Abstract
In many species of social Hymenoptera, unmated workers can lay eggs that will produce males by parthenogenesis. Nevertheless, in queenright honey bee colonies (Apis mellifera), worker reproduction is low. One possible mechanism for this difference is worker policing, the removal of worker-laid eggs by other workers. This behavior can evolve in species in which queens are multiply mated, where workers are more closely related to the sons of their mother than those of their sisters. Another possible mechanism of the low level of worker reproduction is worker-laid eggs being less viable than queen-laid eggs. We show that this difference in quality is the case for honey bees.
Worker reproduction is low in honey bee (Apis mellifera) colonies with a queen (1, 2), because a suite of pheromones derived from the queen and the brood inhibits ovarian development in workers (3). Moreover, workers with developed ovaries are attacked by other workers (4). Nevertheless, a considerable proportion (≈4%) of workers can have functional ovaries (5) and can lay a substantial number (7%) of male eggs (6). Therefore, a crucial factor restricting successful worker reproduction in honey bees seems to be the removal of worker-laid eggs by other workers (worker policing) (7). Worker policing has been the focus of many theoretical and empirical studies in a wide range of species of social Hymenoptera (7–12). This behavior is considered adaptive in species in which queens mate multiply, causing workers to be on average more closely related to the son's of the queen than with sons of other workers (8, 9).
It has been postulated that queen honey bees mark their eggs with a queen-specific pheromonal label, providing the proximate cue for worker discrimination between queen-laid and worker-laid eggs (13). However, neither the source nor chemical nature of this postulated label has yet been identified (14–16). In contrast, the removal of diseased and dead brood by workers (hygienic behavior) is more fully understood (17). Workers remove dead brood within a few hours of death, an important factor in resisting disease (18). If there is a high mortality rate in worker-laid eggs, this alone would plausibly explain the removal of worker-laid eggs.
The question of a difference in the viability of queen-laid and worker-laid male eggs has been addressed in an in vitro incubation experiment (7) and yielded no significant differences between the two types of eggs. However, the egg viability was extremely, and probably abnormally, low for both types of eggs because of the in vitro experimental conditions in this study (7). This low viability may have masked normal differences in egg viability. We therefore examined the relative viability of queen-laid and worker-laid male eggs in vivo in natural brood nests of honey bee colonies. We also compared our viability data with egg removal data for queen-laid and worker-laid male eggs in the same colonies. The data show a striking correspondence between egg viability and egg removal, suggesting that hygienic behavior may be the prime factor driving the removal of worker-laid eggs.
Materials and Methods
Egg Viability. Unrelated, noninbred, equally strong A. mellifera carnica colonies (≈15,000 bees each) were used for the experiments. Worker-laid eggs, typically haploid (which would give males), were obtained from three queenless colonies, and queen-laid male eggs (haploid) were obtained from three queenright colonies. Combs containing eggs freshly laid (0–24 h) (7) were obtained from each of the source colonies. The eggs were counted in the combs, their positions were recorded on transparent plastic sheets, and the combs were covered with a mesh to prevent worker access to the eggs. Two mesh-covered test combs (one with queen-laid male eggs and one with worker-laid male eggs) were placed next to each other in the center of the brood nest in each of three queenright test colonies. After 96 h, the test combs were removed and the hatched larvae were counted. All larvae that did not hatch after the 96-h test period were considered dead, because honey bee larvae normally hatch after 72 h at most (19, 20). Because workers and queens lay different numbers of eggs, the number of eggs per test comb was between 32 and 575 (queen-laid eggs, 193 ± 152 per comb; worker-laid eggs, 219 ± 237 per comb).
Egg Removal. Following standard methods (7, 12, 21), egg-removal studies were performed simultaneously by using the same egg source colonies and the same queenright test colonies as in the egg-viability experiment. Twenty queen-laid and 20 worker-laid male eggs were transferred from each of the egg-source colonies into drone cells of test combs. The combs were not covered with wire mesh, thereby allowing worker access to the eggs. The combs were introduced into the brood nest area of each of the queenright test colonies (n = 60 queen-laid eggs and n = 60 worker-laid eggs) by placing them next to the viability test combs. Before the experiment started the brood nests of all three test colonies were moved above a queen excluder to prevent any interference by a laying queen. These policing test combs were briefly removed after 2, 4, and 24 h to count the number of remaining queen-laid and worker-laid eggs.
Results
Both studies, on egg viability and removal, performed simultaneously in the same colonies, gave significant differences between queen-laid and worker-laid eggs.
Egg viability was studied under natural honey bee colony conditions. We compared the viability of worker-laid male eggs and queen-laid male eggs by counting the number of larvae hatched within a 96-h time window after introducing the eggs. We found about four times as many larvae from queen-laid eggs compared with worker-laid male eggs (Fig. 1).
