Abstract
N-(Adamantan-1-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide (STS-135) is a new synthetic cannabinoid in herbal incense products discussed on internet drug user forums and identified in police seizures. To date, there are no STS-135 clinical or in vitro studies identifying STS-135 metabolic profiles. However, characterizing STS-135 metabolism is critical because synthetic cannabinoid metabolites can possess pharmacological activity and parent compounds are rarely detectable in urine.
To characterize the metabolite profile, human hepatocytes were incubated with 10 μmol/L STS-135 for up to 3 h. High-resolution mass spectrometry with software-assisted data mining identified 29 STS-135 metabolites. Less than 25% of STS-135 parent compound remained after 3 h incubation. Primary metabolites were generated by mono-, di- or trihydroxylation with and without ketone formation, dealkylation and oxidative defluorination of N-fluoropentyl side chain or possible oxidation to carboxylic acid, some of them further glucuronidated. Hydroxylations occurred mainly on the aliphatic adamantane ring and less commonly on the N-pentyl side chain. At 1 h phase I metabolites predominated, while at 3 h phase II metabolites were present in higher amounts. The major metabolites were monohydroxy STS-135 (M25) and dihydroxy STS-135 (M21), both hydroxylated on the adamantane system. Moreover, metabolic stability of STS-135 (1 μmol/L) was assessed in human liver microsomes experiments. The in vitro half-life of STS-135 was 7.2±0.6 min and intrinsic clearance (CLint) was 93.6 mL·min−1·kg−1.
This is the first report characterizing STS-135 hepatic metabolic pathways. These data provide potential urinary targets to document STS-135 intake in clinical and forensic settings and potential candidates for pharmacological testing.
Keywords: STS-135, high resolution mass spectrometry, human hepatocytes, metabolism, synthetic cannabinoids
INTRODUCTION
Synthetic cannabinoids are cannabinoid CB1 and/or CB2 receptor agonists whose sale and consumption have grown greatly in the last few years [1-2]. Although synthetic cannabinoids were originally synthesized as pharmacological tools for investigating the endocannabinoid system, clandestine drug manufacturers exploited their cannabimimetic properties for recreational abuse [3]. They are usually sold as herbal mixtures labeled “not for human consumption,” on the internet or in local head shops [4] and initially offered a legal alternative to cannabis. In 2010, the United States emergency-scheduled the early generation synthetic cannabinoids JWH-018, JWH-073, HU-210, CP-47,497 and CP-47,497-C8[5]. Additional new synthetic cannabinoids were scheduled upon enactment of the Synthetic Drug Abuse Prevention Act in July 2012 and May 2013 [6-7]. A major reason synthetic cannabinoids are consumed is that they are not detected in routine cannabinoid immunoassays, therefore, avoiding detection in private sector, military and criminal justice drug testing programs [8].
Many synthetic cannabinoids share common structural features (Figure 1), such as the central indole ring that can be attached to a naphthyl, phenyl, tetramethylcyclopropyl, adamantyl or quinoline ring by either a carbonyl, carboxamide or ester linkage. As manufacturers try to circumvent scheduling efforts, new structural elements are constantly introduced, e.g. a halogenated terminal carbon on the alkyl side chain led to increased receptor binding in the brain, as occurred with AM-2201 [9], a fluorinated analog of JWH-018, and XLR-11 [10], a fluorinated analog of UR-144. STS-135 (N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide) is the 5-fluoro analog of APICA and also structurally similar to AKB-48 that was recently scheduled by the US Drug Enforcement Administration (DEA). A recent study identified STS-135 in herbal smoking blends in Germany [11], but to date there are no published clinical data. The structurally similar AKB-48 binds with stronger affinity to CB2 than CB1 receptors (430 nM vs. 824 nM) [12], but there are no binding affinity data for STS-135 yet. Proof of synthetic cannabinoid intake can be challenging for analytical laboratories, mainly because new compounds constantly emerge on the market with unknown urinary metabolite targets. Many liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS) methods were published for detecting synthetic cannabinoids in human plasma, whole blood, oral fluid, hair and urine [13-19], with the latter remaining the most common screening matrix because it is easy to obtain non-invasively and allows relatively simple extraction procedures. However, parent compounds are seldom eliminated in urine [20], necessitating targeting of metabolites to document synthetic cannabinoid intake. Thus, metabolism studies elucidating metabolic profiles for newly emerging synthetic cannabinoids are required. In vitro studies are critical since clinical studies cannot be conducted yet due to the lack of available safety data to obtain an investigational new drug application by the Food and Drug Administration (FDA).
Figure 1.
Chemical structures of STS-135 and selected synthetic cannabinoids
Metabolism studies were performed for a variety of synthetic cannabinoids including JWH-018 [20-24], JWH-073 [20, 22, 24-25], JWH-081 [20], JWH-122 [20], JWH-200 [26], JWH-210 [20], JWH-250 [20, 27], AM694 [28], AM2201 [23, 29-30], RCS-4 [20, 31], RCS-8 [32], AB-001 [33], UR-144 [29, 34], AKB48 [35], XLR-11 [36], PB-22 and 5F-PB-22 [37]. These studies showed that oxidative phase I metabolism is common; carboxylation of the alkyl side chain (if contained in the parent structure) is also common and oxidative defluorination on the alkyl side chain of fluorinated compounds occurs. Oxidated and carboxylated metabolites are further conjugated via glucuronidation.
Little is understood about many emerging synthetic cannabinoids’ pharmacologic actions and potential toxicity, as preclinical and clinical drug administration studies have not yet been conducted. Case reports suggest that synthetic cannabinoids produce not only behavioral [4] but also renal [38-40] and cardiovascular toxicities in humans [41-43] but the mechanisms of action are not known. In vitro studies showed that primary metabolites of JWH-018, JWH-073 and AM2201 retained strong binding affinity and potency towards CB1 and CB2 receptors [23, 43-45]. Thus, complete characterization of metabolic profiles of new synthetic cannabinoids is imperative, not only for identifying targets of drug intake, but also to determine potentially active or toxic metabolites.
We incubated STS-135 with human hepatocytes for up to 3 h and identified metabolites with high resolution mass spectrometry (HRMS) and data mining software. We conducted our experiments with primary human hepatocytes because they contain physiological levels of phase I and II drug metabolizing enzymes, bile canalicular membrane, and uptake and efflux drug transporters providing a more clinically relevant metabolic profile than typically achieved with human liver microsomes (HLM). Usually, glucuronide metabolites predominate in human urine, further highlighting the importance of conducting hepatocyte incubations or special HLM procedures to identify phase II metabolites. As opposed to unit resolution mass spectrometry, HRMS with a quadrupole/time-of-flight mass (Q-TOF) spectrometer can identify drug metabolites in complex biological matrices with more streamlined workflows. Using predefined peak selection criteria for information dependent acquisition (IDA), high resolution MS and MS/MS spectra are acquired throughout the run.. Post acquisition data processing with software such as MetabolitePilot™ simplifies metabolite identification.
In vitro studies with HLM are other useful and well-established tools to predict in vivo pharmacokinetic behavior of a compound. Therefore, in addition to the hepatocyte study, we conducted experiments with human liver microsomes to determine the metabolic stability of STS-135. Metabolic stability is defined as the susceptibility of a drug to biotransformation and is expressed as in vitro half-life (T1/2) and intrinsic clearance (CLint). These enzyme kinetic parameters can be used to predict in vivo hepatic clearance, in vivo half-life and bioavailability with different models [46].
