Abstract
The CRISPR-associated RNA-guided nuclease Cas9 has emerged as a powerful tool for genome engineering in a variety of organisms. To achieve efficient gene targeting rates in Drosophila, current approaches require either injection of in vitro transcribed RNAs or injection into transgenic Cas9-expressing embryos. We report a simple and versatile alternative method for CRISPR-mediated genome editing in Drosophila using bicistronic Cas9/sgRNA expression vectors. Gene targeting with this single-plasmid injection approach is as efficient as in transgenic nanos-Cas9 embryos and allows the isolation of targeted knock-out and knock-in alleles by molecular screening within 2 months. Our strategy is independent of genetic background and does not require prior establishment of transgenic flies.
Keywords: Drosophila, CRISPR, Cas9, HDR
The generation of targeted mutations and the precise modification of an organism’s genome sequence are powerful approaches to characterize the function of genes and regulatory elements. A prerequisite to achieve these goals efficiently is the induction of targeted double-strand breaks (DSBs) in the genome (Rouet et al. 1994). DSBs are typically repaired by error-prone nonhomologous end-joining (NHEJ), which often generates short insertions and deletions (indels), thereby causing frame-shift mutations (Bibikova et al. 2002). In the presence of a homologous donor, DSBs can also be repaired via homology-directed repair (HDR), a pathway that can be exploited to introduce specific nucleotide changes or generate defined insertions or deletions (Bibikova et al. 2003; Chen et al. 2011).
Of the various programmable nuclease platforms available for the generation of sequence-specific DSBs, the prokaryotic type II clustered regularly interspaced short palindromic repeat (CRISPR) adaptive immune system has attracted considerable attention for its versatility and ease of use (Sander and Joung 2014; Hsu et al. 2014). The CRISPR-associated Cas9 endonuclease can recognize and cleave target DNA in combination with a single chimeric guide RNA (sgRNA), where the first 20 nucleotides of the RNA provide sequence-specificity by guiding Cas9 to complementary DNA molecules (Jinek et al. 2012; Gasiunas et al. 2012). By replacing these 20 nucleotides of the sgRNA, this simple two-component system can be readily reprogrammed to target a sequence of choice, making it ideally suited for genome engineering purposes (Jinek et al. 2012).
Although the molecular details of the CRISPR/Cas9 mechanism were elucidated only recently, this RNA-guided nuclease (RGN) has already been applied to edit the genomes in a wide variety of organisms (Sander and Joung 2014; Hsu et al. 2014). Previous attempts of Cas9-mediated gene targeting in Drosophila initially achieved low mutation rates (Gratz et al. 2013) and required in vitro transcription of Cas9 and the sgRNA (Yu et al. 2013; Bassett et al. 2013; Yu et al. 2014) or injecting into transgenic Cas9 expressing embryos (Kondo and Ueda 2013; Ren et al. 2013; Sebo et al. 2014; Gratz et al. 2014; Port et al. 2014) to achieve feasible targeting rates.
We describe a highly efficient, fast, and versatile method to generate targeted mutations and perform precise genome engineering in less than 2 months. Our strategy is independent of genetic background and based on injecting a bicistronic plasmid that contains both components required to induce targeted DSBs, Cas9 and the sgRNA, under the control of constitutive Drosophila promoters.
Materials and Methods
Generation of pDCC plasmids
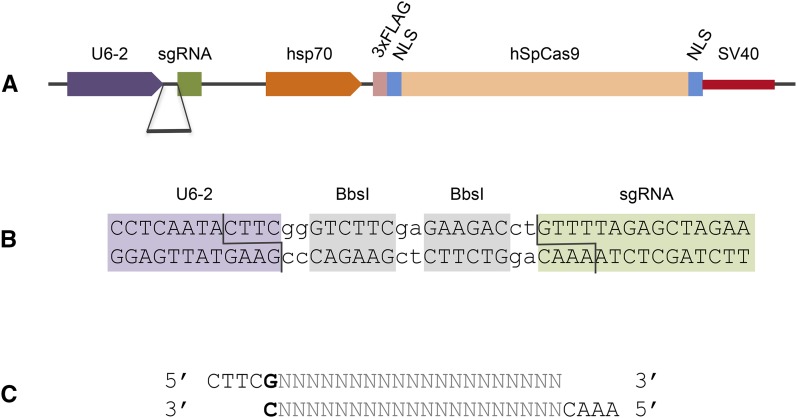
The human codon-optimized Streptococcus pyogenes Cas9D10A nickase (hSpCas9D10A) was cloned from pX335 (Cong et al. 2013) into the pHW Drosophila Gateway vector and the BbsI site in the SV40 3′-UTR mutated by PCR. The sgRNA expression cassette was then inserted into the NotI site of pHW-Cas9D10A by In-Fusion cloning (Clontech) of the U6:96Ab promoter amplified from genomic DNA and the chimeric sgRNA scaffold from pX335, resulting in plasmid pDCC1. To generate pDCC2, the N-terminus of Cas9D10A in pDCC1 was replaced with an AgeI-ApaI fragment of wild-type Cas9 from pX330 (Cong et al. 2013). Finally, we replaced the hsp70Ab promoter between the XbaI and HindIII sites in pDCC1 and pDCC2 with a 350-bp hsp70Bb promoter fragment to obtain plasmids pDCC5 and pDCC6, respectively (Figure 1A and Supporting Information, Figure S1).
