Abstract
One of the most efficient mechanisms to optimize signal-to-noise ratios is the Lombard effect – an involuntary rise in call amplitude due to ambient noise. It is often accompanied by changes in the spectro-temporal composition of calls. We examined the effects of broadband-filtered noise on the spectro-temporal composition of horseshoe bat echolocation calls, which consist of a constant-frequency component and initial and terminal frequency-modulated components. We found that the frequency-modulated components became larger for almost all noise conditions, whereas the bandwidth of the constant-frequency component increased only when broadband-filtered noise was centered on or above the calls' dominant or fundamental frequency. This indicates that ambient noise independently modifies the associated acoustic parameters of the Lombard effect, such as spectro-temporal features, and could significantly affect the bat's ability to detect and locate targets. Our findings may be of significance in evaluating the impact of environmental noise on echolocation behavior in bats.
KEY WORDS: Acoustic communication, Audio-vocal integration, Bat echolocation, Lombard effect, Signal masking, Vocalization
INTRODUCTION
Acoustic communication signals invariably face the challenge of being masked by ambient noise. To facilitate signal transmission, animals have therefore evolved several strategies to increase the signal-to-noise ratio (SNR). One of the most efficient mechanisms in this context is the so-called Lombard effect, i.e. the involuntary rise in call amplitude in response to masking ambient noise (for review, see Brumm and Zollinger, 2011). First described in human communication, it has since also been found in birds and mammals, including bats. In both human speech and animal vocalizations, the Lombard effect is often accompanied by several additional changes in vocal parameters, such as rises in fundamental frequency and/or their spectro-temporal composition (for a review, see Hotchkin and Parks, 2013). However, it is still largely unknown how the adaptive changes in call amplitude are related to these associated vocal changes and how the underlying mechanisms are linked.
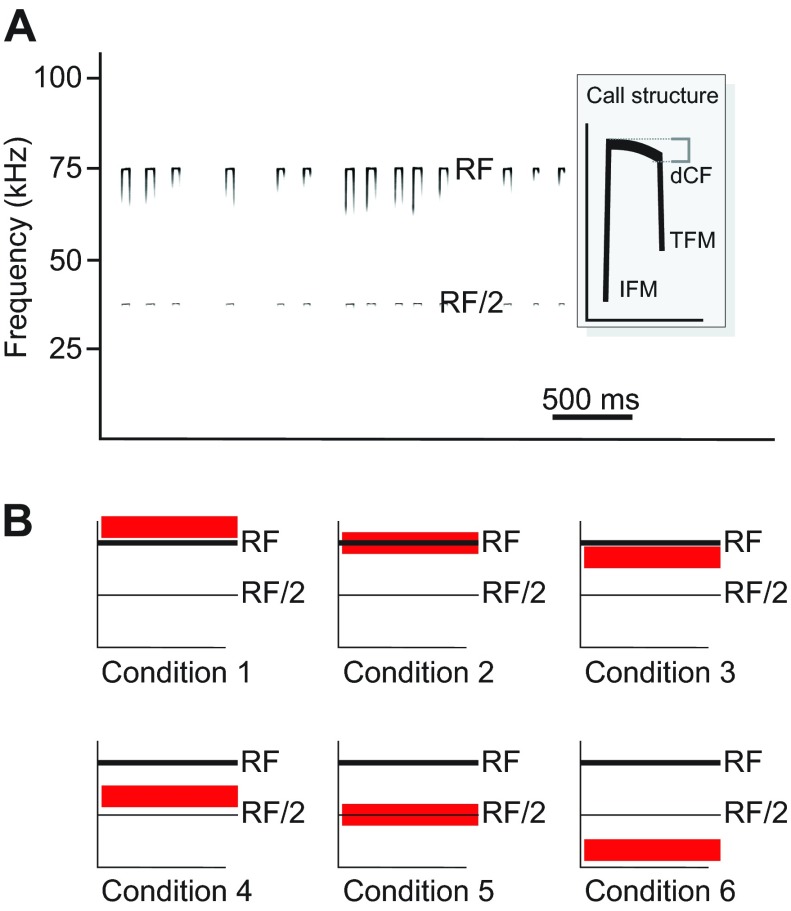
In a recent study on the Lombard effect in echolocating horseshoe bats (Hage et al., 2013), we showed that shifts in amplitude and frequency of their echolocation calls were controlled independently depending on which frequency band within the bat's hearing range was masked by bandpass-filtered noise (BFN; bandwidth 20 kHz). In horseshoe bats, echolocation calls are characterized by a long constant-frequency (CF) component, which normally represents the second harmonic of the calls (see Fig. 1A). The CF frequency emitted while the bat is perched (‘at rest’) is called the resting frequency (RF). This dominant CF portion is normally framed by two brief frequency-modulated (FM) components: an initial upward (IFM) and a terminal downward FM (TFM), each extending 5 to 15 kHz below the CF component (see Fig. 1A). During echolocation, FM portions serve in measuring target distance and location (Schnitzler, 1968), whereas the long CF components enable the bats to detect the rhythmic frequency modulations caused by the wing beats of flying insect prey (reviewed by Schnitzler and Denzinger, 2011; Fenton et al., 2012). During flight, the CF components of the echoes increase as a result of Doppler effects. Horseshoe bats compensate for these shifts by lowering the frequency of the subsequent calls. This so-called Doppler-shift compensation (DSC) behavior (Schnitzler, 1968) ensures that the echoes remain within the bat's best hearing range (Schnitzler and Denzinger, 2011).
