Abstract
Aim
Gestational diabetes mellitus (GDM) is associated with cardiovascular diseases; however, the relationship between epicardial fat thickness (EFT) and GDM remains unclear. The present study evaluates and compares EFT using transthoracic echocardiography in pregnant women with GDM.
Materials and methods
This cross-sectional study included 129 pregnant women in the third trimester: 65 with GDM (GDM group) and 64 with uncomplicated pregnancies (control group). As defined by the World Health Organization, the diagnosis of GDM was based on an abnormal 2-h oral glucose tolerance test (OGTT) results. We used echocardiography to measure EFT in blood samples for all the participants.
Results
The postprandial blood glucose level was significantly higher in the GDM group than in the control group (P < 0.001). There were no significant differences in BMI, heart rate, systolic and diastolic blood pressure or lipid parameters between the groups. In the GDM group, isovolumic relaxation time (IVRT) parameters were significantly higher than in the control group. EFT was significantly higher in the GDM group (P < 0.001) and was correlated with postprandial glucose, BMI, age, and heart rate in both the groups. Only postprandial glucose and BMI remained significantly associated with EFT after multiple stepwise regression analysis.
Conclusion
Echocardiographically measured EFT was significantly higher in the patients with GDM. The findings show that EFT was strongly correlated with postprandial glucose.
Keywords: Atherosclerosis, Diabetes mellitus, Transthoracic echocardiography
Introduction
Gestational diabetes mellitus (GDM) is impairment in carbohydrate tolerance that begins or is recognized for the first time during pregnancy [1]. It has an incidence of 1-14% [2, 3]. GDM is not just a pregnancy disorder, but patients with GDM are also at risk of developing type 2 diabetes mellitus (DM) postpartum [4]. The relationship between type 2 DM and cardiovascular diseases is well-known [5–8]. Since, the preclinical markers of the atherosclerotic process are observed just before the development of type 2 DM, cardiovascular risk in females can be complicated by GDM.
Epicardial fat tissue is an active tissue that originates from the same embryogenic layer as visceral adipose tissue and contributes to the energy supply of the heart and its surrounding tissues, and secretes hormones adiponectin and leptin. Epicardial fat thickness (EFT) is associated with the thickness of visceral fat tissue [9]. In addition to being an anatomical barrier, epicardial fat tissue is also a local reserve for proinflammatory cytokines [10] and is related to obesity, hypertension, insulin resistance and coronary artery disease [11–16]. Currently, EFT measurement is performed via transthoracic echocardiography (TTE), magnetic resonance imaging (MRI) and multi-slice computed tomography (CT) [17]. Measurement of EFT via TTE is quite easy and is used for cardiovascular risk assessment in routine practice [18].
The increase in EFT is associated with an increase in the incidence of insulin resistance and DM [11]; however, the relationship between GDM and EFT has not yet been evaluated. Since, GDM is associated with a decrease in insulin sensitivity or increase in insulin resistance, EFT measurement during the pregnancy can be a significant determinant of GDM. The present study aimed to evaluate and compare EFT using transthoracic echocardiography in pregnant women with and without GDM.
Materials and methods
The subjects were selected from pregnant women referred to our clinics between January 2013 and August 2013 for routine pregnancy monitoring and were diagnosed with GDM. OGTT was conducted in patients with blood glucose levels over 140 mg/dL during 24–28 gestational weeks. OGTT was measured one hour after the patients were given water with 50 g glucose. Gestational diabetes was diagnosed if any two of the following values were found: fasting blood glucose levels ≥92 mg/dL, blood glucose levels ≥180 mg/dL in the first hour and ≥153 mg/dL during the second hour [19].
This study was approved by the local ethical committee. All subjects who participated in the study were informed about the study procedures and they signed an informed consent. We excluded women with a history of any previous glucose impairment (type 1 diabetes, type 2 diabetes, or gestational diabetes), previous or current blood pressure disorders and heart arrhythmia. Moreover, subjects with a history of the use of corticosteroids in the previous three months, tocolytic drugs, and serious maternal illnesses such as cancer, endocrinological disorders, chronic inflammation (systemic lupus erythematosus and rheumatoid arthritis), and those who were carrying more than one fetus, or had a liver or renal disease were also excluded.
