Abstract
The endogenous cannabinoid anandamide is removed from the synaptic space by a high-affinity transport system present in neurons and astrocytes, which is inhibited by N-(4-hydroxyphenyl)-arachidonamide (AM404). After internalization, anandamide is hydrolyzed by fatty-acid amide hydrolase (FAAH), an intracellular membrane-bound enzyme that also cleaves AM404. Based on kinetic evidence, it has recently been suggested that anandamide internalization may be mediated by passive diffusion driven by FAAH activity. To test this possibility, in the present study, we have investigated anandamide internalization in wild-type and FAAH-deficient (FAAH–/–) mice. Cortical neurons from either mouse strain internalized [3H]anandamide through a similar mechanism, i.e., via a rapid temperature-sensitive and saturable process, which was blocked by AM404. Moreover, systemic administration of AM404 to either wild-type or FAAH–/– mice enhanced the hypothermic effects of exogenous anandamide, a response that was prevented by the CB1 cannabinoid antagonist rimonabant (SR141716A). The results indicate that anandamide internalization in mouse brain neurons is independent of FAAH activity. In further support of this conclusion, the compound N-(5Z, 8Z, 11Z, 14Z eicosatetraenyl)-4-hydroxybenzamide (AM1172) blocked [3H]anandamide internalization in rodent cortical neurons and human astrocytoma cells without acting as a FAAH substrate or inhibitor. AM1172 may serve as a prototype for novel anandamide transport inhibitors with increased metabolic stability.
The endogenous cannabinoid anandamide is inactivated in the brain through a two-step process consisting of internalization into neurons and astrocytes followed by intracellular hydrolysis (for review, see refs. 1 and 2). Anandamide internalization is thought to require a carrier-mediated transport system for four reasons: (i) plots of the initial rates of [3H]anandamide uptake in rat brain neurons and astrocytes yield apparent Michaelis constants (KM) consistent with a saturable process (3, 4) and comparable to the KM values of brain amine or amino acid transporters (5); (ii) neurons and astrocytes in culture internalize [3H]anandamide but not structurally related fatty-acid ethanolamides (3, 6, 7); (iii) [3H]anandamide uptake is inhibited by certain arachidonic acid derivatives, such as N-(4-hydroxyphenyl)-arachidonamide (AM404) and N-(3-furylmethyl)-arachidonamide (3, 8); and (iv) chiral analogs of anandamide compete with [3H]anandamide for transport in a stereoselective manner, suggesting that the compounds may interact with a common macromolecular target (7). Nevertheless, the molecular identity of the putative transport system responsible for anandamide internalization remains unknown.
In brain neurons, the intracellular hydrolysis of anandamide is primarily catalyzed by fatty-acid amide hydrolase (FAAH) (9–11). This enzyme is widely distributed in the rodent central nervous system, where its presence in dendrites adjacent to axon terminals containing CB1 cannabinoid receptors implies a role in the deactivation of neurally released anandamide (12, 13). Such a role is further supported by genetic and pharmacological experiments showing that disruption of FAAH activity enhances anandamide-mediated signaling in vivo (11, 14).
Recent kinetic studies in neuroblastoma and astrocytoma cells have led to the proposal that anandamide may be internalized through passive diffusion driven by FAAH activity rather than carrier-mediated transport (15). In this context, the ability of AM404 to prevent anandamide internalization was attributed to an inhibitory interaction of the compound with FAAH (15). Here, we have reexamined the role of FAAH in anandamide transport by using three complementary approaches: (i) we have asked whether genetic deletion of FAAH affects [3H]anandamide internalization in mouse brain neurons; (ii) we have conducted a comparative investigation of the pharmacological properties of AM404 in wild-type and FAAH-deficient (FAAH–/–) mice; and (iii) we have developed a hydrolysis-resistant analog of AM404 N-(5Z, 8Z, 11Z, 14Z eicosatetraenyl)-4-hydroxybenzamide (AM1172), which inhibits anandamide internalization without interacting with FAAH.
Materials and Methods
Chemicals. Anandamide and other fatty acid ethanolamides were synthesized in the laboratory (7). AM404 and capsazepine were obtained from Tocris (Ellisville, MO) and rimonabant (SR141716A) was from Research Triangle Institute (Research Triangle Park, NC). All other chemicals were purchased from Sigma.