Fig. 1.
Echolocation call characteristics and stimulus presentation. (A) Spectrograms of horseshoe bat echolocation calls showing the characteristic constant-frequency (CF) and frequency-modulated (FM) components without broadband-filtered noise (BFN) presentation (data from bat 2). Echolocation calls start with a brief initial FM (IFM) component and end with a brief terminal FM (TFM) component. The FM components surround a long (~40 ms) CF component, typically between 65 and 80 kHz in greater horseshoe bats. (B) Frequency ranges covered by BFN (red horizontal blocks; conditions 1–6). RF, resting frequency; RF/2, fundamental resting frequency. Condition 1: BFN extended 20 kHz above RF +500 Hz; condition 2: BFN centered around RF; condition 3: BFN extends 20 kHz below RF −500 Hz; condition 4: BFN centered 20 kHz below RF; condition 5: BFN centered around RF/2; condition 6: BFN presented between 10 and 30 kHz. This design allowed us to test the effects of noise on different portions of the bat's hearing range, such as RF (condition 2), the range in which Doppler-shifted echo frequencies occur (conditions 1 and 2), echolocation calls emitted during Doppler-shift compensation (conditions 3 and 4), communication calls (condition 5), and low-frequency ambient noise, such as noise caused by raindrops falling on vegetation or other surfaces as well as urban noise (condition 6).
So far, it was unknown how ambient noise affected the FM components and whether such noise-induced changes depended on the frequency bands masked. We therefore analyzed the effects of BFN on the spectro-temporal composition of greater horseshoe bat [Rhinolophus ferrumequinum (Schreber 1774)] echolocation calls by masking different frequencies within the bats' hearing range. Studying the effects of such masking of different frequency bands on the spectral composition of calls and how they may interact with each other and other call parameters, such as call duration, rate or amplitude, may yield a better understanding of both the phenomenology and the underlying neurobiological mechanisms (Brumm and Zollinger, 2011).
RESULTS AND DISCUSSION