Detailed physical examination was performed, blood pressure measurements were obtained and body mass index (BMI) was calculated in all subjects. Echocardiographic analyses were performed and blood samples were obtained from all the subjects.
Echocardiographic investigation
All of the participants underwent TTE using a VIVID 3 (General Electric) device. The patients were evaluated while in the left lateral decubitus position following a five-minute rest. As recommended by the American Society of Echocardiography [20], left ventricle end-diastolic and end-systolic diameters, and the left ventricle ejection fraction (LVEF) were measured via M-mode echocardiography using standard windows and the parasternal long-axis view. Left ventricle systolic functions, left ventricle wall movements, and mitral, aortic, tricuspid, and pulmonary valve structures and insufficiency were evaluated with a 2-dimensional color Doppler ultrasonography. While examining diastolic functions, transmitral flow samples were written at a rate of 100 mm s-1 by placing the Pulse Wave (PW) Doppler cursor 1 cm above the mitral annular line in apical four-chamber sections.
EFT was defined as the echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium. EFT was measured perpendicularly from the free wall of the right ventricle at end-diastole in three cardiac cycles, as previously described [21, 22]. The maximum EFT was measured from the point on the free wall of the right ventricle along the midline of the ultrasound beam perpendicular to the aortic annulus. For mid-ventricular parasternal short-axis assessment, maximum EFT was measured from the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the intraventricular septum at the mid-choral level and the tip of the papillary muscles as the anatomic landmark. The intraobserver variability coefficient was calculated as 0.95.
Blood collection and measurement
Blood samples from All the participants were obtained between 0800 and 1000 following 8–12 h of fasting. We used 8-mL vacuum tubes with gel to collect blood samples from antecubital veins. The samples were then centrifuged at 4000 × g for 10 min to separate the serum. Biochemical parameters were measured using an Abbott ARCHITECT c8000 (Abbott Laboratories, USA) auto analyzer and commercial kits. Hematological parameters were examined using an Abbott CellDyn 3700 (Abbott Laboratories, USA) device via the laser and impedance method.
Statistical analysis
Statistical analyses were carried out using the Statistical package for Social Sciences for Windows version 15.0 (SPSS, Chicago, IL, USA). Descriptive statistics for each variable were determined. Results for continuous variables were demonstrated as mean ± standard deviation. Statistically significant differences between groups were determined by the chi-square test for categorical variables and unpaired Student’s t-test for continuous variables. Associations between the variables were explored using the Pearson correlation and Spearman’s rho (for data that were not normally distributed). A multiple stepwise line regression analysis was used to determine the contribution of various factors to EFT. Univariate analysis was performed and variables with p < 0.10 were entered into a backward stepwise multivariate logistic regression analysis to assess independent predictor for the presence of GDM. A p-value less than 0.05 was considered significant.
Results
The study included 129 pregnant women: 65 in the GDM group and 64 in the control group. Mean age in the GDM group was 29.7 ± 5.4 years versus 30.3 ± 4.4 years in the control group (p = 0.65). Heart rate, BMI and systolic and diastolic blood pressure measurements were not significantly different between the GDM and control groups. Fasting and postprandial blood glucose levels were significantly higher in the GDM group than in the control group (p <0.001); however, there were not any significant differences in lipid parameters between the groups (Table 1).
Table 1.
Baseline clinical and laboratory characteristics of study population and comparison between the groups
| Variables | GDM group (n: 65) | Control group (n: 64) | p-value |
|---|---|---|---|
| Age (year) | 29.7 ± 5.4 | 30.3 ± 4.4 | 0.651 |
| Body mass index (kg/m2) | 27.4 ± 3.4 | 26.3 ± 3.4 | 0.082 |
| Heart rate (beats/minute) | 89.3 ± 11.5 | 88. 9 ± 7.3 | 0.695 |
| Mean systolic blood pressure (mmHg) | 112.3 ± 12.8 | 112.6 ± 13.1 | 0.551 |
| Mean diastolic blood pressure (mmHg) | 71.0 ± 9.7 | 69.7 ± 11.1 | 0.991 |
| Serum glucose (mg/dL) | 98.8 ± 14.8 | 89.1 ± 9.3 | <0.001 |
| Postprandial serum glucose (mg/dL) | 192.4 ± 37.4 | 132.2 ± 8.7 | <0.001 |
| Triglyceride (mg/dL) | 184 ± 116 | 168 ± 58.5 | 0.411 |
| Low-density lipoprotein cholesterol (mg/dL) | 103.3 ± 36.7 | 105.3 ± 35.7 | 0.574 |
| High-density lipoprotein cholesterol (mg/dL) | 35.9 ± 7.6 | 36.2 ± 8.7 | 0.934 |
| Total cholesterol (mg/dL) | 179.2 ± 40.6 | 177.4 ± 37.9 | 0.891 |
Abbreviations: GDM Gestational diabetes mellitus.
