocked with 1X casein solution (Vector Laboratories, Burlingame, CA) and incubated with FLM (diluted 1:300) or 498 (diluted 1:2000) at 4°C overnight. After brief washing and subsequent incubation with the secondary anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Dallas, TX), the blots were developed using an enhanced chemiluminescence reaction (Amersham Pharmacia, Piscataway, NJ).
Cell Block Studies
HEK 293 cells were transfected with menin as previously described (35). Briefly, the cells (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (Life Technologies, Carlsbad, CA) containing high glucose and glutamine supplemented with 10% fetal bovine serum (Gemini Bio Products, West Sacramento, CA), and a 1X concentration of antibiotic-antimycotic (Life Technologies, Carlsbad, CA), in a humidified incubator at 5% CO2 at 37°C. Transient transfection with pCMV-Sport-menin was carried out with Superfect (Qiagen, Germantown, MD). Both transfected and non-transfected cells were pelleted and processed into an FFPE cellblock.
Immunoelectron Microscopy
Tissue pieces were removed from a paraffin block, deparaffinized in xylenes, placed in absolute ethanol, and embedded in LR White (SPI, West Chester, PA). Ultrathin sections were mounted on 150-mesh uncoated nickel grids. Grids were floated on blocking solution (PBS, 0.1% Tween 20, 0.5% cold-water fish gelatin [Ted Pella, Inc., Redding, CA]) for 20 min, incubated for one hour with a 1:100 dilution of the FLM antibody, rinsed in blocking buffer for 5 min, blocked with 2% goat serum, rinsed with blocking buffer, then incubated with 10 nm gold-conjugated secondary goat antibody (Ted Pella, Inc., Redding, CA), rinsed in PBS, and air dried. Sections were stained with aqueous uranyl acetate and examined with a Phillips CM10 electron microscope.
Results
Six separate anti-menin antibodies were produced in rabbits and selected for study based on recognition of the protein on an immunoblot (Table). One antibody was raised against full-length menin (FLM) and five were against peptides, including N-terminal (GPN), mid-protein (GEE), and C-terminal regions (SQV, 498, 620).
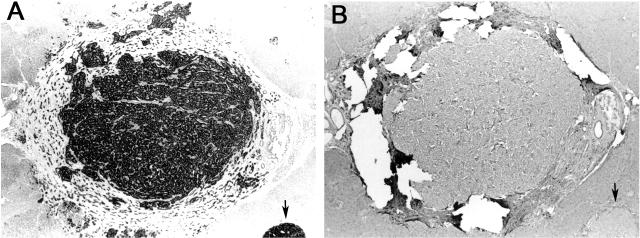
Overall, four of the antibodies (GPN, GEE, SQV, 620) did not generate consistent IHC results in islet or exocrine cells in archival clinical pancreas specimens and were not pursued further. In contrast, the FLM and 498 antibodies showed unexpected, punctate cytoplasmic staining that was uniquely observed in a subset of cells in all islets (over 100 total examined) in 10 cases of normal pancreas. The staining was present with both antibodies, but the quality and reproducibility varied. The FLM antibody provided clear, reproducible, and definitive results that were minimally affected by experimental conditions (Figure 1, Panels A, B), with mostly peripheral localization of immunoreactive cells in islets and strong positivity. Immunoelectron microscopy performed with the FLM antibody showed the staining was localized to secretory granules (Figure 1, Panel C). Adjacent islet cells, exocrine pancreas, and nuclei were all negative by EM, consistent with the IHC findings.
Figure 1. Menin IHC in normal human pancreas.
Panels A, B - FLM antibody results. Light microscopy demonstrates an islet with approximately 50% immunoreactive cells seen in perivascular and/or peripheral locations. The immunostaining is cytoplasmic and granular (Original magnification ×400). Panel C - Immunoelectron microscopy with FLM antibody reveals 10 nm gold particles (arrowheads) in and near secretory granules (G). No gold particles are seen in the nucleus (N) (Original magnification ×12,000). Panel D-F - 498 antibody demonstrates islet immunostaining results generally similar to FLM (D, E), but with less intense staining and in a smaller subset of islet cells. Other 498 antibody preparations produced more equivocal staining (F) (Original magnification ×400). Panel G - Immunoblot of a normal human pancreas lysate using the FLM antibody shows a single band at the predicted size of menin.
