Abstract
Classical Chinese pharmacopeias describe numerous excellent herbal formulations, and each prescription is an outstanding pool of effective compounds for drug discovery. Clarifying the bioactivity of the combined mechanisms of the ingredients in complex traditional Chinese medicine formulas is challenging. A classical formula known as Qingfei Xiaoyan Wan, used clinically as a treatment for prevalent chronic lung disease, was investigated in this work. A mutually enhanced bioactivity-guided ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS) characterization system was proposed, coupled with a dual-luciferase reporter assay for β2AR-agonist cofactor screening. Arctiin, arctigenin, descurainoside and descurainolide B, four lignin compounds that showed synergistic bronchodilation effects with ephedrine, were revealed. The synergistic mechanism of arctigenin with the β2ARagonist involved with the reduction of free Ca2+ was clarified by a dual-luciferase reporter assay for intracellular calcium and the Ca2+ indicator fluo-4/AM to monitor changes in the fluorescence. The relaxant and contractile responses of airway smooth muscle are regulated by crosstalk between the intracellular cAMP and calcium signaling pathways. Our data indicated the non-selective βAR agonist ephedrine as the principal bronchodilator of the formula, whereas the lignin ingredients served as adjuvant ingredients. A greater understanding of the mechanisms governing the control of these pathways, based on conventional wisdom, could lead to the identification of novel therapeutic targets or new agents for the treatment of asthma and COPD.
Introduction
In recent decades, biotechnology has provided novel approaches to drug development and generated new classes of biological therapeutics. Researchers have focused on drug discovery, using an important group of complementary and alternative therapeutics, herbal medicines and botanical sources [1], [2]. Traditional Chinese medicines (TCMs) have attracted increased global attention because they are outstanding pools of effective compounds for drug discovery with long clinical use and reliable therapeutic efficacy [3]. Classical Chinese pharmacopeias describe many excellent herbal formulations used to treat various diseases, particularly chronic conditions [4]. TCM formulas are prescribed so that each herb is used to its greatest potential, thus improving the treatment results and reducing any adverse effects caused by combined herbal drugs [5]. The efficacy of TCM is attributed to the complex mixture of chemical compounds present in the various herbs. The principal ingredient is the substance that provides the main therapeutic effect and is included in the monarch drugs. The second principal ingredient enhances or assists the actions of the first and is generally a minister or assistant drug [6]. Combinatorial medicines have been gaining acceptance in the West, and examples of these combinations include the drug cocktails used to treat acquired immunodeficiency syndrome and cancer, as well as complex antibiotic medications [7], [8]. These therapeutics contain a limited number of pure compounds that have been well characterized. TCMs are notably more challenging because of the variability of the individual herbs and the chemical complexities of the formulations. Isolation methodologies and the characterization of bioactive compounds from plant resources have recently undergone rapid development [9], [10]. Combined with a target-based reporter gene assay, bioactivity-guided ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS) has been applied to screen receptor agonists or inhibitors from botanical drugs [11], [12]. Compared to conventional methods, this powerful tool facilitates the screening and identification of potential lead compounds in complex herbal extracts. The synergistic interaction between TCM ingredients has not been revealed by these strategies.
Asthma and chronic obstructive pulmonary disease (COPD) are the two most common chronic lung diseases worldwide, and they could become the third leading cause of death by 2030 [13], [14]. The pharmacological treatments, as summarized in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for managing stable COPD, include bronchodilators, β2-adrenergic receptor (β2AR) agonists and inhaled glucocorticosteroids [15]. Modulating multiple biological targets together, rather than regulating a single-target, could be beneficial for the treatment of diseases with complex etiologies. Concurrently, complementary and alternative therapies are increasingly important in treating complex diseases because they could act on multiple targets in the disease network [16], [17]. TCMs differ from therapeutics based on single-chemical entities. A number of herbal medicines and ancient TCM prescriptions are novel therapeutic regimens against COPD or asthma [18]–[21]; naturalβ2AR agonists or antagonists participate in the regulation process, and TCM prescription compatibility enhances the regulation pathway more effectively [22].
Qingfei Xiaoyan Wan (QFXY) evolved from a classical TCM prescription that has been used to treat pulmonary diseases since 200 B.C., known as Maxing Shigan decoction. QFXY consists of the following eight herb preparations: Ephedra Herba as a monarch drug, Saigae Tataricae Cornu, Pheretima, Arctii Fructus, Lepidii Semen, Bovis Calculus Artifactus, Armeniacae Semen Amarum, and Gypsum Fibrosum as minister or assistant drugs. In clinical practice, QFXY has a good clinical therapeutic effect against COPD, asthma, and lung inflammation because it relieves various respiratory symptoms. In a previous work, a UPLC/Q-TOF-MS system using two-cell-based dual-luciferase reporter assays was established to screen for NF-κB inhibitors and β2AR agonists, and four types of compounds (arctigenin derivatives, cholic acid derivatives, chlorogenic acid, and sinapinic acid) with anti-inflammatory activity as well as one β2AR agonist, ephedrine, were identified [23]. Although the bronchodilation effect of QFXY could be partially blocked by a classicalβ receptor antagonist, propranolol (Pro), the smooth muscle relaxant effect of QFXY exceeds that of an identical dose of ephedrine, possibly because the biological activities of QFXY result from a mixture of active compounds rather than from ephedrine alone. A mutually enhanced bioactivity-guided UPLC/Q-TOF-MS characterization system for β2AR-agonist cofactors was proposed in this paper to determine the rationale for the formula of QFXY. Several ingredients that have synergistic effects with ephedrine on the β2AR/cAMP signal pathway were suggested, and the efficacies and mechanisms were tested.