This is the first characterization of human STS-135 metabolism, identifying predominant metabolites to be targeted in biological matrices and providing metabolic stability data. These data enable development of analytical methods to identify STS-135 intake. In addition, these data will also guide future research on the pharmacology, toxicology and pharmacokinetic behavior of STS-135 and its metabolites.
MATERIALS AND METHODS
Chemicals and reagents
Cryopreserved human hepatocytes, human liver microsomes, thawing and incubation media and NADPH regenerating system solutions were purchased from BioreclamationIVT (Baltimore, MD). STS-135 was obtained from Cayman Chemicals (Ann Arbor, MI). LC-MS-grade acetonitrile and water were supplied by Sigma-Aldrich (St. Louis, MO).
Incubation of STS-135 with cryopreserved primary human hepatocytes
Cryopreserved primary human hepatocytes pooled from 10 donors were thawed in a water bath set at 37°C. One vial of hepatocytes was immersed into hepatocyte thawing medium to wash and remove any dead cells by centrifugation (50 g, 5 min at room temperature). Supernatant medium was aspirated without disturbing the hepatocyte pellet. The pellet was reconstituted in 2 mL of pre-warmed Krebs-Henseleit buffer and the tube gently shaken to homogenously re-suspend the pellet. Cell viability was assessed with Trypan blue (0.4% v/v) exclusion dye method assuring greater than 80% viability. STS-135 (10 μmol/L final concentration) dissolved in DMSO was incubated with primary human hepatocytes (6.25 × 105 cells/0.5mL/well) in a covered 24-well non-tissue coated cell culture plate (BD Biosciences, San Diego, CA). The plate was placed in a sterile incubator with constant horizontal shaking at 37°C. Diclofenac (CYP2C9 substrate, 10 μmol/L) was incubated as a positive control to ensure metabolic capability under our experimental conditions. Reactions were stopped with an equal volume of ice-cold acetonitrile (250 μL) and supernatant was removed after 0, 1 and 3 h. Samples were stored at −80°C for further analysis.
Sample preparation
Samples were spun at 15,000 g, 4°C for 5 min in a 5804r bench top centrifuge (Eppendorf, Hamburg, Germany) to remove cell debris or particulate matter. Eighty microliters of supernatant was diluted with 80 μL mobile phase (50:50 A:B) and 10 μL was injected on the LC-MS/MS system.
Metabolic stability of STS-135 in human liver microsomes
Duplicate 1 mg/mL human liver microsomal suspensions (50 donor pool) consisting of 50 mmol/L potassium phosphate buffer (pH 7.4) and NADPH regenerating system (glucose-6-phosphate, MgCl2 and glucose-6-phosphate dehydrogenase) in a final volume of 1 mL were incubated with 1 μmol/L STS-135 at 37°C for up to 1 h in a shaking water bath. One hundred microliters of sample were collected after 0, 3, 8, 13, 20, 30, 45 and 60 min and added to 100 μL of ice cold acetonitrile containing 6% glacial acetic acid. Samples were centrifuged at 15,000 g, 4°C for 5 min and supernatants were stored at −20°C before analysis.
Chromatographic instrumentation and analysis
Chromatographic separation was performed on a Shimadzu Prominence™ HPLC system consisting of two LC-20ADXR pumps, a DGU-20A5R degasser, a SIL-20ACXR auto sampler and a CTO-20AC column oven (Shimadzu Corp., Columbia, MD). Chromatographic separation was performed on a Kinetex™ C18 column (100 mm × 2.1 mm, 2.6 μm) fitted with a KrudKatcher Ultra HPLC in-line filter (0.5 μm × 0.1 mm ID) (Phenomenex, Torrance, CA). The HPLC mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B), respectively. Gradient elution was performed with 10% B for 0.3 min, increased to 20% B at 0.5 min, then ramped to 80% B at 20 min, increased to 95% B at 20.1 min and held until 21.9 min before reequilibrating the column to 10% B at 22 min for a total run time of 25 min. Column and autosampler temperatures were maintained at 40 and 4°C, respectively.
MS instrumentation and analysis
Mass spectrometric analysis was achieved on a 5600+ TripleTOF mass spectrometer (AB Sciex, Redwood City, CA) with data acquired via AB Sciex Analyst TF v.1.6. Positive electrospray ionization (ESI) mode was used to acquire MS and MS/MS data with information-dependent acquisition (IDA) methods and with dynamic background subtraction (DBS) feature turned on. The data were acquired with and without mass defect filter (MDF) to ensure that potential metabolites were not missed during data acquisition. IDA criteria were as follows: signals in the TOF mass spectra (TOF-MS) survey scan exceeding 500 cps were selected for the dependent scan, isotopes within 3 Da were excluded and mass tolerance was ±50 mDa. Mass defect filters are shown in Table I. TOF-MS were acquired scanning a mass range of m/z 100-950 followed by product ion scanning from m/z 60-950 with accumulation times of 0.1 and 0.075 s, respectively. Declustering potential and collision energy were optimized by infusing STS-135. Optimized declustering potential was 140 V; optimized collision energy was 40 eV with a collision energy spread of ±15 eV. An automated calibrant delivery system was used to maintain mass calibration throughout the batch.
Table I.
Multiple mass defect filtering: Mass defect filters that were used as selection criteria during the Information Dependent Acquisition (IDA) for triggering product ion spectra of phase I and II STS-135 metabolites. The width is a window of ±50 Da around the molecular weight (MW) of the proposed metabolite, mass defect is the non-integral portion of the mass-to-charge ratio m/z in mDa.
| Possible biotransformation | Formula | m/z | Width (Da) | Mass Defect (mDa): |
|---|---|---|---|---|
| STS-135 precursor ion | C24H31N2OF | 383.2493 | 100 | 249.3 |
| Glucuronidation | C30H39N2O7F | 559.2814 | 100 | 281.4 |
| Bis-Glucuronidation | C36H47N2O13F | 735.3135 | 100 | 313.5 |
| Glutathione | C34H48N5O7FS | 690.3331 | 100 | 333.1 |
| Sulfate | C24H31N2O4FS | 463.2062 | 100 | 206.2 |
| Loss of C5H9F | C19H22N2O | 295.1805 | 100 | 180.5 |
| Loss of C10H15N | C14H16NOF | 234.1289 | 100 | 128.9 |
| Loss of C14H14NOF | C10H17N | 152.1434 | 100 | 143.4 |
| Loss of C10H14 | C14H17N2OF | 249.1398 | 100 | 139.8 |
| Loss of C14H15N2OF | C10H16 | 137.1325 | 100 | 132.5 |
Data analysis
Mass spectra acquired by Analyst were analyzed with MetabolitePilot™ v.1.5 (AB Sciex, Redwood City, CA) utilizing different peak finding algorithms, e.g. common product ion and neutral loss scanning, MDF, and generic peak finding. The total ion chromatogram peak intensity threshold was set at 1400 cps, MS peak intensity at 400 cps, and MS/MS peak intensity at 100 cps, respectively. The raw list of potential metabolites was checked for possible false positives generated by adduct formation or in-source collision-induced dissociation or water loss, which were eliminated. Potential STS-135 metabolites were evaluated based on mass shift, MS/MS fragmentation patterns and plausible retention time.