We recorded echolocation pulses from three horseshoe bats emitted at rest under various masking conditions (see Fig. 1B) and examined the resulting changes in each of the frequency components of the echolocation calls, i.e. the bandwidths of IFM, TFM and CF (dCF). We compared dCFs between all conditions and selected 423 calls (bat 1: 137; bat 2: 140; bat 3: 146) that were randomly chosen from all conditions tested (mean ± s.e.=20±1 calls for each condition and bat). Median dCFs in the control condition (no BFN noise) were 450 Hz (bat 1), 525 Hz (bat 2) and 405 Hz (bat 3) and showed significant inter-individual differences in their ranges (Bartlett multiple-sample test, P<0.001) but not in their medians (Kruskal–Wallis test, P>0.05, d.f.=2, χ2=5.17). Median dCFs were significantly different between all conditions for all bats (see
List of abbreviations
- BFN
bandpass-filtered noise
- CF
constant frequency
- dCF
bandwidth of the constant frequency
- DSC
Doppler-shift compensation
- FM
frequency modulated
- IFM
initial frequency modulated
- RF
resting frequency
- SNR
signal-to-noise ratio
- TFM
terminal frequency modulated
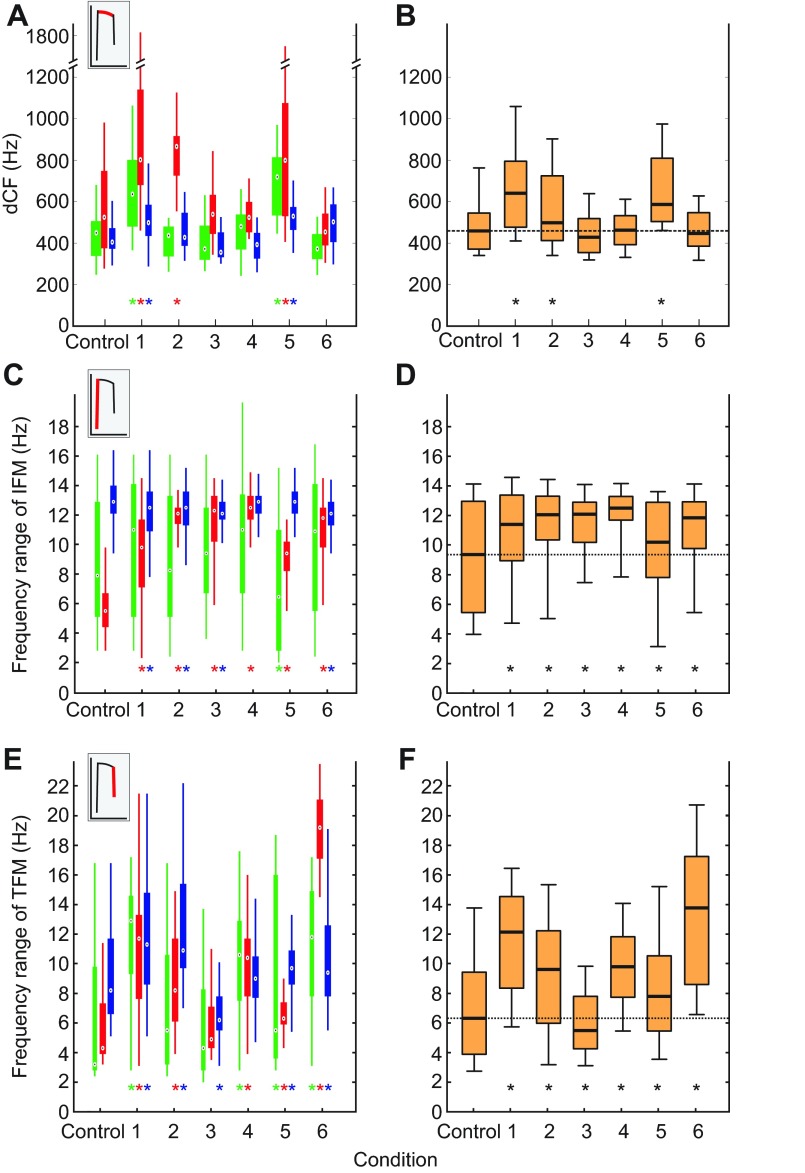
supplementary material Table S1). dCF widened in all bats when BFN was presented immediately above RF (condition 1) and when it was centered on the fundamental RF (RF/2; condition 5; Fig. 2A). In one bat, dCF also widened when BFN was centered directly on RF (condition 2). When averaged for all three bats, dCFs were significantly different between all conditions (see Fig. 2B and supplementary material Table S1). They widened when BFN was presented immediately above RF (condition 1) and when it was centered on the fundamental RF (RF/2; condition 5).
Fig. 2.
Distribution of CF bandwidth (dCF) and FM components in response to different BFN conditions and the control. (A) Distribution of dCF for all three bats (green, bat 1; red, bat 2; blue, bat 3) and (B) averaged for all three bats. (C) Distribution of IFM bandwidth for all three bats and (D) averaged for all three bats. (E) Distribution of TFM bandwidth for all three bats and (F) averaged for all three bats. Insets in A, C and E show appropriate call component analyzed. Medians, white dots (A,C,E) and horizontal bars (B,D,F) inside boxes; 1st and 3rd quartiles, upper and lower margins of boxes, respectively; 5% and 95% quantiles, end of vertical bar (A,C,E) and small vertical bars (B,D,F) above and below boxes, respectively. Asterisks indicate significant differences between the appropriate BFN conditions and the control.