Figure 1.
A bicistronic Drosophila CRISPR/Cas9 vector. (A) Schematic map of pDCC6, with the sgRNA cassette under the control of the Drosophila U6:96Ab (U6-2) promoter as well as an hsp70Bb promoter driving Cas9 expression. (B) gRNA sequences are inserted between the U6 promoter and the sgRNA scaffold via two BbsI sites. (C) gRNAs are cloned as complementary oligonucleotide pairs with suitable overhangs and an additional G (bold, required for RNA PolIII transcription) preceding the target-specific 20 nt protospacer (gray).
gRNA cloning and embryo injections
Genomic regions downstream of the start codon (for NHEJ mutagenesis) or close to the stop codon (for HDR) were sequenced in the injection stocks to avoid SNPs. The sequences were then scanned for NGG protospacer adjacent motifs (PAM) and suitable gRNAs were checked for potential off-targets with the CRISPR Optimal Target Finder (Gratz et al. 2014).
All gRNAs were preceded by a 5′-G required for RNA Pol III transcription from the U6 promoter and cloned as complementary oligonucleotides with suitable overhangs for the BbsI site (Figure 1). The oligos were phosphorylated with polynucleotide kinase (Thermo Scientific), annealed, and ligated into BbsI-digested and dephosphorylated pDCC6 or pU6-BbsI-chiRNA vectors. Sequence-verified plasmid DNA (Midi-Prep, Qiagen) was injected into dechorionated embryos at 100 ng/µl as described (Ringrose 2009). For HDR, ultramer DNA oligos (IDT) were co-injected at 150 ng/µl (ham) or 250 ng/µl (dpn, mγ).
Fly crosses
pDCC6-e was injected into isogenized w1118 embryos, and pU6-e was injected into nanos-Cas9 (Port et al. 2014) and vasa-Cas9 (Gratz et al. 2014). Hatched G0 flies were crossed to TM3, Sb, e/TM6, Hu, Tb, e, and dark flies were counted in the next generation. pDCC6-y and pDCC6-w were co-injected into Canton-S embryos and subsequently crossed to y, w mutant flies. When targeting ebony as well as yellow and white, two rounds of injections were performed on different days, and the results from both injections were combined (Table 1 and Table 2).
Table 1. Germline transmission rates for NHEJ-mediated mutagenesis and ssODN-mediated HDR.
| Male Crosses |
Female crosses |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Construct | Host | ssODN | Embryos | % (No.) Founders | % (No.) Progeny | % (No.) Founders | % (No.) Progeny | % F1 |
| DCC6-e | w1118 | — | 189 | 89 (17/19) | 14 (386/2802) | 57 (26/46) | 12 (536/4312) | 13 |
| U6-e | nos-cas9 | — | 192 | 53 (9/17) | 5.9 (68/1143) | 64 (9/14) | 16 (153/954) | 11 |
| U6-e | vas-cas9 | — | 201 | 83 (10/12) | 29 (362/1270) | 94 (32/34) | 49 (1765/3572) | 44 |
| DCC6-MED27 | FRT82 | — | 75 | 43 (3/7) | 20 (7/35) | 0 (0/5) | 0 (0/25) | 12 |
| DCC6-ham | w1118 | V5 | 85 | 14 (3/22) | 2.7 (3/110) | 6.7 (1/15) | 6.7 (4/60) | 4.1 |
| DCC6-dpn | w1118 | V5 | 126 | 11 (1/9) | 2.8 (2/72) | 13 (2/15) | 2.5 (3/120) | 2.6 |
| DCC6-mγ | w1118 | V5 | 191 | 15 (3/20) | 11 (11/100) | 5.9 (1/17) | 5.9 (5/85) | 8.6 |
Table 2. Mutation rates for simultaneously targeting yellow and white.
| G0 Founders |
F1 Mutants |
|||||||
|---|---|---|---|---|---|---|---|---|
| Construct | Host | Embryos | y− | w− | y− w− | y− w+ | y+ w− | y− w− |
| DCC6-y + DCC6-w | Canton-S | 167 | 31/35 | 26/35 | 20/35 | 23% | 4.7% | 15% |
pDCC6-MED27 was injected into FRT82B embryos and G0 flies crossed to Ly/TM3, Sb. From each fertile G0 cross, five Ly males were individually crossed to Df(Exel7283)/TM3, Sb virgins.