Interventricular septum thickness (IVS), posterior wall thickness (PW), left ventricular end-systolic diameter (LVESD), left ventricle end-diastolic diameter (LVEDD), LA diameter and LVEF (p > 0.05) were not significantly different between the GDM and control groups. IVRT was significantly extended in the GDM group compared to the control group (80.8 ± 26.4 vs. 71.59 ± 17.5; p = 0.020); however, E, A, and E/A rate, E/E’ rate and deceleration times were significantly different (Table 2).
Table 2.
Comparison of echocardiographic parameters between the groups
| Variables | GDM group (n: 65) | Control group (n: 64) | p-value |
|---|---|---|---|
| Left ventricle end-diastolic diameter (mm) | 46.0 ± 5.1 | 46.6 ± 4.5 | 0.675 |
| Left ventricle end-systolic diameter (mm) | 28.8 ± 6.6 | 30.4 ± 3.0 | 0.224 |
| Left ventricular ejection fraction (%) | 64.6 ± 5.1 | 62.9 ± 4.5 | 0.205 |
| Interventricular septum (mm) | 8.8 ± 1.4 | 8.7 ± 1.6 | 0.682 |
| Posterior wall (mm) | 8.9 ± 1.2 | 9.0 ± 1.5 | 0.823 |
| Right ventricular diameter (mm) | 27.4 ± 2.6 | 27.5 ± 2.4 | 0.654 |
| Left atrium diameter (mm) | 32.9 ± 3.8 | 32.8 ± 2.7 | 0.784 |
| E wave | 0.75 ± 0.2 | 0.77 ± 0.2 | 0.685 |
| A wave | 0.59 ± 0.1 | 0.57 ± 0.1 | 0.065 |
| Deceleration time | 192.6 ± 47.3 | 181.4 ± 25.6 | 0.112 |
| isovolumetric relaxation time | 80.8 ± 26.4 | 71.59 ± 17.5 | 0.020 |
| E/A | 1.29 ± 0.4 | 1.38 ± 0.3 | 0.141 |
| E/E’ | 6.7 ± 1.8 | 6.1 ± 2.3 | 0.193 |
| Epicardial fat thickness (mm) | 7.2 ± 2.5 | 5.6 ± 1.7 | <0.001 |
Simple logistic regression analysis revealed that post-prandial glucose (mg/dL) (OR = 1.566, 95% CI: 0.815-3.236; p < 0.001) and epicardial fat thickness (mm) (OR = 1.624, 95% CI: 0.815-3.236; p < 0.001 showed a trend (p < 0.10) toward an association with the presence of GDM (Table 3). We entered these variables into a backward stepwise multivariate logistic regression model, which demonstrated that post-prandial glucose and epicardial fat thickness were significant and independent predictors of GDM(OR = 1.625, 95% CI: 0.988-2.786; p < 0.001 and OR = 1.524, 95% CI: 0. 0.715-2.958; p < 0.001) (Table 3).
Table 3.