In vitro microsomal half-life (T1/2) and intrinsic clearance (CLint, micr) of STS-135 were calculated based on the model described in [46]. The microsomal intrinsic clearance was scaled to whole liver dimensions according to [47] yielding intrinsic clearance CLint. Human hepatic clearance (CLH) and extraction ratio (ER) were calculated [46] without considering plasma protein binding.
RESULTS
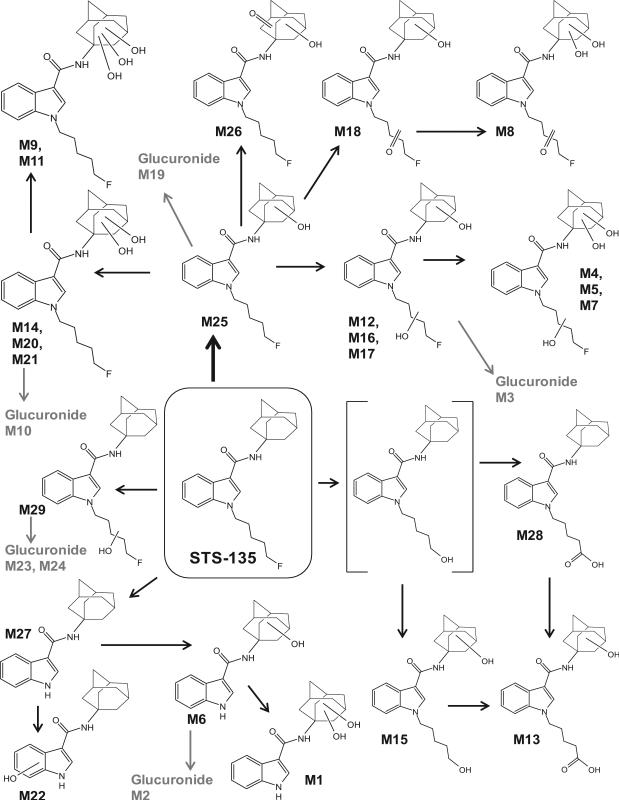
More than 90% decrease in diclofenac with a corresponding increase in 4’-hydroxydiclofenac and diclofenac β-D acyl glucuronide peak areas after 3 h incubation with the human hepatocytes confirmed hepatocyte metabolic activity. STS-135 was rapidly metabolized leading to a significant drop in parent compound concentration after 3 h incubation, with only 25% parent compound remaining. A total of twenty-nine metabolites were identified after STS-135 incubation with human hepatocytes. Table II lists all metabolites with retention time, observed m/z, suggested metabolic reaction, elemental composition, peak areas and diagnostic fragment ions. STS-135 underwent monohydroxylation with (M19, M23) and without glucuronidation (M25, M29); dihydroxylation with (M3, M10, M24) or without glucuronidation (M12, M14, M16, M17, M20, M21); trihydroxylation (M4, M5, M7, M9, M11); mono- (M18, M26) or dihydroxylation with ketone formation (M8); dealkylation (M27) with mono- (M6, M22) or dihydroxylation (M1); N-defluoropentylation with monohydroxylation and glucuronidation (M2); oxidative defluorination to carboxylic acid with monohydroxylation (M13) or oxidative defluorination with monohydroxylation (M15); and oxidative defluorination to carboxylic acid (M28). No glutathione or sulfate conjugates were observed. Based on our results we propose a hepatic metabolic scheme for STS-135, depicted in Figure 2.
Table II.
Retention time, accurate mass molecular ion m/z, elemental composition, diagnostic product ions, mass error and MS peak areas (1 and 3 h) of STS-135 and its metabolites. All values are given for the 3 h sample (except M29).
| Peak ID | Metabolic reaction | RT (min) | Molecular ion (m/z) | Elemental composition | Diagnostic product ions (m/z) | Mass error (ppm) | MS Area 1 h | MS Area 3 h |
|---|---|---|---|---|---|---|---|---|
| M1 | Dealkylation/Dihydroxylation | 4.37* | 327.1708 | C19H22N2O3 | 118, 144, 149, 161, 167 | 1.5 | 2.86E+04 | 2.45E+05 |
| M2 | Dealkylation/Monohydroxylation/Glucuronidation | 4.41 | 487.2074 | C25H30N2O8 | 133, 144, 150, 293, 311 | −0.3 | ND | 3.98E+04 |
| M3 | Dihydroxylation/Glucuronidation | 5.10 | 591.2706 | C30H39N2O9F | 133, 151, 222, 248, 415 | −1.1 | ND | 2.83E+04 |
| M4 | Trihydroxylation | 5.70 | 431.2344 | C24H31N2O4F | 144, 149, 167, 204, 222, 248 | 0.8 | ND | 7.77E+04 |
| M5 | Trihydroxylation | 5.94 | 431.2346 | C24H31N2O4F | 149, 167, 204, 222, 248 | 1.1 | 3.84E+04 | 1.69E+05 |
| M6 | Dealkylation/Monohydroxylation | 6.02 | 311.1760 | C19H22N2O2 | 133, 144, 151 | 1.9 | 3.65E+05 | 5.57E+05 |
| M7 | Trihydroxylation | 6.15 | 431.2342 | C24H31N2O4F | 144, 149, 167, 204, 222, 248 | 0.3 | 2.59E+04 | 1.12E+05 |
| M8 | Dihydroxylation/Ketone formation | 6.57 | 429.2183 | C24H29N2O4F | 149, 167, 220, 246 | −0.3 | ND | 1.73E+04 |
| M9 | Trihydroxylation | 7.18 | 431.2341 | C24H31N2O4F | 144, 183, 206, 232, 249 | 0.1 | 1.83E+04 | 7.65E+04 |
| M10 | Dihydroxylation/Glucuronidation | 7.23 | 591.2712 | C30H39N2O9F | 144, 149, 206, 232, 379, 415 | −0.1 | 8.41E+04 | 5.54E+05 |
| M11 | Trihydroxylation | 7.34 | 431.2344 | C24H31N2O4F | 132, 144, 183, 206, 232 | 0.7 | 5.45E+04 | 1.66E+05 |
| M12 | Dihydroxylation | 7.36 | 415.2398 | C24H31N2O3F | 133, 151, 204, 222, 248 | 1.6 | 1.24E+05 | 1.29E+05 |
| M13 | Oxidative defluorination to carboxylic acid/Monohydroxylation | 7.43 | 411.2285 | C24H30N2O4 | 133, 151, 200, 218, 244, 393 | 1.5 | ND | 5.93E+05 |
| M14 | Dihydroxylation | 7.48 | 415.2394 | C24H31N2O3F | 144, 149, 167, 206, 232, 249 | 0.6 | 6.40E+04 | 1.09E+05 |
| M15 | Oxidative defluorination/Monohydroxylation | 7.56 | 397.2487 | C24H32N2O3 | 133, 151, 186, 204, 230, 247 | 0.4 | 3.35E+04 | 1.08E+04 |
| M16 | Dihydroxylation | 7.64 | 415.2396 | C24H31N2O3F | 133, 151, 204, 222, 248, 265 | 1.2 | 7.61E+04 | 1.10E+05 |
| M17 | Dihydroxylation | 7.84 | 415.2396 | C24H31N2O3F | 133, 151, 204, 222, 248, 265 | 1.2 | 1.19E+05 | 9.47E+04 |
| M18 | Monohydroxylation/Ketone formation | 8.40 | 413.2242 | C24H29N2O3F | 133, 151, 220, 246 | 1.7 | 9.89E+03 | 1.17E+04 |
| M19 | Monohydroxylation/Glucuronidation | 8.52 | 575.2769 | C30H39N2O8F | 133, 151, 206, 232, 381, 399 | 1.0 | 1.17E+05 | 1.79E+05 |
| M20 | Dihydroxylation | 8.70 | 415.2391 | C24H31N2O3F | 144, 149, 167, 206, 232, 249 | −0.1 | 3.73E+04 | 1.94E+04 |
| M21 | Dihydroxylation | 9.04 | 415.2394 | C24H31N2O3F | 144, 149, 167, 206, 232 | 0.7 | 1.48E+06 | 1.44E+06 |
| M22 | Dealkylation/Monohydroxylation | 9.43 | 311.1752 | C19H22N2O2 | 135, 159, 160 | −0.7 | 1.23E+04 | 1.51E+04 |
| M23 | Monohydroxylation/Glucuronidation | 10.10 | 575.2762 | C30H39N2O8F | 135, 144, 177, 222, 248, 265, 399, 424 | −0.2 | 3.58E+04 | 4.14E+04 |
| M24 | Dihydroxylation/Glucuronidation | 10.44 | 591.2710 | C30H39N2O9F | 135, 177, 238, 264, 415 | −0.4 | 7.34E+04 | 7.36E+04 |
| M25 | Monohydroxylation | 11.00 | 399.2447 | C24H31N2O2F | 133, 144, 151, 206, 232 | 1.2 | 1.22E+06 | 3.81E+05 |
