Abstract
Despite the prevalence of sex in animal kingdom, we have only limited understanding of how sex is determined and evolved in many taxa. The mollusc Pacific oyster Crassostrea gigas exhibits complex modes of sexual reproduction that consists of protandric dioecy, sex change, and occasional hermaphroditism. This complex system is controlled by both environmental and genetic factors through unknown molecular mechanisms. In this study, we investigated genes related to sex-determining pathways in C. gigas through transcriptome sequencing and analysis of female and male gonads. Our analysis identified or confirmed novel homologs in the oyster of key sex-determining genes (SoxH or Sry-like and FoxL2) that were thought to be vertebrate-specific. Their expression profile in C. gigas is consistent with conserved roles in sex determination, under a proposed model where a novel testis-determining CgSoxH may serve as a primary regulator, directly or indirectly interacting with a testis-promoting CgDsx and an ovary-promoting CgFoxL2. Our findings plus previous results suggest that key vertebrate sex-determining genes such as Sry and FoxL2 may not be inventions of vertebrates. The presence of such genes in a mollusc with expression profiles consistent with expected roles in sex determination suggest that sex determination may be deeply conserved in animals, despite rapid evolution of the regulatory pathways that in C. gigas may involve both genetic and environmental factors.
Keywords: sex determination, doublesex, Sry, FoxL2, oyster, Mollusca
Sexual reproduction is one of the most prevailing and remarkable phenomena in biology. It is found in almost all groups of multicellular animals. The differentiation of two sexes, sometimes with spectacular dimorphism, has long fascinated human curiosity. How sex is determined and evolved remains a major question in modern biology. Why sex is necessary at all given the high cost of producing males has not been adequately answered (Hartfield and Keightley 2012). Assuming sexual production provides critical benefits, it is intriguing that sex is not always genetically determined to ensure a 1:1 sex ratio. In many organisms, sex may be determined by environmental factors or modified by hermaphroditism or sex change (Guo et al. 1998; Devlin and Nagahama 2002; Merchant-Larios and Diaz-Hernandez 2013). These variations could be adaptations for reducing the cost of sex while preserving meiosis, but testing this and other hypotheses on the evolution of sex requires a good understanding of molecular mechanisms of sex determination in diverse taxa.
Studies in model organisms have revealed key genes and complex pathways involved in sex determination and differentiation. In mammals, male determination begins with the expression of Sry (Sex-determining region on Y), which suppresses ovarian promoting genes and turns on Sox9 (Sry-box 9) as part of the testis-determining cascade leading to the activation of doublesex/MAB-3 related transcription factor 1 (Dmrt1) and differentiation of Sertoli cells (Veitia 2010). In females, a forkhead box transcription factor (FoxL2), β-catenin and Wnt4 are expressed to promote and maintain ovarian development while suppressing Sox9. Dmrt1 contains a DNA-binding motif (DM), and DM domain containing genes and their role in testis differentiation are deeply conserved in diverse organisms from invertebrates to mammals (Matson and Zarkower 2012). Although DM domain genes are deeply conserved in metazoans, up-stream sex regulators such as Sry, Sox, and FoxL2 are thought to be recent inventions in vertebrates or placental mammals (Gamble and Zarkower 2012; Matson and Zarkower 2012). In Caenorhabditis elegans, an X chromosome to autosome (X:A) ratio of 0.5 triggers expression of a cascade genes that act on three DM domain genes to initiate male development (Gamble and Zarkower 2012). In Drosophila, sex is also determined by the X:A ratio through alternative splicing of doublesex (dsx), a DM domain gene, into dsxF and dsxM to initiate female and male differentiation, respectively (Hempel and Oliver 2007; Rideout et al. 2010; Robinett et al. 2010).
Mollusca is a major branch of lophotrochozoan where diverse modes of sexual reproduction are observed. In bivalve molluscs, there are dioecious and hermaphroditic species, as well as species capable of sex change, and sex determination can be genetic, environmental, or both (Coe 1943; Guo and Allen 1994; Chavez-Villalba et al. 2011). Oysters in particular have a complex and dynamic sex determination system. Certain species, including many within the genus Ostrea, are functional hermaphrodites, whereas others such as Crassostrea species may exhibit protandric dioecy, hermaphroditism and sex change within the same species (Coe 1943). In the Pacific oyster Crassostrea gigas, most individuals mature as males first and may subsequently change to females, and females may change to males as well (Amemiya 1929). Warm and nutritious conditions may favor the development of females (Quayle 1988). Genetic determinants of sex in oysters also have been demonstrated through analysis of family sex ratios, and genetic models have been proposed without any knowledge of molecular mechanisms of sex determination (Haley 1979; Guo et al. 1998; Hedrick and Hedgecock 2010).
Studies on sex determination genes and pathways in bivalves and other molluscs are few and limited. Several genes homologous to sex-determining pathway genes in model species have been identified in bivalve molluscs, although their expression profile is often inconsistent with expected roles in sex determination. DM domain genes have been identified in C. gigas, scallop Chlamys farreri, and Chlamys nobilis, but they are not specifically expressed in testis (Naimi et al. 2009a; Feng et al. 2010; Shi et al. 2014). Homologs of Dmrt did show high or specific expression in the pearl oyster Pinctada martensii, the blacklip pearl oyster Pinctada margaritifera, and the abalone Haliotis asinina (Klinbunga et al. 2009; Yu et al. 2011; Teaniniuraitemoana et al. 2014). Sox genes have been reported in C. farreri and C. gigas, but their expression is not restricted to testis (He et al. 2013; Santerre et al. 2014). Cg-FoxL2 and Cg-β-catenin showed high but not specific expression in female gonad (Naimi et al. 2009b; Santerre et al. 2014), although FoxL2 was later found specifically expressed in female gonad of C. gigas and P. margaritifera (Dheilly et al. 2012; Teaniniuraitemoana et al. 2014). Despites the identification of some candidate genes, sex-determining pathways in molluscs remain elusive. Genome-wide studies have been limited due to a lack of reference genomes (Ghiselli et al. 2012; Dheilly et al. 2012; Teaniniuraitemoana et al. 2014).
The availability of the C. gigas genome (Zhang et al. 2012) provides an opportunity for a comprehensive analysis of sex-determining pathways in this species that has an interesting sex determination system. In this study, we investigated genes related to sex determination by sequencing and analyzing transcriptomes of female and male gonads of C. gigas. Our analysis reveals that several key genes of the vertebrate sex-determining pathway are present in C. gigas with expression profiles consistent with roles in sex determination, and the C. gigas pathway appears more similar to that of vertebrates than to that of worms and flies.
Methods
To discover genes related to sex-determining pathways, we sequenced transcriptomes of mature gonads from one female (F3) and two male (M2 and M3) Pacific oysters. In addition to the three transcriptomes obtained in this study, we also included gonadal transcriptomes of two females (F1 and F2) and one male (M1) that were generated in our previous study (Zhang et al. 2012). Thus, a total of three female and three male gonadal transcriptomes were included in this analysis. To establish expression profile and infer function of selected genes, we consulted seven somatic transcriptomes from six organs (gill, outer and pallial mantle, adductor muscle, digestive gland, labial palp, and hemocytes) and transcriptomes from 38 developmental stages that were obtained in our previous study (Zhang et al. 2012).
All animals used for gonadal transcriptome sequencing in this and the previous study were mature 2-yr-old oysters obtained from Laodong Aquaculture Breeding Company, Qingdao, China. Sex of oysters was determined by observing the presence of eggs or sperm under microscope. Gonadal tissues were dissected, frozen in liquid nitrogen and stored at −80° before RNA extraction. RNA extraction, cDNA synthesis, library construction, and transcriptome sequencing were performed in the same way as in our previous study (Zhang et al. 2012). In brief, total RNA was extracted using TRIzol reagents and protocol (Invitrogen). Poly-A RNA was isolated with oligo-dT-coupled beads and sheared for first strand cDNA synthesis with random hexamers and Superscript II reverse transcriptase (Invitrogen). The second strand was synthesized with Escherichia coli DNA PolI (Invitrogen). Double-stranded cDNA was purified with the Qiaquick PCR purification kit (QIAGEN, Gaithersburg, MD). After end repair and addition of a 3′ dA overhang, the cDNA was ligated to Illumina adapters and size selected to about 200 bp by gel purification. The selected fragments were amplified for 15 polymerase chain reaction cycles and sequenced for 49 bp single-end reads using Illumina sequencing platform. After cleaning, RNA-seq reads were mapped to the oyster genome with Tophat (Trapnell et al. 2009). Expression level for each gene was measured with reads per kilobase per million (RPKM). Only genes with a RPKM value larger than 3 in at least 1 of the 13 transcriptomes (6 gonad and 7 somatic) were used for further analysis.