Materials and Methods
1. Reagents and Materials
HPLC-grade solvents were purchased from Tedia (Fairfield, CA, USA). Deionized water was purified using a Milli-Q system (Millipore Laboratory, Bedford, MA, USA). Commercial QFXY (lot no. 5230139) was purchased from Darentang Pharmaceutical Company (Tianjin, China). Ephedra herbal samples (lot no. 1105139131) were purchased from Anguo Changan Limited Company (Anguo, Hebei, China). A standard arctigenin (Atg) sample was obtained from Tianjin Institute of Pharmaceutical Research (Tianjin, China). Ephedrine hydrochloride was purchased from Yifang S&T (Tianjin, China). Salbutamol was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fluo-4, in the form of acetoxymethyl ester (Fluo-4/AM), and Lipofectamine 2000, a transfection reagent, were purchased from Invitrogen (Carlsbad, CA, USA). Two Luc2p reporter plasmids, pGL4.29 and pGL4.30, and a Renilla luciferase reporter vector, pRL-TK plasmid, were obtained from Promega (Madison, WI, USA). The β2AR-transfected human embryonic kidney 293 (β2AR-HEK 293) cells were grown in our laboratory [24]. Hanks' balanced salt solution (HBSS) (Ca2+ and Mg2+ free), Dulbecco's modified Eagle medium (DMEM), and fetal bovine serum (FBS) reagents for the cell culture were purchased from HyClone (UT, USA). The remaining reagents were of analytical grade.
2. Sample Preparation and UPLC Separation
The ephedra herbal samples (100 g) were powdered and soaked in 1 L of 0.1 mol/L HCl overnight; after a 30-min ultrasonic treatment, the supernatant was filtered and adjusted to a pH of 11. Subsequently, the samples were passed through a column (2×20 cm) containing D151 macroporous resin and eluted with 0.1 mol/L HCl. The eluate was freeze-dried to provide 0.388 g of ephedra extract (EE). QFXY (1 g) were crushed and dissolved in 10 ml of methanol under ultrasonic conditions. After passage through a 0.22-µm filter, the filtrate was frozen at −20°C for 12 hours and centrifuged at 13,000 rpm for 10 min at 4°C to remove the excipient polyethylene glycol precipitation; the supernatant was used for the analysis.
3. Ethics Statements
The animal experiments were strictly performed under the guide lines for the treatment of laboratory animals of Nankai University, and the animal protocols were approved by the Institute Research Ethics Committee at Nankai University. To minimize animal suffering, the test animals were sacrificed by cervical dislocation after the experiments.
4. Histamine-Induced Asthma Model in Guinea Pigs
A histamine (His)-induced guinea pig asthma model was developed using standard methods [25]. Thirty male Hartley strain guinea pigs (approximately 300 g) with identical latent periods of His-stimulated asthma were divided into 6 groups during the preliminary experimental period. During the experiment, the animals were kept in an inhalation cage consisting of 3 boxes. An animal was placed into box A for drug administration using an ultrasonic nebulizer to atomize the test drug solutions. The drug was atomized at 0.5 ml/min and disseminated into box A. The animal was kept in box A for 1 min under spontaneous breathing during the administration of the atomized test drugs or normal saline. Box B served as a sluice through which the animal was passed into box C. In box C, the animal was exposed to an atomized 0.1% His solution for 20 s. His was atomized at 0.5 ml/min and disseminated into box C. The animal was subsequently withdrawn from the inhalation cage. The time that elapsed until the appearance of an asphyxial convulsion was considered the latent asthma period. The increasing rate of the latent period was calculated using Vogel's method, and the response of the pre-experimental group was recorded as 100%. The rate of increase in the latent period (%) for the test group was used to evaluate the activity.
5. The Relaxant Test on the Guinea Pig Tracheal Muscle
In vitro spasmolytic activity tests on isolated tracheas were conducted, as previously described, with a slight modification [26]. The tracheal strips were mounted vertically in a 20-ml water-jacketed organ bath filled with Krebs-bicarbonate buffer under 95% O2 and 5% CO2 at 37°C. Before each experiment, each strip was subjected to a 1 g load for at least 1 h with frequent changes of the Krebs-bicarbonate buffer until a stable baseline tension was obtained. The strips were washed thoroughly with Krebs-bicarbonate buffer immediately after the peak tension developed and remained unstimulated until a stable baseline tension was obtained. In every experiment, 200 µL of each drug was added to the organ bath, and all of the drug concentrations were expressed as the final concentration; then the test samples were added at 10 min per series of acetylcholine (Ach) concentrations. The peak contractile response was recorded as 100%. The half-maximal effective concentration values of Ach for the tensions were expressed as 50% of the peak contractile response (EC50) and were used to evaluate the activity.