Cell Cultures. We purchased CCF-STTG1 astrocytoma cells from American Type Culture Collection. The cells were seeded at a density of 2 × 105 per well in Corning 24-well dishes and grown until confluent in RPMI medium 1640 (GIBCO/BRL) supplemented with FBS (10%) and penicillin/streptomycin (1%). Cortical neurons were prepared as described (16, 17). Briefly, cerebral cortices from 18-day-old embryos of Wistar rats, wild-type (C57BL/6) mice, or FAAH–/– mice were dissected and the neurons dissociated mechanically. The cells were plated in 24-well dishes (0.5 × 106 cells per ml; 0.5 ml per well) coated with poly(l)-ornithine (10 μg/ml; Mr, 30,000–70,000) and poly(dl)-lysine (100 μg/ml; Mr, 30,000–70,000). The culture medium was composed of B27-supplemented Neurobasal (GIBCO/BRL) containing l-glutamine (0.5 mM), l-glutamic acid (24 μM), streptomycin (5 μg/ml), and penicillin (10 units/ml). After 3 days, the medium was replaced with B27-supplemented Neurobasal (0.5 ml) containing l-glutamine (0.5 mM), streptomycin (5 μg/ml), and penicillin (10 units/ml). The cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Transport Assays. We incubated the cells for 0–10 min at 37°C in Tris–Krebs buffer (0.5 ml) containing [3H]anandamide (30 nM, 10 × 104 dpm per ml). The cultures were rinsed twice and then scraped into 0.1 M NaOH (0.5 ml). Radioactivity was measured by liquid scintillation counting. Drugs were added to the cultures 10 min before [3H]anandamide.
FAAH Assays. We measured FAAH activity at 37°C for 30 min in 0.5 ml of Tris buffer (50 mM, pH 7.5) containing fatty acid-free BSA (0.05%), brain membrane protein (27,000× g, 50 μg of protein), and anandamide[ethanolamine-3H] (10,000 dpm; American Radiolabeled Chemicals, St. Louis). After stopping the reactions with chloroform/methanol (1/1, 1 ml), we measured radioactivity in the aqueous layers by liquid scintillation counting.
Metabolic Stability of AM404 and AM1172. We incubated membrane fractions (27,000× g, 50 μg of protein) from the brains of wild-type or FAAH–/– mice at 37°C for 30 min in Tris buffer (0.5 ml, 50 mM, pH 7.5) containing AM404 or AM1172 (0.1–1 nmol) and fatty acid-free BSA (Sigma fraction V powder, 0.05%). After stopping the reactions with chloroform/methanol (2 ml), we collected the organic layers and measured AM404 or AM1172 by HPLC/MS, as described below. Extraction recovery was ≈60% for both compounds.
HPLC/MS Analyses. We quantified anandamide and other fatty-acid ethanolamides by isotope dilution (18) and AM404 and AM1172 by using an external standard method (19). For AM404, we selectively monitored the ion m/z = 418, and for AM1172, the ion m/z = 432.
Pharmacological Assays in Vitro. We measured CB1 receptor binding using rat cerebellar membranes (27,000× g,20 μg of protein) and 10 nM [3H]Win-55212–2 (40–60 Ci/mmol, DuPont/NEN) as a ligand. CB2 receptor binding was determined by using the same ligand and membranes from CB2-overexpressing Chinese hamster ovary cells (Receptor Biology, Perkin–Elmer) (20). The binding of [35S]GTP-γ-S to rat cerebellar membranes was measured as described (20).
Body Temperature. We measured rectal temperature using a thermocouple probe (Physitemp, Clifton, NJ). All drugs were dissolved in a vehicle of saline/Tween 80/polyethylene glycol (90/5/5) and administered by i.p. injection. AM404 was injected 20 min before anandamide; rimonabant and capsazepine were injected 30 min before anandamide.
Statistical Analyses. Results are expressed as means ± SEM. Statistical significance was evaluated by using Student's t test or, when appropriate, one-way ANOVA followed by Dunnett's test.