Fig. 1.
Proportions (mean ± SD) of hatched larvae from queen-laid and worker-laid male eggs in the three test colonies. There was no significant difference between the test colonies in the proportion of hatched larvae. Significantly more larvae hatched from queen-laid eggs (n = 581) than from worker-laid eggs (n = 1,317; χ2 = 122.94, df = 2, P < 0.001).
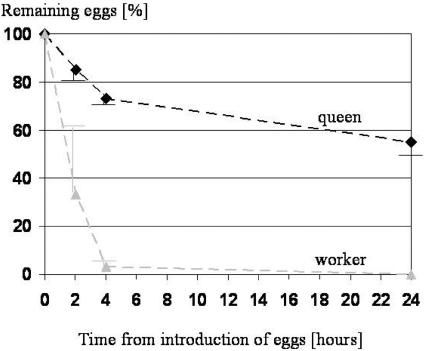
Queen-laid male eggs were removed significantly less than worker-laid male eggs (Fig. 2). All worker-laid eggs were removed after 24 h when 50–60% of the queen-laid eggs were still present. After introducing the eggs into the test colonies, the majority of both types of eggs were removed within the first 4 h. At the first observation point,2hafteregg introduction, 80–90% of queen-laid eggs and 25–40% of worker-laid eggs were left. The apparently different variance in the number of removed eggs could be the result of the variance in age within a time window of 24 h (eggs were laid between 0 and 24 h before introduction into the test colony).
Fig. 2.
Remaining queen-laid (diamonds) and worker-laid (triangles) male eggs (mean ± SD) after 0, 2, 4, and 24 h in the three test colonies. After 24 h, significantly more queen-laid eggs than worker-laid eggs remained (χ2 = 82.5, df = 2, P < 0.001).
Discussion
Our data strongly support earlier findings (22) of low viability of worker-laid eggs in the honey bee. Caste differences between queens and workers may underlie the obvious differences in egg viability. Honey bee queens are much more extensively fed with a protein-rich diet than are laying workers (23, 24), and an augmented protein intake has been shown to significantly enhance embryo development in honey bees (25). The difference in diet could also result in differences in the eggs abilities to resist dehydration (22) and, hence, viability. Ovarian development in queens is greatly superior to that in laying workers (24, 26) and may also foster high egg viability. Furthermore, in contrast to queens, workers often show imperfect oviposition (27), which perhaps damages the eggs (21). We cannot exclude the probability that some honey bee workers may lay eggs that fail to develop, similar to the trophic eggs common to other social insect species (28). In both cases this would lead to nonviable eggs.
Still another possibility could be that the competition among workers in the queenless colonies caused damage to the worker-laid eggs in these colonies. All of the worker-laid eggs are removed after 24 h, but only 80% of the removed eggs within our data set can be explained by the viability of the eggs. However, we only investigated the viability of the worker-derived brood until the end of the egg stage. Because the viability of larvae derived from worker-laid eggs is low and only 20% of the hatched larvae reach the pupal stage (29), egg and larva viability alone plausibly explains the “policing” phenomenon that only 0.1% (1) of the adult male offspring are worker-derived.
In light of the differential viability between queen and worker-laid eggs, we argue that the most parsimonious explanation for low levels of worker reproduction in queenright honey bee colonies is the worker hygienic behavior. Indeed, there are other clear descriptions of “worker policing” phenomenon when there are no relatedness benefits involved, e.g., in the Cape honey bee, where laying workers produce clonal female offspring (21), or when only single matings occur (Diacamma sp.) (30). Obviously, factors other than colony kin structure can govern worker reproduction in a wide variety of social insect species (21, 30–32).
We do not wish to, and cannot, exclude the existence of a potential honey bee queen egg-marking pheromone (13–15) acting as an honest signal for queen fertility (33), which would facilitate the removal of worker laid eggs. Such a signal could enhance colony efficiency (21) with respect to both hygiene and the production of males. However, to achieve an efficient removal of worker-laid eggs in a honey bee colony, it appears to be sufficient for a honey bee worker to simply discriminate between dead and live eggs.
Acknowledgments
We thank H. Velthuis and J. Gadau for fruitful discussions and C. Hepburn for comments on an earlier version of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (P.N. and R.F.A.M.) and the Bavarian government (C.W.W.P. and J.T.).
References
- 1.Visscher, P. K. (1989) Behav. Ecol. Sociobiol. 25, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Page, R. E. & Erickson, E. H. (1988) Behav. Ecol. Sociobiol. 23, 117–126. [Google Scholar]
- 3.Slessor, K. N., Foster, L. J. & Winston, M. L. (1998) in Pheromone Communication in Social Insects, eds. Vander Meer, R. K., Breed, M. D., Winston, M. L. & Espelie, K. E. (Westview, Boulder, CO), pp. 331–344.