6. UPLC/Q-TOF Analysis
A Waters Acquity UPLC instrument system (Waters Co., USA) equipped with a photo diode array detector (DAD) (190–400 nm) was used for the analysis, and the system was controlled by MassLynx V4.1 software (Waters Co., USA). The separations were performed using a Waters Acquity BEH C18 column (2.1 mm×100 mm, 1.7 µm). A gradient elution of 1% formic acid solution (A) and CH3CN (B) was performed as follows: 2% B maintained from 0–10 min, 10–40% B from 10–15 min, 40–95% B from 15–26 min, and 95–100% from 26–35 min. The flow rate was 0.40 ml/min, and the column temperature was 30°C. The injection volume was 1.0 µL. The UPLC effluent was split 1∶9 after the substance separation. The 10% fraction was directed toward the Q-TOF-MS for the structural analysis. The 90% fraction was directed toward the diode array detector and was collected into a 96-deep-well plate (2.2 ml) every 0.5 min. After being evaporated to dryness in a 40°C vacuum drying oven, the residues were dissolved in cell culture medium (100 µL) for the luciferase reporter activity assay.
Accurate mass measurements and MS/MS were performed using a Waters Q/TOF Premier with an ESI system (Waters, Manchester, UK). The ESI-MS spectra were acquired in the negative and positive ionization modes. The capillary voltage was 3.0 kV for the negative mode and 2.5 kV for the positive mode. The sample cone voltage during the positive ion mode was set to 30 V, whereas that during the negative ion mode was set to 45 V; high-purity nitrogen was used as the nebulization and auxiliary gas. The nebulization gas was set to 600 L/h at 350°C, the cone gas was set to 50 L/h, and the source temperature was 100°C. The Q/TOF Premier acquisition rate was 0.1 s with a 0.02-s inter-scan delay. Argon was utilized as the collision gas at 5.3×10−5Torr. The instrument was operated with the first resolving quadrupole in the wide-pass mode (50–1200 Da), whereas the collision cell operated at two alternative energies (i.e., 5 and 30 eV). Leucine encephalin amide acetate was used as the lock mass ([M−H]− = 553.2775, [M+H]+ = 555.2931) at a concentration of 200 pg/µL and was added at 20 µL/min.
7. Luciferase Reporter Assay
The β2AR-HEK 293 cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL) containing 10% fetal bovine serum (FBS) (Gibco BRL), 100 U/ml penicillin and 0.1 mg/ml streptomycin at 37°C and 5% CO2. For the β2AR/cAMP activity analysis, the culture medium was replaced after 24 h, and the cells were co-transfected with 100 ng/well pCRE-Luc reporter plasmid pGL4.29 and 16 ng/well Renilla luciferase reporter vector pRL-TK plasmid, as previously described [27]. For the intracellular calcium concentration detection, the β2AR-HEK 293 cells were co-transfected with 100-ng/well pGL4.30 Luc plasmid and 10 ng/well Renilla luciferase reporter vector pRL-TK plasmid, according to the manufacturer's protocol. After 24 h of transfection, the cells were then pretreated with different drugs and stimulated by ionomycin (1 mmol/L) combined with 12-myristate 13-acetate (PMA, 1 mg/ml) for 9 h for the cell bioactivity assay. The cells were washed, lysed, and assayed for luciferase activity using a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. The relative luciferase activity was obtained by normalizing the firefly luciferase activity against the activity of the internal Renilla luciferase control (Modulus, Turner BioSystems, USA). The ratio of the firefly luciferase activity to the renilla luciferase activity was used to normalize the differences in the transfection efficiency.
8. Apparent Embodiment of Intracellular Ca2+
Human bronchial smooth muscle cells (HBSMC) were obtained from Sciencell (cat. #3400) and cultured in DMEM supplemented with 10% FBS. The HBSMCs were loaded with Ca2+ indicator dye Fluo-4/AM according to the manufacturer's instructions. The cells were incubated in 1 ml of HBSS (Ca2+ and Mg2+ free) containing 5 µM Fluo-4/AM for 30 min at 37°C. After loading, to de-esterify the Fluo-4/AM, the cells were washed with HBSS to remove excess dyes and then equilibrated for 30 min. The changes in the fluorescence intensity of the Fluo-4-Ca2+ complex were monitored by confocal microscopy (TCS SP5, Leica, Germany). The stained cells were treated with samples immediately before the confocal microscopy, and the intracellular [Ca2+]i changes at 494/516 nm (Ex/Em) were recorded. The [Ca2+]i curve was analyzed in the single-cell mode.
9. Statistical Analysis
The results are expressed as the standard error of the mean (SEM). Multiple comparisons were performed using ANOVA, followed by Bonferroni's post hoc test. For single comparisons, the significant differences between the means were determined using Student's t-test. P<0.05 was considered statistically significant.
Results and Discussion
1. The Bronchodilator Effect of QFXY
The guinea pig asthma model was established to validate the bronchodilation effect of high, middle and low doses of QFXY (397.5, 132.5, and 44.1 mg/100 g, respectively) and EE (0.146 mg/100 g)which containing the same dose of ephedrine (about 21.9 µg) with the middle dose QFXY. As shown in Figure 1A, 10−6 mol/L salbutamol (Sal) used as a positive control prolonged the latent period by approximately 1.48-fold. QFXY inhibited asphyxial convulsions in a concentration- dependent manner. The increasing rates of the high, middle and low doses were approximately 1.76-, 1.07-, and 0.22-fold, respectively. The EE contained the identical dose of ephedrine as the middle dose of QFXY and prolonged the latent period only 0.46-fold. Additionally, the identical effect was observed on the guinea pig tracheal muscle relaxant test, and the EC50 of the middle QFXY dose was more effective than the EE extract (Figure 1B). Differing from Sal and the EE extract, the relaxant effect of QFXY was not completely inhibited by 10−7 mol/L Pro. As a result, QFXY was more effective for reducing the severity of bronchoconstriction after exposing His or Ach stimulation than when the ephedrine extract was used alone. In addition to the monarch drug (Herba Ephedrae), other ingredients played a role in the anti-asthmatic action.