Results
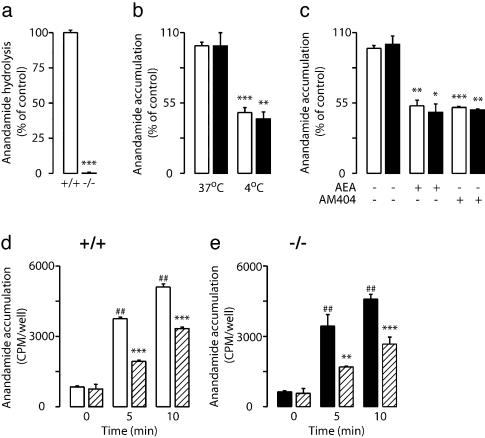
Anandamide Transport Is Preserved in FAAH–/– Neurons. To test the role of FAAH in anandamide internalization, we used primary cultures of neurons prepared from the cortex of wild-type or FAAH–/– mice (11). Despite an essentially complete lack of [3H]anandamide hydrolysis (Fig. 1a), FAAH–/– neurons were indistinguishable from wild-type cells in their ability to accumulate exogenous [3H]anandamide. Irrespective of the genotype, [3H]anandamide internalization was temperature-dependent (Fig. 1b), was saturated by excess nonradioactive anandamide (100 μM) (Fig. 1c), and reached 50% of its maximum within ≈3 min of incubation with the radiotracer (Fig. 1 d and e). Moreover, kinetic analyses revealed no significant difference in Michaelis constant (Km) or maximum velocity (Vmax) between the two genotypes (Km in μM; wild-type, 1.1 ± 0.1; FAAH–/–, 1.3 ± 0.1; Vmax in pmol per min per mg of protein; wild-type, 151 ± 8; FAAH–/–, 157 ± 15; P > 0.05; n = 3). Finally, in both wild-type and FAAH–/– neurons, the temperature-dependent component of [3H]anandamide accumulation was inhibited by AM404 (10 μM) (Fig. 1 c–e). The results suggest that anandamide hydrolysis does not provide the driving force for anandamide internalization in mouse brain neurons. They further indicate that AM404 prevents internalization by acting at a molecular site distinct from FAAH.
Fig. 1.
Anandamide transport in primary cultures of FAAH–/– neurons. (a)[3H]Anandamide hydrolysis in homogenates of brain neurons from wild-type mice (open bars) and FAAH–/– mice (filled bars) (mean ± SEM, n = 6). (b) Accumulation of [3H]anandamide in wild-type and FAAH–/– neurons is temperature-dependent (mean ± SEM, n = 4). (c) Accumulation of [3H]anandamide in wild-type (open bars) and FAAH–/– (filled bars) neurons is inhibited by nonradioactive anandamide (AEA, 100 μM) or AM404 (10 μM) (*, P < 0.05, **, P < 0.01, ***, P < 0.001; n = 3). Time course of [3H]anandamide accumulation in wild-type (+/+) (d) and FAAH–/– (–/–)(e) neurons in the absence or presence of AM404 (10 μM) (hatched bars) (**, P < 0.01, ***, P < 0.001 vs. control; ##, P < 0.01 vs. 0 time point; n = 3).
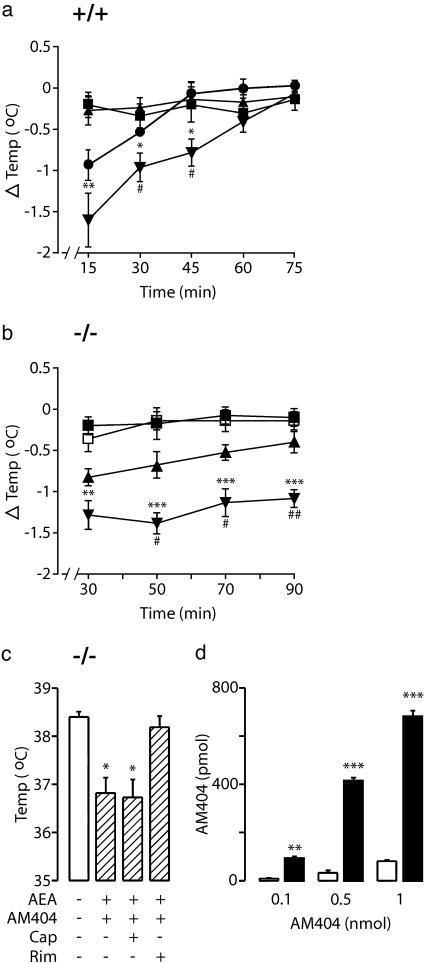
AM404 Inhibits Anandamide Deactivation in FAAH–/– Mice. In wild-type mice, AM404 (10 mg/kg, i.p.) did not change body temperature when administered alone but increased the hypothermic effects of exogenous anandamide (5 mg/kg, i.p.) (Fig. 2a). To determine whether this enhancement was due to inhibition of FAAH activity (15), we tested the effects of AM404 in FAAH–/– mice. The drug produced a modest decrease in body temperature when injected alone and dramatically intensified and prolonged the hypothermic response to a low dose