Abstract
Identifying effective treatment combinations for MS patients failing standard therapy is an important goal. We report the results of a phase II open label baseline-to-treatment trial of a humanized monoclonal antibody against CD25 (daclizumab) in 10 multiple sclerosis patients with incomplete response to IFN-β therapy and high brain inflammatory and clinical disease activity. Daclizumab was very well tolerated and led to a 78% reduction in new contrast-enhancing lesions and to a significant improvement in several clinical outcome measures.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the CNS with suspected autoimmune pathogenesis. The available treatments for MS are only partially effective, and considerable numbers of treated patients retain inflammatory CNS activity with contrast-enhancing MRI lesions (CEL) and continue to accumulate clinical disability.
Limiting T cell expansion by blocking IL-2 signaling by means of its high-affinity receptor that is expressed on activated T cells (i.e., blocking IL-2Rα-chain, CD25) inhibits solid-organ graft rejection (1–3) and helps to restore tolerance in immune-mediated uveitis (4). Based on analogies of pathogeneses between these conditions and aberrant T cell activity in MS, we tested the effect of add-on therapy of daclizumab in MS patients with incomplete clinical and MRI response to IFN-β therapy.
Materials and Methods
Trial Design. Eleven patients with relapsing-remitting (RR) or secondary progressive (SP) MS were treated in this open-label baseline vs. treatment phase II trial (Table 1). Inclusion criteria included the following: age 18–65 yr and expanded disability status scale (EDSS) (5) 1.0–6.5. Exclusion criteria included the following: primary-progressive MS and concurrent medical conditions that could influence the immune system or accumulation of disability. Patients who were previously treated with therapies other than IFN-β had to discontinue these therapies for at least 12 weeks. Failure to respond to IFN-β was defined as follows: at least one MS exacerbation or progression of sustained disability by at least 1 EDSS point during the preceding 18 months on therapy. Patients were followed by monthly clinical and MRI examinations on IFN-β monotherapy for 4 months. To initiate daclizumab dosing, we stipulated at least 0.67 new CEL/month during this baseline period. Daclizumab was administered i.v. at 1mg/kg/dose 2 weeks apart for the first 2 doses (month 0 & 0.5) and every 4 weeks thereafter for a total of seven infusions. MS exacerbations were defined by Schumacher's criteria (6) and treated by i.v. methylprednisolone (IVMP) therapy (1g/day × 5 days). MRI scans and clinical ratings within 28 days of IVMP were disregarded and substituted by data from the following month. Both baseline and treatment phases were extended appropriately by 1 month per MS exacerbation to yield 4 baseline and 6.5 treatment months that were not affected by IVMP.
Table 1. Patient characteristics: demographic data, clinical data, and adverse events.
| Adverse events during baseline
|
Adverse events during therapy
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient code | Sex/race/age | MS type* | IFN-β† formulation/neutral. Ab | MS duration, y‡ | End-baseline EDSS§ | End-therapy EDSS§ | Baseline NRS (mean)¶ | Therapy NRS (mean)¶ | Baseline exac. rate No./mo∥ | Therapy exac. rate No./mo∥ | I** | L** | O** | I** | L** | O** |
| MS-Z1 | F/W/38 | SP | A/- | 8.0 | 4.5 | 5.0 | 68.3 | 63.6 | 0/4 | 0/6.5 | 1 | 1 | ||||
| MS-Z2 | F/W/27 | SP | A/- | 3.3 | 2.5 | 2.0 | 81.5 | 84.9 | 1/5 | 0/6.5 | ||||||
| MS-Z3 | M/W/36 | SP | B/- | 9.3 | 6.0 | 6.0 | 60.0 | 81.8 | 2/6 | 0/6.5 | 1 | |||||
| MS-Z4 | F/W/49 | RR | B/+ | 24.0 | 3.5 | 3.0 | 66.3 | 78.3 | 2/6 | 1/7.5 | 1 | 1 | 1 | |||
| MS-Z5 | F/B/51 | SP | B/- | 10.3 | 6.0 | 6.0 | 56.5 | 59.0 | 0/4 | 1/7.5 | 1 | |||||
| MS-Z6 | F/W/42 | RR | B/- | 7.9 | 3.0 | 3.0 | 83.5 | 86.6 | 2/6 | 0/6.5 | 1 | |||||
| MS-Z7 | M/W/33 | RR | B/- | 1.9 | 2.5 | 0.0 | 85.0 | 97.0 | 0/4 | 0/6.5 | 1 | 1 | 1 | |||
| MS-Z8†† | F/W/48 | SP | B/- | 6.0 | 3.5 | 3.5 | 69.7 | 72.4 | 0/4 | 1/7.5 | ||||||
| MS-Z9 | M/W/23 | RR | B/- | 1.0 | 2.0 | 2.0 | 94.8 | 89.6 | 2/6 | 0/6.5 | 1 | 1 | ||||
| MS-Z10 | F/W/29 | RR | A/- | 5.5 | 3.0 | 1.5 | 62.7 | 89.9 | 1/5 | 0/6.5 | 1 | |||||
| MS-Z11 | F/W/40 | RR | B/+ | 6.7 | 1.5 | 1.0 | 93.3 | 98.0 | 0/4 | 0/6.5 | 2 | |||||
| Group medians or cumulative values | 7.6 | 3.0 | 2.5 | 74.9 | 85.8 | 10/50 | 2/67 | 1 | 2 | 2 | 5 | 2 | 4 | |||
RR, relapsing-remitting MS; SP, secondary progressive MS.