Ovary-specific genes were identified by comparing expression in three female gonadal transcriptomes with that in seven somatic and three testis transcriptomes and vice versa for testis-specific genes. To qualify as ovary or testis-specific genes, genes should satisfy the following criteria: 1) All three ovary or testis samples had greater RPKM values than any of the other 10 samples; 2) The RPKM mean of the three ovary or testis samples should be at least fivefold of that in other 10 samples. For a general characterization of ovary- and testis-specific genes, Gene Ontology (GO) term enrichment analysis was conducted with the Fisher’s exact test in classic algorithm of topGO (Alexa and Rahnenfuhrer 2010). KEGG enrichment was conducted with the algorithm implemented in GOstats (Falcon and Gentleman 2007). The Benjamini-Hochberg false discovery rate control was used to adjust the P value (Benjamini and Hochberg 1995).
Genes belonging to sex-determining pathways in model species (see the Results) were collected from the literature and searched against the oyster genome and our transcriptome dataset. Homologs were identified and their expression profiles in ovary, testis, somatic tissues, and at various developmental stages were extracted to infer function in C. gigas. Although the published oyster genome provided annotation for most genes, genes of interest were manually checked with ESTs and RNA-seq reads for possible assembly errors and characterized by searching against databases, including InterPro (Hunter et al. 2009), GO (Ashburner et al. 2000), SWISS-PROT (Magrane and Consortium 2011), TrEMBL (Magrane and Consortium 2011), and KEGG (Kanehisa and Goto 2000). Domain structures of selected genes were determined using SMART (http://smart.embl-heidelberg.de/). To confirm homology of selected genes, conserved domain sequences were identified, aligned, and compared. Unrooted maximum likelihood phylogenetic trees of protein sequences were constructed with RAxML (Stamatakis 2014), with the model PROTGAMMAJTT and bootstrap for 1000 replications. Genes in the oyster genome are coded by a prefix of “CGI_100” plus a five-digit unique gene number and for brevity especially in figures, the prefix of “CGI_100” was replaced with “Cg.”
Results and Discussion
Ovary- and testis-specific genes
Comparative analyses of RNA-seq data between gonad and somatic organs identified 621 genes that were specifically expressed in female gonad, and 552 specifically expressed in male gonad (Supporting Information, File S1). The finding of more ovary-specific than testis-specific genes is in agreement with the finding of 1570 ovary-enriched and 1370 testis-enriched genes in zebrafish (Santos et al. 2007), but differs from other studies where more genes are found in testis (Rinn et al. 2004; Small et al. 2009). Analyses of these ovary- and testis-specific genes reveal some similarities and interesting differences between two sexes. Genes related to meiosis are overrepresented in both ovary- and testis-specific genes. Among ovary-specific genes, GO terms related to DNA replication, nucleic acid metabolism, DNA repair, chromosome organization, cell cycle, gene expression regulation, DNA recombination, and telomere maintenance are significantly enriched (Table 1). Among testis-specific genes, GO terms related to protein phosphorylation and dephosphorylation, protein metabolism, and sex differentiation are significantly enriched. KEGG enrichment analysis shows similar patterns that pathways related to DNA replication, DNA repair, nucleotide metabolism, recombination, oocyte maturation, cell cycle, and proteolysis are enriched in ovary-specific genes, whereas KEGG pathways related to protein digestion and absorption, protein interaction, flagellar assembly, proteolysis, and focal adhesion are enriched in testis-specific genes (Table 1). These findings suggest that ovary-specific genes are more likely involved in DNA replication, DNA metabolism, DNA repair, DNA organization, and DNA transcription, whereas testis-specific genes are primarily involved in protein phosphorylation, protein interactions and protein metabolism.
Table 1. GO terms and KEGG pathways enriched in ovary- and testis-specific genes of C. gigas.
| ID Code | Term | Genes | Expected | P value |
|---|---|---|---|---|
| GO: ovary | ||||
| GO:0006260 | DNA replication | 27 | 1.7 | 3.90E-26 |
| GO:0090304 | Nucleic acid metabolic process | 76 | 22.7 | 1.40E-23 |
| GO:0006281 | DNA repair | 12 | 2.2 | 1.80E-06 |
| GO:0008152 | Metabolic process | 137 | 108.0 | 6.60E-06 |
| GO:0006996 | Organelle organization | 16 | 4.6 | 1.30E-05 |
| GO:0000278 | Mitotic cell cycle | 6 | 0.6 | 2.40E-05 |
| GO:0051276 | Chromosome organization | 10 | 2.2 | 5.40E-05 |
| GO:0071103 | DNA conformation change | 6 | 1.5 | 0.00369 |
| GO:0007017 | Microtubule-based cellular movement | 8 | 2.7 | 0.00527 |
| GO:0016070 | RNA metabolic process | 28 | 17.1 | 0.00621 |
| GO:0043687 | Posttranslational protein modification | 4 | 1.0 | 0.01488 |
| GO:0006950 | Response to stress | 15 | 8.1 | 0.01497 |
| GO:0010468 | Regulation of gene expression | 18 | 11.4 | 0.03546 |
| GO:0006310 | DNA recombination | 2 | 0.3 | 0.04507 |
| GO:0000723 | Telomere maintenance | 1 | 0.1 | 0.04857 |
| GO: testis | ||||
| GO:0006468 | Protein phosphorylation | 15 | 4.6 | 4.50E-05 |
| GO:0055085 | Transmembrane transport | 14 | 6.5 | 0.00517 |
| GO:0019538 | Protein metabolic process | 30 | 19.2 | 0.00542 |
| GO:0044765 | Single-organism transport | 20 | 11.8 | 0.01116 |
| GO:0016999 | Antibiotic metabolic process | 1 | 0.0 | 0.02340 |
| GO:0006470 | Protein dephosphorylation | 5 | 1.8 | 0.03155 |
| GO:0007548 | Sex differentiation | 1 | 0.0 | 0.03490 |
| GO:0050953 | Sensory perception of light stimulus | 1 | 0.1 | 0.04627 |
| KEGG: ovary | ||||
| 03030 | DNA replication | 21 | 1.5 | 7.04E-18 |
| 04110 | Cell cycle | 29 | 3.6 | 7.04E-18 |
| 04113 | Meiosis, yeast | 19 | 1.9 | 8.06E-14 |
| 03420 | Nucleotide excision repair | 15 | 1.9 | 1.49E-09 |
| 00240 | Pyrimidine metabolism | 18 | 3.3 | 1.11E-08 |
| 03440 | Homologous recombination | 12 | 1.4 | 2.41E-08 |
| 00230 | Purine metabolism | 22 | 6.4 | 6.67E-07 |
| 04914 | Progesterone-mediated oocyte maturation | 12 | 1.9 | 6.67E-07 |
| 03430 | Mismatch repair | 8 | 1.2 | 3.98E-05 |
| 00785 | Lipoic acid metabolism | 4 | 0.3 | 0.00038 |
| 04115 | P53 signaling pathway | 8 | 1.9 | 0.00065 |
| 04114 | Oocyte meiosis | 12 | 3.9 | 0.00067 |
| 00670 | One carbon pool by folate | 3 | 0.6 | 0.01936 |
| 04120 | Ubiquitin mediated proteolysis | 12 | 6.4 | 0.02939 |
| 00624 | Polycyclic aromatic hydrocarbon degradation | 3 | 0.7 | 0.03771 |
| KEGG: testis | ||||
| 04113 | Meiosis, yeast | 5 | 0.9 | 0.01878 |
| 04962 | Vasopressin-regulated water reabsorption | 4 | 0.7 | 0.02478 |
| 04974 | Protein digestion and absorption | 6 | 2.1 | 0.03946 |
| 04512 | ECM-receptor interaction | 9 | 4.0 | 0.03946 |
| 02040 | Flagellar assembly | 1 | 0.0 | 0.03946 |
| 00230 | Purine metabolism | 7 | 3.1 | 0.03946 |
| 04120 | Ubiquitin mediated proteolysis | 7 | 3.1 | 0.03946 |
| 04140 | Regulation of autophagy | 2 | 0.3 | 0.03946 |
| 04510 | Focal adhesion | 12 | 6.8 | 0.03946 |
GO, Gene Ontology; ECM, extracellular matrix; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Protein phosphorylation is important for sperm capacitation in mammals (Visconti and Kopf 1998). In mammalian testis, abnormal or damaged spermatozoa are ubiquitinated for proteolytic destruction (Sutovsky et al. 2002; Purdy 2008). The fact that ubiquitin mediated proteolysis is enriched among testis-specific genes suggests ubiquitination may play a role in maintaining sperm quality in oysters also. GO terms related to cellular component movement, RNA metabolism, and response to stress are enriched in ovary-specific genes, suggesting these pathways are also important to ovary biology. Overall, it seems that ovary-specific genes are enriched for more diverse pathways than testis-specific genes (Table 1), which may indicate that the production of large and yolk-containing oocytes involves more metabolic pathways than the production of sperm.