| M26 | Monohydroxylation/Ketone formation | 11.20 | 413.2236 | C24H29N2O3F | 144, 206, 232, 249, 367, 395 | 0.3 | 3.57E+04 | 2.46E+04 |
| M27 | Dealkylation | 12.47 | 295.1808 | C19H22N2O | 79, 135, 144 | 1.1 | 1.22E+05 | 5.71E+04 |
| M28 | Oxidative defluorination to carboxylic acid | 13.02 | 395.2330 | C24H30N2O3 | 135, 200, 218, 226, 244, 377 | 0.1 | 1.33E+05 | 6.90E+04 |
| M29 | Monohydroxylation | 13.63 | 399.2442 | C24H31N2O2F | 135, 222, 248 | −0.1 | 7.18E+04 | ND |
| STS-135 | 17.01 | 383.2495 | C24H31N2OF | 135, 144, 206, 232 | 0.6 | 2.41E+06 | 1.54E+06 |
ND, not detected
RT in 1 h sample was 4.28 min
Figure 2.
Proposed human hepatic metabolic pathway of STS-135 with metabolite identification; ambiguous assignments of functional groups are shown as Markush structures
After 3 h incubation, more than 75% of parent compound was metabolized. As expected, STS-135 peak area after 3 h was less than after 1 h incubation with increased metabolite peak areas after 3 h. There were no significant differences between the number of metabolites found in the data acquired with and without MDF: At 1 h, 23 metabolites were identified with MDF compared to 22 metabolites without MDF (M18 was missed); at 3 h, 28 metabolites were identified with MDF compared to 27 without MDF (M13 was missed). Only one metabolite (M29) was unique to the 1 h sample, whereas five additional metabolites (M2, M3, M4, M8 and M13) were observed after 3 h. The 20-min-gradient enabled baseline chromatographic separation of all metabolites eluting between 4.37 and 13.02 min with a mass error < ±2 ppm; STS-135 parent eluted at 17.01 min. The in vitro half-life (T1/2) of STS-135 in HLM was 7.2±0.6 min, in vitro microsomal intrinsic clearance CLint, micr was 0.096 mL·min−1·mg−1 and intrinsic clearance CLint was 93.6 mL·min−1·kg−1. Hepatic clearance CLH was calculated to 16.5 mL·min−1·kg−1 and extraction ratio ER to 0.82.
DISCUSSION
Characteristic STS-135 fragmentation
The STS-135 product ion spectrum (STS-135, MH+, m/z 383.2495) revealed a base peak at m/z 135 consistent with the adamantyl ion. This ion was clearly the most intense fragment ion. Cleavage of the bond between the carboxamide carbon and nitrogen atom generated an N-fluoropentylindole acylium ion at m/z 232. Further loss of the fluorinated N-pentyl side chain resulted in an indole acylium ion at m/z 144. Product ions at m/z 79, 93 and 107 were confirmed as break-down ions from the adamantane ring. Figure 3 shows product ion spectrum and structure of STS-135 with proposed fragmentation.
Figure 3.
Product ion spectrum and structure of STS-135 with assigned fragmentation pattern
Identification of mono-, di- and trihydroxylated STS-135 metabolites
Oxidation was the predominant biotransformation, occurring alone and in combination with other metabolic reactions. This observation is consistent with published findings for other synthetic cannabinoids with mono- or dihydroxylation of the N-pentyl side chain or the indole moiety (e.g. JWH-018, JWH-073, JWH-081, JWH-122, JWH-210, JWH-250, or RCS-4 [20, 48]) as well as hydroxylation of the aliphatic adamantane ring for AB-001 [33] or AKB-48 [35]. Glucuronidation is the major phase II biotransformation for synthetic cannabinoids studied to date [21, 28, 35-36, 49-50]. We identified 2 mono-, 6 di- and 5 trihydroxylated STS-135 metabolites, as well as 2 monohydroxylated/glucuronidated and 3 dihydroxylated/glucuronidated metabolites (Figure 4A).
Figure 4.
Combined extracted ion chromatogram of STS-135 and its hydroxylated metabolites shown in the 3 h sample, M29 is not shown because it was only found in the 1 h sample (A); product ion spectrum and structure of M25, a monohydroxylated metabolite (B); of M12, M16, and M17, dihydroxylated metabolites (C); of M14, M20 and M21, dihydroxylated metabolites (D);; of M4, M5 and M7, trihydroxylated STS-135 metabolites (E).
M25 was identified as a monohydroxylated metabolite of STS-135 with a protonated molecule at m/z 399 (Figure 4B). Product ion spectra indicated that hydroxylation occurred on the aliphatic adamantane ring (m/z 151) with an unaltered N-fluoropentylindole moiety (m/z 144, 206 and 232). This metabolite was the second most intense metabolite after 1 h incubation. The other monohydroxylated metabolite, M29, produced different fragment ions indicating hydroxylation on the fluoropentylindole system (m/z 248) leaving the adamantane ring fragment ion unchanged (m/z 135). This metabolite was found in the 1 h sample only. M19 with a protonated molecule at m/z 575 was a monohydroxylated/glucuronidated metabolite of STS-135 with product ions similar to that of M25. The fragment ions at m/z 144, 151, 206 and 232 in addition to m/z 399, which is associated with the complete core structure of the aglycone, indicate hydroxylation and subsequent glucuronidation on the adamantane ring. In contrast to that, M23 – another monohydroxylated and glucuronidated metabolite – was hydroxylated on the N-fluoropentyl side chain (m/z 222 and 248) containing an unaltered adamantane ring (m/z 135) and indole moiety (m/z 144). It is possible that M19 is generated from M25, and that M23 is derived from M29, but it is impossible to prove that with the available mass spectrometric data.
