Abstract
Presynaptic acetylcholine (ACh) synthesis and release is thought to be sustained by a hemicholinium-3-sensitive choline transporter (CHT). We disrupted the murine CHT gene and examined CHT–/– and +/– animals for evidence of impaired cholinergic neurotransmission. Although morphologically normal at birth, CHT–/– mice become immobile, breathe irregularly, appear cyanotic, and die within an hour. Hemicholinium-3-sensitive choline uptake and subsequent ACh synthesis are specifically lost in CHT–/– mouse brains. Moreover, we observe a time-dependent loss of spontaneous and evoked responses at CHT–/– neuromuscular junctions. Consistent with deficits in synaptic ACh availability, we also observe developmental alterations in neuromuscular junction morphology reminiscent of changes in mutants lacking ACh synthesis. Adult CHT+/– mice overcome reductions in CHT protein levels and sustain choline uptake activity at wild-type levels through posttranslational mechanisms. Our results demonstrate that CHT is an essential and regulated presynaptic component of cholinergic signaling and indicate that CHT warrants consideration as a candidate gene for disorders characterized by cholinergic hypofunction.
Acetylcholine (ACh) serves important roles as a neurotransmitter of both the central and peripheral nervous systems. ACh is released at the neuromuscular junction (NMJ), as well as at autonomic synapses, and also modulates a variety of central circuits that support arousal, attention, reward, learning, and memory (1–4). Lethal deficits in cholinergic function occur with exposure to irreversible acetylcholinesterase (AChE) inhibitors (5). Genetic and autoimmune impairments in ACh synthesis or responsiveness at the NMJ trigger myasthenic syndromes (6), whereas degeneration of basal forebrain cholinergic neurons occurs in Alzheimer's disease and may contribute to dementia (7). In turn, both myasthenias and symptoms of dementia are relieved by reversible AChE inhibitors (8).
Within presynaptic terminals, ACh is synthesized from choline and acetyl-CoA by the enzyme choline acetyltransferase (ChAT) (9, 10), and this ACh synthesis is thought to be limited by choline availability (11, 12). With respect to sources of choline, pathways exist within the brain for the de novo synthesis of phosphatidylcholine from phosphatidylethanolamine (13), and the release of choline by the action of phospholipase D can support ACh synthesis (14). However, cytoplasmic synthesis of ACh is believed to depend predominantly on the acute uptake of extracellular choline across the presynaptic plasma membrane (12, 15). Two major neuronal transport mechanisms for choline have been described: a Na+-dependent, hemicholinium-3 (HC-3)-sensitive, high-affinity choline uptake (HACU; Km = 1–5 μM) process associated with cholinergic presynaptic terminals and a more ubiquitous mechanism of HC-3-insensitive, Na+-independent, choline transport having a lower affinity for choline (Km ≈ 100 μM) (16, 17). High-affinity HC-3-sensitive choline transport is believed to sustain ACh synthesis in presynaptic terminals (15, 16, 18). Indeed, the dynamic regulation of HACU in response to changes in cholinergic neuronal activity appears to match presynaptic ACh synthesis to the rate of ACh release (19).
Despite several decades of research on presynaptic cholinergic mechanisms, the identity of the choline transporter (CHT) has only recently become clear (20). Okuda and coworkers (21), as well as our own group (22, 23), have shown that the transfection of cloned CHT cDNAs elicits Na+/Cl–-dependent HC-3-sensitive HACU in cultured cells. Additionally, in rodents (24, 25) and humans (26), CHT proteins are found in all major cholinergic nuclei and colocalize with the vesicular ACh transporter (VAChT) at presynaptic terminals (25). In conjunction with evidence that HC-3 treatment of animals suppresses ACh synthesis (27, 28), CHT's synaptic localization argues that the transporter plays a critical role in sustaining cholinergic signaling.
To evaluate the in vivo contributions of CHT to cholinergic neurotransmission, we targeted the CHT gene in mice by homologous recombination in embryonic stem (ES) cells. CHT–/– mice exhibited deficits in cholinergic function and died neonatally. In contrast, CHT+/– mice maintain wild-type levels of HC-3-sensitive presynaptic choline uptake despite a significant reduction in CHT protein levels. Our results demonstrate that CHT plays an essential and regulated role in sustaining cholinergic neurotransmission.
Methods
Approval was obtained from our respective Institutional Animal Care and Use Committees for experiments involving mice. A portion of the mouse CHT gene beginning at the start codon in exon 2 and extending into intron 4 was deleted by homologous recombination in embryonic stem cells. A full description of methods for the targeting of the CHT gene as well as for immunofluorescence and histochemical analyses can be found in the Supporting Text, which is published as supporting information on the PNAS web site.
Immunoblot Analysis. Freshly dissected brain tissue was subjected to immunoblot analysis as per our previously established protocols for CHT detection (25). Anti-vesicular ACh transporter immunoblots used a goat polyclonal antibody from Chemicon.
NMJ Electrophysiology. Embryonic day 19 (E19) mice were anesthetized by i.p. injection of 0.05 ml of ketamine (17.4 mg/ml; Phoenix Pharmaceuticals, St. Joseph, MO). Sternomastoid muscle with attached nerve was removed and pinned out in a Sylgard-lined Petri dish and superfused with oxygenated normal saline solution (pH 7.4) containing 137.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2,10mM d-glucose, and 10 mM Hepes. Sharp glass microelectrodes (1.0-mm o.d.; W-P Instruments, New Haven, CT) were pulled (50–70 MΩ) and filled with 3 M KCl, and single muscle fibers were impaled near the motor end plate. Evoked end-plate potentials (EPPs) and miniature EPPs (MEPPs) were amplified by using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA), low-pass filtered at 1 kHz, and digitized at 10 kHz by using an analog-to-digital converter (DigiData; Axon Instruments) and interactive software (axoscope 9.0; Axon Instruments). Intracellular recordings were obtained from muscles incubated with 1.8 mM Ca2+ (Ringer's solution) and in the presence of 2 mM d-tubocurarine. MEPPs (100–200) were recorded in high-Mg2+ (12 mM), low-Ca2+ (1 mM) saline solution over a 2- to 4-min recording period.