Sex-determining pathway genes
Clearly, not all ovary- and testis-specific genes are involved in sex-determining pathways. To identify genes related to sex-determining pathways, we searched the oyster genome for such genes previously identified in model organisms and examined their expression profiles in gonadal transcriptomes of C. gigas. Of the 24 genes examined, homologs were found for nine genes or gene families (Table 2). Among these nine genes, only three genes (CgDsx, SoxH or Sry-like and FoxL2) showed sex-specific expression as expected for sex-determining genes. The other six genes (Fem, Gata4, Wnt4, beta-catenin, Run, Sox9) did not show sex-specific expression in our samples (Table 2), suggesting that they may not be involved in sex determination or maintenance in mature gonads. We cannot rule out the possibility that they may have sex-specific expression at an earlier stage.
Table 2. Presence and sex-specific expression of key sex-determining pathway genes from Caenorhabditis elegans, Drosophila melanogaster and Mus musculus.
| Name of genes | Caenorhabditis elegans | Drosophila melanogaster | Mus musculus | Crassostrea gigas |
|---|---|---|---|---|
| Xol-1 | +/+ | −/− | −/− | −/− |
| Sdc | +/+ | +/− | +/− | −/− |
| Her | +/+ | +/− | −/− | −/− |
| Tra | +/+ | +/+ | +/− | −/− |
| Fem | +/+ | +/− | +/− | +/− |
| Fog | +/+ | +/− | +/+ | −/− |
| Fru | −/− | +/+ | −/− | −/− |
| Sis | −/− | +/+ | −/− | −/− |
| Run | +/− | +/+ | +/− | +/− |
| Sxl | −/− | +/+ | −/− | −/− |
| Doa | −/− | +/+ | −/− | −/− |
| Gata4 | −/− | −/− | +/+ | +/− |
| Wt1 | −/− | −/− | +/+ | −/− |
| M33 | −/− | −/− | +/+ | −/− |
| Sf1 | −/− | −/− | +/+ | −/− |
| Mis | −/− | −/− | +/+ | −/− |
| Dax1 | −/− | −/− | +/+ | −/− |
| Sry/Sox30/SoxH | −/− | −/− | +/+ | +/+ |
| Sox9/SoxE/sox100B | −/− | +/− | +/+ | +/− |
| MAB-3/dsx/Dmrt1 | +/+ | +/+ | +/+ | +/+ |
| FoxL2 | −/− | −/− | +/+ | +/+ |
| Rspo1 | −/− | −/− | +/+ | −/− |
| Wnt4 | −/− | +/− | +/+ | +/− |
| Beta-catenin | −/− | +/− | +/+ | +/− |
The first + sign indicates presence and the second + sign indicates sex-specific expression or confirmed role in sex-determining pathways.
Doublesex and MAB-3 related transcription factor 1 (Dmrt1):
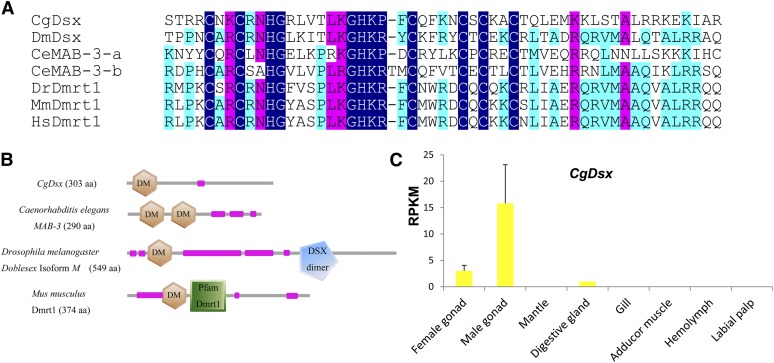
Dmrt1 is a transcription factor that contains a characteristic zinc finger DM domain and plays deeply conserved roles in sex determination and differentiation (Kopp 2012). Members of the family include the doublesex (dsx) gene in fruit fly, MAB-3 in C. elegans and the Dmrt1 in vertebrates, all of which promote male-specific development. In Drosophila melanogaster, dsx is alternatively spliced to produce male- and female-specific isoforms in male and female gonads, respectively (Burtis and Baker 1989). MAB-3 in C. elegans and Dmrt1 in vertebrates are exclusively expressed in testis and promote male-specific development (Yi and Zarkower 1999; Smith et al. 2009; Kopp 2012). The oyster genome encodes three DM domain containing genes: Cg19568, Cg01830, and Cg15952, compared with 11 in nematodes, 4 in flies, and 7 in human (Matson and Zarkower 2012). We named one of the three genes, Cg19568, as CgDsx (GenBank accession No. KJ489413) after manual correction to remove three misassembled exons. It contains a DM domain showing closest homology (45%, E-value = 9e-13) to Dsx isoform A found in D. melanogaster (Figure 1A). CgDsx has three exons, but it shows no sex-specific alternative splicing as described in D. melanogaster. Similar to MAB-3 from C. elegans, CgDsx does not contain any other recognizable c-terminal domains that are found in Dsx of Drosophila or in vertebrate Dmrt1 (Figure 1B).
Figure 1.
A DM domain gene in C. gigas (CgDsx) and its male-specific expression profile. (A) Alignment of CgDsx and other DM domain containing proteins involved in sex determination from model species. (B) Domain structure of CgDsx and selected DM domain genes from model species (purple designates low complexity regions). (C) Expression profile of CgDsx in different adult organs with standard deviation as error bars (n = 3). Species names are abbreviated as Cg for Crassostrea gigas, Dm for Drosophila melanogaster, Ce for Caenorhabditis elegans, Dr for Danio rerio, Mm for Mus musculus, and Hs for Homo sapiens. Accession numbers: CgDsx KJ489413; DmDsx NP_731197.1, CeMAB-3-a and CeMAB-3-b CAB16489.1, DrDmrt1 AAQ04555.1, MmDmrt1 AAF12826.1, and HsDmrt1 AAD40474.1.
CgDsx is exclusively expressed in gonads with virtually no expression in somatic organs (0.2 RPKM). Its expression in testis (15.8 RPKM) is 5.3-fold greater than that in ovary (3 RPKM) (Figure 1C). The high expression in testis supports a possible role for CgDsx in determining or promoting male-specific development. The finding of low levels of CgDsx transcripts in female gonad may indicate that dormant male germline cells exist in female gonad, permitting sex change in coming seasons. Oyster gonads are known to contain both male and female germline cells possibly as a prerequisite for sex change (Cole 1941; Guo et al. 1998).