For HDR, pDCC6 plasmids and donor oligos were co-injected into an isogenized w1118 stock and hatched G0 flies crossed to balancer flies appropriate for the target chromosome. Between four and eight males from each fertile cross were individually crossed to balancer virgins again. In the next generation, males were collected for PCR.
PCR screening
Flies were homogenized in a 96-well format (1–3 flies per well) together with 100 µl squishing buffer (Gloor et al. 1993) and a 5-mm steel bead (Qiagen) by vigorous shaking in a TissueLyser II (Qiagen) for 10 min at 28 Hz. The plates were then incubated at 65° for 2 hr, followed by an inactivation step at 94° for 2 min; 1 µl of the supernatant was used for PCR (25 µl volume, 35 cycles). For HDR screening, undiluted PCR samples were analyzed with a high-resolution 96-capillary fragment analyzer (Advanced Analytical).
Prior to sequencing, 6 µl of each PCR were enzymatically purified with 0.5 µl of Illustra ExoProStar 1-Step enzyme mix (GE Healthcare) at 37° for 30 min, followed by inactivation at 80° for 20 min.
Results
An efficient CRISPR vector for Drosophila
To adapt the CRISPR/Cas9 system for gene targeting in Drosophila, we generated the plasmid pDCC6 (Figure 1), which contains the Cas9 gene from S. pyogenes under the control of the hsp70Bb promoter (Gratz et al. 2013) and a sgRNA cassette downstream of the U6:96Ab promoter (Gratz et al. 2013; Kondo and Ueda 2013; Ren et al. 2013; Xue et al. 2014).
As an initial test, we targeted the ebony (e) gene on the third chromosome, which when mutated leads to a darker pigmentation of the adult cuticle that is simple to score. A gRNA target site was identified just downstream of the start codon (Port et al. 2014) and cloned into pDCC6 as a pair of oligonucleotides. Flies injected with pDCC6-e were crossed to homozygous e1 mutants and the number of dark offspring were counted in the next generation. From 65 fertile G0 flies, 43 gave rise to ebony mutants (66% founders), with 13% of all F1 flies being ebony (Table 1). Sequencing of genomic DNA from randomly chosen ebony flies revealed indels leading to frame-shifts close to the gRNA target site (File S1), characteristic of DSB repair by NHEJ.
Comparison with transgenic Cas9 flies
Several laboratories have recently generated transgenic flies that express Cas9 under the control of either germline-specific or ubiquitous promoters (Kondo and Ueda 2013; Sebo et al. 2014; Ren et al. 2013; Gratz et al. 2014; Port et al. 2014). To compare the targeting rates of the bicistronic pDCC6 plasmid with sgRNA plasmid injections into published transgenic Cas9 flies, we cloned the same gRNA that targets ebony into pU6-BbsI-chiRNA (Gratz et al. 2013). This vector also makes use of the U6:96Ab promoter to drive sgRNA expression but lacks the Cas9 gene. The resulting pU6-e plasmid was injected into nanos-Cas9 (Port et al. 2014) and vasa-Cas9 (Gratz et al. 2014) expressing embryos. After crossing G0 flies to e1 mutants, 11% from the nanos-Cas9 injections had a dark body color, a slightly lower frequency than what we observed after pDCC6-e injection into w1118 embryos (13%). In contrast, injection of vasa-Cas9 flies resulted in a mutation rate of 44%, a 3.4-fold increase over our bicistronic vector (Table 1).
Mutagenesis of an essential gene
Using the bicistronic pDCC6 vector, we next aimed to generate mutations in an essential gene whose loss does not result in a visible phenotype and chose to target subunit 27 of the mediator complex (MED27). Because MED27 is located at cytological position 83B, pDCC6-MED27 was injected into FRT82B embryos, which would subsequently allow the generation of mitotic clones without having to recombine the mutant alleles onto the FRT chromosome.