The metabolites M12, M14, M16, M17, M20, and M21 with a protonated molecule at m/z 415 were identified as dihydroxylated metabolites of STS-135. M21 was the most intense metabolite after 1 h and 3 h incubation. Product ion spectra of M12, M16 and M17 contained an intense fragment ion at m/z 151 indicating monohydroxylation on the adamantane ring and fragment ions at m/z 144, 222 and 248 indicating another hydroxyl group located on the N-fluoropentyl side chain (Figure 4C). In contrast to that, the product ion spectra of M14, M20 and M21 showed characteristic fragment ions at m/z 144, 206 and 232, which are associated with an unaltered N-fluoropentylindole moiety, and at m/z 167 indicating dihydroxylation on the adamantane ring (Figure 4D).
Some dihydroxylated metabolites underwent subsequent glucuronidation to form M3, M10 and M24, which showed a protonated molecule at m/z 591 and a product ion at m/z 415 corresponding to the dihydroxylated aglycone moiety of STS-135. Retention times varied from 5.10, 7.23 to 10.44 min for M3, M10 and M24, respectively. M24 eluted even later than many other unconjugated dihydroxylated metabolites. The different locations of the two hydroxyl groups and the glucuronic acid moiety likely produce varying polarities: Product ion spectra of M3, detected only after 3 h incubation, indicated monohydroxylation on the adamantane ring (m/z 151) and the N-pentyl side chain (m/z 222 and 248), whereas for M10 the product ion at m/z 167 indicated dihydroxylation on the adamantane ring, and for M24 both hydroxyl groups were attached on the N-fluoropentyl side chain (m/z 238 and 264) with unaltered adamantane ring (m/z 135).
Five STS-135 metabolites (M4, M5, M7, M9, and M11) were generated by trihydroxylation and showed a protonated molecule at m/z 431. Product ion spectra of M4, M5 and M7 indicated that two hydroxyl groups were attached to the adamantane ring (m/z 167) and one on the N-fluoropentyl side chain (m/z 222 and 248) with unaltered indole moiety (m/z 144) (Figure 4E). Product ion spectra of M9 and M11 indicated that all three hydroxyl groups were attached to the adamantane ring (m/z 186) with unaltered N-fluoropentylindole moiety (m/z 144, 206 and 232). M4 was only present in the 3 h sample. Due to increased polarity by three hydroxyl groups the five trihydroxylated metabolites eluted early in the gradient – between 5.70 and 7.34 min – with M4, M5, M7 (two hydroxyl groups on the adamantane ring and one on the N-pentyl side chain) eluting earlier than M9 and M11 (all three hydroxyl groups on the adamantane ring). Trihydroxylated metabolites did not produce any glucuronide conjugates.
We observed a cluster of two or three peaks with a difference of 18 Da corresponding to a water loss for all metabolites that were mono- or dihydroxylated on the adamantyl ring. The dihydroxylated adamantyl product ion (m/z 167) generated product ions at m/z 149 and 131, which can be found in all corresponding MS/MS spectra; the monohydroxylated adamantyl product ion (m/z 151) generated an additional fragment ion at m/z 133.
We did not detect any sulfate conjugates. However, the ionization of a potential sulfate conjugate depends on the chemical nature of the drug moiety, the sulfate group and the mobile phase conditions. The STS-135 parent compound was efficiently protonated under our LC/MS conditions. Given STS-135's efficient positive mode ionization and our acidic mobile phase composition, the sulfate group likely retains its proton; therefore we expect that we should have been able to detect sulfated STS-135 metabolites.
Identification of STS-135 metabolites with ketone formation
We identified 3 STS-135 metabolites with ketone formation (M8, M18 and M26). M18 and M26 (depicted in Figure 5A) were identified with a protonated molecule at m/z 413 that is consistent with monohydroxylation and ketone formation. The M18 product ion spectrum indicated monohydroxylation occurring on the adamantane ring (m/z 151) and ketone formation on the N-fluoropentyl side chain (m/z 220 and 246), respectively (Figure 5B). The M26 product ion spectrum suggests that both the hydroxyl and keto groups were located on the adamantane ring (m/z 165) and that the N-fluoropentylindole moiety remained unchanged (m/z 144, 206 and 232). M8 with a protonated molecule at m/z 429 is a dihydroxylated metabolite with both hydroxy groups on the adamantane ring (m/z 167) and a keto group located on the N-fluoropentyl side chain (m/z 220 and 246). This metabolite is depicted in blue in Figure 4A and a proposed structure is shown in Figure 5C. Since an unambiguous assignment of the locations of all functional groups is not possible, it remains unclear if M8 is generated from M26.
Figure 5.
Combined extracted ion chromatograms of mono- (M18, M26) or dihydroxylated metabolites (M8) with ketone formation after a 3 h hepatocyte incubation (A); product ion spectrum and structure of M18 (B) and M8 (C)
Identification of STS-135 metabolites that underwent oxidative defluorination
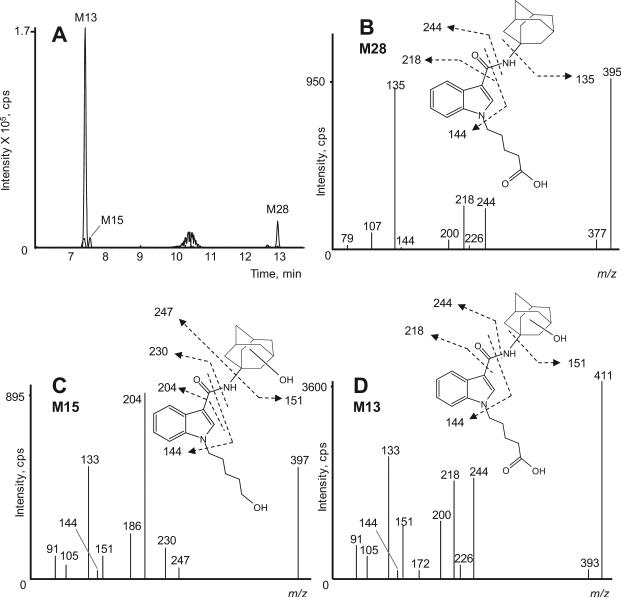
Oxidative defluorination can occur on fluorinated alkyl chains, as was previously shown for the fluorinated synthetic cannabinoids AM2201 [29], XLR-11 [36] and 5F-PB-22 [37]. Consequently, these compounds share metabolites with the unfluorinated analogs JWH-018, UR-144 and PB-22. STS-135 also underwent oxidative defluorination, with 3 oxidative defluorinated STS-135 metabolites identified: M13 (the predominant metabolite), M15 and M28 (Figure 6A). The oxidative defluorination mechanism involves the exchange of the fluoro atom by a hydroxyl group on the terminal carbon atom of the STS-135 N-pentyl chain.
Figure 6.