Figure 1. The bronchodilator effect of QFXY on an atomized His-induced guinea pig asthma model (A) and the guinea pig tracheal muscle relaxant test (B).
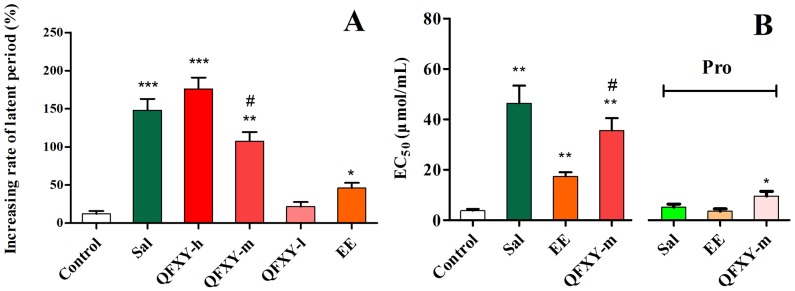
The values are presented as the mean ± SEM (n = 5). *P<0.05, **P<0.01, ***P<0.001, compared to the control group; # P<0.05, middle dose QFXY compared to the EE group.
2. Screening Synergistic Bronchodilators from QFXY
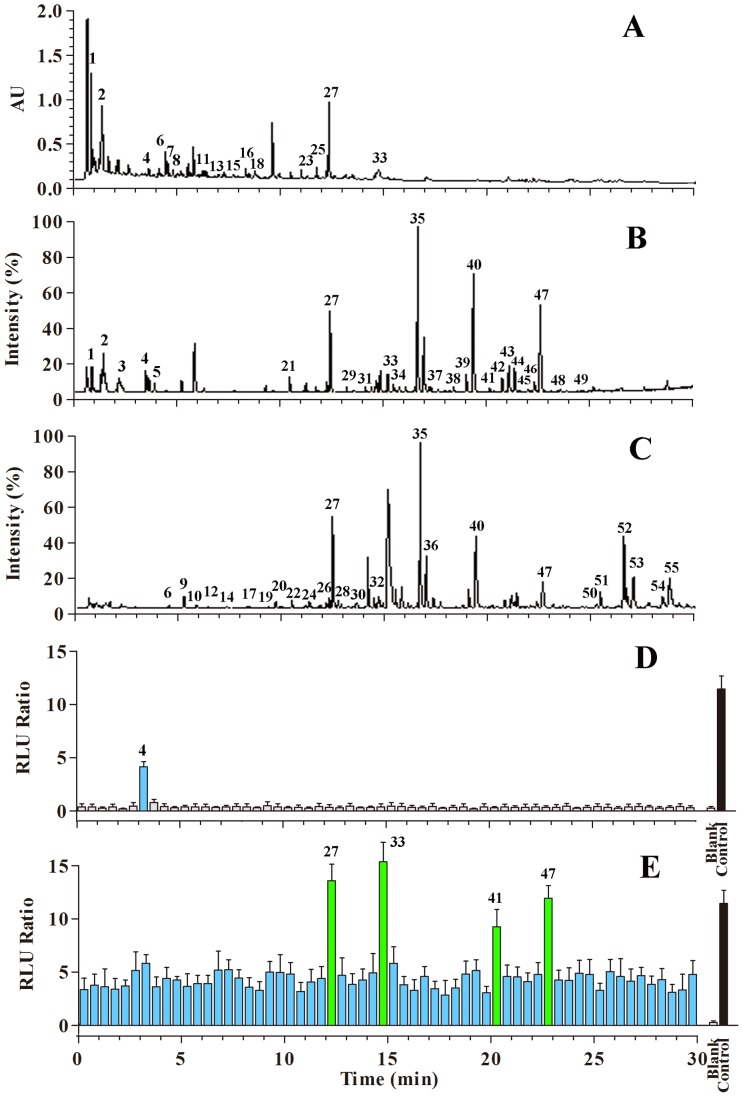
The optimal UPLC conditions were applied for purifying the components of QFXY (Figure 2A). The total ion current chromatograms collected in the positive and negative ESI modes are shown in Figure 2B and 2C, respectively. The [M+H]+ and [M−H] − ions were obtained with as much relevant information as possible to confirm the molecular weight and structure of the constituents. Finally, 55 compounds were identified in QFXY (the data are not shown). As shown in Figure 2D, only peak no. 4 (ephedrine, Eph) has been identified as a β2AR agonist; the synergistic ingredient for bronchodilation remains unknown.
Figure 2. UPLC/Q-TOF-MS and synergic β2AR activation-bioactivity analysis of QFXY.
(A) The UPLC/UV chromatograms of QFXY observed at 254 nm; (B, C) The TIC chromatograms in (B) the positive and (C) negative ESI modes; (D) The bioactivity chromatograms obtained using the dual-luciferase reporter assay system for β2AR activation; (E) The mutually enhanced bioactivity chromatograms obtained using the dual-luciferase reporter assay system for β2AR activation.