of anandamide (2 mg/kg, i.p.) (Fig. 2b). This effect was blocked by the CB1 antagonist rimonabant (0.3 mg/kg, i.p.) but not by the vanilloid (VR1/TRPV1) antagonist capsazepine (30 mg/kg, i.p.) (Fig. 2c). A higher dose of anandamide (5 mg/kg, i.p.) produced profound hypothermia, which was not intensified by AM404 (peak response at 30 min; anandamide, –1.4 ± 0.1°C; anandamide plus AM404, –1.3 ± 0.2°C; P > 0.05; n = 5), suggesting that exogenous anandamide may saturate the transport system in FAAH–/– mutants at lower doses than it does in wild-type mice. Noteworthy, AM404 had a longer duration of action in FAAH–/– than in wild-type mice (Fig. 2 a and b), which implies that the compound may serve as a substrate rather than an inhibitor of FAAH activity. That brain membranes from wild-type mice rapidly degraded AM404, whereas those of FAAH–/– mice did not (Fig. 2d), confirm this conclusion.
Fig. 2.
Administration of AM404 enhances the effects of exogenous anandamide in both wild-type and FAAH–/– mice. AM404 increases anandamide-induced hypothermia in wild-type (+/+) mice (a)(▪, vehicle; •, anandamide, 5 mg/kg; ▴, AM404, 10 mg/kg; ▾, AM404 plus anandamide) and FAAH–/– (–/–) mice (b) (▪ vehicle; □ anandamide, 2 mg/kg; ▴ AM404, 7 mg/kg; ▾ AM404 plus anandamide) (*, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. vehicle; #, P < 0.05, ##, P < 0.01 vs. anandamide alone; n = 9). (c) The CB1 antagonist rimonabant (Rim, 0.3 mg/kg, i.p.) but not the vanilloid antagonist capsazepine (Cap, 30 mg/kg, i.p), prevents the combined effect of AM404 (7 mg/kg) plus anandamide (AEA, 2 mg/kg) in FAAH–/– mice (*, P < 0.05; mean ± SEM). (d) AM404 levels after a 30-min incubation with brain membranes of wild-type (open bars) or FAAH–/– (filled bars) mice. **, P < 0.01; ***, P < 0.001; n = 5; mean ± SEM.
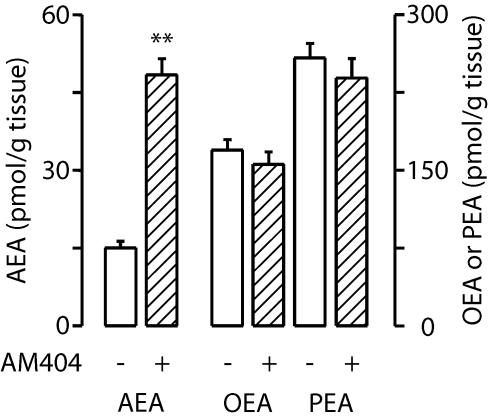
Together, our in vitro and in vivo results indicate that AM404 magnifies the actions of anandamide by targeting the deactivating transport of this endocannabinoid substance into neurons and glia. A corollary of this hypothesis is that systemic administration of AM404 should raise endogenous anandamide levels without affecting the levels of oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), two fatty-acid ethanolamides that are metabolized by FAAH (11, 14) but are not substrates for transport (3, 6, 7). Consistent with this prediction, administration of AM404 (10 mg/kg, i.p.) to wild-type mice selectively increased brain anandamide content while having no effect on OEA or PEA levels (Fig. 3).
Fig. 3.
Endogenous levels of anandamide (AEA), oleoylethanolamide (OEA), and palmitoylethanolamide (PEA) in the brains of wild-type mice 1 h after administration of AM404 (10 mg/kg, i.p.). **, P < 0.01; n = 5.
