Background
Adherence is important for women with breast cancer because it is a primary determinant of treatment effectiveness and optimum clinical benefit. Adherence is described as the extent to which an individual’s behavior coincides with medical health advice.1,2 Racial differences exist for adherence in women with breast cancer who initiate and complete chemotherapy. African-American women are more likely to be diagnosed and treated at a later stage of breast cancer than Caucasian women due to delays in the diagnosis and initiation of treatment.3–5 Evidence also reveals racial disparities in the receipt of chemotherapy where African-American women are less likely to receive chemotherapy treatment when compared to their white counterparts after a diagnosis of a stage 1a or higher hormone receptor negative breast tumor (67% versus 78%; P < .01).6 However, the extent to which racial differences exist in adherence to intravenous chemotherapy is inconsistent and largely understudied.
Non-adherence to breast cancer treatments and treatment delays have been purported to partially explain worse breast cancer outcomes in African-American women.6,7 However, it is important to note these findings conflict with other studies where no significant differences between races were found to chemotherapy adherence rates.8–11 Inconsistencies in the relationship between race and chemotherapy adherence described in the literature, indicate a need for more research to establish the role race plays in chemotherapy adherence. Furthermore, the frequency, amount, and type of chemotherapy treatment is significantly different for early and advanced stage breast cancer which can potentially cause a different decision making process of risks and benefits seen in women with a more terminal diagnosis. Survival for early stage breast cancer can be as high as 98%, within all races, due to the advancement in the treatment and management of early stage breast cancer.12 This recognition is important because it suggests that breast cancer survival disparities can be decreased through clinical interventions that increase adherence to treatment. This information is crucial for the development and testing of population specific interventions that have the potential for addressing disparities in breast cancer treatment adherence and survival rates between African-Americans and Caucasians. Thus, the purpose of this study is to examine the variables that influence the decision to adhere to chemotherapy in a sample of African-American and Caucasian women and to identify other relevant factors that may be associated with chemotherapy adherence in a sample of women with early stage breast cancer.
Outcomes of adherence may include decrease in health care costs; decrease disease exacerbations, crisis or relapse, increase in patient quality and preservation of life.2,13,14 These benefits are not achievable if the patient does not adhere to medication or treatment. Adherence to intravenous chemotherapy for primary breast cancer is significantly associated with improved outcomes, when indicated, offers greater survival and lower recurrence.15,16 An early systematic review of 133 randomized trials showed highly significant reduction in the annual rate of recurrence and death rates as a result of intravenous chemotherapy.17 An overview of 18,000 women provided statistically definitive evidence that some form of chemotherapy can affect survival and recurrence with an odds reduction of 21% for recurrence and 11% for mortality. This review demonstrated that the effect of chemotherapy on annual death rates after five years was highly significant with a 28% reduction in recurrence rates and 16% reduction in mortality rates in the first five years.17
In order to receive maximum benefits from adjuvant treatment, patients must adhere to chemotherapy treatment regimens. Early studies found that patients receiving less than 85% of total intravenous chemotherapy had a poorer clinical course than those receiving complete therapy.15 A significant reduction in the 5-year relapse free survival was seen in women with stage II breast cancer on intravenous chemotherapy who received less than 65% of the planned drug dose.15 However, even when faced with a potentially life-threatening illness such as breast cancer, it cannot be assumed that the patient will adhere to intravenous chemotherapy. An early study found 21% of the patients actually received more than 85% of the prescribed chemotherapy dose, 34% of the patients received 66–84% of the prescribed dose, and 45% received less than 65% of the dose prescribed.18
There is a growth of literature that examines the role race plays in predicting adherence to cancer therapy7,11,19 but these studies fail to examine or adjust for socioeconomic status. Yet, it is important to note, that many factors that determine socioeconomic states are closely related to race. Minority groups make up a majority of the lower socioeconomic status in America, so it is hard to tease out the effects of poverty from the effects of race, which may contribute to inconsistences in racial differences in adherence. For example, a study of 51 participants with early stage breast cancer found non-adherent patients were of a significantly lower socioeconomic status, but the study did not report findings based on racial differences.10 However, Hershman et al found African-American women were more likely than Caucasian women to terminate intravenous chemotherapy treatment prematurely and were twice as likely to die as Caucasian women.7 Only 68% of African-American patients, compared to 76% of Caucasian patients, completed all prescribed cycles of intravenous chemotherapy. Of the 270 Caucasian women in the study, 93% were still living five years after diagnosis, and of the 202 African-American women, 81% were living five years after diagnosis.7 This study was the first study to find an association between early termination of chemotherapy and racial disparities in breast cancer outcomes. The current study will further elucidate the role of sociodemographic, social, and behavioral factors on chemotherapy adherence in African-American and Caucasian women with breast cancer.
Theoretical Framework
The study used an adaptation of the Health Decisions Model (HDM) for the conceptual basis for the study. The HDM is a revised version of the Health Belief Model where it incorporates strengths of the Health Belief Model and modifying factors of patient preferences to provide various predictors of adherence.20 The HDM consists of the following six key interrelated components that predict the health decision to adherence: sociodemographic, social interaction, experience, knowledge, general and specific health beliefs, and patient preferences.
The HDM model provides the most appropriate and comprehensive framework to explore the predictors that best influence the decision to adhere to chemotherapy in women with breast cancer. For the purpose of this study which is based on a sound review of the literature, sociodemographic, social interaction, knowledge, personal experience, and specific health beliefs were used in the study as the best predictors of adherence to chemotherapy. These five factors have been identified in the literature as predictors of cancer treatment and general medical adherence in various populations. Based from the HDM, the roles of socio-demographic factors (age, race, and access to care), social interaction factors (support mechanisms, i.e., social support and religious coping), cancer experience (chemotherapy side effects and depression), breast cancer knowledge, and health beliefs (perceived susceptibility, severity, motivation, benefits and barriers of the disease and cancer fatalism) were examined as predictors to treatment decision making (see Figure)20.