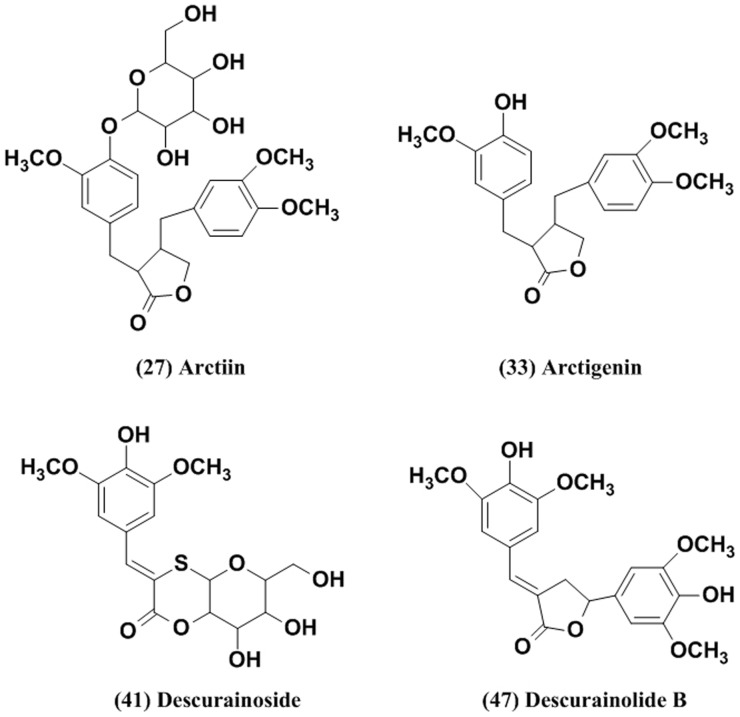
To reveal the cofactors, a modified system incorporating a dual-luciferase reporter validation system was established. After incubation with every UPLC effluent sample for 3 hours, the identical dose of Eph (10−5 mol/L) was added to each well; the samples were incubated 3 hours and subjected to the luciferase reporter activity assay. Consequently, four fractions (corresponding peak no. 27, 33, 41 and 47) with significant synergistic effects for the β2AR agonist were identified (Figure 2E). Peak 47 (22.425 min) was selected to illustrate the identification approach. The base peak in the positive ESI mode was m/z 403.1401 and was confirmed to be [M+H]+. The elemental and possible molecular compositions were deduced using the exact molecular weight. These molecular compositions were assessed using Ref. [28], and only descurainolide B (C21H22O8) was revealed as the most probable compound from Lepidii Semen. The other constituents were identified as arctiin and Atg (from Arctii Fructus) as well as descurainoside (from Lepidii Semen) using the identical approach; the detailed fragment information is listed in Table 1.
Table 1. The MS/MS data in both ESI modes and the identification of the compounds in QFXY possessing synergic effects on the β2AR-signaling pathway.
| Peak No. | t R(min) | Identification | Mode | m/z | MS2 | Composition | Herb |
| 4 | 3.985 | Ephedrine | Pos | 166.1235 | 331[2M+H]+,166[M+H] +,148[M+H-H2O]+,117[M+H-H2O-NHCH3]+ | C10H15NO | EH |
| 27 | 12.444 | Arctiin | Pos | 535.2139 | 535[M+H]+,491[M+H-CO2]+,373[M+H-Glu]+,339[M+H-Glu-2OH]+ | C27H34O11 | AF |
| 33 | 15.256 | Arctigenin | Pos | 373.1644 | 373[M+H]+,355[M+H-H2O]+,337[M+H-2H2O]+,324[M+H-H2O-OCH3]+ | C21H24O6 | AF |
| 41 | 20.412 | Descurainoside | Pos | 401.0911 | 401[M+H]+,383[M+H-H2O]+,365[M+H-2H2O]+,333[M+H-2H2O-CH3OH]+ | C17H20O9S | LS |
| 47 | 22.425 | Descurainolide B | Pos | 403.1401 | 403[M+H]+,371[M+H-CH3OH]+,353[M+H-CH3OH-H2O]+,321[M+H-2CH3OH-H2O]+ | C21H22O8 | LS |
Descurainia Sophia L. is widely distributed in northeastern China, and its seeds (Lepidii Semen) are used to relieve coughing, prevent asthma, reduce edema, promote urination and induce a cardiotonic effect. Biological screening of the alcoholic extract revealed that the plant is highly safe and has analgesic, antipyretic and anti-inflammatory effects [29]. Arctiin and its aglucone, Atg, are found in the fruits of Arctiumlappa L (ArctiiFructus). Arctiin has anti-inflammatory, anti-oxidant, antibacterial, and antiviral effects in vitro [30], [31]. The plasma pharmacokinetics and tissue distribution of arctiin and its major metabolite Atg in rats have been validated [32]. Recently, Atg was used as an antitumor agent that killed tumor cells via glucose deprivation by inhibiting cellular energy metabolism [33], [34]. The four ingredients were lignin compounds identified from two herbs (Figure 3). There has been a lack of studies regarding the effects of these compounds on β2AR agonist synergistic activity.
Figure 3. Structures of the QFXY constituents exhibiting a synergic effect on the β2AR-signalling pathway.