Cytoplasmic islet cell staining was also observed using the 498 antibody; however, in contrast to FLM, the 498 staining was somewhat equivocal. The quality and reproducibility of the signal varied (sometimes showing cytoplasmic staining in islet cells and other times weak or non-interpretable staining with a diffuse brown background) depending on the experimental conditions and the particular 498 antibody-rabbit preparation (Figure 1, Panels D-F).
Control experiments for the FLM and 498 antibodies included competitive inhibition experiments using a 50X molar ratio of full-length menin or one of three menin peptides. Blocking of the FLM and 498 antibodies with whole menin and 498-peptide, respectively, eliminated islet cell staining, thus confirming the IHC results were due to a specific antibody-antigen reaction (results not shown). Islet staining with both antibodies was also blocked by an independent menin C-terminal peptide, but was not affected by a non-C-terminal peptide at the same concentration, indicating that both the 498 antibody (as expected) and the FLM antibody recognize the C-terminus of the protein. Immunoblotting of a human pancreatic lysate with the FLM antibody showed a single band at approximately 63 kDa (Figure 1, panel G), supporting the notion that the observed IHC staining was due to menin.
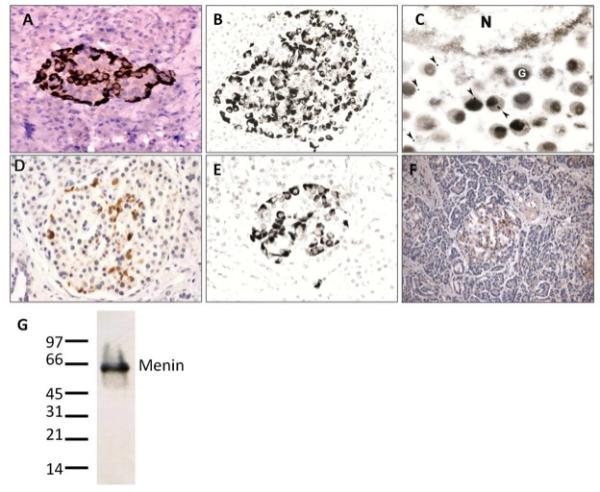
Examination of pancreatic specimens from MEN1 patients showed a generally similar immunostaining pattern of unaffected islets as in normal control tissue, except for a more peripheral location of positive cells in some otherwise normal appearing islets (Figure 2, A, B). A spectrum of additional pathologic MEN1-related changes was also present and further highlighted by menin IHC staining, including: An increase in menin-positive islet precursor cells budding off pancreatic ducts; hyperplastic islets with increased numbers of meninimmunoreactive cells; and, endocrine tumorlets/microadenomas with diffuse cytoplasmic menin staining that suggests an alteration in menin amount or sub-cellular location in these lesions (Figure 2, C, D). An MEN1 islet cell tumor (glucagonoma; Figure 3), three MEN1 parathyroid tumors, and one MEN1 pulmonary carcinoid with known biallelic MEN1 gene inactivation (see Materials and Methods) were negative for menin staining with both the 498 and FLM antibodies, consistent with a two-hit loss of a tumor suppressor protein, as the majority of MEN1 tumors delete the wild-type allele (Table) (33).
Figure 2. FLM staining in normal and MEN1 specimens.
Panel A - Section of normal pancreas showing evenly distributed and regularly sized islets with approximately 50% immunoreactive cells using the FLM antibody (Original magnification ×40). Panel B - Section of pancreatic tissue surrounding a neuroendocrine tumor in a resection from an MEN1 patient. IHC staining demonstrates FLM-immunoreactive cells located at the periphery of islets that appear unremarkable when examined by light microscopy (Original magnification ×40). Panel C - Section from a pancreatectomy specimen of an MEN1 patient demonstrates somewhat enlarged islets with peripheral FLM-immunoreactive cells, and a hyperplastic islet in the center with FLM-immunoreactive cells (Original magnification ×40). Panel D - Section of pancreatic tissue from an MEN1 patient demonstrates increased numbers of periductal endocrine cells and a small islet budding from the duct (arrow), enlarged hypertrophic islets, and a small tumorlet that are all immunoreactive with FLM (Original magnification ×100).