A, Avonex; B, Betaserone; neutral. Ab, neutralizing antibodies; -, negative; +, positive.
Disease duration in years was calculated from the first symptom attributable to MS.
EDSS at the end of baseline or treatment period that was sustained > 3 months = measure of sustained disability.
Average Scripps NRS score from baseline and treatment periods = clinical measure that includes monthly variations in clinical status.
Exacerbation rate (exac. rate) is displayed as number of exacerbations per number of months during baseline or treatment periods. Note that, when exacerbation occurred, the baseline or treatment period was extended by 1 month per 1 exacerbation because the MRI and clinical values within 28 days of IVMP therapy were excluded from the analysis and were replaced by the next month's values.
I, infections (four urinary tract infections, two upper respiratory tract infections); L, abnormal laboratory values (transient elevation of LFTs); O, others (transient headache, constipation, breast tenderness, iron-deficiency anemia, exacerbation of ongoing depression, surgery for kidney stones).
Patient MS-Z8 received higher dose of daclizumab (2mg/kg i.v. q 2w) and was excluded from final analysis.
Primary outcome measures were new CEL and total number of CEL at baseline (IFN-β) vs. combination therapy (IFN-β plus daclizumab). Secondary outcomes (MRI) were as follows: T2 lesion volume (T2LV), volume of CEL, and T1-hypointensities [black hole volume (BHV)]. Secondary outcomes (clinical measures) were as follows: exacerbation rate (cumulative number of exacerbations/cumulative baseline or treatment months), change in EDSS and Scripps Neurological Rating Scale (Scripps NRS) (7), change in ambulation index, timed 25-foot walk, and 9-hole peg test (9-HPT), all baseline vs. treatment. The trial was approved by the National Institute of Neurological Disorders and Stroke institutional review board, and informed consent was obtained from every patient.
MRI Collection and Analysis. Contiguous axial MRI images (3 mm × 42 axial slices) were acquired at 1.5 Tesla with T2-weighted/proton density (PD)/fast spin echo (FSE), fluid attenuation inversion recovery (FLAIR)-, and T1-weighted sequences before and after contrast (Magnevist 0.1 mmol/kg; Berlex Laboratories, Cedar Knolls, NJ) administration as described (8). CEL were recorded on hard copy films by consensus of two neuroradiologists. T2 lesion volume was determined by a semiautomated thresholding technique (PV-WAVE) (9). Black hole volume and volume of CEL were determined from registered images (10) by using a semiautomated thresholding program (Jeff Solomon, mrips, National Institutes of Health) on medx (Sensor Systems, Sterling, VA) applied to postcontrast T1WI, after verification of lesion colocalization on T2WI or FLAIR images.
Statistical Analysis. Statistics were based on nonparametric comparisons of group medians by Signed Rank Test with predetermined P value < 0.05 for statistical significance.
Results
The primary trial objective was to assess safety and tolerability of daclizumab in MS. A total of 11 screened patients proceeded to the dosing phase, and all received seven doses of daclizumab without any serious adverse event (Table 1). We observed an increase in the number of infections during the treatment phase (1 in 50 at baseline vs. 5 in 67 cumulative months at treatment), but all of these were mild urinary and upper respiratory tract infections that are common in MS patients. Furthermore, 2 transient elevations of liver function tests (LFTs) and bilirubin occurred on daclizumab therapy. Other adverse events (transient headache, constipation, breast tenderness, iron-deficiency anemia, exacerbation of ongoing depression and surgery for kidney stones) were either mild and did not require therapy or were deemed unlikely to be due to daclizumab dosing. Overall, the drug was very well tolerated, and all 11 patients requested continued daclizumab therapy had this been allowed by the protocol.