3. The Synergistic Effect of Eph and Atg on the cAMP Response and Tracheal Muscle
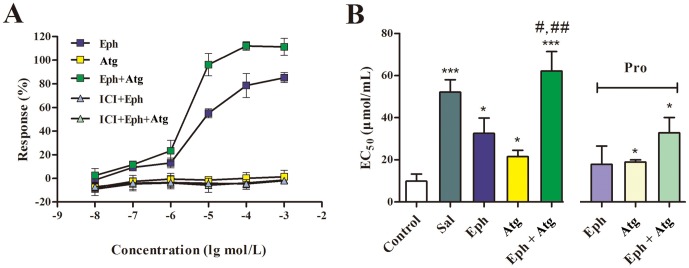
In this study, Atg was selected to validate its synergistic effect with the β2AR agonist using a dual-luciferase reporter assay for the β2AR/cAMP signal pathway. As shown in Figure 4A, Atg alone did not exhibit activity. However, compared to Eph alone, Atg could significantly enhance the cAMP response of ephedrine by shifting the dose-response curve with the EC50 value from 1.463 to 9.331 mol/L. ICI 118551 (10 µmol/L), a selective β2AR inhibitor, could completely block Eph plus Atg and Eph alone. The cAMP synergistic effect of Atg on Eph was β2AR dependent.
Figure 4. The synergistic effects of Eph and Atg on β2AR activation evaluated by the dual luciferase reporter assay system (A) and the guinea pig tracheal muscle relaxant test (B).
Each bar represents the mean ± SEM, n = 5 per group, and the EC50 values are expressed as the mean. *P<0.05, **P<0.01, ***P<0.001, compared to the control group; # P<0.05, compared to the Eph group; ## P<0.01, compared to the Atg group.
The in vitro tracheal muscle relaxant test, treatment with 10−5 mol/L Eph or Atg, significantly inhibited the tracheal contractions induced by a series of ACh concentrations in a dose-dependent manner, and the EC50 values were 32.6 and 21.5 µmol/L, respectively (Figure 4B). When combined with the above-mentioned dose of Eph and Atg, the EC50 value increased to 62.1 µmol/L and exceeded that of the 10−8 mol/L Sal positive control group (52.1 µmol/L). Blocking the β2ARs with 10−7 mol/L Pro significantly affected the inhibitory effect exerted by an identical dose of Eph; however, there was little effect on the Atg or Atg plus Eph (32.8 µmol/L). Atg could inhibit tracheal contractions independently; however, the synergistic relaxant effect was based on the β2AR/cAMP pathway.
4. The Synergistic Mechanism of Atg in Reducing Intracellular Calcium Concentration
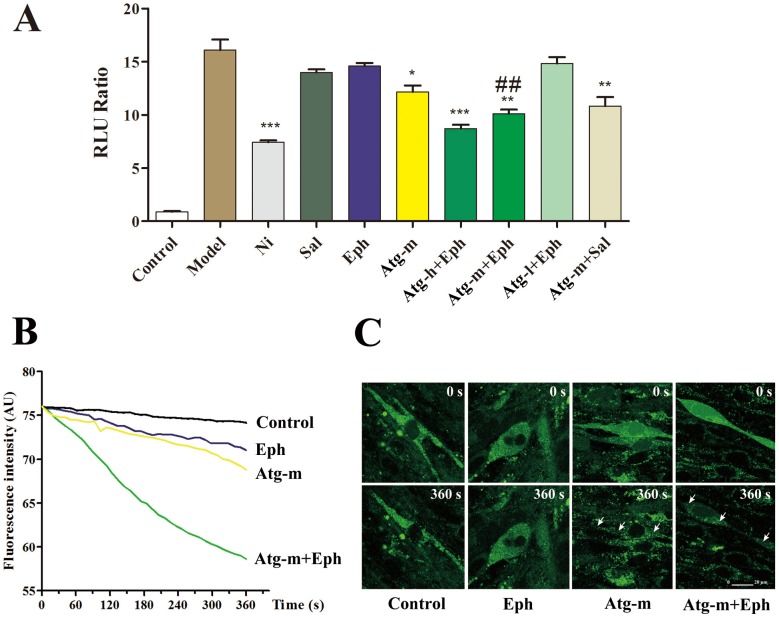
The major pathway that mediates airway smooth muscle constriction is the activation of phospholipase C, with the release of inositol 1,4,5-triphosphate and the elevation of intracellular calcium levels [35]. Ach and His could increase the intracellular free Ca2+ by the activation of the muscarinic M3 receptor and the histamine H1 receptor and a phospholipase C-dependent mechanism [36]. Complex cAMP and calcium crosstalk occurs between these pathways and leads to the careful regulation of airway smooth muscle tone [37]; however, cytoplasmic Ca2+ concentrations [Ca2+]i and myosin light chain phosphorylation are considered key elements [38]. It was reported recently that Atg could regulate human bronchial smooth muscles by affecting transmembrane Ca2+ flow [39]. In this paper, the crosstalk between a β2AR agonist with intracellular free Ca2+ was investigated. Compared with treatment with10−8 mol/L Sal or 10−5 mol/L Eph, 10−5 mol/L Atg-m reduced the intracellular free calcium concentration; however, the reduction was less than that by the calcium channel blocker nimodipine (10−5 mol/L) (Figure 5A). When Eph (10−5 mol/L) combined with high, middle and low doses of Atg (10−4, 10−5 and 10−6 mol/L, respectively), the intracellular calcium levels decreased in a dose-dependent manner. The identical effect was observed in the Sal combination group. The cytosolic [Ca2+]i components of the HBSMCs were detected with confocal microscopy. As shown in Figure 5B, compared with the control or Eph (10−5 mol/L) group, Atg-m (10−5 mol/L) clearly promoted Ca2+ efflux during a time interval of 360 s. In the Atg-m plus Eph group, the [Ca2+]i changed more obviously; [Ca2+]i intensity images in the single-cell mode treated with Atg-m and Eph at 0 s and 360 s are shown in Figure 5C and present a significant decrease. The mechanism of Atg as a synergistic bronchodilator that exhibited a relaxation effect in the airway smooth muscle by reducing the intracellular free calcium was clarified.