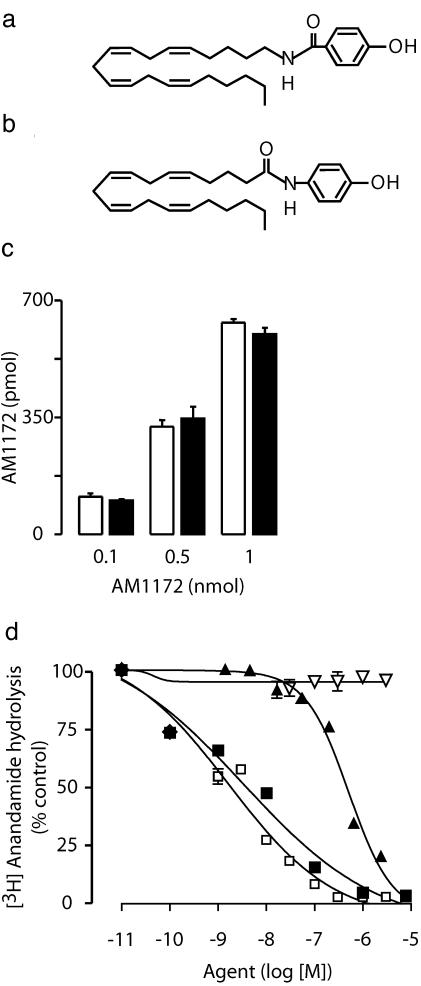
AM1172, an Anandamide Transport Inhibitor That Does Not Interact with FAAH. To further examine the role of FAAH in anandamide transport, we designed a series of previously undescribed anandamide analogs that retain the key pharmacophoric features necessary for transport inhibition (7), but in which the orientation of the carboxamide moiety is inverted to achieve metabolic stability. The compound AM1172, the synthesis of which will be described elsewhere, provides an example of this design (Fig. 4 a and b).
Fig. 4.
AM1172, a metabolically stable inhibitor of anandamide transport. Chemical structures of AM1172 (a) and AM404 (b). Note the reverse-amide moiety in AM1172, which may confer stability toward FAAH hydrolysis. (c) AM1172 levels after a 30-min incubation with brain membranes from wild-type (open bars) or FAAH–/– (filled bars) mice. (d) AM1172 (▿) does not inhibit FAAH activity in rat brain membranes; also shown are the effects of AM404 (▴) and the FAAH inhibitors URB597 (□) and AM374 (▪). Results are from one experiment, performed in triplicate, representative of three.
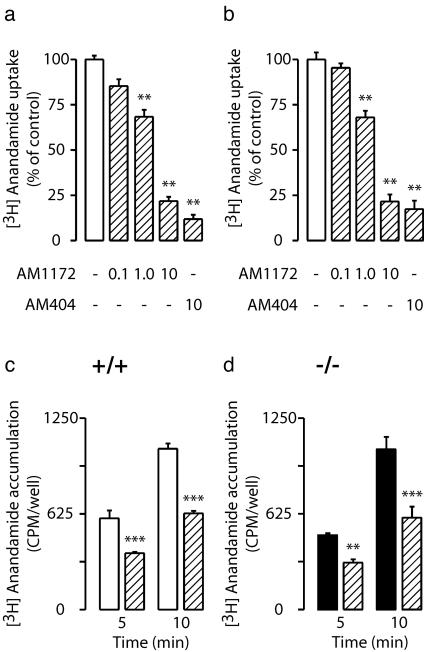
Unlike AM404 (21), AM1172 did not serve as a substrate for brain FAAH activity (Fig. 4c) and did not compete with [3H]anandamide for FAAH-mediated hydrolysis at any of the concentrations tested (Fig. 4d). Nevertheless, the compound inhibited [3H]anandamide transport in human CCF-STTG1 astrocytoma cells and rat cortical neurons in culture with IC50 values of 2.5 ± 0.9 and 2.1 ± 0.4 μM, respectively (Fig. 5 a and b). Furthermore, AM1172 prevented [3H]anandamide internalization in neurons prepared from either wild-type or FAAH–/– mice (Fig. 5 c and d).
Fig. 5.
Effects of AM1172 and AM404 (0–10 μM) on [3H]anandamide transport in human astrocytoma cells (a) and rat cortical neurons (b) in culture. Time course of [3H]anandamide accumulation in neurons from wild-type mice (+/+)(c) or FAAH–/– (d)(–/–) mice in the absence or presence of AM1172 (10 μM) (hatched bars). **, P < 0.01; ***, P < 0.001 vs. control; n = 4.