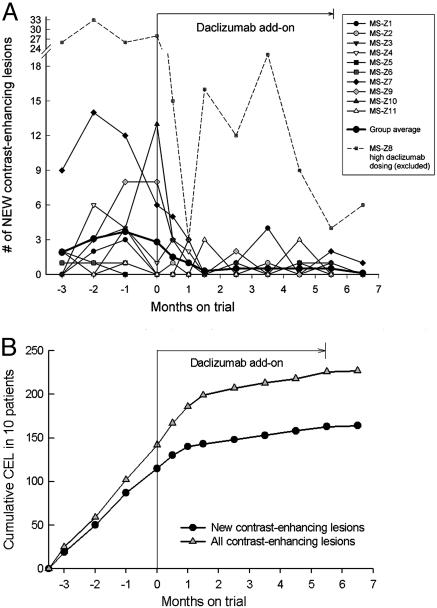
The secondary objective of the trial was to explore the efficacy of daclizumab on brain inflammatory activity in MS. Enrolled patients were characterized by high inflammatory and persistent clinical activity despite therapy with IFN-β, and, in view of the profound effect of IFN-β on CEL (11), these patients were considered to have failed treatment. Based on previous experiences with this trial design (12), we estimated that the combination therapy would have to lead to >60% decrease in CEL to reach statistical significance in a cohort of 10 patients. As demonstrated in Table 2 and Fig. 1, daclizumab therapy led to 78% decrease in new CEL and 70% decrease in total CEL as compared with baseline. The cumulative lesion analysis (Fig. 1B) demonstrates that this decline in CEL was not immediate but developed gradually over 1.5–2 months.
Table 2. Results for the primary and secondary outcome measures.
| Outcome measures | Baseline* group median average ± SD | Treatment* group median average ± SD | P value† % improvement‡ |
|---|---|---|---|
| Primary outcome measures: brain MRI | |||
| Number of new CEL | 1.38 | 0.75 | P = 0.004 |
| 2.92 ± 3.16 | 0.63 ± 0.48 | 78.42% | |
| Number of total CEL | 1.50 | 1.00 | P = 0.002 |
| 3.58 ± 3.95 | 1.06 ± 1.22 | 70.39% | |
| Secondary outcome measures: brain MRI | |||
| Volume of CEL (GdLV) | 0.121 | 0.043 | P = 0.014 |
| 0.231 ± 0.302 | 0.063 ± 0.080 | 72.72% | |
| Volume of T2 hyperintense lesions | 4.195 | 3.471 | NS |
| 4.793 ± 3.554 | 4.654 ± 3.414 | 2.90% | |
| Volume of T1 hypointense lesions (black hole volume) | 1.443 | 1.393 | NS |
| 1.912 ± 1.879 | 1.907 ± 1.980 | 0.26% | |
| Secondary outcome measures: clinical measures | |||
| Exacerbation rate (no. of exacerbations per patient/year) | 2.40 | 0.00 | P = 0.047 |
| 1.92 ± 1.78 | 0.37 ± 0.78 | 80.72% | |
| Kurtzke Expanded Disability Status Scale (EDSS; from 0 = best to 10 = worst) | 2.94 | 2.72 | NS |
| 3.31 ± 1.72 | 3.07 ± 1.90 | 7.25% | |
| Scripps NRS (from 100 = best to 0 = worst) | 74.88 | 85.63 | P = 0.048 |
| 75.48 ± 13.79 | 82.54 ± 13.22 | 9.35% | |
| Ambulation index (from 0 = best to 10 = worst) | 1.25 | 1.06 | NS |
| 1.80 ± 1.61 | 1.58 ± 1.45 | 12.22% | |
| Timed 25-foot walk, s (average of two attempts per each clinical visit) | 4.33 | 4.31 | NS |
| 5.09 ± 2.13 | 4.86 ± 1.82 | 4.52% | |
| 9-hole peg test, (s) | 22.07 | 20.65 | P = 0.006 |
| 22.96 ± 5.27 | 21.86 ± 5.26 | 4.79% |
For each patient, an average of 4 baseline months is compared with an average of 6.5 treatment months. MRI and clinical data collected within 28 days of IVMP therapy were disregarded and substituted by subsequent month data. The baseline and therapy periods were extended appropriately to yield full 4 months vs. 6.5 months comparison for each patient.