Figure.
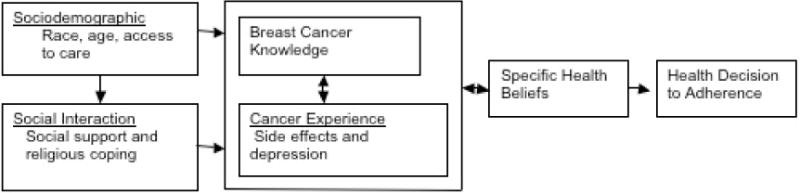
Factors That Influence Decision Making in Women with Breast Cancer as Adapted from the Health Decision Model.
Methods
Sample Recruitment
The sample size for this study was estimated using the Power Analysis and Sample Size (PASS) statistical software for calculation.21 Since the most statistically complex analysis that will be used in this study is multiple linear regressions, sample size was calculated using this statistical procedure. It was calculated that a sample size of 120 is needed to achieve 80% power to detect an R-Squared of 0.10 attributed to 6 independent variable(s) using an F-Test with a significance level (alpha) of 0.05. A preliminary analysis of the data revealed a rather homogeneous sample in regards to adherence. A dominantly adherent group lacked the variation needed to compare differences between those who continued or discontinued chemotherapy. The preliminary analysis revealed a sample size of n=90 would produce an effect size of .299 while a sample of n=120 would produce an effect size of .258. Due to the small variation between the effect size of a sample of 90 participants and 120 participants, recruitment efforts ended at n=99 participants.
The study enrolled 48 Caucasian and 51 African-American women diagnosed with early stage breast cancer whose ages ranged between 26 and 86 years. Additional sample characteristics are presented in Table 1. Early stage breast cancer was defined as having a primary diagnosis of Stage I, II, or IIIa breast cancer. Recruitment sites that served low, middle, and/or high socioeconomic populations were targeted to ensure the availability of a heterogeneous sample. The sample was recruited from a large public hospital and a private academic hospital in the metropolitan Atlanta, Georgia area. Eligible participants volunteered to participate into the study. At the completion of the study, each participant was given a $10 gift card to a local grocery store for compensation of her time. Approval from a university’s institutional review board (IRB), and from the hospital research oversight committee for the clinic providing care were granted for the study.
Table 1.
Characteristics of the Sample
| Characteristic (n/%) | Total Sample (N=99) | Caucasian (η=48) | African American (η=51) | |||||
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| 45 or younger | 35 (35.4%) | 18 (51.4%) | 17 (48.6%) | |||||
| 46–55 years old | 28 (28.3%) | 11 (39.3%) | 17 (60.7%) | |||||
| 56 years and older | 36 (36.4%) | 19 (52.8%) | 17 (47.2%) | |||||
| Mean | 51.83 | 52.75 | 50.96 | |||||
| Total household incomea | ||||||||
| < $10,000 | 17 (17.9%) | 5 (5.3%) | 12 (12.6%) | |||||
| $10,000– $19,999 | 8 (8.4%) | 3 (3.2%) | 5 (5.3%) | |||||
| $20,000– $29,999 | 12 (12.6%) | 3 (3.2%) | 9 (9.5%) | |||||
| $30,000– $39,999 | 5 (5.3%) | 0 (0%) | 5 (5.3%) | |||||
| $40,000– $49,999 | 3 (3.2%) | 2 (2.1%) | 1 (1.1%) | |||||
| $50,000– $59,999 | 3 (3.2%) | 2 (2.1%) | 1 (1.1%) | |||||
| $60,000– $69,999 | 7 (7.4%) | 3 (3.2%) | 4 (4.2%) | |||||
| $70,000– $79,999 | 6 (6.3%) | 4 (4.2%) | 2 (2.1%) | |||||
| $80,000– $89,999 | 10 (10.5%) | 6 (6.3%) | 4 (4.2%) | |||||
| $90,000– $99,999 | 4 (4.2%) | 1 (1.1%) | 3 (3.2%) | |||||
| $100,000 or more | 20 (21.1%) | 17 (17.9%) | 3 (3.2%) | |||||
| Highest level of education | ||||||||
| <12th grade | 3 (3%) | 2 (2%) | 1 (1%) | |||||
| 12th grade | 16 (16.2%) | 4 (4%) | 12 (12.2%) | |||||
| Vocational/ trade school | 10 (10.1%) | 6 (6.1%) | 4 (4%) | |||||
| >1 of junior college | 12 (12.1%) | 2 (2%) | 10 (10.1%) | |||||
| Associate’s degree | 7 (7.1%) | 4 (4%) | 3 (3%) | |||||
| Baccalaureate degree | 30 (30.3%) | 19 (19.2%) | 11 (11.1%) | |||||
| Master’s degree | 19 (19.2%) | 9 (9.1%) | 10 (10.1%) | |||||
| Doctorate/ Law degree | 2 (2%) | 2 (2%) | 0 (0%) | |||||
| Marital status | ||||||||
| Now married | 46 (46.5%) | 35 (35.4%) | 11 (11.1%) | |||||
| Domestic partner | 1 (1%) | 1 (1%) | 0 (0%) | |||||
| Single/ never married | 22 (22.2%) | 4 (4%) | 18 (18.2%) | |||||
| Divorced | 19 (19.2%) | 7 (7.1%) | 12 (12.1%) | |||||
| Separated | 2 (2%) | 0 (0%) | 2 (2%) | |||||
| Widowed | 9 (9.1%) | 1 (1%) | 8 (8.1%) | |||||
| Spouse or partner employed | ||||||||
| Not applicable | 47 (47.5%) | 10 (10.1%) | 37 (37.4%) | |||||
| Yes | 36 (36.4%) | 30 (30.3%) | 6 (6.1%) | |||||
| No | 16 (16.2%) | 8 (8.1%) | 8 (8.1%) | |||||
| Living arrangements | ||||||||
| Lives alone | 23 (23.2%) | 8 (8.1%) | 15 (15.2%) | |||||
| Lives with spouse | 40 (40.4%) | 30 (30.3%) | 10 (10.1%) | |||||
| Lives with domestic partner | 5 (5.1%) | 3 (3%) | 2 (2%) | |||||
| Lives with children | 19 (19.2%) | 1 (1%) | 18 (18.2%) | |||||
| Lives with family member | 12 (12.2%) | 6 (6.1%) | 6 (6.1%) | |||||