Logistic regression analysis for the presence of GDM in pregnancy
| Simple regression OR (95% CI) | p-value | Multiple regression OR (95% CI) | p-value | |
|---|---|---|---|---|
| Age (years) | 0.979 (0.915-1.048 | 0.538 | ||
| Post-prandial glucose (mg/dL) | 1.566 (0.965-2.862) | <0.001 | 1.625 (0.988-2.786) | <0.001 |
| Body mass index (kg/m2) | 1.125 (0.848-1.494) | 0.414 | ||
| Heart rate (beats/minute) | 1.095 (1.011-1.186) | 0.266 | ||
| Epicardial fat thickness (mm) | 1.624 (0.815-3.236) | <0.001 | 1.524 (0.715-2.958) | <0.001 |
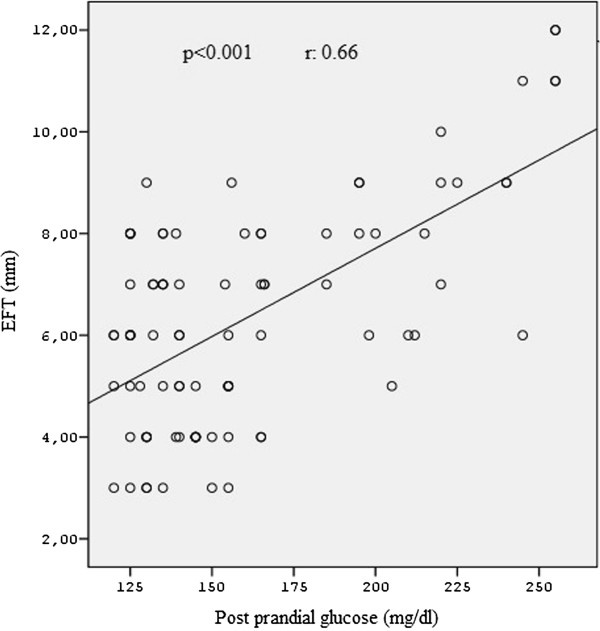
Pearson’s correlation analysis showed that EFT was positively correlated with the post-prandial glucose level (p < 0.001) and BMI in both the groups (p < 0.01) (Figures 1 and 2). Correlation between EFT and other variables are shown in Table 4. Multiple stepwise regression analysis showed that only post prandial glucose (r = 0.627, p < 0.001) and BMI (r = 0.264, p < 0.01) significantly associated with serum EFT.
Figure 1.
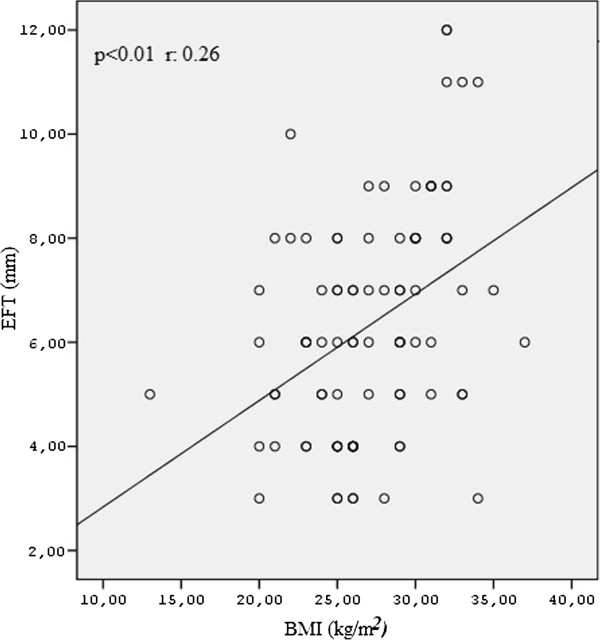
The correlation analysis of postprandial glucose levels and epicardial fat thickness in both the groups.
Figure 2.
The correlation analysis of BMI levels and epicardial fat thickness in both the groups.
Table 4.
The univariate correlations of the epicardial fat thickness
| Variables | r-value | p-value |
|---|---|---|
| Postprandial glucose (mg/dL) | 0.66 | <0.001 |
| Body mass index (kg/m2) | 0.26 | <0.01 |
| Heart rate (beats/minute) | 0.25 | 0.01 |
| Age (years) | 0.19 | 0.04 |
Abbreviations: BMI Body mass index.
Discussion
We used TTE to measure EFT in pregnant women with and without GDM and observed two significant findings: EFT was significantly higher in the GDM group, and it was significantly correlated with the post-prandial glucose level.
Among the factors associated with GDM, visceral obesity is a major risk factor. Progressive increase in intra-abdominal fat tissue causes insulin resistance in liver and adipose tissue, and, as a result, causes glucose intolerance, low-level HDL, elevated triglycerides and hypertension [23, 24]. Since, epicardial fat tissue originates from visceral fat tissue, the present study was conducted to investigate the hypothesis that EFT might be elevated in patients with GDM. Several methods are used to measure EFT in systole and diastole. Some researchers favor investigator-dependent methods such as echocardiography, whereas others prefer investigator-independent methods such as multislice computed tomography. In the present study, we used TTE to measure EFT.