AM404 does not productively interact with G protein-coupled CB1 or CB2 receptors (3, 22) but activates VR1/TRPV1 receptor channels in vitro (23). Target selectivity assays revealed that AM1172 has significant, albeit moderate, affinities for both CB1 (IC50 = 271 ± 51 nM, n = 5) and CB2 (IC50 = 189 ± 69 nM, n = 5) and stimulates [35S]GTP-γ-S binding to rat cerebellar membranes as a partial agonist with an EC50 value of 86 ± 11 nM (n = 2). However, the compound does not stimulate vanilloid receptor activity in Xenopus oocytes injected with receptor mRNA. These results define AM1172 as a metabolically stable inhibitor of anandamide transport with partial agonist activity at CB1 receptors.
Discussion
Although anandamide can diffuse passively through lipid membranes, this process appears to be accelerated in the brain by an energy independent but highly selective transport system (3, 4, 6, 7, 24). Experiments in human astrocytoma cells indicate that this putative carrier preferentially recognizes compounds with a polar nonionizable head group of defined stereochemical configuration. The experiments further suggest that transport activity is optimal with substrates containing a minimum of four cis nonconjugated double bonds (7). Both anandamide and 2-arachidonoylglycerol, another endocannabinoid ligand (25, 26), meet these requirements and serve as substrates for transport, whereas closely related lipids such as arachidonic acid and oleoylethanolamide do not (3, 7). Similar selectivity profiles have been documented in rat cortical astrocytes in culture (3) and in freshly dissected adult rat brain slices (22).
Pharmacological inhibitors have provided important insight into the properties of anandamide transport. Compounds such as AM404 (3) and N-(3-furylmethyl)-arachidonamide (8) have not only aided in the functional characterization of anandamide internalization in vitro but have also helped reveal possible functions of the endocannabinoid system in the modulation of neurotransmitter activity and synaptic plasticity (22, 27). Moreover, the pharmacological properties of these inhibitors suggest that anandamide transport might offer a drug target in disease states, such as motor dyskinesias (28, 29) and opiate withdrawal (30), in which endocannabinoid signaling is thought to play a protective role.
The impact of these theories is limited, however, by the fact that the putative transport system responsible for anandamide internalization remains unknown. Indeed, the existence of such a system has been recently questioned, based on the observation that [3H]anandamide uptake in astrocytoma and neuroblastoma cells is saturable at longer (>5 min) but not at shorter (<40 sec) incubation times (15). This result was interpreted to indicate that FAAH may be responsible for the apparent saturation of uptake observed with longer incubations. Although the presence of a high concentration of serum albumin in these assays (0.4%) may well account for these negative results (1, 6, 31), in the present study, we have further tested the role of FAAH in anandamide internalization by asking two complementary questions: whether deletion of FAAH activity affects anandamide transport in brain neurons, and whether AM404 enhances the pharmacological effects of exogenous anandamide in FAAH–/– mutants, as it does in wild-type mice (3, 32).
We found that FAAH–/– neurons internalize anandamide as efficiently as do wild-type cells and that AM404 inhibits this process. The results indicate that FAAH does not provide the driving force for anandamide internalization and rule out this enzyme as a primary target for AM404. In vivo experiments further support these conclusions. Indeed, that AM404 potentiates the actions of exogenous anandamide more effectively in FAAH–/– mutants than in wild-type mice implies that AM404 acts not as a FAAH inhibitor, as has been proposed (15), but as a FAAH substrate. Evidence for this possibility has been provided (21) and is confirmed here by demonstrating that brain membranes from wild-type mice degrade AM404, whereas those from FAAH–/– mutants do not. If FAAH does not provide the driving force for anandamide internalization, which is known to be an energy-independent process (3, 4), what does? Although we cannot yet answer this question, one possibility is that anandamide associates intracellularly with binding components (e.g., a lipid-binding protein) en route to hydrolysis (1). If such a component were selective for anandamide and 2-arachidonoylglycerol, it might directly participate in the internalization process or serve as a target for AM404 and other transport inhibitors. This scenario is comparable to fatty acid transport into cells, which also requires the cooperation of membrane transporters and cytosolic fatty-acid-binding proteins (33).