| Employment status | ||||||||
| Unemployed | 25 (25.3%) | 11 (11.1%) | 14 (14.1%) | |||||
| Full-time | 32 (32.3%) | 17 (17.2%) | 15 (15.2%) | |||||
| Part-time | 11 (11.1%) | 8 (8.1%) | 3 (3%) | |||||
| Retired | 13 (13.1%) | 9 (9.1%) | 4 (4%) | |||||
| Medical leave/ disability | 18 (18.2%) | 3 (3%) | 15 (15.2%) | |||||
| Type of health insuranceb | ||||||||
| None | 3 (3.1%) | 1 (1%) | 2 (2.1%) | |||||
| Private | 53 (54.6%) | 36 (37.1%) | 17 (17.5%) | |||||
| Medicare | 2 (2.1%) | 1 (1%) | 1 (1%) | |||||
| Medicaid | 26 (26.8%) | 3 (3.1%) | 23 (23.7%) | |||||
| Combination | 13 (13.4%) | 6 (6.2%) | 7 (7.2%) | |||||
Note.
4 missing cases
1 missing case
Sample Inclusion/Exclusion Criteria
Inclusion criteria for participation were: 1) self-reported African-American/black or Caucasian/white woman; 2) diagnosed with early stage (I, II, or IIIa) breast cancer; 3) completed two or less chemotherapy treatments; 4) able to read, write, and speak English; 5) initial and primary diagnosis of breast cancer documented in the medical charts; 6) recommended to receive an intravenous non-hormonal chemotherapy regimen for treatment; 7) provides voluntary consent to participate in the study; and 8) over the age of 21 years. Exclusion criteria for the study were: 1) advanced stage breast cancer (stage IIIb or IV); 2) chart documented of a major mental disorder; 3) unable to read or write English. The frequency, amount, and type of chemotherapy treatment are different for early and advanced stage breast cancer; these facts can potentially cause a difference in the decision-making process in women with a terminal diagnosis. Thus, the study only included women with a diagnosis of early stage breast cancer as documented in their medical charts. All participants were requested to read and speak English in order to read, comprehend, and complete the questionnaires. Women treated with hormonal and self-administered oral chemotherapy were excluded because those treatments produce different costs and benefits to patients and may produce different predictors to treatment adherence. The study excluded women with mental disorders, in order to control for the risk of non-adherence due to poor mental health or lack of understanding of the study or her recommended treatment.
Measures
Adherence
The outcome of interest was non-adherence to intravenous chemotherapy among African-American and Caucasian women with early stage breast cancer. A medical chart review was used to measure chemotherapy treatment adherence. A patient’s missed appointment due to not showing up for an appointment, cancellation, or refusal of chemotherapy was documented in her medical records. Since prior studies found that patients receiving less than 85% of total chemotherapy had a poorer clinical course than those receiving complete therapy, the original measure for adherence was based on a cut-off point of 85 percent. However, preliminary analysis of the data revealed a generally highly adherent sample so the cutoff point was changed from those who were 100% adherent to chemotherapy to those who were less than 100% adherent. The reclassification was done to allow for adequate statistical analysis. Adherence was calculated by dividing the number of prescribed chemotherapy sessions, by the number of appointments attended. Adherence was dichotomized as a “yes” or “no” variable where patients that attended 100% of their chemotherapy sessions were considered adherent (“yes”) and those completing <100% were considered non-adherent (“no”).
A second measure of adherence in this study was “days from diagnosis to treatment.” This was a proxy measure for adherence to treatment recommendations and to explore the length of time that lapsed for the woman to initiate recommended treatment. “Days from diagnosis to treatment,” was measured by the number of days from the pathology reported diagnosis of early stage breast cancer to the initiation of cancer treatment (either surgery or chemotherapy) as documented in the medical chart.
Sociodemographic Factors
A demographic questionnaire was compiled by the principal investigator to collect demographic information from each subject including age, race, education, combined household income, living arrangements, employment, stage of disease at diagnosis, type of health insurance, and usual transportation to appointments. Access to health insurance served as an indicator for SES, where subjects were placed into lower or working/middle class based on health insurance coverage. Participants with no health insurance coverage or with Medicaid were categorized as lower socioeconomic class. Those with private insurance or Medicare coverage were considered working/middle socioeconomic class.