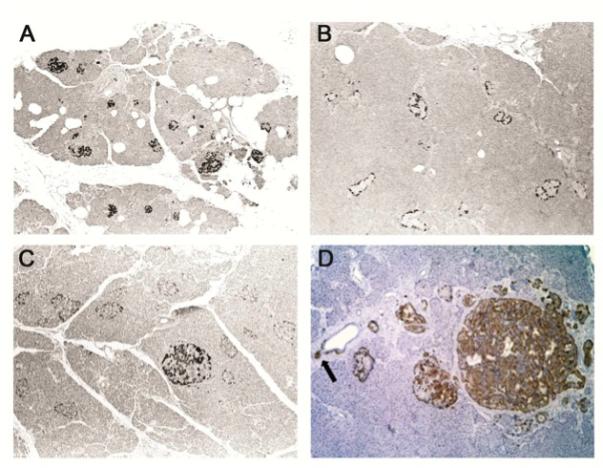
Figure 3. Lack of FLM staining of an MEN-1 glucagonoma with germline mutation K119del known tumor LOH.
Panel A - Centrally located tumor surrounded by a thick fibrous capsule and a smaller tumor at the low right corner (arrow) are strongly positive for glucagon (original magnification ×40). Panel B - Consecutive histological section stained with FLM demonstrates lack of immunoreactivity in both tumors. Note - tears in the section resulted from rupture of the thick fibrous tumor capsule during the antigen retrieval procedure (FLM at 1:300, original magnification ×40).
As a control experiment to assess the staining sensitivity of the FLM antibody, HEK 293 cells with and without menin transfection were processed into a cellblock via a standard FFPE method and analyzed by IHC. Non-transfected cells were negative for menin (Figure 4, A) even though the cells showed the presence of the protein on an immunoblot (35). In contrast, greater than 50% of transfected cells showed strong nuclear staining, suggesting the protein is only detectable by IHC when present above a certain threshold (Figure 4, B).
Figure 4. FLM staining results in HEK 293 cells.
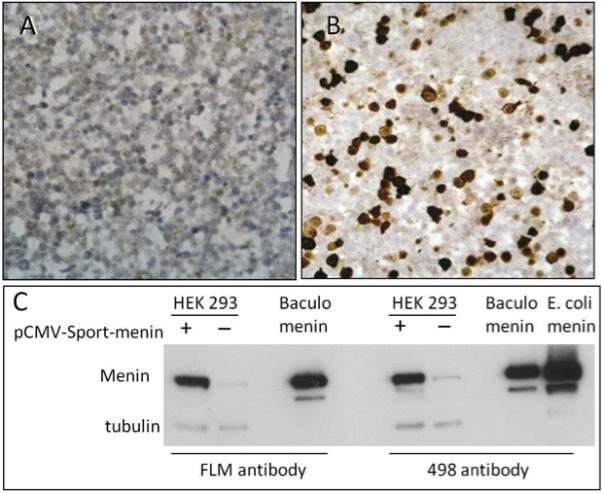
Panels A, B – Histological sections from an HEK 293 FFPE cellblock stained with FLM antibody show no immunoreactivity in untransfected cells (A) and strong nuclear staining in cells transfected with full length MEN1 mRNA (B) (Original magnification x400). Corresponding immunoblot results with the FLM and 498 antibodies and controls are shown in panel C.
Discussion
Previous biochemical studies suggest menin resides in the cell nucleus and implicates transcription factor and other nuclear binding partners in the protein's function (20, 35-37). In situ analysis performed by several groups also demonstrate nuclear localization of the protein, although the results depend on the particular antibody and/or technique employed (immunohistochemistry versus immunofluorescence) and the biological source of the sample (mouse versus human) (35, 38-40). Alternatively, some published IHC reports describe cytoplasmic menin staining or a shuttling of the protein amongst cellular compartments (41-43).