Epicardial adipose tissue is not only a physiological barrier, but is also a bioactive organ that produces several inflammatory cytokines and adipokines [25, 26]. These inflammatory cytokines trigger physiopathological mechanisms such as insulin resistance, endothelial dysfunction, atherosclerosis, and obesity. There is a relationship between EFT, abdominal visceral obesity, subclinical atherosclerosis, impaired fasting glucose, type 1 DM, and hypertension [27–31]. The negative glucose profile in epicardial fat tissue is affected by the levels of glucose transporter-4 and retinol-binding protein [32]. Moreover, mRNA expression is related to epicardial fat, plasma insulin, and insulin-resistance-related molecule resistance [25]. The negative effects of EAT on glucose metabolism are also associated with the studies performed in patients with DM [11].
Caliskan et al. [33] showed that patients who previously had GDM showed elevated EAT compared to patients in the control group. Moreover, the authors concluded that patients with a history of GDM also had atherosclerosis.
Elevated EFT can further complicate GDM because EFT is also a risk factor for coronary artery disease. Kim et al. [34] used cardiovascular magnetic resonance (CMR) to show that an increase in EFT is an independent risk factor for coronary artery stenosis in patients with asymptomatic type 2 DM. Wang et al. [35] used cardiac multislice CT to describe the relationship between EFT volume and metabolic syndrome and coronary atherosclerosis. In another study, Bachar et al. [36] used a multidetector CT to investigate EFT, coronary calcium score and coronary artery stenosis, and reported that EFT is a strong predictor of coronary artery disease.
DM affects the diastolic functions of the heart, and uncontrolled hyperglycemia provokes the diastolic left ventricular dysfunction in type 2 diabetic patients without cardiac involvement [37, 38]. Left ventricular diastolic function changes are associated with fasting plasma glucose levels and glycohemoglobin concentrations under the diabetes threshold [39]. The IVRT elongation observed in the GDM group in the present study can be attributed to the negative effects of hyperglycemia on the diastolic functions of the heart.
In the present study, we observed a strong positive correlation between EFT and postprandial glucose levels. Echocardiography and CT studies show a strong correlation between fasting plasma glucose and EFT [40]. In the present study, we showed a relationship between the postprandial glucose level and EFT, which might have been due to the relationship between EFT and hyperglycemia-insulin resistance, as described above. Our study corroborated the results of a previous study that showed that BMI and EFT have a weak correlation. Bettencourt et al. [41] showed that EFT is directly related to gender, age, BMI, waist circumference, abdominal visceral fat tissue, and atherosclerotic load. EFT is associated with BMI in females with DM [11] and was higher in females with a BMI >27 kg m-2 [16]. Moreover, EFT is an independent predictor of intra-abdominal visceral fat tissue [42–44]. The correlation between EFT and BMI is an expected finding, as EFT is a parameter indicative of adipose tissue. However, our study showed that BMI is weakly correlated with EFT. This can be attributed to the weight gain and the changes in body composition during pregnancy. These changes include the changes in adipose tissue and RBC mass, body water, uterine and breast tissue, and the products of conception including the fetus, placenta and amniotic fluid [45]. The increased visceral adipose tissue has a decreased effect on weight gain during pregnancy, which may cause the weak correlation between BMI and epicardial adipose tissue.
Limitations of the study
Measurement of EFT is routinely used during echocardiographic examination. This is an observational study. In addition to the adipose tissue, pregnancy-associated weight gain and significant changes in body composition affect EFT related parameters such as waist circumference, anthropometric measurements and BMI. Studies involving pre- and post-pregnancy follow-ups may help us understand the cause and effect relationship.
Conclusion
This study shows that TTE measured EFT is higher in patients with GDM. Moreover, a strong correlation between EFT and the postprandial glucose levels was found. These findings can be helpful to understand the physiopathology of GDM; however, further studies are necessary to support this conclusion.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GN, GA, OKU and SI researched data and designed the study. GN and SI wrote the manuscript. SI and KS performed statistical analyses and prepared all the tables and figures. GA, OKU and KS reviewed the manuscript and contributed to discussion. RN performed all laboratory investigations. All authors read and approved the final manuscript.