To test for BFN-dependent changes in the FM components, we analyzed IFM and TFM bandwidths of 2434 calls (bat 1: 854; bat 2: 813; bat 3: 767) chosen randomly from all conditions tested (116±2 calls for each condition and bat). The median values for IFM and TFM, respectively, were 7900 and 3400 Hz for bat 1, 5500 and 4300 Hz for bat 2 and 12,900 and 8200 Hz for bat 3. Notably, the medians and ranges of both IFM and TFM exhibited significant inter-individual differences for the control condition (medians: Kruskal–Wallis test, P<0.001, IFM, χ2=174.5; TFM, χ2=70.7; ranges: Bartlett multiple-sample test, P<0.001, IFM and TFM). The medians of IFMs and TFMs were significantly different between all bats across all BFN conditions (see Fig. 2C,E and supplementary material Table S1).
When we compared the medians of the IFM bandwidths, we found that the median of IFM bandwidths increased significantly for all BFN conditions only in the bat that exhibited the lowest median IFM for the control (bat 2). The other two bats that already showed large bandwidths of IFMs for the control did not exhibit significant increases in IFM for all of the BFN conditions.
In all bats, the median TFMs increased when BFN was presented just above the RF (condition 1), centered on the RF/2 (condition 5), and far below the RF/2 (centered at 20 kHz; condition 6; post hoc Wilcoxon rank sum test). When BFN was centered on the RF (condition 2) or 20 kHz below the RF (condition 4), TFM increased in two bats (bats 2 and 3). BFN centered 10.5 kHz below the RF (condition 3) increased TFM only in bat 3.
In summary, we found that dCF increased when BFN masked frequencies immediately above the RF, within the range of Doppler-shifted echoes, but also when it was centered on the RF/2. The FM components increased their median bandwidths in almost all conditions, including low-frequency BFN noise (below 30 kHz).
When averaged for all three bats, the medians of IFMs and TFMs were significantly different across all BFN conditions as well (see Fig. 2D,F, supplementary material Table S1). Here, the median of both IFM and TFM bandwidths increased significantly for all but one BFN condition and decreased only for TFM bandwidths when we presented BFN below the RF.
Next, we tested the relationships between the dCF and the range of the IFM and TFM components to investigate whether changes in these components varied with each other or independently. We did not find any significant correlation between any of the investigated call components (Pearson's correlation; supplementary material Fig. S1A–C), indicating that the changes in the dCF as well as of the range of the IFM and TFM components occurred independent of each other.
Because IFM and TFM bandwidths correlate strongly with call duration (Schnitzler, 1968), the increased bandwidth might merely result from shorter signal durations produced during masking rather than be a direct response to BFN. Changes in call rate may also similarly affect the FM components. We therefore analyzed the call duration and rate of 2100 calls chosen randomly from all BFN conditions tested (100 calls for each condition and bat). Median values of duration and rate showed significant inter-individual differences for the control condition (medians: Kruskal–Wallis test, duration, P<0.001, χ2=100.7; rate, χ2=28.8; ranges: Bartlett multiple-sample test, duration and rate, P<0.001). Although median duration and rate differed significantly for some BFN conditions (see supplementary material Fig. S2, Table S1), the differences were small (mean: 4.8±1.1% of the median values) and inconsistent between the three bats. The population effect size (Hage et al., 2013), however, yielded shifts in duration and rate that were not different from the control condition except for duration in bat 3. Next, we tested the relationships between the spectral component bandwidths (CF, IFM and TFM) and duration and rate. We also investigated the relationship between the spectral component bandwidths and call amplitude to test for any potential effects of changes in SNR on FM component bandwidths [data of call amplitudes taken from Hage et al. (Hage et al., 2013)]. However, we did not find any significant correlation between any of the investigated call components (see supplementary material Fig. S1D–L).
In the current study, we examined the effect of noise on the spectro-temporal composition of echolocation calls in horseshoe bats by analyzing how BFN presented at different frequency bands within the bats' hearing range altered the spectro-temporal composition of their calls. We found that different noise conditions affected different call components (CF, IFM and TFM) differently, suggesting frequency-specific effects of auditory feedback for call production. Specifically, we found that the bandwidths of CF, IFM and TFM widened particularly when we masked the frequency range that is essential for DSC (conditions 1, 2 and 5). The magnitude of the observed changes in CF, IFM and TFM were virtually independent of each other. We therefore concluded that these changes were affected differently by the BFN conditions presented and are not merely the result of behavioral alterations caused by changes in the animals' attention. Interestingly, the effects of BFN on the various call components analyzed here were generally not correlated with changes in duration and rate. In addition, IFMs and TFMs were also affected by other frequencies, such as those far below the fundamental frequency components (below 30 kHz), which are often part of anthropogenic noise (e.g. Schaub et al., 2008).