FAAH-mediated hydrolysis limits both the efficacy and duration of action of AM404. To overcome this limitation, several AM404 analogs have been synthesized that are poor FAAH substrates in vitro (8, 34). In the present study, we have used a reverse-amide design to generate a previously undescribed transport inhibitor, AM1172, which is resistant to degradation and does not interfere with FAAH-mediated anandamide hydrolysis. That AM1172 effectively inhibits anandamide internalization in brain neurons and astrocytoma cells underscores the notion that membrane transport and intracellular hydrolysis are two discrete steps in anandamide deactivation.
Conclusion
Our results confirm the hypothesis that brain cells internalize anandamide through a selective transport mechanism, which is inhibited by AM404 (3, 4, 6). The reverse amide, AM1172, may serve as a scaffold for the development of metabolically stable inhibitors of anandamide internalization.
Acknowledgments
We thank Dr. Benjamin Cravatt (The Scripps Research Institute, La Jolla, CA) for the generous gift of FAAH–/– mice and Dr. M.-L. Solbrig for critical reading of the manuscript. This work was supported by National Institute on Drug Abuse Grants 12447 and 3412 (to D.P.) and DA3801, DA09185, and DA00493 (to A.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FAAH, fatty acid amide hydrolase; AM404, as N-(4-hydroxyphenyl)-arachidonamide; AM1172, N-(5Z, 8Z, 11Z, 14Z eicosatetraenyl)-4-hydroxybenzamide.
See Commentary on page 8512.
References
- 1.Hillard, C. J. & Jarrahian, A. (2003) Br. J. Pharmacol. 140, 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freund, T. F., Katona, I. & Piomelli, D. (2003) Physiol. Rev. 83, 1017–1066. [DOI] [PubMed] [Google Scholar]
- 3.Beltramo, M., Stella, N., Calignano, A., Lin, S. Y., Makriyannis, A. & Piomelli, D. (1997) Science 277, 1094–1097. [DOI] [PubMed] [Google Scholar]
- 4.Hillard, C. J., Edgemond, W. S., Jarrahian, A. & Campbell, W. B. (1997) J. Neurochem. 69, 631–638. [DOI] [PubMed] [Google Scholar]
- 5.Masson, J., Sagne, C., Hamon, M. & El Mestikawy, S. (1999) Pharmacol. Rev. 51, 439–464. [PubMed] [Google Scholar]
- 6.Di Marzo, V., Fontana, A., Cadas, H., Schinelli, S., Cimino, G., Schwartz, J. C. & Piomelli, D. (1994) Nature 372, 686–691. [DOI] [PubMed] [Google Scholar]
- 7.Piomelli, D., Beltramo, M., Glasnapp, S., Lin, S. Y., Goutopoulos, A., Xie, X. Q. & Makriyannis, A. (1999) Proc. Natl. Acad. Sci. USA 96, 5802–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Rodriguez, M. L., Viso, A., Ortega-Gutierrez, S., Lastres-Becker, I., Gonzalez, S., Fernandez-Ruiz, J. & Ramos, J. A. (2001) J. Med. Chem. 44, 4505–4508. [DOI] [PubMed] [Google Scholar]
- 9.Schmid, P. C., Zuzarte-Augustin, M. L. & Schmid, H. H. (1985) J. Biol. Chem. 260, 14145–14149. [PubMed] [Google Scholar]
- 10.Cravatt, B. F., Giang, D. K., Mayfield, S. P., Boger, D. L., Lerner, R. A. & Gilula, N. B. (1996) Nature 384, 83–87. [DOI] [PubMed] [Google Scholar]
- 11.Cravatt, B. F., Demarest, K., Patricelli, M. P., Bracey, M. H., Giang, D. K., Martin, B. R. & Lichtman, A. H. (2001) Proc. Natl. Acad. Sci. USA 98, 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsou, K., Nogueron, M. I., Muthian, S., Sañudo-Peña, M. C., Hillard, C. J., Deutsch, D. G. & Walker, J. M. (1998) Neurosci. Lett. 254, 137–140. [DOI] [PubMed] [Google Scholar]
- 13.Egertova, M., Cravatt, B. F. & Elphick, M. R. (2003) Neuroscience 119, 481–496. [DOI] [PubMed] [Google Scholar]