Social Support
The Norbeck’s Social Support Questionnaire (NSSQ) was utilized to measure the perceived social support of the participants22. This instrument measures the types (affect, affirmation, and aid) and sources of social support through a 6-item and 3-situation specific item questionnaire using a 5-point rating scale from 0 (not at all) to 4 (a great deal). Because the NSSQ is not a summative-type instrument, Pearson correlations among the items and subscales were calculated to test internal consistency reliability.22 Each of the two items for each subscale was highly correlated: Affect, .97; Affirmation, .96; and Aid, .89. The test-retest correlations were Affect, .89; Affirmation, .88; and Aid, .86. Validity of the NSSQ was supported by concurrent and construct validity, and the response bias of social desirability, which was ruled out.23 Concurrent validity was tested with the Social Support Questionnaire (SSQ), developed by Cohen and Lazarus.24 The affirmation and affect scale of the NSSQ was moderately associated with the SSQ measure of informational support (r=.33) and emotional support (r=.51), respectively.23 Construct validity was assessed using the Fundamental Interpersonal Relations Orientation (FIRO-B) measure.25 Construct validity was demonstrated by significant associations between FIRO-B’s need for inclusion and affection scales to NSSQ’s functional subscales (r= .18 to .27) and to most of the NSSQ’s network scales (r= .17 to .23).23
Religious Coping
The Pargament Religious Coping Scale, known as the RCOPE26, measures the ability to adapt to a life-changing event through the belief in a higher being and a range of religious coping strategies. This instrument is a 63-item questionnaire that measures both helpful and harmful religious coping methods26. Respondents are asked to reflect on the role religion played as a form of coping during a specified event such as chemotherapy sessions for women in this study. Each respondent is asked to answer each question on the extent to which there is agreement with each statement using a Likert scale of 1 (not at all) to 4 (a great deal). Positive religious coping subscales (e.g. spiritual support, benevolent religious reframing, collaborative religious coping, congregational support) ranges from 3 to 12 and the overall positive religious coping scale ranges from 36 (low) to 144 (high). Questions that constituted the negative religious coping subscales (e.g. spiritual discontent, punitive religious reframing, self-directing religious coping, congregational discontent) ranges from 3 to 12 where the overall negative religious coping score ranges from 27 (low) to 108 (high). If the respondent is not religious, he or she is asked to substitute “religion” with “spirituality” and “God” with a “higher being or force” or to simply mark “not at all” if neither applied26.
Factor analysis largely validated the conceptualization and the construction of the subscales and provided evidence of high internal consistency and incremental validity. All but three subscales (Reappraisal of God’s Power, Marking Religious Boundaries, and Interpersonal Religious Discontent) had alphas of .65 or greater, and seven subscales had alphas of .80 or greater for internal consistency subscales, confirming generally high reliability estimates26. Cronbach’s alpha levels (>0.75) calculated for the RCOPE is acceptable. The RCOPE has performed well in predicting physical and psychological adjustment to life crises when compared to a measure of Global Religiosity in other studies.27,28
Knowledge
The participant’s understanding of information relevant to the breast cancer diagnosis, including the risks, treatment options, and side effects that encompass the disease was measured by the Comprehensive Breast Cancer Related Knowledge Test.29 This scale is a 20-question true-false scale consisting of two subscales (general knowledge and curability) that assesses the knowledge or risk factors for breast cancer, symptoms of breast cancer, side effects of treatment, treatment efficacy, and methods of treatment. Correct answers were summed to produce a score that ranged from 0 to 12 for the general knowledge subscale and 0 to 8 for the curability subscale and an overall score ranging from 0 to 20. The internal consistency reliability for the post-tested general knowledge subscale was 0.60 and for the curability subscale was 0.62, which is acceptable. The overall alpha coefficient was 0.71.29
Side Effects
The Memorial Symptom Assessment Scale Short Form (MSAS-SF)30 was used to measure the participant’s symptoms experienced during chemotherapy treatments. The MSAS-SF is an abbreviated version of the Memorial Symptom Assessment Scale developed to provide multidimensional information about common symptoms experienced in oncology populations.30 The MSAS-SF instrument captures 28 prevalent symptoms of cancer therapies and assesses the patient’s rated severity, frequency, and distress associated with the symptoms. The respondent is asked to mark the experiences they experienced during chemotherapy and then rate how bothersome or distressful the symptom was. Distress is rated on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much).
Psychometric properties of the scale consisted of a Cronbach’s alpha coefficient that ranged from 0.80–0.87 for each subset of symptoms. The one day test-retest coefficient ranged from 0.86 to 0.94 and the one week test-retest ranged from 0.40–0.8430. The MSAS-SF subscales were assessed against the subscales of the Functional Assessment Cancer Therapy (FACT-G)31 to determine criterion validity. Correlation coefficients between the MSAS-SF and FACT-G subscales ranged from −.74 to −.68.30
Depression
Depression was measured by the Center for Epidemiologic Studies-Depression Scale (CES-D).32 This instrument is a 20-item self-report scale designed to survey six components of depression: depressed mood; feelings of guilt and worthlessness; feelings of helplessness and hopelessness; psychomotor retardation; loss of appetite; and sleep disturbance. Rated on a 4-point scale (0 = rarely or none at all; 3= most or all of the time), respondents indicate how often within the last week they experienced each symptom. The scores for the 20 items are added and result in an overall score that ranges from 0 to 60. It is important to note, the CES-D is not a diagnostic tool and is only a measure of depressive symptomology where a score equals or is greater than 16 is indicative of positive symptomology over the past week. Respondents who indicated high scores on the CES-D had their primary care provider notified for further evaluation.