The present study revealed strong granular cytoplasmic menin immunoreactivity in a subset of cells in normal human islets. Nuclear menin staining was not observed in any endocrine, exocrine or stromal cells, nor in non-transfected HEK cells, suggesting that IHC staining using our antibodies was not sensitive enough to measure endogenous nuclear menin in FFPE-processed cultured cells or clinical samples. In contrast, the cytoplasmic staining observed in patient specimens by IHC appears due to menin expressed at a high level in secretory granules, similar to insulin, glucagon, and other hormones that are abundant in islets.
IHC is an important technology for semi-quantitative measurement of a protein across a histological section; however, data must be interpreted conservatively as hybridization of an antibody to a surface-bound tissue matrix is a complex interaction. Thus, we approached the present IHC results with some caution, particularly since cytoplasmic menin expression in pancreatic endocrine cells was unexpected. To fully interrogate the IHC results, we performed all appropriate control experiments, including competitive inhibition, and these data were consistent with menin as the source of the immunoreactivity. Yet it's important to note that these controls do not rule out the possibility of cross-reaction with a protein other than menin, even though several observations argue against this possibility. For example, although the cellular and subcellular menin staining was similar to that of glucagon, strongly positive glucagon endocrine tumors were completely negative for menin (Figure 3). Moreover, selective staining of only a subset of islet cells, as well as lack of tumor cell staining (tumors retain structurally normal granules), indicate it's unlikely our antibodies react with constitutive endocrine granule proteins since all islet cells and tumors should be positive if this were true. Cross-reaction with one of the other pancreatic hormones (somatostatin, pancreatic polypeptide, ghrelin) is a theoretical possibility, but immature endocrine cells (budding off ducts) that do not produce these hormones were positive for menin. Finally, an immunoblot of frozen pancreatic tissue showed a single band corresponding to the known molecular weight of menin.
None of the anti-menin peptide antibodies, except 498 to some extent, produced strong, reliable, and non-equivocal results in FFPE samples. It is worth noting, at least in our experience, that antibodies produced against full-length proteins generally produce superior IHC staining compared to anti-peptide antibodies, particularly for low abundant proteins measured in archival specimens. Thus our generally poor results with the anti-peptide menin antibodies, including the batch-to-batch variation seen with 498, were not entirely unexpected. In contrast, we are confident the IHC staining pattern from the FLM antibody is accurate and reproducible since the experiments were performed numerous times, on multiple different normal and MEN1-derived tissue specimens, by several investigators, under various technical conditions, and all gave the identical result.
Islet cell hyperplasia, dysplasia, and ductulo-insular complexes in MEN1 have been previously described (44). Our study identified a spectrum of pancreatic endocrine histopathology in MEN1 specimens and included increased numbers of menin-positive cells budding off ducts; menin-positive pre-neoplastic lesions (hyperplastic islets and tumorlets/microadenomas); and, menin-negative endocrine tumors. Overall these changes can be explained by a continuum model of tumor suppression (45) in which reduction in tumor suppressor gene dosage (haploinsufficiency) confers a new phenotype, and “one-hit” premalignant changes are a part of tumor progression. A recent report on changes in global gene expression observed in endocrine pancreas of young mice heterozygous for Men1 supports the notion that the gene acts as a haploinsufficient suppressor (46).