Noise-dependent effects on the spectral composition of vocalizations have to date been investigated only in a few vertebrates, such as birds and bats, and are therefore still rather poorly understood. In birds, one study reported a general noise-dependent decrease of song complexity (McLaughlin and Kunc, 2013), and in echolocating free-tailed bats, the bandwidth of their FM calls decreased when 5 kHz noise masked the terminal call frequencies (Tressler and Smotherman, 2009). While it is unclear whether these changes significantly deteriorated signal transmission in these bats, a recent study reported noise-related deterioration in echolocation performance in greater mouse-eared bats (e.g. Schaub et al., 2008), although it remains unclear whether this was due to masking effects or a general distraction of the bat's attention. However, noise may have induced a deterioration of signal transmission in birds: the degree of song complexity changes in response to different noise levels was directly correlated with the likelihood of the bird of moving away from the noise source (McLaughlin and Kunc, 2013). The noise-dependent changes in the spectro-temporal composition of echolocation calls, however, could significantly affect the bat's ability to detect and locate targets. Horseshoe bats use the CF component to detect the frequency modulations produced by wing beats of insect prey (reviewed by Schnitzler and Denzinger, 2011; Fenton et al., 2012). In contrast, they use FM components to determine target distance and location (Schnitzler, 1968). Any noise-induced changes in these call components could therefore affect the bats' echolocation performance. Hence, were the observed changes in the composition of the calls detrimental to echolocation performance or did they somehow improve the SNR? Widening of the dCF would presumably lower their chances of detecting the presence of wing-beating insects; its behavioral significance is therefore unclear. In contrast, widening TFMs in noisy environments may perhaps make these insects more conspicuous and increase SNRs. Additionally, widening IFMs in such masking conditions may even further improve SNR by adding yet another marker for target measurements.
Nevertheless, only further tests will reveal whether noise-induced changes in echolocation calls indeed affect echolocation performance. Furthermore, it remains to be seen whether noise-induced spectrotemporal changes in echolocation calls also occur in bats that produce brief FM calls with low repetition rates, so-called low duty cycle echolocators (Fenton et al., 2012), and how it affects their echolocation performance. Preliminary evidence suggests that, at least in free-tailed bats, masking noise may have some effect on echolocation call structure (Tressler and Smotherman, 2009). Recently developed telemetry systems that allow recording echolocation behavior ‘on-board’ (Hiryu et al., 2010) in several freely flying bat species may aid in tackling this question.
Conclusions
The spectro-temporal compositions of echolocation calls experienced significant changes when masked by BFN. Most notably, we found that CF and FM components were affected differently by different BFN conditions. While bandwidths of the FM components increased for almost all BFN conditions, the bandwidth of the CF component increased only when BFN was centered on or above the calls' dominant or fundamental frequency. These different effects of BFN on CF and FM components of the bats' calls indicate that not only the Lombard effect itself, but also its associated acoustic parameters, are controlled by different neuronal mechanisms or circuits.
MATERIALS AND METHODS
Animals
We used three greater horseshoe bats, Rhinolophus ferrumequinum (two male, one female), collected in the People's Republic of China. All procedures were in accordance with National Institutes of Health guidelines for experiments involving vertebrate animals and were approved by the University of California Los Angeles Animal Research Committee.
Experimental design
Animals were acoustically stimulated with continuous BFN (bandwidth 20 kHz, 100 dB SPL) as described previously (Hage et al., 2013). Briefly, BFN was produced by digitally band-pass filtering broadband noise to a bandwidth of 20 kHz (Tucker-Davis Technologies) with sharp flanks (bandwidth of BFN stimuli at −10 dB: <21 kHz). To cover most of the bats' hearing range, the 20 kHz BFN noise stimuli were centered around different frequencies as indicated in Fig. 1B. BFN stimuli had amplitudes of 100 dB SPL. All BFN stimuli were presented under acoustic free-field conditions through an electrostatic speaker (ED1, Tucker-Davis Technologies), which was placed approximately 20 deg laterally and 10 cm in front of the bat's left ear. Echolocation calls emitted when no noise was presented served as controls.