The other two DM domain genes (Cg15952 and Cg01830) both have a DmrtA domain c-terminal to the DM domain and show the highest homology to DmrtA2 (aka Dmrt5) from many species (identity 38–83%, E-value < e-40). They are expressed in all organs and appeared to be unrelated to sex determination or differentiation. Cg15952 has been previously identified as Cg-DM1 and shown to be related to gonad development by Naimi et al. (2009a). In our data, however, Cg-DM1 is primarily expressed in gill, labial palp, and mantle (61.5 RPKM) rather than in female (5.3 RPKM) or male (19.6 RPKM) gonads. Our results suggest that Cg-DM1 may have broad functions in organ development and may not be involved in sex determination in C. gigas. Cg01830 has low expression in gonads (2.9 RPKM), and its greatest expression is at the umbo larval stages (35.6 RPKM) and in adult labial palp (15.1 RPKM), suggesting it is also unrelated to sex determination. Our analysis indicates that this novel CgDsx is a DM domain gene and a close relative of Dsx that may be involved in promoting male development in C. gigas. This finding along with reports of testis-specific Dmrt-like genes in other molluscs (Klinbunga et al. 2009; Yu et al. 2011; Teaniniuraitemoana et al. 2014) supports the idea that the sex-determining role of DM domain genes is deeply conserved in evolution (Matson and Zarkower 2012).
Sox genes:
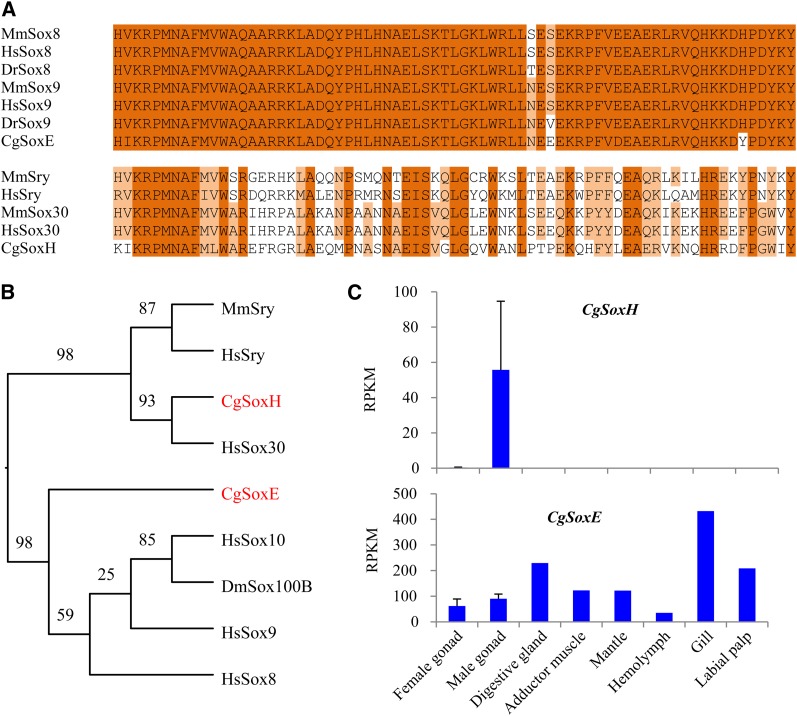
Sox (Sry-related HMG box) proteins are a family of transcription factors that possess a DNA binding HMG-box (high mobility group) domain. Sox genes are highly conserved in animal kingdom and play key roles in determining cell fate in development and differentiation (Lefebvre et al. 2007). Some members of the Sox family function in sex determination and differentiation, including the founding member Sry (sex-determining region on the Y-chromosome) and Sox9 from mammals (Kent et al. 1996; Koopman 2001; Kashimada and Koopman 2010). Expression of Sry activates Sox9 and the male determining pathway leading to Dmrt1 (Koopman 2005). Sox9 inhibits ovary development through the induction of anti-Müllerian hormone in Sertoli cells and promotes male sex-development through the activation of glia-activating factor 9 (Schepers et al. 2003). It is specifically expressed in testis germ cells in humans (Su et al. 2004).
The C. gigas genome encodes 32 proteins containing the HMG domain, and 10 of them can be classified as Sox genes (Table 3). One of the oyster Sox genes (Cg22931) recently has been identified as a member of the SoxE family (Cg-SoxE) that includes the sex-determining Sox9 from mammals (Santerre et al. 2014). Its HMG domain shares high similarity (86.1%) to that of Sox8 and Sox9 in vertebrates (Figure 2, A and B). However, Cg-SoxE is probably not involved in sex determination. In Santerre et al.’s (2014) study, Cg-SoxE was expressed in both male and female gonads, higher at undifferentiated than mature stages. In our transcriptome data, it was mostly expressed in somatic organs (averaging 197 RPKM) and at gastrula stage (194 RPKM); its expression was lower in male (90 RPKM) and female (61 RPKM) gonads (Figure 2C). These results suggest that the primary function of Cg-SoxE may not be related to sex determination or differentiation in C. gigas.
Table 3. Sox genes in C. gigas genome and their expression in gonads.
| Gene ID | Homolog | E-value | Gonad-specific |
|---|---|---|---|
| Cg27966 | Sox4, Columba livia, EMC90062.1 | 5e-49 | No |
| Cg10085 | Sox2, Pinctada fucata, AGS18764.1 | 2e-124 | No |
| Cg03991 | Sox7, Homo sapiens, NP_113627.1 | 7e-32 | No |
| Cg05643 | SoxB2, Azumapecten farreri, AGY14563.1 | 2e-109 | No |
| Cg22931 | Sox9 (Cg-SoxE), Pinctada fucata, AGI96396.1 | 8e-161 | No |
| Cg21811 | SoxC, Platynereis dumerilii, CAY12635.1 | 4e-91 | No |
| Cg05340 | Sox2,Caenorhabditis remanei, XP_003118339.1 | 6e-08 | No |
| Cg06950a | Sox30 (group H), Homo sapiens, NP_848511.1 | 5.8e-14 | Testis |
| Cg03963 | Sox1 (groupB1), Xenopus laevis, NP_001079136.2 | 9e-79 | No |
| Cg27723 | Sox7-like, Saccoglossus kowalevskii, NP_001158464.1 | 3e-85 | No |
CgSoxH or Sry-like, specifically expressed in testis.
Figure 2.
SoxE and SoxH genes identified in C. gigas and their expression profile. (A) Alignment of HMG domains of CgSoxE, CgSoxH, and homologs from selected vertebrates. (B) Phylogenetic tree of protein sequences of CgSoxE, CgSoxH, and selected genes. (C) Expression profile of CgSoxE and CgSoxH in adult organs of C. gigas with standard deviation as error bars (n = 3). Species names are abbreviated as Cg for Crassostrea gigas, Dm for Drosophila melanogaster, Dr for Danio rerio, Mm for Mus musculus, and Hs for Homo sapiens. Numbers in the tree represent bootstrap values. Accession numbers: CgSoxE EKC31659.1, CgSoxH EKC38002.1, DmSox100B AAF57112.2, MmSry AAI11529.1, HsSry AFG33955.1, HsSox8 AAH31797.1, HsSox9 CAA86598.1, HsSox10 CAG30470.1, and HsSox30 BAA37146.1.
Among all Sox genes that we identified in C. gigas, only one showed testis-specific expression in our transcriptome data. This novel Sox gene Cg06950, classified as CgSoxH, is closely related to Sox30, a member of SoxH. Within family identity in vertebrate Sox gene families usually ranges between 70 and 95% (Lefebvre et al. 2007), and the classification of CgSoxH is tentative and needs to be confirmed in future studies. Nevertheless, phylogenetic analysis shows CgSoxH is a close relative of Sox30 and Sry of vertebrates (Figure 2B). CgSoxH encodes a protein of 1283 aa divided into eight exons, whereas Sox30 in mammals has five exons alternatively spliced producing two polypeptides of 753 and 501 amino acid residues (Osaki et al. 1999). Its HMG domain shares a 54% identity (e-value = 1.4e−15) with Sox30 from human as well as a 49% identity (e-value = 2e−21) to Sry from Mus musculus domesticus (Figure 2A). Phylogenetic analysis also shows that CgSoxH is clustered with Sox30 and then with Sry (Figure 2B). At four positions, CgSoxH shared identical amino acids with Sry instead of Sox30 so it may be considered as an Sry-like gene. It is possible that CgSoxH may be closely related to the ancestral gene before the divergence of Sry and Sox30.