Similar to many familial cancer syndromes, affected members of MEN1 kindreds harbor a gene mutation in each cell in their body yet only develop tumors in certain organs (1, 2). A simple mechanistic explanation is that normal physiological expression of menin is limited to precursor neoplastic cells, thus functional inactivation affects growth and produces tumors only in target endocrine glands. However, this is not what is observed experimentally as the MEN1 gene is ubiquitously expressed in cells and tissues at both the transcript and protein level. The etiology of inherited, organ-specific endocrine tumors appears complex, likely with at least two cellular roles for menin, one that is involved in all cells and a second that is unique to those that form neoplasms. Recent data on the three-dimensional structure of menin demonstrates that along with sites interacting with MLL, JunD and other nuclear proteins, it contains a helical palm domain with three TPR (tetratrico peptide repeat) motifs predicting wide versatility of binding partners and corresponding cellular processes (47). To date, there is no mechanistic explanation for the pattern of organ involvement observed in MEN1 patients, and endocrine cell-specific functions of menin have not been identified, thus the present IHC data point to potential new, non-nuclear functions involved in tumorigenesis.
The secretory granule localization of menin observed by immunoelectron microscopy suggests the protein might function outside of the cell, or regulates hormones that do so. At first glance, a secretory/extracellular role for menin seems unusual and differs significantly from most known tumor suppressor genes. However, endocrine hormones have multiple physiological effects on target cells, including growth, so a hormone-related tumor suppressor activity for menin is not necessarily implausible, although the mechanism whereby archetypal, two-hit inactivation of the MEN1 gene and corresponding loss of menin in secretory granules, in a single islet cell, results in endocrine tumorigenesis may not be simple or straightforward. One can hypothesize that autocrine or paracrine growth inhibition is the normal physiological role of secreted menin, thus either haploinsufficiency or complete loss of function negates a normal feedback loop and produces increased cell division in the affected or neighboring cell(s). Or alternatively, menin may regulate other secreted growth-influencing hormones in granules and its loss disrupts normal homeostasis in the islet more generally. As an example of this possibility, preliminary results suggest that menin interacts with prohormone convertase (PC1), a known regulator of insulin and other hormones that is also located in endocrine cell secretory granules (unpublished data). Further evaluation of the putative cytoplasmic form of menin and its role in endocrine cell tumorigenesis is warranted to address these and other questions.
Acknowledgements
Funding Support
This research was supported (in part) by the Intramural Research Program of the NIH (National Cancer Institute, Center for Cancer Research; National Institute for Diabetes, Digestive and Kidney Disease), Bethesda, MD. We thank Dr. Alison Hickman, NIDDK, NIH for providing purified menin, Dr. Leticia Quintanilla-Fend for consultation on immunohistochemistry protocols, and Dr. Siradanahalli Guru for help with cell transfections and antibody purifications.
Footnotes
Conflict-of-Interest
The authors report no conflicts of interest.
References
- 1.Marx SJ, Agarwal SK, Kester MB, et al. Multiple endocrine neoplasia type 1: clinical and genetic features of the hereditary endocrine neoplasias. Recent Prog Horm Res. 1999;54:397–438. [PubMed] [Google Scholar]
- 2.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1). Best practice & research. Clinical endocrinology & metabolism. 2010;24:355–70. doi: 10.1016/j.beem.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Perrier ND, Villablanca A, Larsson C, et al. Genetic screening for MEN1 mutations in families presenting with familial primary hyperparathyroidism. World journal of surgery. 2002;26:907–13. doi: 10.1007/s00268-002-6617-9. [DOI] [PubMed] [Google Scholar]
- 4.Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). The Journal of clinical endocrinology and metabolism. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 5.Wermer P. Genetic aspects of adenomatosis of endocrine glands. The American journal of medicine. 1954;16:363–71. doi: 10.1016/0002-9343(54)90353-8. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 7.Larsson C, Skogseid B, Oberg K, et al. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–7. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- 8.Debelenko LV, Brambilla E, Agarwal SK, et al. Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Human molecular genetics. 1997;6:2285–90. doi: 10.1093/hmg/6.13.2285. [DOI] [PubMed] [Google Scholar]