Construct validity was evaluated in sample of women diagnosed with breast cancer by comparing the CES-D with the Profile of Mood State Fatigue Scale (POMS-F)33, the State version of the State–Trait Anxiety Inventory (STAI-S)34, and the Mental Health Summary Scale from the Short-Form 36 Health Survey (SF-36 Vitality scale).35 Construct validity was demonstrated by moderate to high correlations with measures of the POMS (r= 0.66), STAI (r= 0.77), and the SF-36 Vitality scale (r= −0.65).32 The CES-D was found to have good internal consistency with alpha coefficients >0.85 in a group of women with breast cancer as well as adequate test-retest reliability.32
Health Beliefs
The Champion’s Health Belief Model Scale (CHBMS)36 measures five concepts of the Health Belief Model: perceived susceptibility, perceived seriousness, perceived benefit, perceived barriers, and motivation. Each respondent is asked to rate how much she agrees or disagrees with each statement using a 5-point Likert scale of 1 (strongly disagree) to 5 (strongly disagree). The instrument subscales assess beliefs related to susceptibility to breast cancer before diagnosis, seriousness of her breast cancer diagnosis, benefits of chemotherapy treatments, suspected barriers to chemotherapy treatments, and motivation for good health.
The test-retest correlations ranged from 0.47 to 0.86.36 Factor analysis of the measure revealed statistical evidence for the independence of constructs. Principal components factor analysis for all items ranged from 0.36 to 0.75. Internal consistency of the factors ranged from 0.36 to 0.78. Cronbach’s alpha reliability coefficients for subscales ranged from 0.80 to 0.93.36 A multiple regression analysis of the subscales revealed a multiple R of 0.51 with 26% of variance accounted for in the model, which also demonstrates construct validity.36
Cancer Fatalism
The Powe Fatalism Inventory (PFI)37 was used to measure the fatalistic belief that death is inevitable with breast cancer. Items address fear, predetermination, pessimism, and inevitability of death through 15 “yes” or “no” questions. “Yes” responses are summated to produce a PFI score. Scores ranging from 0 to 8 denote low fatalism attitudes and scores of 9 to 15 denote high fatalism. In a sample of African-American women, the PFI has a reported internal consistency reliability ranging from 0.84 to 0.87.37 Validity and factor analysis of the PFI is acceptable37. Factor analysis resulted in all items loading on one factor. Fourteen of the items revealed Eigen values > 0.30. The coefficient alpha for internal consistency reliability was 0.87.
Data Collection
To help alleviate the possible burden of completing several questionnaires among this population, data collection consisted of two time points: time point one (T1), at enrollment, and time point two (T2), at the end of the participant’s chemotherapy sessions. Upon enrollment in the study or at the initiation of intravenous chemotherapy (T1), four questionnaires were administered: 1) Demographics measure; 2) CES-D; 3) PFI; and 4) Champion’s Health Belief Model Scale. The timing of administration of these questionnaires was considered as the appropriate baseline information each participant. The PFI and Champion’s Health Belief Model Scale were used to determine attitudes, beliefs, and feelings about breast cancer that existed at initiation of the chemotherapy treatment regimen that might predict the participant’s decision to adhere or discontinue chemotherapy. Depression scores were also obtained at baseline and T2 to detect for change in mood.
The duration of recommended chemotherapy for early breast cancer varied between women, ranging from one to six months. The second time point was at the end of the prescribed intravenous chemotherapy therapy (T2) and the following five questionnaires were administered: 1) RCOPE; 2) MSAS-SF; 3) CES-D; 4) Norbeck’s Social Support Questionnaire; and 5) the Breast Cancer Related Knowledge Measure. The RCOPE was administered at T2 to capture social support that the participant used throughout the entire (before and during) chemotherapy regimen. The MSAS-SF was used to identify symptoms the woman experienced during chemotherapy treatment. The CES-D was used at this time point to capture depression that may have occurred during chemotherapy. The knowledge measure was administered at T2 to measure the amount of knowledge the participant acquired during her chemotherapy experience. The woman’s adherence to chemotherapy was assessed at the second time point via a medical chart review. Contact of the participant was approved by an IRB, so if the PI was unable to meet the participant at the end of her chemotherapy treatments, the participant was contacted and asked permission to mail the last set of questionnaires along with her a SASE.
Statistical Analysis
The Statistical Package for the Social Sciences 19.038 was employed for data analysis. Double entry and double-checking were performed to decrease data entry errors. Data analyses consisted of descriptive statistics, correlations, and regression coefficients. Descriptive statistics such as means, frequencies, and standard deviations were employed to examine the sample’s demographic data. Univariate analysis was performed with chi-squared and Fisher’s exact probability for categorical variables and student’s T test for continuous variables. Significant variables were inputted in a model using logistic regressions to predict adherence to treatment. An alpha level of 0.05 was selected as the statistical criterion for significance.
Results
Adherence to Chemotherapy
Ninety percent (n= 84) of the sample was adherent to their chemotherapy regimen and 10% (n= 9) of the sample discontinued chemotherapy prior to completion. For the 44 Caucasian participants, 87.5% (n=42) were adherent and 4.3% (n=2) were non-adherent. For the 49 African-America participants, 82.4% (n=42) were adherent and 13.7% (n=7) were non-adherent. No racial difference was found in adherence to chemotherapy between African-American and Caucasian women (χ2= 2.627, p= .10).
The sample was grouped into 100% adherent (n= 79) and <100% adherent (n= 19) group for further statistical analysis. Between these two groups, those who were <100% adherent to chemotherapy regimens reported lower income (p <.001). Approximately 66% of those who were 100% adherent had private health insurance or a combination of private insurance and Medicare. Whereas, only 3% of the women who were <100% adherent reported private or combination health insurance (p < .001). For access to transportation to and from treatment sessions, 71% of those who were 100% adherent reported reliable access all the time while 11% of women who were <100% adherent reported reliable transportation all the time (p = .016).