In our data, CgSoxH is exclusively expressed in testis (Figure 2C). Its expression in testis is 55.7 RPKM compared with <1 RPKM in all other transcriptomes, including ovary, all somatic organs, and at all developmental stages. In mammals, Sry is the primary male-determining gene, and Sox30 is exclusively expressed in normal testis, but not in maturing germ cell-deficient testis, suggesting a role in differentiation of male germ cells (Osaki et al. 1999). The homology between CgSoxH and male-determining Sox30 and Sry and the fact that CgSoxH is exclusively expressed in testes suggest that CgSoxH may play a key role in determining or promoting male sex development in oysters. As far as we can determine, CgSoxH is the first Sry-like gene with possible roles in male-determination identified in a mollusc. A member of SoxE family (Sox100B) has been found in D. melanogaster showing male-specific expression, although it is clearly not a homolog of Sry (Figure 2, A and B). SoxE genes have been identified in molluscs but without male-specific expression or roles in sex determination (He et al. 2013; Santerre et al. 2014, this study). It has been suggested that Sry and Sox9 assumed their roles in sex determination in vertebrates (Matson and Zarkower 2012). The finding of an Sry-like gene in C. gigas with testis-specific expression suggests that Sry and its role in sex determination may not be inventions specific to vertebrates but deeply rooted in evolution. It is possible that an Sry-like gene existed in the common ancestor of bilaterians and had a role in sex determination already. The gene may have been lost in some lineages (such as worms and flies) but conserved in others (molluscs and vertebrates).
All other oyster Sox genes are not specifically expressed in gonads. Many of them are highly expressed at certain embryonic stages, suggesting that they may play roles in determining cell fate during early development.
Fox genes:
Fox (forkhead-box) proteins are a family of transcription factors with a characteristic DNA-binding forkhead domain. They regulate gene expression and play roles in diverse biological processes including development, differentiation, metabolism and immunity. One member of the Fox gene family, FoxL2, is a key gene involved in ovarian determination in vertebrates (Uhlenhaut and Treier 2006). In mammals, FoxL2 is expressed in the ovary and promotes ovarian development while suppressing the key male promoting Sox9 gene (Crisponi et al. 2001; Ottolenghi et al. 2007; Uhlenhaut et al. 2009).
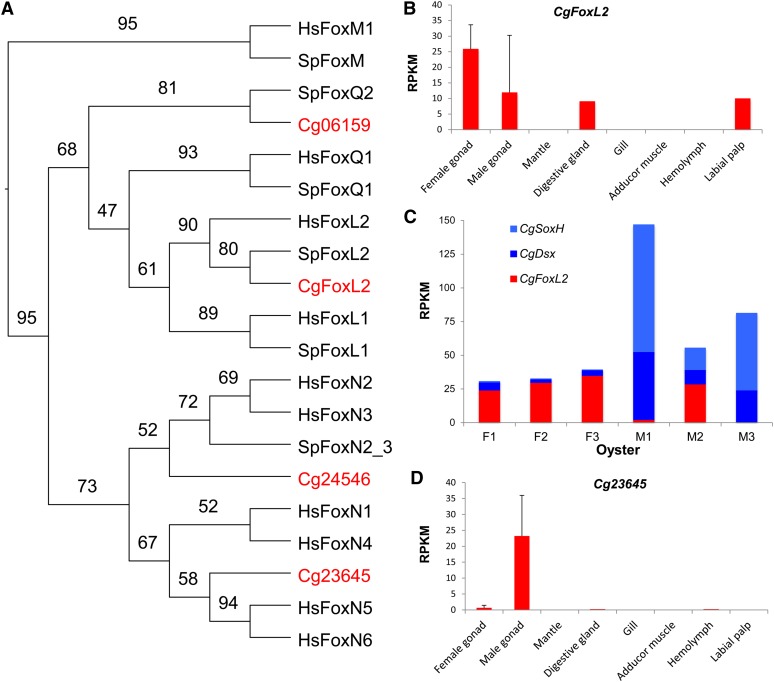
The C. gigas genome encodes 26 Fox genes (Table 4), compared with more than 40 found in human (Katoh and Katoh 2004a). All 26 Fox genes showed evidence of expression in our transcriptome data with two (Cg06159 and Cg24546) specifically expressed in ovary. We initially suspected that these two ovary-specific Fox genes might function like FoxL2 and determine ovarian development in C. gigas, however their expression profiles suggested otherwise. These two Fox genes had their greatest expression in oocytes, which progressively declined during embryonic development, to undetectable levels by late D-stage (Cg24546) or juvenile stage (Cg06159). They likely play important roles in embryogenesis, but seem unrelated to sex-determining pathways. Cg06159 is a homolog of FoxQ2 (Figure 3A). In sea urchin, FoxQ2 is progressively restricted to the animal plate during cleavage stage and provides the linkage of the primary animal-vegetal and secondary oral-aboral axes (Yaguchi et al. 2008). Hydrozoan has two FoxQ genes: FoxQ2a is expressed in early embryos and maintained through larval stages, while FoxQ2b is not expressed in embryos or larvae or polyp, but specially expressed in the gonad of medusa (Chevalier et al. 2006). Cg24546 is closely related to FoxN2 (Figure 3A), which in murine is involved in differentiation of multiple tissues during embryogenesis (Tribioli et al. 2002). In sea urchin, FoxN2/3 is a key gene involved in the formation of the larval skeleton (Rho and Mcclay 2011).
Table 4. Fox genes identified in C. gigas genome and their expression in gonads.
| Gene ID | Homolog | E-value | Gonad specific |
|---|---|---|---|
| Cg25509 | Fork head protein, Patella vulgate, CAD45552.1 | 1e-115 | No |
| Cg11405 | FoxB1, Saccoglossus kowalevskii, NP_001158435.1 | 8e-78 | No |
| Cg11631 | FoxA/B, Saccoglossus kowalevskii, NP_001164676.1 | 1e-71 | No |
| Cg17698 | FoxC-like protein, Saccoglossus kowalevskii, NP_001158465.1 | 5e-97 | No |
| Cg11851 | FoxD, Saccoglossus kowalevskii, NP_001164677.1 | 3e-69 | No |
| Cg06006 | FoxE1, Saccoglossus kowalevskii, NP_001158436.1 | 4e-37 | No |
| Cg08560 | FoxF, Patella vulgate, CBI70345.1 | 5e-88 | No |
| Cg28651 | FoxG, partial, Terebratalia transversa, AEZ03828.1 | 8e-79 | No |
| Cg21832 | FoxJ1, Saccoglossus kowalevskii, NP_001158438.1 | 3e-66 | No |
| Cg19731 | FoxJ2/3, Saccoglossus kowalevskii, ADB22670.1 | 6e-105 | No |
| Cg26255 | FoxK1, Branchiostoma floridae, ACE79146.1 | 8e-175 | No |
| Cg17701 | Fox/forkhead, Capitella teleta, ADC35033.1 | 1e-65 | No |
| Cg11004 | FoxL2, Azumapecten farreri, AFB35647.1 | 4e-132 | Noa |
| Cg06326 | FoxM1, partial, Columba livia, EMC82562.1 | 2e-28 | No |
| Cg14633 | FoxN1, Saccoglossus kowalevskii, NP_001158439.1 | 3e-60 | No |
| Cg11126 | FoxN2/3, Branchiostoma floridae, ACE79140.1 | 2e-91 | No |
| Cg24546 | FoxN2, Bos mutus, ELR49084.1 | 8e-15 | Ovaryb |
| Cg07980 | FoxO, Blattella germanica, CCF23214.1 | 1e-96 | No |
| Cg14285 | FoxP, Octopus vulgaris, ACN38054.1 | 2e-113 | No |
| Cg06159 | FoxQ2-like, Saccoglossus kowalevskii, NP_001161546.1 | 5e-51 | Ovaryb |
| Cg02561 | Foxl1-ema, Chelonia mydas, EMP25610.1 | 4e-46 | No |
| Cg03726 | FoxQ/D-like protein, Saccoglossus kowalevskii, NP_001161545.1 | 9e-74 | No |
| Cg12628 | FoxH1, Dicentrarchus labrax, CBN81873.1 | 2e-27 | No |
| Cg01578 | FoxH1, Oryzias latipes, NP_001153943.1 | 8e-07 | No |
| Cg23645 | Fox protein, Glarea lozoyensis, EPE29336.1 | 3e-06 | Testis |
Highly expressed in ovary, although technically not classified as ovary-specific because of abnormally high expression in one male.