- 9.Debelenko LV, Emmert-Buck MR, Zhuang Z, et al. The multiple endocrine neoplasia type I gene locus is involved in the pathogenesis of type II gastric carcinoids. Gastroenterology. 1997;113:773–81. doi: 10.1016/s0016-5085(97)70171-9. [DOI] [PubMed] [Google Scholar]
- 10.Heppner C, Kester MB, Agarwal SK, et al. Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet. 1997;16:375–8. doi: 10.1038/ng0897-375. [DOI] [PubMed] [Google Scholar]
- 11.Marx SJ, Agarwal SK, Kester MB, et al. Germline and somatic mutation of the gene for multiple endocrine neoplasia type 1 (MEN1). Journal of internal medicine. 1998;243:447–53. doi: 10.1046/j.1365-2796.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Z, Ezzat SZ, Vortmeyer AO, et al. Mutations of the MEN1 tumor suppressor gene in pituitary tumors. Cancer research. 1997;57:5446–51. [PubMed] [Google Scholar]
- 13.Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Human molecular genetics. 1997;6:1169–75. doi: 10.1093/hmg/6.7.1169. [DOI] [PubMed] [Google Scholar]
- 14.Villablanca A, Wassif WS, Smith T, et al. Involvement of the MEN1 gene locus in familial isolated hyperparathyroidism. European journal of endocrinology / European Federation of Endocrine Societies. 2002;147:313–22. doi: 10.1530/eje.0.1470313. [DOI] [PubMed] [Google Scholar]
- 15.Bergman L, Teh B, Cardinal J, et al. Identification of MEN1 gene mutations in families with MEN 1 and related disorders. British journal of cancer. 2000;83:1009–14. doi: 10.1054/bjoc.2000.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoo J, Bee YM, Giraud S, et al. Novel association of thymic carcinoid with a germline mutation in a kindred with multiple endocrine neoplasia 1 (MEN1). Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2012;120:257–60. doi: 10.1055/s-0032-1309012. [DOI] [PubMed] [Google Scholar]
- 17.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Human mutation. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 18.Hannan FM, Nesbit MA, Christie PT, et al. Familial isolated primary hyperparathyroidism caused by mutations of the MEN1 gene. Nature clinical practice Endocrinology & metabolism. 2008;4:53–8. doi: 10.1038/ncpendmet0718. [DOI] [PubMed] [Google Scholar]
- 19.Tham E, Grandell U, Lindgren E, et al. Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. The Journal of clinical endocrinology and metabolism. 2007;92:3389–95. doi: 10.1210/jc.2007-0476. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SK, Guru SC, Heppner C, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–52. doi: 10.1016/s0092-8674(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 21.Balogh K, Patocs A, Hunyady L, et al. Menin dynamics and functional insight: take your partners. Molecular and cellular endocrinology. 2010;326:80–4. doi: 10.1016/j.mce.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Liu R, Jiang X, et al. Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin. Molecular and cellular biology. 2009;29:5477–87. doi: 10.1128/MCB.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Ozawa A, Zaman S, et al. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer research. 2011;71:371–82. doi: 10.1158/0008-5472.CAN-10-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuescher L, Angevine K, Hinds T, et al. Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. American journal of physiology Endocrinology and metabolism. 2011;301:E474–83. doi: 10.1152/ajpendo.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensah-Osman E, Zavros Y, Merchant JL. Somatostatin stimulates menin gene expression by inhibiting protein kinase A. American journal of physiology. Gastrointestinal and liver physiology. 2008;295:G843–54. doi: 10.1152/ajpgi.00607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Egido J, Cunningham J, Berg M, et al. Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity. Experimental cell research. 2002;278:175–83. doi: 10.1006/excr.2002.5575. [DOI] [PubMed] [Google Scholar]