Combined extracted ion chromatograms of metabolites that underwent oxidative defluorination (M13, M15 and M28) in the 3 h sample (A); product ion spectrum and structure of M28, an oxidatively defluorinated and carboxylated STS-135 metabolite (B); of M15, an oxidatively defluorinated/monohydroxylated metabolite (C) and M13, an oxidatively defluorinated/carboxylated metabolite (D)
M28 with a protonated molecule at m/z 395 was generated by oxidative defluorination followed by carboxylation. The product ion spectrum indicated an unaltered adamantane ring (m/z 135) and carboxylated N-pentyl chain (m/z 218 and 244), while the indole ring remained unchanged (m/z 144). Product ion spectrum and proposed structure with fragmentation are depicted in Figure 6B. M15 with a protonated molecule at m/z 397 was identified as an oxidative defluorinated and monohydroxylated metabolite of STS-135. Presence of m/z 151, 204 and 230 in the M15 product ion spectrum suggests that one hydroxyl group was attached to the adamantane ring while the other is clearly – due to the reaction mechanism – located on the terminal carbon atom of the N-pentyl side chain (Figure 6C). M13 with a protonated molecule at m/z 411 was generated by subsequent monohydroxylation of M28 on the adamantane ring (m/z 151); product ion spectrum and structure are depicted in Figure 6D. M13 was not detected after 1 h, but showed the second most intense signal of all metabolites after 3 h incubation.
There are no metabolism studies for the unfluorinated analog of STS-135, APICA, yet. However, it is likely, that the APICA molecule follows the common metabolic pathways of related synthetic cannabinoids. If this is true, APICA would be oxidized at the pentyl chain to form a 5-hydroxypentyl (other isomers are also possible) and a pentanoic acid metabolite with likely further hydroxylations at the adamantyl ring system and would share M13, M15 and/or M28 with STS-135. Distinguishing between STS-135 and APICA intake during clinical and forensic urine testing will likely be confounded by common metabolites shared by both compounds.
Identification of dealkylated STS-135 metabolites
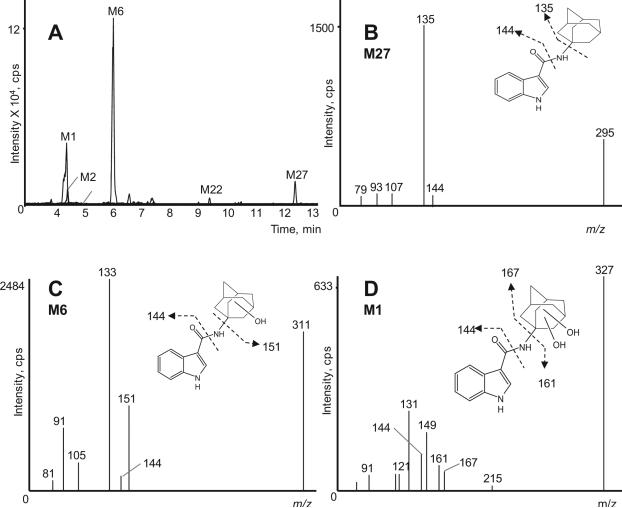
N-dealkylation was described for various synthetic cannabinoids, e.g. JWH-018 [24], JWH-250 [27], AM2201 [29], RCS-4 [31], UR-144 [29] and AB-001 [33], but could not be detected for many others – although structurally similar – like RCS-8 (in press), AM-694 [28], AKB-48 [35], XLR-11 [36], PB-22 and 5F-PB-22 [37]. To date, it is unclear which structural elements limit dealkylation. We identified five N-dealkylated STS-135 metabolites that could be further metabolized. We did not detect N-dealkylated metabolites in our recent AKB-48 study, a novel synthetic cannabinoid also containing an adamantyl ring system and a carboxamide linkage, but has an indazole core and a fluorinated N-pentyl side chain. Figure 7A depicts an extracted ion chromatogram of STS-135 dealkylated metabolites.
Figure 7.
Combined extracted ion chromatograms of dealkylated STS-135 metabolites (M1, M2, M6, M22 and M27) in the 3 h sample (A); Product ion spectrum of M27, dealkylated metabolite (B); of M6, dealkylated and monohydroxylated metabolite (C) and of M1, dealkylated and dihydroxylated metabolite (D)
N-defluoropentylation of STS-135 without any further modifications led to M27, which showed a protonated molecule at m/z 295. The product ion spectrum is depicted in Figure 7B. After loss of the alkyl chain characteristic product ions are m/z 135 and 144 associated with an unaltered adamantane ring and indole ring. All other dealkylated metabolites are derived from M27. Subsequent monohydroxylation produced M6 and M22 with a protonated molecule at m/z 311. In M6 (Figure 7C), the hydroxyl group is located on the adamantane ring – as indicated by m/z 151 – whereas in M22 it is located on the indole ring (m/z 160). Subsequent dihydroxylation of M27 generated M1 with a protonated molecule at m/z 327. Both hydroxyl groups were attached on the adamantane ring (m/z 167). Product ion spectrum and structure are depicted in Figure 7D. Further glucuronidation of a dealkylated and monohydroxylated metabolite generated M2 with a protonated molecule at m/z 487 that was only present in the 3 h sample. The product ion spectrum contained an m/z 311 fragment ion, corresponding to the aglycone moiety of M2, and at m/z 151 indicating monohydroxylation on the adamantane ring.
Major STS-135 metabolites
MS peak areas provide only semi-quantitative results due to varying ionization efficiencies of different metabolite ions; however we assume that the most predominant metabolites are most likely major human STS-135 metabolites and could be promising targets for analytical methods. The most intense metabolite in our human hepatocyte samples was M21, a dihydroxylated metabolite with two hydroxyl groups on the adamantane ring, which showed the highest signal in the 1 and 3 h sample. The second highest signal in the 1 h sample belonged to M25, a monohydroxylated metabolite from which M21 probably is generated. Other intense metabolites after 1 h incubation were M6 (dealkylated/monohydroxylated), M28 (defluorinated/carboxylated), M27 (dealkylated), M19 (monohydroxylated/glucuronidated), M17 and M12 (two other dihydroxylated metabolites). These results suggest that hydroxylation is the predominant phase I biotransformation but that dealkylation and oxidative defluorination also play an important role. This hypothesis is supported by the findings in the 3 h sample where M13 (defluorinated/carboxylated/monohydroxylated metabolite derived from M28), M6, M10 (dihydroxylated/glucuronidated), M25 and M1 (dealkylated/dihydroxylated) showed intense signals. Sequential metabolism likely occurs during incubation, producing a general trend towards metabolites with multiple phase I biotransformations and/or subsequent conjugation with glucuronic acid over incubation time. Peak areas for metabolites whose generation involved one step only, for instance M25, M29 and M27, decreased whereas peak areas of metabolites that were formed in multiple steps, like all trihydroxylated metabolites, increased over time. Similarly, signals of all glucuronides were higher after 3 h than after 1 h incubation. Since exact locations of functional groups are often unclear, it is difficult to define which metabolites are linked and are generated from each other. However, we are confident that M27 is interconnected with M1, M2, M6 and M22 and that M28 is further metabolized to M13. For these two examples a plausible decrease of the reactant signal (M27 and M28, respectively) and a corresponding increase in the product signals was observed.