Five males from each of the 12 fertile G0 crosses were individually mated to deficiency virgins uncovering the MED27 locus. Of the 60 crosses analyzed, seven did not complement the deficiency (11.7%) (Table 1). Sequencing of the gRNA target site in these flies revealed indels leading to frame-shifts in the MED27 coding sequence (File S1). It is thus feasible to isolate loss-of-function alleles of an essential gene at frequencies high enough for fast screening in the absence of a visible phenotype and in a genetic background of choice.
Generation of double mutants
The high mutation rates achieved when targeting single genes led us to investigate whether we could mutate two genes simultaneously. For this purpose, a mix of two pDCC6 plasmids that target the body color gene yellow (y) and the eye color gene white (w) on the X chromosome were co-injected into wild-type embryos. Screening F1 flies, we observed targeting rates of 38% and 20% for yellow and white, respectively, and 15% of all offspring were phenotypically both yellow and white (Table 2). Thus, it is possible to multiplex our vector to generate double mutants with high efficiency in a wild-type background.
HDR with oligonucleotide donors
The use of single-stranded oligodeoxynucleotides (ssODN) as HDR donors is a simple, yet powerful, approach to introduce precise changes in the genome (Chen et al. 2011). We set out to add a V5 epitope tag to the C-terminus of the transcription factors Hamlet (Ham), Deadpan (Dpn), and E(spl)mγ-HLH (mγ) with oligos containing the 42 nucleotide V5 sequence, flanked by homology arms ranging from 61 to 71 nucleotides in length. Each ssODN was co-injected into w1118 embryos with a pDCC6 plasmid that targets the corresponding gene close to the stop codon (File S1).
Using PCR primers distal to the homology arms, the genomic region surrounding the target site was amplified from heterozygous F2 flies. High-resolution capillary gel electrophoresis identified 7/170 (ham), 5/190 (dpn), and 16/185 (mγ) that had amplified a fragment of wild-type length as well as an additional fragment approximately 45 bp larger, indicative of V5 integration via HDR (Table 1, Figure S2). For 3/7 (ham), 5/5 (dpn), and 6/16 (mγ) lines, the integrity of the tag could be confirmed by DNA sequencing. The remaining lines lacked one or two nucleotides within or close to the V5 sequence, probably due to shorter donor molecules present in the injection mix, which is common for synthesized oligos of that length.
Using our bicistronic vector, we could demonstrate that it is feasible to identify HDR-mediated targeting events in the absence of a visible marker by PCR screening in a standard genetic background that is independent of transgenic Cas9 expression.
Discussion
Whereas previous reports of using CRISPR/Cas9 in Drosophila required the injection of in vitro transcribed RNA or injecting into transgenic Cas9-expressing embryos to achieve feasible gene targeting rates (Yu et al. 2013; Bassett et al. 2013; Kondo and Ueda 2013; Sebo et al. 2014; Ren et al. 2013; Gratz et al. 2014; Yu et al. 2014; Port et al. 2014), we chose a plasmid-based approach and reasoned that having both Cas9 and the sgRNA on a single vector might enhance targeting efficiencies (Cong et al. 2013; Wang et al. 2013). We obtained germline transmission rates of >10% for mutations in ebony and the essential gene MED27, as well as for simultaneously targeting yellow and white. Using three ssODN donors, we identified HDR events in 2.6–8.6% of F1 flies, similar to frequencies reported with a dsRed-marked plasmid donor injected into vasa-Cas9 embryos (Gratz et al. 2014).
Although mutations in MED27 were isolated by screening for lethality, detecting mutant alleles by phenotype is not feasible for every gene. Because mutation rates are usually above 10% following pDCC6 injection, we now routinely sequence PCR products amplified from 50 to 100 heterozygous F2 flies to identify those with frame-shift mutations, or from 200 flies for ssODN-mediated HDR.
Transgenic flies that constitutively express Cas9 have recently been used to increase targeting frequencies (Kondo and Ueda 2013; Ren et al. 2013; Sebo et al. 2014; Gratz et al. 2014; Port et al. 2014). However, we did not observe higher mutation rates when targeting ebony in nanos-Cas9 flies over our single-plasmid injection approach, but we obtained a 3.4-fold increase with vasa-Cas9 embryos. For many applications, the ability to induce mutations in a genetic background of choice will outweigh the increased targeting rates in vasa-Cas9 flies. In addition, vasa-Cas9 expression is not restricted to the germline (Port et al. 2014), and sgRNA injected vasa-Cas9 embryos were reported to have low viability and fertility rates, especially when targeting essential genes (Sebo et al. 2014; Port et al. 2014). In contrast, we did not notice patches of mutant tissue in adult G0 flies when targeting ebony or yellow, indicating that mutations in somatic cells after pDCC6 injection are rare, despite efficient targeting in the germline. Another advantage of our bicistronic vectors is that they are independent of host genotypes, which we have exploited to mutate genes on FRT chromosomes. For such cases, it would be time-consuming and laborious either to first cross in a Cas9 transgene or to recombine the mutant alleles afterward.