Contributor Information
Gökay Nar, Email: gokay_nar@yahoo.com.
Sinan Inci, Email: doktorsinaninci@gmail.com.
Gökhan Aksan, Email: Aksan55@yahoo.com.
Oguz Kağan Unal, Email: ohu@yahoo.com.
Rukiye Nar, Email: rukiye_nar@yahoo.com.
Korhan Soylu, Email: korhansoylu@yahoo.com.
References
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 1998;2:161–167. [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:62–67. doi: 10.2337/dc09-S062. [DOI] [Google Scholar]
- 3.Noussitou P, Monbaron D, Vial Y, Gaillard RC, Ruiz J. Gestational diabetes mellitus and the risk of metabolic syndrome: a population based study in Lausanne, Switzerland. Diabetes Metab. 2005;31:361–369. doi: 10.1016/S1262-3636(07)70205-7. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21:103–113. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. J Am Med Assoc. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 6.Ferraz TB, Motta RS, Ferraz CL, Capibaribe DM, Forti AC, Chacra AR. C-reactive protein and features of metabolic syndrome in Brazilian women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78:23–29. doi: 10.1016/j.diabres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Hak AE, Stehouwer CD, Bots ML, Polderman KH, Schalkwijk CG, Westendorp IC, Hofman A, Witteman JC. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986–1991. doi: 10.1161/01.ATV.19.8.1986. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. J Am Med Assoc. 2004;291:1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 9.Malavazos AE, Ermetici F, Coman C, Corsi MM, Morricone L, Ambrosi B. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int J Cardiol. 2007;121:132–134. doi: 10.1016/j.ijcard.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 10.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Cetin M, Cakici M, Polat M, Suner A, Zencir C, Ardic I. Relation of epicardial fat thickness with carotid intima-media thickness in patients with type 2 diabetes mellitus. Int J Endocrinol. 2013;2013:769175. doi: 10.1155/2013/769175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–209. doi: 10.1016/S0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 13.Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, Prokop M, Visseren FL. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–385. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample the framingham heart study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 15.Okyay K, Balcioglu AS, Tavil Y, Tacoy G, Turkoglu S, Abaci A. A relationship between echocardiographic subepicardial adipose tissue and metabolic syndrome. Int J Cardiovasc Imaging. 2008;24:577–583. doi: 10.1007/s10554-008-9295-3. [DOI] [PubMed] [Google Scholar]
- 16.Altun B, Tasolar H, Eren N. Echocardiography. 2013. Epicardial adipose tissue thickness in hemodialysis patients. [DOI] [PubMed] [Google Scholar]
- 17.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes 2011. Diabetes Care. 2011;34:11–64. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/S0894-7317(89)80013-6. [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometricand clinical parameters of metabolic syndrome: a new indicator ofcardiovascular risk. The J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis G, Assael F, Ribaudo M, Zappaterreno A, Alessi G, Di Mario U, Leonetti F. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. Bunting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 24.Pascot A, Despres JP, Letnieux I, Bergeron J, Nadeau A, Prud'homme D, Tremblay A, Lemieux S. Contribution of visceral obesity to the deterioration of the metabolic risc profile in men with impaired glucose tolerance. Diabetologia. 2000;43:1126–1135. doi: 10.1007/s001250051503. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Liu Q, Liu C, Sun L, Li D, Liu A, Jia R. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease in patients with acute myocardial infarction. Echocardiography. 2014;19:12545. doi: 10.1111/echo.12545. [DOI] [PubMed] [Google Scholar]