- 14.Kathuria, S., Gaetani, S., Fegley, D., Valino, F., Duranti, A., Tontini, A., Mor, M., Tarzia, G., La Rana, G., Calignano, A., et al. (2003) Nat. Med. 9, 76–81. [DOI] [PubMed] [Google Scholar]
- 15.Glaser, S. T., Abumrad, N. A., Fatade, F., Kaczocha, M., Studholme, K. M. & Deutsch, D. G. (2003) Proc. Natl. Acad. Sci. USA 100, 4269–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewer, G. J. (1995) J. Neurosci. Res. 42, 674–683. [DOI] [PubMed] [Google Scholar]
- 17.Evans, M. S., Collings, M. A. & Brewer, G. J. (1998) J. Neurosci. Methods 79, 37–46. [DOI] [PubMed] [Google Scholar]
- 18.Giuffrida, A., Rodríguez de Fonseca, F. & Piomelli, D. (2000) Anal. Biochem. 280, 87–93. [DOI] [PubMed] [Google Scholar]
- 19.Giuffrida, A., Rodriguez de Fonseca, F., Nava, F., Loubet-Lescoulie, P. & Piomelli, D. (2000) Eur. J. Pharmacol. 408, 161–168. [DOI] [PubMed] [Google Scholar]
- 20.Tarzia, G., Duranti, A., Tontini, A., Spadoni, G., Mor, M., Rivara, S., Vincenzo Plazzi, P., Kathuria, S. & Piomelli, D. (2003) Bioorg. Med. Chem. 11, 3965–3973. [DOI] [PubMed] [Google Scholar]
- 21.Lang, W., Qin, C., Lin, S., Khanolkar, A. D., Goutopoulos, A., Fan, P., Abouzid, K., Meng, Z., Biegel, D. & Makriyannis, A. (1999) J. Med. Chem. 42, 896–902. [DOI] [PubMed] [Google Scholar]
- 22.Beltramo, M., de Fonseca, F. R., Navarro, M., Calignano, A., Gorriti, M. A., Grammatikopoulos, G., Sadile, A. G., Giuffrida, A. & Piomelli, D. (2000) J. Neurosci. 20, 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralevic, V., Kendall, D. A., Jerman, J. C., Middlemiss, D. N. & Smart, D. (2001) Eur. J. Pharmacol. 424, 211–219. [DOI] [PubMed] [Google Scholar]
- 24.Jarrahian, A., Manna, S., Edgemond, W. S., Campbell, W. B. & Hillard, C. J. (2000) J. Neurochem. 74, 2597–2606. [DOI] [PubMed] [Google Scholar]
- 25.Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N. E., Schatz, A. R., Gopher, A., Almog, S., Martin, B. R., Compton, D. R., et al. (1995) Biochem. Pharmacol. 50, 83–90. [DOI] [PubMed] [Google Scholar]
- 26.Sugiura, T., Kondo, S., Sukagawa, A., Nakane, S., Shinoda, A., Itoh, K., Yamashita, A. & Waku, K. (1995) Biochem. Biophys. Res. Commun. 215, 89–97. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, R. I. & Nicoll, R. A. (2001) Nature 410, 588–592. [DOI] [PubMed] [Google Scholar]
- 28.Lastres-Becker, I., Hansen, H. H., Berrendero, F., De Miguel, R., Perez-Rosado, A., Manzanares, J., Ramos, J. A. & Fernandez-Ruiz, J. (2002) Synapse 44, 23–35. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer, B., Asbrock, N., Kathuria, S., Piomelli, D. & Giuffrida, A. (2003) Eur. J. Neurosci. 18, 1607–1614. [DOI] [PubMed] [Google Scholar]
- 30.Del Arco, I., Navarro, M., Bilbao, A., Ferrer, B., Piomelli, D. & Rodriguez De Fonseca, F. (2002) Eur. J. Pharmacol. 454, 103–104. [DOI] [PubMed] [Google Scholar]
- 31.Ligresti, A., Morera, E., Van Der Stelt, M. M., Monory, K., Lutz, B., Ortar, G. & Di Marzo, V. (2004) Biochem. J., in press. [DOI] [PMC free article] [PubMed]
- 32.Calignano, A., La Rana, G., Beltramo, M., Makriyannis, A. & Piomelli, D. (1997) Eur. J. Pharmacol. 337, R1–R2. [DOI] [PubMed] [Google Scholar]
- 33.Black, P. N. & DiRusso, C. C. (2003) Microbiol. Mol. Biol. Rev. 67, 454–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortar, G., Ligresti, A., De Petrocellis, L., Morera, E. & Di Marzo, V. (2003) Biochem. Pharmacol. 65, 1473–1481. [DOI] [PubMed] [Google Scholar]