Psychosocial Variables
Women who were <100% adherent had a higher mean depression score on the CES-depression (12.71 [SD, 9.62]) at the end of end of their chemotherapy (T2) than those who were 100% adherent (20.62 [SD, 10.69], p = .009). No significant difference in depression scores was found at baseline. Additionally, the <100% adherent group had a higher fatalistic view of cancer than the 100% adherent group (4.76 [SD, 3.23] versus 3.27 [SD, 2.62], p < .05).
Knowledge and Health Beliefs
Women who were 100% adherent to chemotherapy were more knowledgeable about general breast cancer facts. The average score for this group was 8.72 (SD, 1.43) compared to 7.54 (SD, 1.71) for women who were <100% adherent (p = .01). Using Champion’s Health Beliefs Measure, women who were 100% adherent also reported higher motivation to maintain their health (32 [SD, 5.51]) than women who were <100% adherent (26.24 [SD, 6.05], p <.001).
Symptom Experiences
Women who were <100% adherent reported experiencing more symptoms and more severe symptoms than women who were 100% adherent. Women who were <100% adherent experienced a mean of 17.33 (SD, 6.54) symptoms while the adherent group reported a mean of 12.94 (SD, 5.65) symptoms (p = .018). The average severity score for women who were <100% adherent was 50.77 (SD,20.22) and 31.32 (SD,16.10) for women who were 100% adherent (p < .001).
Days from Diagnosis to Treatment
The exploration of the variable, days from diagnosis to treatment (delays to treatment), as a proxy for adherence to treatment recommendations, revealed meaningful outcomes. The number of days from diagnosis to treatment in the overall sample ranged from 7 to 564 days. A participant who delayed treatment >500 days did not return to start chemotherapy and was omitted from the analysis. The mean days from diagnosis to treatment was 59.69 days and delays ranged from 44 to 74 days. The median days to treatment was 42 days and ranged from 35 to 48 days. Women who were <100% adherent were more likely to delay starting chemotherapy. The mean number of days from diagnosis to treatment in the <100% group was 100.53 days (SD, 96.07) versus 45.96 (SD, 36.68) days in the adherent group (p = .041).
Multivariate analysis
Variables that were significant at p<0.10 in the univariate analysis were included for a more robust selection into the multivariate model to predict non-adherence (<100%) to chemotherapy. Bivariate analysis revealed health insurance and income were highly correlated, thus income was not included for selection into the final model (r= .664, p=.000). Health insurance (OR: .071, p= .001), days to treatment (OR: .980, p= .028), depression score at T1 (OR: .938, p= .083), change in depression score (OR: .923, p= .023), Powe’s fatalism inventory score (OR: .827, p= .050), curability knowledge (OR: 1.75, p= .072), health motivation (OR: 1.20, p= .001), symptom severity (OR: .915, p= .000) were entered into the logistic model and met the criteria (p< 0.10) for inclusion in the initial adjusted logistic regression model. Using backwards stepwise approach, the final model revealed days to treatment (OR: .982, p=.058), health insurance (OR: .121, p= .016), change in depression (OR: .935, p= .118), and symptom severity (OR: .950, p= .038) were independently associated with non-adherence to chemotherapy (See Table 2).
Table 2.
Final Model of Logistic Regression Analysis (Hierarchal Backwards Stepwise Procedure) for Factors Predicting Adherence (N=73a)
| Predictors | B | SE | Wald | Adjusted OR |
p-value |
|---|---|---|---|---|---|
| Constant | 6.019 | 1.578 | 14.553 | 411.073 | .000 |
| Days to treatment | −.018 | .010 | 3.606 | .982 | .058 |
| Health insurance | −2.111 | .879 | 5.765 | .121 | .016 |
| Change in depression score | −.067 | .043 | 2.449 | .935 | .118 |
| Symptom severity | −.051 | .025 | 4.289 | .950 | .038 |
Note. Univariate variables p<.10 were included for selection in the final model.
26 missing cases
Discussion
The study did not confirm racial differences in rates of non-adherence to recommended chemotherapy regimens. In fact, 90% of the sample was adherent to their chemotherapy, which could indicate a selection bias where highly motivated individuals who were more likely to adhere to treatment consented to be in the study. This study is consistent with other studies where no racial difference to chemotherapy adherence was found. Andic et al. found no difference by race in the completion of neoadjuvant chemotherapy for inflammatory breast cancer (Caucasians 84% and African-Americans 86.7%) and no difference in the median length of time to completion of treatment (Caucasians 263 days and African-Americans 262 days).11 Libscomb et al. did not find that African- American women with breast cancer living in the rural South were more likely to discontinue chemotherapy.39 In fact, African-Americans completed chemotherapy at rates that equal or exceeded their Caucasian counterparts.39
The study found once a woman started chemotherapy, she completed treatment as recommended by her health care providers. However, some women experienced considerably more delays from diagnosis to treatment. A longer time to starting treatment after diagnosis it was revealed to be a predictor for treatment non-adherence. The median start day was 42 days (range: 7–564 days) where women who were <100% adherent were more likely to have experienced treatment delays. Hershman et al found that 60% of their sample (n= 344) experienced treatment delays in excess of 7 days receiving chemotherapy for early stage breast cancer but the study did not test if treatment delays influenced adherence to treatment.7 Thus, our findings suggests that it is important to acknowledge that the decision making process for adherence starts at the diagnosis of breast cancer. Prior studies defined treatment delay as the time from which a woman finds an abnormality to the time she seeks medical attention. The study is unique where it examined the time period between diagnosis and initiating treatment. Therefore, this finding reveals another important and vulnerable time period to intervene to potentially decrease treatment delay and maximize breast cancer outcomes. Vigorous follow up could potentially lessen delays and can play a role in improving adequate follow-up to treatment.