- 26.Baker AR, da Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1–7. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacobellis G, Pellicelli AM, Sharma AM, Grisorio B, Barbarini G, Barbaro G. Relation of subepicardial adipose tissue to carotid intima-media thickness in patientswith human immunodeficiency virus. Am J Cardiol. 2007;99:1470–1472. doi: 10.1016/j.amjcard.2006.12.082. [DOI] [PubMed] [Google Scholar]
- 28.Yazıcı D, Ozben B, Yavuz D, Deyneli O, Aydın H, Tarcin Ö, Akalın S. Epicardial adipose tissue thickness in type 1 diabetic patients. Endocrine. 2011;40:250–255. doi: 10.1007/s12020-011-9478-x. [DOI] [PubMed] [Google Scholar]
- 29.Colak Y, Karabay CY, Tuncer I, Kocabay G, Kalayci A, Senates E, Ozturk O, Doganay HL, Enc FY, Ulasoglu C, Kiziltas S. Relation of epicardial adipose tissue and carotid intima-media thickness in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24:613–618. doi: 10.1097/MEG.0b013e3283513f19. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, Barbaro G, Gerstein HC. Relationship of epicardial fat thickness and fasting glucose. Int J Cardiol. 2008;128:424–426. doi: 10.1016/j.ijcard.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 31.Eroglu S, Sade L, Yıldırır A, Demir O, Müderrisoğlu H. Association of epicardial adipose tissue thickness by echocardiography and hypertension. Arch Turkish Soc Cardiol. 2013;41:115–122. doi: 10.5543/tkda.2013.83479. [DOI] [PubMed] [Google Scholar]
- 32.Salgado-Somoza A, Teijeira-Fernandez E, Rubio J, Couso E, González-Juanatey JR, Eiras S. Coronary artery disease is associated with higher epicardial renitol binding protein 4 (RBP4) and lower glucose transporter (GLUT) 4 levels in epicardial and subcutaneous adipose tissue. Clin Endocrinol. 2012;76:51–58. doi: 10.1111/j.1365-2265.2011.04140.x. [DOI] [PubMed] [Google Scholar]
- 33.Caliskan M, Caklili OT, Caliskan Z, Duran C, Ciftçi FC, Avci E, Güllü H, Kulaksizoglu M, Koca H, Muderrisoglu H. Echocardiography. 2014. Does gestational diabetes history increase epicardial fat and carotid intima media thickness? [DOI] [PubMed] [Google Scholar]
- 34.Kim HM, Kim KJ, Lee HJ, Yu HT, Moon JH, Kang ES, Cha BS, Lee HC, Lee BW, Kim YJ. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiacmagnetic resonance. Cardiovasc Diabetol. 2012;11:83. doi: 10.1186/1475-2840-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 36.Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol. 2012;110:534–538. doi: 10.1016/j.amjcard.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Von Bibra H, Hansen A, Dounis V, Bystedt T, Malmberg K, Rydén L. Augmented metabolic control improves myocardial diastolic function and perfusion in patients with non-insulin dependent diabetes. Heart. 2004;90:1483–1484. doi: 10.1136/hrt.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grandi AM, Piantanida E, Franzetti I, Bernasconi M, Maresca A, Marnini P, Guasti L, Venco A. Effect of glycemic control on the left ventricular diastolic function in type 1 diabetes mellitus. Am J Cardiol. 2006;97:17–76. doi: 10.1016/j.amjcard.2005.07.110. [DOI] [PubMed] [Google Scholar]
- 39.Celentano A, Vaccaro O, Tammaro P, Galderisi M, Crivaro M, Oliviero M, Imperatore G, Palmieri V, Iovino V, Riccardi G, Divitiis O. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am J Cardiol. 1995;76:1173–1176. doi: 10.1016/S0002-9149(99)80330-0. [DOI] [PubMed] [Google Scholar]
- 40.Wang TD, Lee WJ, Shih FY, Huang CH, Chang YC, Chen WJ, Lee YT, Chen MF. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab. 2009;94:662–669. doi: 10.1210/jc.2008-0834. [DOI] [PubMed] [Google Scholar]
- 41.Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, Xará S, Leite-Moreira A, Nagel E, Gama V. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Ateherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, Prokop M, Visseren FL. Quantification of epicardial and pericoronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Fluchter S, Haghi D, Dinter D, Heberlein W, Kühl HP, Neff W, Sueselbeck T, Borggrefe M, Papavassiliu T. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity. 2007;15:870–878. doi: 10.1038/oby.2007.591. [DOI] [PubMed] [Google Scholar]
- 45.Pipe NG, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol. 1979;86:929–940. doi: 10.1111/j.1471-0528.1979.tb11240.x. [DOI] [PubMed] [Google Scholar]