P value is based on nonparametric paired signed rank test.
Percentage of improvement is calculated from group averages.
Fig. 1.
Change in CEL on brain MRI during daclizumab trial: individual patients (A) and cumulative lesion analysis (B). (A) Evolution of new CEL on brain MRI during daclizumab trial. All 11 patients are presented. Group average for each time point is calculated from data on 10 MS patients who received 1 mg/kg daclizumab dosing. (B) Cumulative lesion analysis of new and total CEL during daclizumab trial. Number of new (and total) CEL per each month were added together for 10 MS patients and plotted as a cumulative lesion analysis. There is proportional monthly accumulation of CEL in the whole cohort (as evident from the linear relationship), and daclizumab add-on leads to gradual decrease in cumulative CEL (change in slope) that becomes evident after 1.5–2 months of therapy.
One patient with extraordinarily high MRI inflammatory activity (MS-Z8) was withdrawn from the final analysis because she received higher daclizumab doses (2 mg/kg every 2 weeks) from month 3.5 (Table 1 and Fig. 1A). She initially responded to daclizumab at 1 mg/kg every 2 weeks but experienced a significant rebound in CEL activity and MS exacerbation during the 4-week dosing. We considered it unethical to withhold more aggressive therapy and offered her discontinuation of treatment with daclizumab and change to mitoxantrone vs. an attempt of higher daclizumab dosing. She chose the latter and was given 2 mg/kg every other week under a single patient IRB-approved exemption. At this dose, she reached 68% reduction in new CEL (from an average of 29 new CEL/month at baseline to 9.13 at treatment) and stabilized clinically (Table 1). An additional patient was recruited to reach 10 patients with 1 mg/kg daclizumab dosing.
The results of the secondary outcomes are presented in Table 2. Although all measures improved, the changes in T2 lesion volume, black hole volume, EDSS, and timed 25-foot walk were nonsignificant whereas the volume of CEL (73% reduction), exacerbation rate (81% reduction), Scripps NRS (9%), and 9-hole peg test (5%) improved significantly.
Discussion
According to this open label baseline-to-treatment phase II trial of daclizumab in MS patients with incomplete response to IFN-β therapy, the addition of daclizumab seems safe and effective in blocking inflammatory disease activity of the CNS. Although we observed a slight increase in infections on treatment, the drug was very well tolerated, and the extrapolated infection rate (1 in 13.4 patient-months) was below that reported in MS (1 in 9 patient-months) (13). This result is in agreement with reported data from treatment of uveitis patients, where even long-term administration of daclizumab at equivalent doses did not lead to an increase in infections (14). We selected MS patients with high persistent inflammatory activity (average of 2.92 new CEL/month during IFN-β therapy whereas only 2/11 patients had neutralizing Ab against IFN-β) that are difficult to treat with conventional therapies and eventually need aggressive immunosuppression to slow disease progression. In this patient population, daclizumab add-on therapy led to 78% decrease in new CEL and a stabilization of all markers of disease progression. In contrast to IFN-β (12) or natalizumab treatment (15), the reduction in CEL with daclizumab was not immediate but decreased gradually over 1.5–2 months (Fig. 1B). This finding suggests that daclizumab does not directly target the blood–brain barrier but induces a gradual immunomodulatory change that is responsible for the observed decrease in brain inflammation. Due to the open-label nature of the trial, the effects on clinical scales have to be interpreted with caution because they could be influenced by subjective rating. However, the clear and congruent improvement in objective clinical tests (i.e., 9-hole peg test and timed 25-foot walk) and the positive trend in all outcomes argue against significant subjective bias. Although the impressive decrease in exacerbation rate (80%) may be influenced by regression to the mean in this unusually active MS cohort, the decrease in CEL is not explained by this phenomenon. In this trial design (8, 12, 16) (and Fig. 1B-baseline), regression to the mean does not occur in a cohort of >10 patients when CEL are averaged over 4–6 months.
In conclusion, daclizumab add-on therapy represents a clear alternative to aggressive immunosuppression in MS patients with unusually high brain inflammatory activity that cannot be controlled by conventional immunomodulatory therapy. Positive experience regarding safety and efficacy has also been demonstrated in a separate cohort of secondary progressive-MS patients under open-label therapy.∥ Large, multicentric, placebo-controlled clinical trials are needed to determine the extent of the clinical benefit of daclizumab in typical MS population and whether daclizumab is similarly effective as monotherapy.