Greatest expression is in oocytes.
Figure 3.
Analysis of selected Fox genes identified in C. gigas. (A) Phylogenetic tree of forkhead domains from selected Fox genes from C. gigas and reference species. (B) Expression profile of CgFoxL2 showing high expression in female gonad. (C) Expression of CgFoxL2, CgSoxH, and CgDsx in three females and three males showing possible interaction among the three genes in M2. (D) Male-specific expression of a novel Fox gene Cg23645. Error bars represent standard deviation (n = 3). Species names are abbreviated as Cg for Crassostrea gigas, Hs for Homo sapiens and Sp for Strongylocentrotus purpuratus. Numbers in the tree are bootstrap values. Accession numbers: CgFoxL2 ACN80999.1, Cg06159 EKC20378.1, Cg24546 EKC30312.1, Cg23645 EKC35023.1, SpFoxL1 ABB89488.1, SpFoxL2 ABB89483.1, SpFoxM, ABB89490.1, SpFoxN2/3 ABB89482.1, SpFoxQ1 ABB89489.1, SpFoxQ2 ABB89473.1, SpFoxY AF517552, HsFoxL1 AAG40312.1, HsFoxL2 AAY21823.1, HsFoxM1 NP_973731.1, HsFoxN1 NP_003584.2, HsFoxN2 AAH63305.1, HsFoxN3 AAH07506.1, HsFoxN4 AAI46826.1, HsFoxN5 AAH38969.2, HsFoxN6 AAH12934.1, and HsFoxQ1 AAH53850.1.
A homolog of FoxL2 has previously been identified in C. gigas (Naimi et al. 2009b), which is also identified in the oyster genome assembly (Figure 3A) although the assembled copy (Cg11004) incorrectly included an additional 3′ exon. In Naimi et al.’s study, CgFoxL2 is expressed in both female and male gonads, with a significant increase in females earlier during sexual development. In our transcriptome data, CgFoxL2 is also expressed in both sexes, highly although not specifically expressed in the ovary (due to abnormally high expression in one male) (Figure 3B). Its expression is high in gonads of all three females (24–35 RPKM) and one male (29 RPKM), but low in the other two males (0.3−2.2 RPKM). Without the exceptional male (M2 in Figure 3C), CgFoxL2 would qualify as an ovary-specific gene (Dheilly et al. 2012). Although the number of oysters sampled is small, the large variation in males is consistent with the sex determination model that recognizes two types of males: fake males that change sex and true male that do not (Guo et al. 1998). M2 with high CgFoxL2 expression may be a fake male that has a higher tendency to change sex. Interestingly, the exceptional male (M2) with high CgFoxL2 expression also had exceptionally low expression of male-promoting CgDsx and CgSoxH genes (Figure 3C). It is possible that all three genes collectively and through their interactions make M2 prone to sex change. This is largely speculative at this time but can be tested in future studies.
CgFoxL2 is not expressed in oocytes, early embryos, or somatic organs except at moderate levels in digestive gland (possibly due to contamination by gonad) and labial palp (Figure 3B). A small peak of CgFoxL2 expression is observed at trochophores stage, which together with ovary-specific expression during sexual development, point to a likely role for CgFoxL2 in germline or ovarian determination.
It is interesting to note that one Fox gene (Cg23645) is specifically expressed in testis (Figure 3D). Cg23645 or CgFoxN5 is closely related to FoxN5, which in mouse is expressed in embryonic germ cells and zygote (Katoh and Katoh 2004b). CgFoxN5 is not expressed at embryonic and larval stages, nor in any somatic organs in C. gigas. Its highly specific expression in testis is novel and may point to a possible role for this novel Fox gene in testis development in C. gigas.
A working model for sex determination in C. gigas
The Pacific oyster has a complex sex-determining system that is characterized by protandry, sex change, and rare but consistent hermaphroditism, and how such a dynamic system is controlled and maintained has been the subject of considerable interest (Coe 1943, Haley 1979, Guo et al. 1998, Naimi et al. 2009a,b, Hedrick and Hedgecock 2010, Santerre et al. 2014). We identified possible sex-determining pathway genes in C. gigas based on sequence homology and functions inferred from transcriptome data. The assumption is that if the gene is involved in sex determination in other organisms and it shows sex-specific expression that is consistent with its known function, it may be related to the sex-determining pathway in C. gigas. We recognize that sequence homology and expression data can only identify possible candidates that require further experimental verification. Given the state of knowledge about sex determination in molluscs, identification of candidate genes and working models are necessary steps for further analysis.
Our analyses indicate that CgDsx, CgSoxH, and CgFoxL2 are probably involved in sex determination in C. gigas. All three genes or their close relatives are key elements of sex-determining pathways in vertebrates and exhibited sex-specific expressions. Other than the DM domain containing Dsx that has been shown to have a deeply conserved role in sex determination in both invertebrates and vertebrates, Sry and FoxL2 are thought to be new recruits to sex-determining pathways in vertebrates or placental mammals (Gamble and Zarkower 2012; Matson and Zarkower 2012). The finding of these key vertebrate sex-determining genes with expected expression profile for sex determination in C. gigas is novel and suggests that these vertebrate genes may not be inventions of vertebrates. Their role in sex determination may be deeply rooted in evolution and at minimum conserved in a mollusc, despite rapid evolution of the regulatory pathways that in C. gigas may involve both genetic and environmental factors. Except for the deeply conserved DM domain gene Dsx, sex-determining or regulating genes in C. elegans and D. melanogaster are either not found in C. gigas or without expression profiles expected for sex-determining pathway genes (Table 2 and Figure 4). Our analysis suggests that sex determination in C. gigas may share more similarities with that in vertebrates than with that of worms and flies (ecdyspzoans). Although the three groups of bilaterians, Lophotrochozoa, Ecdysozoa, and Deuterostomia, are well-recognized, their relationship to each other is not clear. Phylogenetic analysis based on whole-genome sequences indicates that although molluscs and annelids (lophotrochozoans) are related to worms and flies within protostomes, their genomes in many aspects are more similar to those of invertebrate deuterostomes (Simakov et al. 2013). Also, molluscs share the same telomeric sequence with vertebrates, but not with worms and flies (Zakian 1995, Guo and Allen 1997, Sakai et al. 2005). The conservation of genes related to sex determination between the oyster and vertebrates provides additional argument that molluscs may be closer to the common bilaterian ancestor than ecdysozoans.
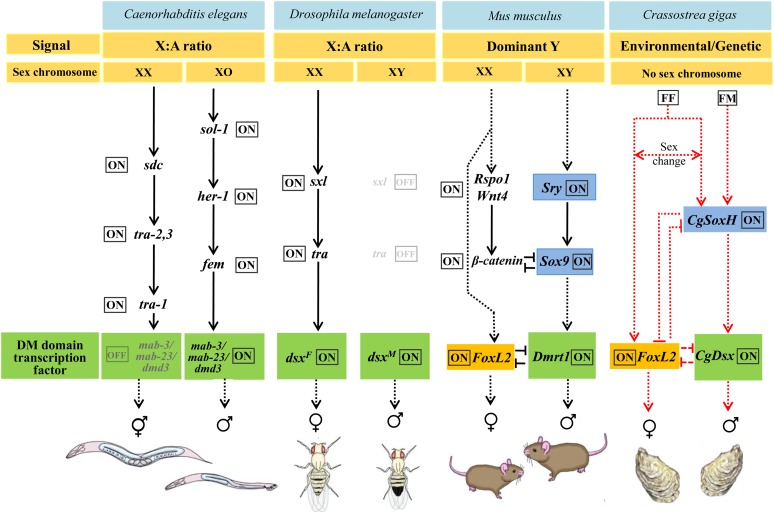
Figure 4.