Effective targets for forensic and clinical analytical methods are metabolites that are specific and can be unambiguously traced back to the consumed parent compound. Therefore, defluorinated or dealkylated metabolites in principle are less suitable because important structural elements are altered or lost. Based on our findings, we recommend targeting dihydroxyadamantyl STS-135 (M21) and monohydroxyadamantyl STS-135 (M25) to prove STS-135 intake. However, it will need additional analytical procedures such as nuclear magnetic resonance spectroscopy or synthesis and subsequent analysis of possible isomers to determine the exact position of the hydroxyl groups in these metabolites. Once reference standards become available, analytical methods can be developed and further studies on the binding affinity and activity at CB receptors as well as the potential toxicity of STS-135 metabolites are possible.
Metabolic stability in human liver microsomes
Human liver microsome samples are a simpler matrix than blood, producing less matrix effect, and enabling confident half-life estimations via peak area determinations without traditional quantitative methods. Therefore, measuring absolute parent peak areas decreases over time to calculate half-life is a suitable approach, widely employed by the pharmaceutical industry in in vitro studies.
In vitro microsomal intrinsic clearance CLint, micr of 0.096 mL·min−1·mg−1 was scaled to whole liver dimensions yielding an intrinsic clearance of 93.6 mL·min−1·kg−1. Without considering plasma protein binding and using a simplified Rowland's equation, we calculated the predicted hepatic clearance CLH to 16.5 mL·min−1·kg−1. The in vitro half-life T1/2 of 7.2±0.6 min and intrinsic clearance CLint of 93.6 mL·min−1·kg−1 indicated that STS-135 is a highly metabolized drug. According to McNaney et al. high-clearance compounds have CLint > ca. 45 mL·min−1·kg−1, intermediate-clearance compounds between ca. 15 to 45 mL·min−1·kg−1 and low-clearance compounds < ca. 15 mL·min−1·kg-1 [47]. Predicted extraction ratio was 0.82, which would classify STS-135 as a high-extraction drug according to Lavé et al. who define an extraction ratio of > 0.7 as high, 0.3 – 0.7 as intermediate and <0.3 as low [51]. T1/2 and CLint can be further used for predicting human pharmacokinetics once plasma protein binding and volume of distribution are determined during preclinical studies.
CONCLUSION
Human hepatocytes have unique advantages over human liver microsomes offering a complete range of metabolizing enzymes (phase I and II) and usually generating a metabolic profile more consistent with in vivo metabolism. Identification of synthetic cannabinoid metabolites is critically important to provide potential targets for analytical methods and candidates to test for pharmacological and toxicological effects. We identified 29 STS-135 metabolites that were generated by mono-, di- and trihydroxylation, dealkylation, oxidative defluorination and glucuronidation. The most intense metabolites in the hepatocyte samples were hydroxyladamantyl STS-135 and dihydroxyladamantyl STS-135, that should be included in analytical methods and spectra libraries to document STS-135 intake during clinical and forensic urine drug testing. In vitro human liver microsomal data suggest that hepatic clearance likely plays a major role in STS-135 clearance from humans, but in vivo clearance studies are needed to confirm this.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
ABBREVIATIONS
- CB
cannabinoid
- CL
clearance
- cps
counts per second
- CYP
cytochrome P450
- DEA
Drug Enforcement Administration
- ER
extraction ratio
- ESI
electrospray ionization
- FDA
Food and Drug Administration
- HLM
human liver microsomes
- HRMS
high-resolution mass spectrometry
- IDA
information dependent acquisition
- LC-MS
liquid chromatography mass spectrometry
- MDF
mass defect filter
- MS
mass spectrometry
- MW
molecular weight
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- QTOF
quadrupole/time-of-flight
- STS-135
N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide
- THC
delta 9-tetrahydrocannabinol
- TOF
time of flight
- XICs
extracted ion chromatograms
Footnotes
Conflicts of interest
None
References
- 1.Gibbons S. ‘Legal highs’ - novel and emerging psychoactive drugs: A chemical overview for the toxicologist. Clin Tox. 2012;50:15. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- 2.Fattore L, Fratta W. Beyond THC: The New Generation of Cannabinoid Designer Drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45:1186. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- 4.Patton AL, Chimalakonda KC, Moran CL, McCain KR, Radominska-Pandya A, James LP, Kokes C, Moran JH. K2 Toxicity: Fatal Case of Psychiatric Complications Following AM2201 Exposure. J Forensic Sci. 2013;58:1676. doi: 10.1111/1556-4029.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Justice, Drug Enforcement Administration Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I (JWH-018, JWH-073, JWH-200, CP-47,497, CP-47,497 C8 homologue). Federal Register. 2011;76:11075. [Google Scholar]
- 6.U. S. Government Synthetic Drug Abuse Prevention Act of 2012 (S.3187) 2012.
- 7.Department of Justice, Drug Enforcement Administration Schedules of Controlled Substances: Temporary Placement of Three Synthetic Cannabinoids Into Schedule I. Federal Register. 2013:78. [PubMed] [Google Scholar]
- 8.Center for Substance Abuse Research Synthetic Cannabinoid Series. CESAR FAX. 2013;22:27. [Google Scholar]
- 9.Makriyannis A, Deng H. Cannabimimetic indole derivatives. 2001 International Patent Application WO 01/28557 A1.
- 10.Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Delta9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer N, Lindigkeit R, Schiebel HM, Ernst L, Beuerle T. Identification and quantification of synthetic cannabinoids in ‘spice-like’ herbal mixtures: A snapshot of the German situation in the autumn of 2012. Drug Test Anal. 2013;6:59. doi: 10.1002/dta.1499. [DOI] [PubMed] [Google Scholar]
- 12.Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int. 2013;227:21. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 13.Kneisel S, Auwarter V, Kempf J. Analysis of 30 synthetic cannabinoids in oral fluid using liquid chromatography-electrospray ionization tandem mass spectrometry. Drug Test Anal. 2012;5:657. doi: 10.1002/dta.1429. [DOI] [PubMed] [Google Scholar]
- 14.Hutter M, Kneisel S, Auwarter V, Neukamm MA. Determination of 22 synthetic cannabinoids in human hair by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;903:95. doi: 10.1016/j.jchromb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Yanes EG, Lovett DP. High-throughput bioanalytical method for analysis of synthetic cannabinoid metabolites in urine using salting-out sample preparation and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;909:42. doi: 10.1016/j.jchromb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Shanks KG, Dahn T, Terrell AR. Detection of JWH-018 and JWH-073 by UPLCMS-MS in postmortem whole blood casework. J Anal Toxicol. 2012;36:145. doi: 10.1093/jat/bks013. [DOI] [PubMed] [Google Scholar]
- 17.Kneisel S, Speck M, Moosmann B, Corneillie TM, Butlin NG, Auwarter V. LC/ESI-MS/MS method for quantification of 28 synthetic cannabinoids in neat oral fluid and its application to preliminary studies on their detection windows. Anal Bioanal Chem. 2013;405:4691. doi: 10.1007/s00216-013-6887-0. [DOI] [PubMed] [Google Scholar]