Figure 5. The synergistic mechanism of Atg and Eph in reducing the intracellular calcium concentration, asevaluated by the dual luciferase reporter assay system (A), confocal microscopy fluorescence intensity analysis (B) and image observations (C).
The white arrowindicates a significant [Ca2+]i intensity decrease in a singleHBSMCcell. The values are presented as the mean ± SEM (n = 5). *P<0.05, **P<0.01, ***P<0.001, compared to the model group (M); ## P<0.01, compared to the Eph group.
Conclusions
The contractile and relaxant responses of airway smooth muscle are regulated by crosstalk between the important intracellular signaling pathways controlling [Ca2+]i and cAMP. For over ten years, evidence-based guidelines for COPD or asthma have recommended β2AR agonists as the principal agents for maintenance pharmacotherapy. In this paper, four lignin ingredients, arctiin, Atg, descurainoside and descurainolide B, which demonstrated synergistic smooth muscle relaxant effects with ephedrine dependent on the β2AR/cAMP signal pathway, were identified from the QFXY prescription. The mechanism of Atg as a β2AR agonist cofactor that could reduce intracellular free calcium was proposed. Additionally, our data indicated the β2AR agonist, ephedrine, as the principal bronchodilator of the QFXY formula, whereas the lignin ingredients that regulated [Ca2+]i served as adjuvant components. A greater understanding of the mechanisms governing the control of these pathways based on conventional wisdom could lead to the discovery of novel therapeutic regimens, which could yield novel agents for the treatment of COPD or asthma.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by a Grant from the National Natural Science Foundation of China (grant no: 81173638 and 81373506), the Specialized Research Fund for the Doctor Program of Higher Education of China (grant no: 20120031110042) and the Key Program of Natural Science of Foundation of Tianjin, China (grant no: 13JCZDJC31400). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Patwardhan B, Vaidya AD, Chorghade M (2004) Ayurveda and natural products drug discovery. Curr Sci India 86: 789–799. [Google Scholar]
- 2. Yuan R, Lin Y (2000) Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther 86: 191–198. [DOI] [PubMed] [Google Scholar]
- 3. Huang X, Kong L, Li X, Chen X, Guo M, et al. (2004) Strategy for analysis and screening of bioactive compounds in traditional Chinese medicines. J Chromatogr B Analyt Technol Biomed Life Sci 812: 71–84. [DOI] [PubMed] [Google Scholar]
- 4. Zhang H, Ho YF, Che CT, Lin ZX, Leung C, et al. (2012) Topical herbal application as an adjuvant treatment for chronic kidney disease-a systematic review of randomized controlled clinical trials. J Adv Nurs 68: 1679–1691. [DOI] [PubMed] [Google Scholar]
- 5. Butler L, Pilkington K (2013) Chinese herbal medicine and depression: the research evidence. Evid Based Complement Alternat Med 2013: 739716 10.1155/2013/739716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L, Zhou GB, Liu P, Song JH, Liang Y, et al. (2008) Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci 105: 4826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radhakrishnan ML, Tidor B (2008) Optimal drug cocktail design: methods for targeting molecular ensembles and insights from theoretical model systems. J Chem Inf Model 48: 1055–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Preissner S, Dunkel M, Hoffmann MF, Preissner SC, Genov N, et al. (2012) Drug cocktail optimization in chemotherapy of cancer. PLoS One 7: e51020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brusotti G, Cesari I, Dentamaro A, Caccialanza G, Massolini G (2014) Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J Pharm Biomed Anal 87: 218–228. [DOI] [PubMed] [Google Scholar]
- 10. Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, et al. (2013) Techniques for extraction of bioactive compounds from plant materials. J Food Eng 117: 426–436. [Google Scholar]
- 11. Jiang M, Zhou M, Han Y, Xing L, Zhao H, et al. (2013) NF-κB inhibitors identification from Xuebijing injection in treatment of sepsis by bioactivity-integrated UPLC-Q/TOF spectrometry. J Ethnopharmacol 147: 426–433. [DOI] [PubMed] [Google Scholar]
- 12. Hou Y, Cao X, Wang L, Cheng B, Dong L, et al. (2012) Microfractionation bioactivity-based ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry for the identification of nuclear factor-κB inhibitors and β2 adrenergic receptor agonists in an alkaloidal extract of the folk herb Alstoniascholaris. J Chromatogr B Analyt Technol Biomed Life Sci 908: 98–104. [DOI] [PubMed] [Google Scholar]
- 13. Carolan BJ, Sutherland ER (2013) Clinical phenotypes of chronic obstructive pulmonary disease and asthma. J Allergy Clin Immunol 131: 627–634. [DOI] [PubMed] [Google Scholar]
- 14. Mathers CD, Loncar D (2006) Protections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3: e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 16. Morphy R, Rankovic Z (2009) Designing multiple ligands-medicinal chemistry strategies and challenges. Curr Pharm Des 15: 587–600. [DOI] [PubMed] [Google Scholar]