Hypothesized sex-determining pathway in C. gigas compared with that in model organisms as summarized by Gamble and Zarkower (2012). For clarity, only selected key sex-specific regulators are shown. Dashed black lines indicate temporal relationships, and dashed red lines indicate hypothetical relationships based on expression data only. [Modified based on Gamble and Zarkower (2012)]. FF genotype permits sex change (Guo et al. 1998).
As a working model, we speculate that CgSoxH may play a leading role in the sex-determining pathway of C. gigas as it is closely related to the up-stream regulator Sry in vertebrates and strictly expressed in testis. It may directly or indirectly activate CgDsx (Figure 4), which as a DM domain gene is a master switch for testis development in all metazoans studied so far. DM domain genes have been suspected for roles in male-determination in bivalve molluscs (Yu et al. 2011). Both CgSoxH and CgDsx may interact or inhibit CgFoxL2, which is specifically expressed in ovaries with the exception of one male (M2 in Figure 3C and partly supported by Naimi et al. 2009b). The abnormally high expression of CgFoxL2 and low expression of CgSoxH and CgDsx in M2 provided preliminary evidence for possible interaction among these male- and female-promoting genes (Figure 3C). This finding is preliminary but consistent with the reported interaction among Sry, Sox9, Dmrt1, and FoxL2 in mammals (Veitia 2010, Matson et al. 2011).
The finding of large variation in expression of sex-determining genes in males supports one of the genetic models of sex determination that recognizes two types of males: fake males (FF) that change sexes and true males (FM) that do not (Guo et al. 1998). Although the variation could be caused by various factors such as different stages of sexual development, it is possible that M2 is a FF male where low expression of male-promoting CgSoxH results in low expression of male-promoting CgDsx and high expression of female-promoting CgFoxL2, which in turn may promote sex change to female. The number of oysters studied here is limited, and further studies are needed. If the proposed model is correct, it would be interesting to ask how the expression of CgSoxH is controlled by cis/trans genetic elements and by environmental factors. The working model and insights provided in this study should stimulate further investigation on sex-determining pathways in molluscs and other invertebrates.
This study identified two novel genes, CgDsx and CgSoxH (or Sry-like), that are likely involved in sex determination in C. gigas and provided supporting data for the involvement of CgFoxL2. The sex-determining functions of Sry and FoxL2 are thought to have emerged late during the evolution of vertebrates. The finding of such genes in C. gigas with sex-specific expression indicates that these vertebrate sex-determining genes may not be inventions of vertebrates as suggested by previous studies. Their role in sex determination may be deeply conserved in evolution, despite rapid evolution of the regulatory pathways that in C. gigas may involve both genetic and environmental factors.
Supplementary Material
Acknowledgments
We thank Guofan Zhang and Li Li for discussions, Yingxiang Li for help with sampling, and Laodong Aquaculture Breeding Company for providing oysters. N.Z. was a visiting student at and supported by Rutgers University. This work was supported in part by Taishan Oversea Scholar Program of Shandong, 973 National Basic Research Program of China Project 2010CB126402 and USDA Project 2009-35205-05052 and NJ32108.
Footnotes
Supporting information is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.114.013904/-/DC1
Communicating editor: B. J. Andrews
Literature Cited
- Alexa, A., and J. Rahnenfuhrer. 2010 TopGO: Enrichment analysis for Gene Ontology. Bioconductor package version 2.6.0. Available at: Accessed September 17, 2014. http://bioc.ism.ac.jp/2.6/bioc/html/topGO.html.
- Amemiya I., 1929. Another species of Monoecious oyster, Ostrea plicata Chemnitz. Nature 123: 874. [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., et al. , 2000. Gene Ontology: tool for the unification of biology. Nat. Genet. 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate —a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. Met. 57: 289–300. [Google Scholar]
- Burtis K. C., Baker B. S., 1989. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively Spliced mRNAs encoding related sex-specific polypeptides. Cell 56: 997–1010. [DOI] [PubMed] [Google Scholar]
- Chavez-Villalba J., Soyez C., Huvet A., Gueguen Y., Lo C., et al. , 2011. Determination of gender in the Pearl oyster Pinctada Margaritifera. J. Shellfish Res. 30: 231–240. [Google Scholar]
- Chevalier S., Martin A., Leclere L., Amiel A., Houliston E., 2006. Polarised expression of FoxB and FoxQ2 genes during development of the hydrozoan Clytia hemisphaerica. Dev. Genes Evol. 216: 709–720. [DOI] [PubMed] [Google Scholar]
- Coe W. R., 1943. Sexual differentiation in mollusks I. Pelecypods. Q. Rev. Biol. 18: 154–164. [Google Scholar]
- Cole H. A., 1941. Sex-ratio in Urosalpinx cinerea, the American oyster drill. Nature 147: 116–117. [Google Scholar]
- Crisponi L., Deiana M., Loi A., Chiappe F., Uda M., et al. , 2001. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27: 159–166. [DOI] [PubMed] [Google Scholar]
- Devlin R. H., Nagahama Y., 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208: 191–364. [Google Scholar]
- Dheilly N. M., Lelong C., Huvet A., Kellner K., Dubos M.-P., et al. , 2012. Gametogenesis in the Pacific oyster Crassostrea gigas: a microarrays-based analysis identifies sex and stage specific genes. PloS one 7: e36353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon S., Gentleman R., 2007. Using GOstats to test gene lists for GO term association. Bioinformatics 23: 257–258. [DOI] [PubMed] [Google Scholar]
- Feng Z. F., Shao M. Y., Sun D. P., Zhang Z. F., 2010. Cloning, characterization and expression analysis of Cf-dmrt4-like gene in Chlamys farreri. J. Fishery Sci. China 17: 930–940. [Google Scholar]
- Gamble T., Zarkower D., 2012. Sex determination. Curr. Biol. 22: R257–R262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli F., Milani L., Chang P. L., Hedgecock D., Davis J. P., et al. , 2012. De novo assembly of the manila clam ruditapes philippinarum transcriptome provides wew insights into expression bias, mitochondrial doubly uniparental inheritance and sex determination. Mol. Biol. Evol. 29: 771–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. M., Allen S. K., 1994. Sex determination and polyploid gigantism in the dwarf surfclam (Mulinia Lateralis Say). Genetics 138: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. M., Allen S. K., 1997. Fluorescence in situ hybridization of vertebrate telomere sequence to chromosome ends of the Pacific oyster, Crassostrea gigas Thunberg. J. Shellfish Res. 16: 87–89. [Google Scholar]
- Guo X. M., Hedgecock D., Hershberger W. K., Cooper K., Allen S. K., 1998. Genetic determinants of protandric sex in the Pacific oyster, Crassostrea gigas Thunberg. Evolution 52: 394–402. [DOI] [PubMed] [Google Scholar]
- Haley L. E., 1979. Genetics of sex determination in the American oyster. Proceedings of the National Shellfisheries Association 69: 54–57. [Google Scholar]
- Hartfield M., Keightley P. D., 2012. Current hypotheses for the evolution of sex and recombination. Integr Zool 7: 192–209. [DOI] [PubMed] [Google Scholar]
- He Y., Bao Z. M., Guo H. H., Zhang Y. Y., Zhang L. L., et al. , 2013. Molecular cloning and characterization of SoxB2 gene from Zhikong scallop Chlamys farreri. Chin. J. Oceanology Limnol. 31: 1216–1225. [Google Scholar]
- Hedrick P. W., Hedgecock D., 2010. Sex determination: genetic models for oysters. J. Hered. 101: 602–611. [DOI] [PubMed] [Google Scholar]
- Hempel L. U., Oliver B., 2007. Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev. Biol. 7: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S., Apweiler R., Attwood T. K., Bairoch A., Bateman A., et al. , 2009. InterPro: the integrative protein signature database. Nucleic Acids Res. 37: D211–D215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S., 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimada K., Koopman P., 2010. Sry: the master switch in mammalian sex determination. Development 137: 3921–3930. [DOI] [PubMed] [Google Scholar]