Data acquisition and analysis
Echolocation pulses were captured by a condenser microphone (4939 with preamplifier 2633, Brüel & Kjær) placed 15 cm ahead of the bat's head. Recorded calls were digitized with a Mikro1401mkII system and Spike2 software (Cambridge Electronic Design; 16 bit resolution, sample rate 200 kHz). Echo mimics were generated by playing back emitted calls with a 4 ms delay and 30 dB attenuation (produced electronically with the Tucker-Davis Technologies system) through an ultrasonic loudspeaker positioned right next to the electrostatic speaker presenting the BFN stimuli. The bats were mildly restrained in a soft body mold with the animals' head remaining mobile, as described previously (Hage et al., 2013). Echolocation pulses were recorded during each of the different noise conditions, which were presented pseudo-randomly for 30 s (one session per day) for four different, non-consecutive days. Groups of calls, so-called ‘doublets’ or ‘triplets’ (Schnitzler, 1968), occurred very rarely throughout the recordings and were excluded from the analysis.
SASLab Pro (Avisoft Bioacoustics) was used to measure bandwidths of CF, IFM and TFM. dCFs were measured 10 dB below the peak frequency of the respective component as determined in the calls' power spectra (frequency resolution: 3 Hz). IFM and TFM bandwidths were measured as follows: peak frequencies at the start, center and end of each echolocation pulse were measured automatically by calculating a fast Fourier transformation (512 points with 87.5% overlap, frequency resolution 391 Hz). Thresholds for component separation were 25 dB below center and 26 dB below start and end peak frequency. Two thresholds were set for the component separation. A threshold of −26 dB was used for component detection and a threshold of −25 dB was used to determine the start and end point of the respective component. Then, the actual bandwidth of IFM and TFM was calculated automatically using the difference between the center and start peak frequency for IFM and the center and end peak frequency for TFM. Custom-written software (MATLAB, MathWorks) was used to detect call onsets and offsets for calculating duration and rate.
Statistical analysis
We used non-parametric tests to analyze significant differences in medians and ranges, respectively, of each of the call components for all BFN conditions and the control (P<0.05 for initial tests, P<0.01 for post hoc tests). The Pearson's correlation yielded correlations between call components (P<0.05).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Brock Fenton and an anonymous reviewer for helpful comments on an earlier version of the manuscript.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
Support was provided by the Deutsche Forschungsgemeinschaft to S.R.H. (Ha 5400/1-1), the Chinese National Natural Science Foundation to J.F. (31030011) and T.L.J. (31100280), and the Chinese Program for Introducing Talents to Universities (B07017) and the National Insitutes of Health to W.M. (DC5400). Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.102855/-/DC1
References
- Brumm H., Zollinger S. A. (2011). The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 148, 1173-1198. [Google Scholar]
- Fenton M. B., Faure P. A., Ratcliffe J. M. (2012). Evolution of high duty cycle echolocation in bats. J. Exp. Biol. 215, 2935-2944. [DOI] [PubMed] [Google Scholar]
- Hage S. R., Jiang T., Berquist S. W., Feng J., Metzner W. (2013). Ambient noise induces independent shifts in call frequency and amplitude within the Lombard effect in echolocating bats. Proc. Natl. Acad. Sci. USA 110, 4063-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiryu S., Bates M. E., Simmons J. A., Riquimaroux H. (2010). FM echolocating bats shift frequencies to avoid broadcast-echo ambiguity in clutter. Proc. Natl. Acad. Sci. USA 107, 7048-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkin C., Parks S. (2013). The Lombard effect and other noise-induced vocal modifications: insight from mammalian communication systems. Biol. Rev. Camb. Philos. Soc. 88, 809-824. [DOI] [PubMed] [Google Scholar]
- McLaughlin K. E., Kunc H. P. (2013). Experimentally increased noise levels change spatial and singing behaviour. Biol. Lett. 9, 20120771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub A., Ostwald J., Siemers B. M. (2008). Foraging bats avoid noise. J. Exp. Biol. 211, 3174-3180. [DOI] [PubMed] [Google Scholar]
- Schnitzler H. U. (1968). [The ultrasonic sounds of horseshoe bats (Chiroptera, Rhinolophidae) in different orientation situations]. Z. Vgl. Physiol. 57, 376-408. [Google Scholar]
- Schnitzler H. U., Denzinger A. (2011). Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. J. Comp. Physiol. A 197, 541-559. [DOI] [PubMed] [Google Scholar]
- Tressler J., Smotherman M. S. (2009). Context-dependent effects of noise on echolocation pulse characteristics in free-tailed bats. J. Comp. Physiol. A 195, 923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.