Acknowledgments
We thank H. Griffith and A. Kokkinis for their expert nursing assistance and patient scheduling, R. Stone for his assistance with clinical database, and B. Lewis, J. Black, and R. Hill (all National Institutes of Health) for assistance in MRI collection. This clinical trial was funded by a National Institutes of Health Bench-to-Bedside Proposal.
Abbreviations: CEL, contrast-enhancing lesions; EDSS, expanded disability status scale; IVMP, intravenous methylprednisolone; MS, multiple sclerosis; NRS, neurological rating scale.
Footnotes
Rose, J. W. (2003) in Proceedings of the 55th Annual Meeting of the American Academy of Neurology, 60, Suppl. 1, A478–A479 (abstr.).
References
- 1.Waldmann, T. A. & O'Shea, J. (1998) Curr. Opin. Immunol. 10, 507–512. [DOI] [PubMed] [Google Scholar]
- 2.Webster, A. C., Playford, E. G., Higgins, G., Chapman, J. R. & Craig, J. C. (2004) Transplantation 77, 166–176. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti, F., Nashan, B. & Light, S. (1998) Transplant. Proc. 30, 2155–2158. [DOI] [PubMed] [Google Scholar]
- 4.Nussenblatt, R. B., Fortin, E., Schiffman, R., Rizzo, L., Smith, J., Van Veldhuisen, P., Sran, P., Yaffe, A., Goldman, C. K., Waldmann, T. A. & Whitcup, S. M. (1999) Proc. Natl. Acad. Sci. USA 96, 7462–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtzke, J. F. (1983) Neurology 33, 1444–1452. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher, G. A. (1965) Ann. N.Y. Acad. Sci. 112, 552–568. [DOI] [PubMed] [Google Scholar]
- 7.Sharrack, B. & Hughes, R. A. (1996) J. Neurol. Sci. 135, 1–9. [DOI] [PubMed] [Google Scholar]
- 8.Bielekova, B., Goodwin, B., Richert, N., Cortese, I., Kondo, T., Afshar, G., Gran, B., Eaton, J., Antel, J., Frank, J. A., et al. (2000) Nat. Med. 6, 1167–1175. [DOI] [PubMed] [Google Scholar]
- 9.DeCarli, C., Maisog, J., Murphy, D. G., Teichberg, D., Rapoport, S. I. & Horwitz, B. (1992) J. Comput. Assist. Tomogr. 16, 274–284. [DOI] [PubMed] [Google Scholar]
- 10.Ostuni, J. L., Levin, R. L., Frank, J. A. & DeCarli, C. (1997) J. Magn. Reson. Imaging 7, 410–415. [DOI] [PubMed] [Google Scholar]
- 11.Paty, D. W. & Li, D. K. (1993) Neurology 43, 662–667. [DOI] [PubMed] [Google Scholar]
- 12.Stone, L. A., Frank, J. A., Albert, P. S., Bash, C. N., Calabresi, P. A., Maloni, H. & McFarland, H. F. (1997) Neurology 49, 862–869. [DOI] [PubMed] [Google Scholar]
- 13.Buljevac, D., Flach, H. Z., Hop, W. C., Hijdra, D., Laman, J. D., Savelkoul, H. F., van Der Meche, F. G., van Doorn, P. A. & Hintzen, R. Q. (2002) Brain 125, 952–960. [DOI] [PubMed] [Google Scholar]
- 14.Nussenblatt, R. B., Thompson, D. J., Li, Z., Peterson, J. S., Robinson, R. R., Shames, R. S., Nagarajan, S., Tang, M. T., Mailman, M., Velez, G., et al. (2003) J. Autoimmun. 21, 283–293. [DOI] [PubMed] [Google Scholar]
- 15.Miller, D. H., Khan, O. A., Sheremata, W. A., Blumhardt, L. D., Rice, G. P., Libonati, M. A., Willmer-Hulme, A. J., Dalton, C. M., Miszkiel, K. A. & O'Connor, P. W. (2003) N. Engl. J. Med. 348, 15–23. [DOI] [PubMed] [Google Scholar]
- 16.Frank, J. A., Richert, N., Lewis, B., Bash, C., Howard, T., Civil, R., Stone, R., Eaton, J., McFarland, H. & Leist, T. (2002) Mult. Scler. 8, 24–29. [DOI] [PubMed] [Google Scholar]