- Katoh M., Katoh M., 2004a Human FOX gene family (review) . Int. J. Oncol. 25: 1495–1500. [PubMed] [Google Scholar]
- Katoh M., Katoh M., 2004b Germ-line mutation of Foxn5 gene in mouse lineage. Int. J. Mol. Med. 14: 463–467. [PubMed] [Google Scholar]
- Kent J., Wheatley S. C., Andrews J. E., Sinclair A. H., Koopman P., 1996. A male-specific role for SOX9 in vertebrate sex determination. Development 122: 2813–2822. [DOI] [PubMed] [Google Scholar]
- Klinbunga S., Amparyup P., Khamnamtong B., Hirono I., Aoki T., et al. , 2009. Isolation and Characterization of Testis-Specific DMRT1 in the Tropical Abalone (Haliotis asinina). Biochem. Genet. 47: 66–79. [DOI] [PubMed] [Google Scholar]
- Koopman, P., 2001 Sry, Sox9 and mammalian sex determination, pp. 25–56 in Genes and Mechanisms in Vertebrate Sex Determination, edited by G. Scherer and M. Schmid. Birkhäuser Verlag Basel, Switzerland. [Google Scholar]
- Koopman P., 2005. Sex determination: a tale of two Sox genes. Trends Genet. 21: 367–370. [DOI] [PubMed] [Google Scholar]
- Kopp A., 2012. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 28: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Dumitriu B., Penzo-Mendez A., Han Y., Pallavi B., 2007. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 39: 2195–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane M., Consortium U., 2011. UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford). 2011: bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson C. K., Zarkower D., 2012. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 13: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson C. K., Murphy M. W., Sarver A. L., Griswold M. D., Bardwell V. J., et al. , 2011. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant-Larios H., Diaz-Hernandez V., 2013. Environmental sex determination mechanisms in reptiles. Sex Dev. 7: 95–103. [DOI] [PubMed] [Google Scholar]
- Naimi A., Martinez A. S., Specq M. L., Diss B., Mathieu M., et al. , 2009b Molecular cloning and gene expression of Cg-Foxl2 during the development and the adult gametogenetic cycle in the oyster Crassostrea gigas. Comp. Biochem. Physiol. B 154: 134–142. [DOI] [PubMed] [Google Scholar]
- Naimi A., Martinez A. S., Specq M. L., Mrac A., Diss B., et al. , 2009a Identification and expression of a factor of the DM family in the oyster Crassostrea gigas. Comp. Biochem. Physiol. A 152: 189–196. [DOI] [PubMed] [Google Scholar]
- Osaki E., Nishina Y., Inazawa J., Copeland N. G., Gilbert D. J., et al. , 1999. Identification of a novel Sry-related gene and its germ cell-specific expression. Nucleic Acids Res. 27: 2503–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi C., Pelosi E., Tran J., Colombino M., Douglass E., et al. , 2007. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 16: 2795–2804. [DOI] [PubMed] [Google Scholar]
- Purdy P. H., 2008. Ubiquitination and its influence in boar sperm physiology and cryopreservation. Theriogenology 70: 818–826. [DOI] [PubMed] [Google Scholar]
- Quayle D. B., 1988. Pacific Oyster Culture in British Columbia. Department of Fisheries and Oceans, Ottawa. [Google Scholar]
- Rho H. K., McClay D. R., 2011. The control of foxN2/3 expression in sea urchin embryos and its function in the skeletogenic gene regulatory network. Development 138: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout E. J., Dornan A. J., Neville M. C., Eadie S., Goodwin S. F., 2010. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J. L., Rozowsky J. S., Laurenzi I. J., Petersen P. H., Zou K., et al. , 2004. Major molecular differences between mammalian sexes are involved in drug metabolism and renal function. Dev. Cell 6: 791–800. [DOI] [PubMed] [Google Scholar]
- Robinett C. C., Vaughan A. G., Knapp J. M., Baker B. S., 2010. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8: e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Okumura S. I., Yamamori K., 2005. Telomere analysis of Pacific abalone Haliotis discus hannai chromosomes by fluorescence in situ hybridization. J. Shellfish Res. 24: 1149–1151. [Google Scholar]
- Santerre C., Sourdaine P., Adeline B., Martinez A. S., 2014. Cg-SoxE and Cg-beta-catenin, two new potential actors of the sex-determining pathway in a hermaphrodite lophotrochozoan, the Pacific oyster Crassostrea gigas. Comp. Biochem. Physiol. A 167: 68–76. [DOI] [PubMed] [Google Scholar]
- Santos E. M., Workman V. L., Paull G. C., Filby A. L., Van Look K. J. W., et al. , 2007. Molecular basis of sex and reproductive status in breeding zebrafish. Physiol. Genomics 30: 111–122. [DOI] [PubMed] [Google Scholar]
- Schepers G., Wilson M., Wilhelm D., Koopman P., 2003. SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J. Biol. Chem. 278: 28101–28108. [DOI] [PubMed] [Google Scholar]
- Shi Y., Wang Q., He M., 2014. Molecular identification of dmrt2 and dmrt5 and effect of sex steroids on their expressions in Chlamys nobilis. Aquaculture 426-427: 21–30. [Google Scholar]
- Simakov O., Marletaz F., Cho S. J., Edsinger-Gonzales E., Havlak P., et al. , 2013. Insights into bilaterian evolution from three spiralian genomes. Nature 493(7433): 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small C., Carney G., Mo Q., Vannucci M., Jones A., 2009. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: Evidence for masculinization of the transcriptome. BMC Genomics 10: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Roeszler K. N., Ohnesorg T., Cummins D. M., Farlie P. G., et al. , 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461: 267–271. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312−1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., et al. , 2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101: 6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P., Neuber E., Schatten G., 2002. Ubiquitin-dependent sperm quality control mechanism recognizes spermatozoa with DNA defects as revealed by dual ubiquitin-TUNEL assay. Mol. Reprod. Dev. 61: 406–413. [DOI] [PubMed] [Google Scholar]
- Teaniniuraitemoana V., Huvet A., Levy P., Klopp C., Lhuillier E., et al. , 2014. Gonad transcriptome analysis of pearl oyster Pinctada margaritifera: identification of potential sex differentiation and sex determining genes. BMC Genomics 15: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribioli C., Robledo R. F., Lufkin T., 2002. The murine fork head gene Foxn2 is expressed in craniofacial, limb, CNS and somitic tissues during embryogenesis. Mech. Dev. 118: 161–163. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut N. H., Treier M., 2006. Foxl2 function in ovarian development. Mol. Genet. Metab. 88: 225–234. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut N. H., Jakob S., Anlag K., Eisenberger T., Sekido R., et al. , 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139: 1130–1142. [DOI] [PubMed] [Google Scholar]
- Veitia R. A., 2010. FOXL2 vs. SOX9: A lifelong “battle of the sexes”. BioEssays 32: 375–380. [DOI] [PubMed] [Google Scholar]
- Visconti P. E., Kopf G. S., 1998. Regulation of protein phosphorylation during sperm capacitation. Biol. Reprod. 59: 1–6. [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Angerer R. C., Angerer L. M., 2008. A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev. Cell 14: 97–107. [DOI] [PubMed] [Google Scholar]
- Yi W. S., Zarkower D., 1999. Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development 126: 873–881. [DOI] [PubMed] [Google Scholar]
- Yu F. F., Wang M. F., Zhou L., Gui J. F., Yu X. Y., 2011. Molecular cloning and expression characterization of Dmrt2 in Akoya Pearl oysters, Pinctada Martensii. J. Shellfish Res. 30: 247–254. [Google Scholar]
- Zakian V. A., 1995. Telomeres - Beginning to Understand the End. Science 270: 1601–1607. [DOI] [PubMed] [Google Scholar]
- Zhang G. F., Fang X. D., Guo X. M., Li L., Luo R. B., et al. , 2012. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490: 49–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.