Inadequate access to health care (i.e. health insurance) served as a barrier to completing 100% treatment in this sample. Participants who were not covered by private insurance or combination insurance (private plus Medicare) were more likely to complete less than 100% of their treatment. Several other studies have found health insurance predicts breast cancer outcomes; patients who are uninsured or covered by Medicare or Medicaid are less likely to be screened with mammography, more likely to delay treatment and be diagnosed at more advanced stage of breast cancer, and have decreased survival rates.40–42 Systematic interventions can improve the delivery of health care, where policies can be implemented to allow equal access to health care and treatment.
Participants who became depressed during treatment or experienced an increase in depression symptomology (change in depression scores) were more likely to not complete 100% of treatment. However, depression at baseline and depression at the end of treatment were not predictors to treatment adherence. Depression can impact cognitive processes and have an effect on patients’ knowledge and understanding of their cancer treatment.43 Emotional distress has been found to interfere with processing accurate information where forgetfulness regarding medical information is increased.43 It is important to recognize the impact depression has on treatment decision-making, where depression compromises physical, emotional, and cognitive functioning, which contributes to ineffective coping, proper cognitive processing of information, maladaptive health beliefs, and finally delays to starting treatment. It is possible the proper assessment and treatment of depression can improve clinical outcomes and can help women cope with the breast cancer experience and improve quality treatment decision-making.
Intravenous chemotherapy improves survival rates; however, not without adverse physical and emotional effects. This study found symptom severity to be an independent predictor to chemotherapy completion. The mean severity score for this sample was 33.9 where participants rated hair loss, lack of energy, difficulty swallowing, changes in the way food tastes, and numbness or tingling in hands and feet as the most severe symptoms. Other studies have found depression, pain, fatigue, and hair loss are the most common distressing side effects experienced by women treated with intravenous treatment for breast cancer.44,45 These side effects threaten functioning, well-being and quality of life.44–48 Distressing physical symptoms can have a significant influence on long-term treatment decisions.49 Several studies found side effects to be the most frequently reported reason for early discontinuation for women taking oral hormonal chemotherapy.50–52 Patients will alter their treatment not recommended by their health care provider to increase coping and to ameliorate symptoms.53 Thus it is important for health care providers to assess symptoms and to provide the proper management and treatment to help ameliorate symptom burden. Proper symptom management can increase coping and quality of life and may increase the likelihood to adhere to recommended treatment recommendations.
Limitations
The first limitation to this study was adherence to intravenous chemotherapy was generally high in this sample, which is clinically advantageous, but statistically problematic when exploring relationships and differences to chemotherapy adherence between groups. Secondly, the study had to change the 85% cut point for statistical analysis which was a change from the original protocol. In addition, the sample included a convenience sample with an extensive inclusion and exclusion criteria, thus findings cannot be generalized to the general populations. The study only included women who were diagnosed with early stage breast cancer due to the decision making process for breast cancer that is still curable is suspected to be different from more advanced breast cancer. Lastly, the final model excluded 26 cases due to missing data which may over or underestimate the final results.
Conclusions
The strengths of this study and the potential implications of the study’s findings are beneficial to current body of cancer nursing literature. Previous studies used large national databases to assess adherence rates between racial groups. The use of large national databases present with limitations such as difficulty to control for extraneous factors or determine specific predictors to treatment decision making. The study identified that delay to treatment, health insurance, depression, and symptom severity as predictors to starting chemotherapy that can be potentially modified with interventions at the clinical setting. Additionally, the findings can be used to spearhead future research such as intervention studies that improve treatment decision making to adherence to treatment recommendations.
Acknowledgments
Disclosure of Funding:The National Institutes of Health, National Institute of Nursing Research National Research Service Award – Grant No. 5 F31 NR 011414-02, American Cancer Society’s Doctoral Degree Scholarship in Cancer Nursing, The Sigma Theta Tau, Alpha Epsilon Chapter.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Berg JS, Dischler J, Wagner D, Raia J, Palmer-Shevlin N. Medication compliance: a healthcare problem. Cincinnati: Harvey Whitney Books Co; 1993. [PubMed] [Google Scholar]
- 2.Sabate E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 3.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88(1):114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Woodward WA, Huang EH, McNeese MD, et al. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107(11):2662–2668. doi: 10.1002/cncr.22281. [DOI] [PubMed] [Google Scholar]
- 5.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Archives of Internal Medicine. 2006;166(20):2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 6.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. Journal of Clinical Oncology. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 7.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. Journal of Clinical Oncology. 2005;23(27):6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 8.Berger D, Braverman A, Sohn CK, Morrow M. Patient compliance with aggressive multimodal therapy in locally advanced breast cancer. Cancer. 1988;61(7):1453–1456. doi: 10.1002/1097-0142(19880401)61:7<1453::aid-cncr2820610729>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Dobie SA, Baldwin L-M, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. Journal of the National Cancer Institute. 2006;98(9):610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebovits AH, Strain JJ, Schleifer SJ, Tanaka JS, Bhardwaj S, Messe MR. Patient noncompliance with self-administered chemotherapy. Cancer. 1990;65(1):17–22. doi: 10.1002/1097-0142(19900101)65:1<17::aid-cncr2820650106>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Andic F, Godette K, O’Regan R, et al. Treatment adherence and outcome in women with inflammatory breast cancer. Cancer. 2010;117(24):5485–5492. doi: 10.1002/cncr.26187. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society. Breast Cancer Facts and Figures 2011–2012. Atlanta, GA: 2012. [Google Scholar]