- 4.Visscher, P. K. & Dukas, R. (1995) Anim. Behav. 49, 542–544. [Google Scholar]
- 5.Jay, S. C. (1968) Can. J. Zool. 46, 345–347. [Google Scholar]
- 6.Visscher, P. K. (1996) Behav. Ecol. Sociobiol. 39, 237–244. [Google Scholar]
- 7.Ratnieks, F. L. W. & Visscher, P. K. (1989) Nature 342, 796–797. [Google Scholar]
- 8.Woyciechowski, M. & Lomnicki, A. (1987) J. Theor. Biol. 128, 317–327. [Google Scholar]
- 9.Ratnieks, F. L. W. (1988) Am. Nat. 132, 217–236. [Google Scholar]
- 10.Foster, K. R. & Ratnieks, F. L. W. (2000) Nature 407, 692–693. [DOI] [PubMed] [Google Scholar]
- 11.Foster, K. R., Gulliver, J. & Ratnieks, F. L. W. (2002) Insectes Soc. 49, 41–44. [Google Scholar]
- 12.Martin, S., Beekman, M., Wossler, T. C. & Ratnieks, F. L. W. (2002) Nature 415, 163–165. [DOI] [PubMed] [Google Scholar]
- 13.Ratnieks, F. L. W. (1995) J. Apic. Res. 34, 31–37. [Google Scholar]
- 14.Katzav-Gozansky, T., Soroker, V. & Hefetz, A. (2002) Behav. Ecol. Sociobiol. 51, 588–589. [Google Scholar]
- 15.Oldroyd, B. P., Ratnieks, F. L. W. & Wossler, T. C. (2002) Behav. Ecol. Sociobiol. 51, 590–591. [Google Scholar]
- 16.Martin, S. J., Jones, G. R., Châline, N., Middleton, H. & Ratnieks, F. L. W. (2002) Naturwissenschaften 89, 528–532. [DOI] [PubMed] [Google Scholar]
- 17.Seeley, T. D. (1985) Honeybee Ecology: A Study of Adaptation in Social Life (Princeton Univ. Press, Princeton)
- 18.Rothenbuhler, W. C. (1964) Am. Zool. 4, 111–123. [DOI] [PubMed] [Google Scholar]
- 19.Nelson, J. A. (1915) The Embryology of the Honey Bee (Princeton Univ. Press, Princeton)
- 20.Wheeler, G. C. & Wheeler, J. (1979) in Social Insects, ed. Hermann, H. R. (Academic, London), pp. 287–338.
- 21.Pirk, C. W. W., Neumann, P. & Ratnieks, F. L. W. (2003) Behav. Ecol. 14, 347–352. [Google Scholar]
- 22.Velthuis, H. H. W., de Araujo Alves, D., Imperatriz Fonseca, V. L. & José, M. (2002) Proc. Exper. Appl. Entomol. NEV 13, 97–102. [Google Scholar]
- 23.Crailsheim, K. (1998) Apidologie 29, 97–112. [Google Scholar]
- 24.Velthuis, H. H. W., Ruttner, F. & Crewe, R. M. (1990) in Social Insects: An Evolutionary Approach to Castes and Reproduction, ed. Engels, W. (Springer, Berlin), pp. 231–243.
- 25.Paulcke, W. (1900) Zool. Jahrb. Abt. Anat. Ontog. Tiere 14, 177–202. [Google Scholar]
- 26.Eckert, J. E. (1937) J. Econ. Entomol. 30, 646–648. [Google Scholar]
- 27.Sakagami, S. F. (1958) Behaviour 13, 280–296. [Google Scholar]
- 28.Hölldobler, B. & Wilson, E. O. (1990) The Ants (Springer, Berlin).
- 29.Woyke, J. (1995) in Proceedings of the First International Electronic Conference on the Cape Bee Problem in South Africa, ed. Magnuson, P. (PPRI, Pretoria), pp. 74–75.
- 30.Kikuta, N. & Tsuji, K. (1999) Behav. Ecol. Sociobiol. 46, 180–189. [Google Scholar]
- 31.Foster, K. R., Ratnieks, F. L. W. & Raybould, A. F. (2000) Mol. Ecol. 9, 735–742. [DOI] [PubMed] [Google Scholar]
- 32.Hammond, R. L., Bruford, M. W. & Bourke, A. F. G. (2003) J. Evol. Biol. 16, 446–455. [DOI] [PubMed] [Google Scholar]
- 33.Gobin, B., Billen, J. & Peeters, C. (1999) Anim. Behav. 58, 1117–1122. [DOI] [PubMed] [Google Scholar]