- 17. Pujol A, Mosca R, Farrés J, Aloy P (2010) Unveiling the role of network and systems biology in drug discovery. Trends in Pharmacol Sci 31: 115–123. [DOI] [PubMed] [Google Scholar]
- 18. Wu L, Chen Y, Xu Y, Guo X, Li X, et al. (2013) Oral huangqi formulae for stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2013: 705315 10.1155/2013/705315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holzinger F, Chenot JF (2011) Systematic review of clinical trials assessing the effectiveness of ivy leaf (hedera helix) for acute upper respiratory tract infections. Evid Based Complement Alternat Med 2011: 382789 10.1155/2011/382789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu R, Fengjie Z, Li Y, Yan S, Miao L, et al. (2013) Modified dachengqi decoction combined with conventional treatment for treating acute exacerbation of chronic obstructive pulmonary disease: a systematic review based on randomized controlled trials. Evid Based Complement Alternat Med 2013: 323715 10.1155/2013/323715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. An X, Zhang AL, May BH, Lin L, Xu Y, et al. (2012) Oral Chinese herbal medicine for improvement of quality of life in patients with stable chronic obstructive pulmonary disease. J Altern Complement Med 18: 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bai G, Hou Y, Jiang M, Gao J (2014) Correlation analysis between visceral manifestation theories on Xuanfa and effect of adrenergic receptors. Chinese Herbal Medicines 6: 85–92. [Google Scholar]
- 23. Cheng B, Hou Y, Wang L, Dong L, Peng J, et al. (2012) Dual-bioactivity-based liquid chromatography-coupled quadrupole time-of-flight mass spectrometry for NF-κB inhibitors and β2AR agonists identification in Chinese Medicinal Preparation Qingfei Xiaoyan Wan. Anal Bioanal Chem 404: 2445–2452. [DOI] [PubMed] [Google Scholar]
- 24. Shi Q, Hou Y, Hou J, Pan P, Liu Z, et al. (2012) Glycyrrhetic acid synergistically enhances β2-adrenergic receptor-Gs signaling by changing the location of Gαs in lipid rafts. PLoS One 7: e44921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu SY, Bian RL, Chen X (2001) Methodology of Pharmacological Experiment (3nd edn). Beijing: People's Medical Publishing House.1368 p. [Google Scholar]
- 26. Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, et al. (2008) Identification of higenamine in Radix AconitiLateralisPreparata as a beta2-adrenergic receptor agonist. Acta Pharmacol Sin 29: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 27. Hou Y, Cao X, Dong L, Wang L, Cheng B, et al. (2012) Bioactivity-based liquid chromatography-coupled electrospray ionization tandem ion trap/time of flight mass spectrometry for β2AR agonist identification in alkaloidal extract of Alstoniascholaris. J Chromatogr A 1227: 203–209. [DOI] [PubMed] [Google Scholar]
- 28. Sun K, Li X, Li W, Wang J, Liu J, et al. (2004) Two new lactones and one new aryl-8-oxa-bicyclo[3,2,1]oct-3-en-2-one from Descurainiasophia. Chem Pharm Bull 52: 1483–1486. [DOI] [PubMed] [Google Scholar]
- 29. Nawal HM, Atta EM (2009) Chemical constituents of Descurainiasophia L. and its biological activity. Rec Nat Prod 3: 58–67. [Google Scholar]
- 30. Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, et al. (2011) A review of the pharmacological effects of Arctiumlappa (burdock). Inflammopharmacology 19: 245–254. [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Shin S, Kim H, Han S, Kim K, et al. (2011) Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-kB pathways. J Inflamm 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He F, Dou DQ, Sun Y, Zhu L, Xiao HB, et al. (2012) Plasma pharmacokinetics and tissue distribution of arctiin and its main metabolite in rats by HPLC-UV and LC-MS. Planta Med 78: 800–806. [DOI] [PubMed] [Google Scholar]
- 33. Gu Y, Qi C, Sun X, Ma X, Zhang H, et al. (2012) Arctigenin preferentially induces tumor cell death under glucose deprivation by inhibiting cellular energy metabolism. Biochem Pharmacol 84: 468–476. [DOI] [PubMed] [Google Scholar]
- 34. Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, et al. (2006) Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res 66: 1751–1757. [DOI] [PubMed] [Google Scholar]
- 35. Ritchie MF, Zhou YD, Soboloff J (2011) Transcriptional mechanisms regulating Ca2+ homeostasis. Cell Calcium 49: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall IP (2000) Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J 15: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 37. Billington CK, Hall IP (2012) Novel cAMP signaling paradigms: therapeutic implications for airway disease. Brit J Pharmacol 166: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong F, Haldeman BD, Jackson D, Carter M, Baker JE, et al. (2011) Biochemistry of smooth muscle myosin light chain kinase. Arch Biochem Biophys 510: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao Z, Yin YQ, Wang ZY, Fang RP, Wu H, et al. (2013) Arctigenin exhibits relaxation effect on bronchus by affecting transmembrane flow of calcium. Biol Trace Elem Res 156: 181–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.