- 18.Wohlfarth A, Scheidweiler KB, Chen X, Liu HF, Huestis MA. Qualitative confirmation of 9 synthetic cannabinoids and 20 metabolites in human urine using LC-MS/MS and library search. Anal Chem. 2013;85:3730. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jager AD, Warner JV, Henman M, Ferguson W, Hall A. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine--an Australian perspective. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;897:22. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Hutter M, Broecker S, Kneisel S, Auwarter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC-MS/MS techniques. J Mass Spectrom. 2012;47:54. doi: 10.1002/jms.2026. [DOI] [PubMed] [Google Scholar]
- 21.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011;83:6381. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH. Cytochrome p450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigoryev A, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, Nosyrev A, Vedenin A, Izotov B, Zabirova I, Rozhanets V. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1126. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, Kornilov AM, Bratton LD, Dobrowolski PJ, Wessinger WD, Fantegrossi WE, Prather PL, James LP, Radominska-Pandya A, Moran JH. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Brabanter N, Esposito S, Tudela E, Lootens L, Meuleman P, Leroux-Roels G, Deventer K, Van Eenoo P. In vivo and in vitro metabolism of the synthetic cannabinoid JWH-200. Rapid Commun Mass Spectrom. 2013;27:2115. doi: 10.1002/rcm.6673. [DOI] [PubMed] [Google Scholar]
- 27.Grigoryev A, Melnik A, Savchuk S, Simonov A, Rozhanets V. Gas and liquid chromatography-mass spectrometry studies on the metabolism of the synthetic phenylacetylindole cannabimimetic JWH-250, the psychoactive component of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2519. doi: 10.1016/j.jchromb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Grigoryev A, Kavanagh P, Melnik A. The detection of the urinary metabolites of 1-[(5-fluoropentyl)-1H-indol-3-yl]-(2-iodophenyl)methanone (AM-694), a high affinity cannabimimetic, by gas chromatography - mass spectrometry. Drug Test Anal. 2012;5:110. doi: 10.1002/dta.1336. [DOI] [PubMed] [Google Scholar]
- 29.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. 2012;4:745. doi: 10.1002/dta.1418. [DOI] [PubMed] [Google Scholar]
- 30.Hutter M, Moosmann B, Kneisel S, Auwarter V. Characteristics of the designer drug and synthetic cannabinoid receptor agonist AM-2201 regarding its chemistry and metabolism. J Mass Spectrom. 2013;48:885. doi: 10.1002/jms.3229. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh P, Grigoryev A, Melnik A, Simonov A. The identification of the urinary metabolites of 3-(4-methoxybenzoyl)-1-pentylindole (RCS-4), a novel cannabimimetic, by gas chromatography-mass spectrometry. J Anal Toxicol. 2012;36:303. doi: 10.1093/jat/bks032. [DOI] [PubMed] [Google Scholar]
- 32.Wohlfarth A, Pang S, Zhu M, Gandhi AS, Scheidweiler KB, Huestis MA. Metabolism of RCS-8, a Synthetic Cannabinoid with Cyclohexyl Structure, in Human Hepatocytes by High-Resolution Mass Spectrometry. Bioanalysis. 2014 doi: 10.4155/bio.14.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigoryev A, Kavanagh P, Melnik A. The detection of the urinary metabolites of 3-[(adamantan-1-yl)carbonyl]-1-pentylindole (AB-001), a novel cannabimimetic, by gas chromatography-mass spectrometry. Drug Test Anal. 2012;4:519. doi: 10.1002/dta.350. [DOI] [PubMed] [Google Scholar]
- 34.Grigoryev A, Kavanagh P, Melnik A, Savchuk S, Simonov A. Gas and liquid chromatography-mass spectrometry detection of the urinary metabolites of UR-144 and its major pyrolysis product. J Anal Toxicol. 2013;37:265. doi: 10.1093/jat/bkt028. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi AS, Zhu M, Pang S, Wohlfarth A, Scheidweiler KB, Liu HF, Huestis MA. First characterization of AKB-48 metabolism, a novel synthetic cannabinoid, using human hepatocytes and high-resolution mass spectrometry. AAPS J. 2013;15:1091. doi: 10.1208/s12248-013-9516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohlfarth A, Pang S, Zhu M, Gandhi AS, Scheidweiler KB, Liu H.-f., Huestis MA. First Metabolic Profile of XLR-11, a Novel Synthetic Cannabinoid, Obtained by Using Human Hepatocytes and High-Resolution Mass Spectrometry. Clin Chem. 2013;59:1638. doi: 10.1373/clinchem.2013.209965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohlfarth A, Gandhi AS, Pang S, Zhu M, Scheidweiler KB, Huestis MA. Metabolism of Synthetic Cannabinoids PB-22 and its 5-Fluoro Analog, 5F-PB-22, by Human Hepatocyte Incubation and High-Resolution Mass Spectrometry. Anal Bioanal Chem. 2014;406:1763. doi: 10.1007/s00216-014-7668-0. [DOI] [PubMed] [Google Scholar]
- 38.Thornton SL, Wood C, Friesen MW, Gerona RR. Synthetic cannabinoid use associated with acute kidney injury. Clin Toxicol. 2013;51:189. doi: 10.3109/15563650.2013.770870. [DOI] [PubMed] [Google Scholar]
- 39.Murphy T. Acute Kidney Injury Associated with Synthetic Cannabinoid Use - Multiple States, 2012. Morbidity and Mortality Weekly Report of the Centers for Disease Control and Prevention. 2013:62. [PMC free article] [PubMed] [Google Scholar]
- 40.Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2013;8:523. doi: 10.2215/CJN.05690612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winstock AR, Barratt MJ. The 12-month prevalence and nature of adverse experiences resulting in emergency medical presentations associated with the use of synthetic cannabinoid products. Hum psychopharmacoly. 2013;28:390. doi: 10.1002/hup.2292. [DOI] [PubMed] [Google Scholar]
- 42.Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:1622. doi: 10.1542/peds.2010-3823. [DOI] [PubMed] [Google Scholar]
- 43.Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajasekaran M, Brents LK, Franks LN, Moran JH, Prather PL. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol. 2013;269:100. doi: 10.1016/j.taap.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, Garberg P, Postlind H. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58:453. [PubMed] [Google Scholar]
- 47.McNaney CA, Drexler DM, Hnatyshyn SY, Zvyaga TA, Knipe JO, Belcastro JV, Sanders M. An Automated Liquid Chromatography-Mass Spectrometry Process to Determine Metabolic Stability Half-Life and Intrinsic Clearance of Drug Candidates by Substrate Depletion. Assay Drug Dev Technol. 2008;6:121. doi: 10.1089/adt.2007.103. [DOI] [PubMed] [Google Scholar]
- 48.Wintermeyer A, Moller I, Thevis M, Jubner M, Beike J, Rothschild MA, Bender K. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem. 2010;398:2141. doi: 10.1007/s00216-010-4171-0. [DOI] [PubMed] [Google Scholar]
- 49.Seely KA, Brents LK, Radominska-Pandya A, Endres GW, Keyes GS, Moran JH, Prather PL. A major glucuronidated metabolite of JWH-018 is a neutral antagonist at CB1 receptors. Chem Res Toxicol. 2012;25:825. doi: 10.1021/tx3000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chimalakonda KC, Bratton SM, Le VH, Yiew KH, Dineva A, Moran CL, James LP, Moran JH, Radominska-Pandya A. Conjugation of synthetic cannabinoids JWH-018 and JWH-073, metabolites by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2011;39:1967. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavé T, Dupin S, Schmitt C, Valles B, Ubeaud G, Chou RC, Jaeck D, Coassolo P. The Use of Human Hepatocytes to Select Compounds Based on Their Expected Hepatic Extraction Ratios in Humans. Pharm Res. 1997;14:152. doi: 10.1023/a:1012036324237. [DOI] [PubMed] [Google Scholar]