- 13.Kane S, Shaya F. Medication non-adherence is associated with increased medical health care costs. Digestive Diseases and Sciences. 2008;53(4):1020–1024. doi: 10.1007/s10620-007-9968-0. [DOI] [PubMed] [Google Scholar]
- 14.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Medical Care. 2005;43(6):521. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 15.Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304(1):10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialist Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialist Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1992;339(8784):10–15. [PubMed] [Google Scholar]
- 18.Lee Y. Adjuvant chemotherapy (CMF) for breast carcinoma: patient’s compliance and total dose achieved. American Journal of Clinical Oncology. 1983;6:25–30. [PubMed] [Google Scholar]
- 19.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. Journal of Clinical Oncology. 2010;28(27):4120. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eraker SA, Kirscht JP, Becker MH. Understanding and improving patient compliance. Annals of Internal Medicine. 1984;100(2):258–268. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- 21.PASS User’s Guide: PASS 2000: Power Analysis and Sample Size for Windows [computer program] Kayville, UT: NCSS; 2000. [Google Scholar]
- 22.Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nursing Research. 1981;30(5):264–269. [PubMed] [Google Scholar]
- 23.Norbeck JS, Lindsey AM, Carrieri VL. Further development of the Norbeck Social Support Questionnaire: Normative data and validity testing. Nursing Research. 1983;32(1):4–9. [PubMed] [Google Scholar]
- 24.Cohen J, Lazarus R. Social support questionnaire. Berkeley: University of California; 1977. [Google Scholar]
- 25.Schutz W. FIRO-B. Consulting Psychologists Press; Palo Alto, CA: 1977. [Google Scholar]
- 26.Pargament KI, Koenig HG, Perez LM, Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol. 2000;56(4):519–543. doi: 10.1002/(sici)1097-4679(200004)56:4<519::aid-jclp6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Koenig HG, Pargament KI, Nielsen J. Religious coping and health status in medically ill hospitalized older adults. The Journal of Nervous and Mental Disease. 1998;186(9):513. doi: 10.1097/00005053-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pargament KI, Smith BW, Koenig HG, Perez L. Patterns of positive and negative religious coping with major life stressors. Journal for the Scientific Study of Religion. 1998:710–724. [Google Scholar]
- 29.Stager JL. The Comprehensive Breast Cancer Knowledge Test: validity and reliability. Journal of Advance Nursing. 1993;18(7):1133–1140. doi: 10.1046/j.1365-2648.1993.18071133.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT. The Memorial Symptom Assessment Scale Short Form (MSAS-SF) Cancer. 2000;89(5):1162–1171. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 32.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) Journal of Psychometric Research. 1999;46(5):437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 33.McNair DM, Lorr M, Droppleman LF. Profile of Mood States, POMS. Educational and industrial testing service; 1981. [Google Scholar]
- 34.Spielberger CD. State-trait anxiety inventory: Corsini Encylcopedia of Psychology. 2005 [Google Scholar]
- 35.Ware JE, Kosinski M, Keller SD. SF-36 physical and mental health summary scales: a users manual. Boston: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 36.Champion VL. Instrument development for health model constructs. Advances in Nursing Science. 1984;6(3):73–85. doi: 10.1097/00012272-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncology nursing forum. 1995;22:1355–1359. [PubMed] [Google Scholar]
- 38.SPSS advanced models 19.0 [computer program] Chicago, IL: SPSS inc; 2011. [Google Scholar]
- 39.Lipscomb J, Gillespie TW, Goodman M, et al. Black–white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Research and Treatment. 2012;133(1):285–296. doi: 10.1007/s10549-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 41.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. Journal of the National Cancer Institute. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 42.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89(11):2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Assessment of anxiety and depression in advanced cancer patients and their relationship with quality of life. Quality of Life Research. 2005;14(8):1825–1833. doi: 10.1007/s11136-005-4324-3. [DOI] [PubMed] [Google Scholar]
- 44.Boehmke MM, Dickerson SS. Symptom, symptom experiences, and symptom distress encountered by women with breast cancer undergoing current treatment modalities. Cancer Nursing. 2005;28(5):382–389. doi: 10.1097/00002820-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. Journal of Pain and Symptom Managment. 1999;18(4):233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 46.Payne R, Medina E, Hampton JW. Quality of life concerns in patients with breast cancer: evidence for disparity of outcomes and experiences in pain management and palliative care among African-American women. Cancer. 2003;97(1 Suppl):311–317. doi: 10.1002/cncr.11017. [DOI] [PubMed] [Google Scholar]
- 47.Badger TA, Braden CJ, Mishel MH. Depression burden, self-help interventions, and side effect experience in women receiving treatment for breast cancer. Oncology nursing forum. 2001;28(3):567–574. [PubMed] [Google Scholar]
- 48.Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107(10):2496–2503. doi: 10.1002/cncr.22274. [DOI] [PubMed] [Google Scholar]
- 49.Revenson TA, Pranikoff JR. A contextual approach to treatment decision making among breast cancer survivors. Health Psychology. 2005;24(4 Suppl):S93–98. doi: 10.1037/0278-6133.24.4.S93. [DOI] [PubMed] [Google Scholar]
- 50.Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient education and counseling. 2005;59(1):97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Research and Treatment. 2006;99(2):215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 52.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. European Journal of Cancer. 2006;42(14):2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Heath KV, Singer J, O’Shaughnessy MV, Montaner JSG, Hogg RS. Intentional nonadherence due to adverse symptoms associated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(2):211–217. doi: 10.1097/00126334-200210010-00012. [DOI] [PubMed] [Google Scholar]