- 27.Nakata Y, Brignier AC, Jin S, et al. c-Myb, Menin, GATA-3, and MLL form a dynamic transcription complex that plays a pivotal role in human T helper type 2 cell development. Blood. 2010;116:1280–90. doi: 10.1182/blood-2009-05-223255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng ZJ, Gao SB, Wu Y, et al. Lung cancer cell migration is regulated via repressing growth factor PTN/RPTP beta/zeta signaling by menin. Oncogene. 2010;29:5416–26. doi: 10.1038/onc.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T, Zhang X, Huang X, et al. Regulation of cyclin B2 expression and cell cycle G2/m transition by menin. The Journal of biological chemistry. 2010;285:18291–300. doi: 10.1074/jbc.M110.106575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walls GV, Reed AA, Jeyabalan J, et al. Proliferation rates of multiple endocrine neoplasia type 1 (MEN1)-associated tumors. Endocrinology. 2012;153:5167–79. doi: 10.1210/en.2012-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls GV, Lemos MC, Javid M, et al. MEN1 gene replacement therapy reduces proliferation rates in a mouse model of pituitary adenomas. Cancer research. 2012;72:5060–8. doi: 10.1158/0008-5472.CAN-12-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stalberg P, Santesson M, Ekeblad S, et al. Recognizing genes differentially regulated in vitro by the multiple endocrine neoplasia type 1 (MEN1) gene, using RNA interference and oligonucleotide microarrays. Surgery. 2006;140:921–9. doi: 10.1016/j.surg.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Emmert-Buck MR, Lubensky IA, Dong Q, et al. Localization of the multiple endocrine neoplasia type I (MEN1) gene based on tumor loss of heterozygosity analysis. Cancer research. 1997;57:1855–8. [PubMed] [Google Scholar]
- 34.Perren A, Anlauf M, Henopp T, Rudolph T, Schmitt A, Raffel A, et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. The Journal of clinical endocrinology and metabolism. 2007;92:1118–28. doi: 10.1210/jc.2006-1944. [DOI] [PubMed] [Google Scholar]
- 35.Guru SC, Goldsmith PK, Burns AL, et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1630–4. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heppner C, Bilimoria KY, Agarwal SK, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene. 2001;20:4917–25. doi: 10.1038/sj.onc.1204529. [DOI] [PubMed] [Google Scholar]
- 37.Kaji H, Canaff L, Lebrun JJ, et al. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3837–42. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbo V, Dalai I, Scardoni M, et al. MEN1 in pancreatic endocrine tumors: analysis of gene and protein status in 169 sporadic neoplasms reveals alterations in the vast majority of cases. Endocrine-related cancer. 2010;17:771–83. doi: 10.1677/ERC-10-0028. [DOI] [PubMed] [Google Scholar]
- 39.Jin S, Zhao H, Yi Y, et al. c-Myb binds MLL through menin in human leukemia cells and is an important driver of MLL-associated leukemogenesis. The Journal of clinical investigation. 2010;120:593–606. doi: 10.1172/JCI38030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavallari I, D'Agostino DM, Ferro T, et al. In situ analysis of human menin in normal and neoplastic pancreatic tissues: evidence for differential expression in exocrine and endocrine cells. The Journal of clinical endocrinology and metabolism. 2003;88:3893–901. doi: 10.1210/jc.2002-021840. [DOI] [PubMed] [Google Scholar]
- 41.Ren F, Xu HW, Hu Y, et al. Expression and subcellular localization of menin in human cancer cells. Experimental and therapeutic medicine. 2012;3:1087–91. doi: 10.3892/etm.2012.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veschi S, Lattanzio R, Aceto GM, et al. Alterations of MEN1 and E-cadherin/beta-catenin complex in sporadic pulmonary carcinoids. International journal of oncology. 2012;41:1221–8. doi: 10.3892/ijo.2012.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang SC, Zhuang Z, Weil RJ, et al. Nuclear/cytoplasmic localization of the multiple endocrine neoplasia type 1 gene product, menin. Laboratory investigation; a journal of technical methods and pathology. 1999;79:301–10. [PubMed] [Google Scholar]
- 44.Mete O, Asa SL. Precursor lesions of endocrine system neoplasms. Pathology. 2013;45:316–30. doi: 10.1097/PAT.0b013e32835f45c5. [DOI] [PubMed] [Google Scholar]
- 45.Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 2011;476:163–9. doi: 10.1038/nature10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lejonklou MH, Barbu A, Stalberg P, et al. Accelerated proliferation and differential global gene expression in pancreatic islets of five-week-old heterozygous Men1 mice: Men1 is a haploinsufficient suppressor. Endocrinology. 2012;153:2588–98. doi: 10.1210/en.2011-1924. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, Gurung B, Wan B, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–6. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]