Co-injecting two pDCC6 plasmids that express different sgRNAs, we were able to simultaneously mutate two genes on the same chromosome. A similar strategy could also be applied to generate large deletions, for which two DSBs are required. In conjunction with the Cas9 nickase (Cas9D10A present in pDCC5), this enables a double-nicking/offset-nicking approach, which has been shown to reduce the indel frequency at off-target sites in mammalian cells (Mali et al. 2013; Ran et al. 2013).
In summary, we describe versatile tools for Cas9-mediated genome editing in Drosophila that are independent of genetic background, yet efficient enough for PCR-based screening to be feasible. For each targeting experiment, one simply needs to clone a pair of oligonucleotides into the targeting vector prior to injection, without the need for in vitro transcription reactions, thus making genome engineering as straightforward as regular transgenesis. Finally, by exchanging the promoters that drive Cas9 and the sgRNA, the single-plasmid approach could be adapted for genome engineering in other species that are amenable to DNA injections.
Supplementary Material
Acknowledgments
We thank our Molecular Biology Services core facility for support with PCR and DNA sequencing, and S. Bhalerao, J. Brennecke, and R. Neumueller for comments on the manuscript. The vectors described in this study (pDCC5 and pDCC6) are available from Addgene and the Vienna Drosophila Resource Center (VDRC). Other CRISPR plasmids were obtained from Addgene, and fly stocks were obtained from the Bloomington Drosophila Stock Center.
Footnotes
Supporting information is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.114.014126/-/DC1
Communicating editor: B. J. Andrews
Literature Cited
- Bassett A. R., Tibbit C., Ponting C. P., Liu J.-L., 2013. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Reports 4: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova M., Beumer K., Trautman J. K., Carroll D., 2003. Enhancing gene targeting with designed zinc finger nucleases. Science 300: 764. [DOI] [PubMed] [Google Scholar]
- Bibikova M., Golic M., Golic K. G., Carroll D., 2002. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Pruett-Miller S. M., Huang Y., Gjoka M., Duda K., et al. , 2011. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods 8: 753–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F. A., Cox D., Lin S., Barretto R., et al. , 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiunas G., Barrangou R., Horvath P., Siksnys V., 2012. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 109: E2579–E2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor G. B., Preston C. R., Johnson-Schlitz D. M., Nassif N. A., Phillis R. W., et al. , 1993. Type I repressors of P element mobility. Genetics 135: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K., et al. , 2013. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194: 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Ukken F. P., Rubinstein C. D., Thiede G., Donohue L. K., et al. , 2014. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196: 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. D., Lander E. S., Zhang F., 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Ueda R., 2013. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics 195: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P., Aach J., Stranges P. B., Esvelt K. M., Moosburner M., et al. , 2013. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F., Chen H.-M., Lee T., Bullock S. L., 2014. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl. Acad. Sci. USA 111: E2967–E2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F. A., Hsu P. D., Lin C.-Y., Gootenberg J. S., Konermann S., et al. , 2013. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154: 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Sun J., Housden B. E., Hu Y., Roesel C., et al. , 2013. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc. Natl. Acad. Sci. USA 110: 19012–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose L., 2009. Transgenesis in Drosophila melanogaster. Methods Mol. Biol. 561: 3–19. [DOI] [PubMed] [Google Scholar]
- Rouet P., Smih F., Jasin M., 1994. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14: 8096–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J. D., Joung J. K., 2014. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebo Z. L., Lee H. B., Peng Y., Guo Y., 2014. A simplified and efficient germline-specific CRISPR/Cas9 system for Drosophila genomic engineering. Fly (Austin) 8: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang H., Shivalila C. S., Dawlaty M. M., Cheng A. W., et al. , 2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153: 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Z., M. Ren, M. Wu, J. Dai, Y. S. Rong et al., 2014 Efficient gene knock-out and knock-in with transgenic Cas9 in Drosophila. G3 (Bethesda) 4: 925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Chen H., Liu J., Zhang H., Yan Y., et al. , 2014. Various applications of TALEN- and CRISPR/Cas9-mediated homologous recombination to modify the Drosophila genome. Biol. Open 3: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Ren M., Wang Z., Zhang B., Rong Y. S., et al. , 2013. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics 195: 289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.