Transport Assays. Crude synaptosomes (P2 fraction) from the brains of newborn mice were prepared as previously described for adult mice (25). Assays of choline transport activity in the brains of newborn mice were performed in triplicate for 5 min at 37°C in Krebs Ringer's Hepes buffer (KRH: 130 mM NaCl/3 mM KCl/2.2 mM CaCl2/1.2 mM MgSO4/1.2 mM KH2PO4/10 mM glucose/10 mM Hepes, pH 7.4) with a final choline concentration of 100 nM (specific activity: 83 Ci/mmol, Amersham Pharmacia; 1 Ci = 37 GBq). HC-3 at 1 μM was used to define CHT-mediated choline uptake. For γ-aminobutyric acid (GABA) uptake assays, samples were incubated in parallel with 50 nM [3H]GABA (93 Ci/mmol, NEN Perkin–Elmer Life Science) at either 37°C or 4°C. Assays were terminated by aspiration and washing onto polyethyleneimine-coated glass fiber filters with a Brandel (Gaithersburg, MD) cell harvester. The low yield of tissue from the newborn mouse brain precluded analysis of saturation kinetics in these samples. Analysis of saturation kinetics for choline uptake in whole brain synaptosomes from the adult mice was performed as previously described (25).
HC-3-Binding Assays. Membrane preparations were prepared by lysing crude synaptosomes in the P2 fraction with a Potter– Elvehjem homogenizer (five strokes at 1,000 rpm) in 5 mM Hepes–NaOH, pH 7.4. Membranes were collected by centrifugation at 15,000 × g for 20 min and resuspended in 50 mM Tris·HCl/200 mM NaCl, pH 8 (Tris/NaCl). The membranes were washed by centrifugation at 15,000 × g and resuspension in Tris/NaCl. The binding assays used 250–400 μg of protein per sample and were performed for 45 min at room temperature in Tris/NaCl in the presence of 10 nM [3H]HC-3 (128 Ci/mmol, NEN Perkin–Elmer Life Science) as previously described (29). Specific binding to CHT was defined by parallel assays that included 1 μM unlabeled HC-3 or 2 mM choline as competitors of [3H]HC-3 binding.
ChAT Activity. Activity of ChAT was measured by the synthesis of [14C]ACh from [acetyl-1-14C]acetyl-CoA and choline (30).
Analysis of ACh Levels. ACh levels in brain tissue were quantified by previously described liquid chromatography/mass spectrometry (LC/MS) methods (31) (Vanderbilt Mass Spectrometry Core Resource). The internal standard propionylcholine was added to brain tissue that was microwaved for acetylcholinesterase inactivation (32), followed by homogenization in acetonitrile, lipid removal with heptane, and vacuum drying. Additional assays achieved similar results by using HPLC methods with electrochemical detection (33).
For the analysis of the conversion of [3H]choline into [3H]ACh in brains of newborn mice, 400-μm slices from the whole brain were preincubated for 10 min at 37°C in KRH with or without 10 μM HC-3 before the addition of [3H]choline (final concentration 100 nM). After 30 min the slices were washed and the newly synthesized [3H]ACh was extracted by sonication in acetonitrile. After lipid removal with heptane and vacuum drying, samples were resuspended in 50 mM H3PO4 and subjected to HPLC to separate ACh from choline, as defined by preinjected standards. Fractions containing the [3H]ACh peak were quantified by liquid scintillation spectrometry.
Results
CHT Is Essential for Neonatal Viability. Validation of our successful disruption of the mouse CHT gene is included in Fig. 5, which is published as supporting information on the PNAS web site. In 35 litters produced from the mating of CHT+/– mice, we obtained the expected Mendelian ratios in the genotypes of the 242 pups analyzed at E19 or the day of birth (55 CHT+/+, 117 CHT+/–, and 70 CHT–/–, P = 0.35 by χ2 analysis). Newborn CHT–/– pups were morphologically normal (Fig. 5A) and equal in weight compared with their healthy CHT+/– and CHT +/+ littermates (1.22 ± 0.02 g versus 1.20 ± 0.03 g, n = 10 and 13, respectively). Further gross analysis of various organs, including brain, heart, stomach, liver, and kidney, revealed no significant differences in weights or morphologies between genotypes. For example, at E19 we observed brain weights of 68 ± 5 mg, 65 ± 4 mg, and 74 ± 3 mg for +/+ (n = 6), +/– (n = 9), and –/– (n = 5) pups (ANOVA P > 0.05). We did not observe the hunched back or other symptoms of flaccid paralysis reported for newborn ChAT–/– mice (9, 10). With respect to the CNS, Nissl staining of brain sections did not reveal any obvious defects in brain development (data not shown).
In the minutes following birth, CHT-null mice became distinguishable from their littermates as they were immobile and could only respond with a limited contraction when touched. Breathing was sporadic and occurred as single gasps separated by bouts of prolonged apnea. These pups became visibly cyanotic and did not often survive beyond the first hour (Fig. 1A). Histological examination of the lungs of the CHT–/– pups revealed fewer aerated alveoli compared with +/+ littermates (Fig. 1B). The diminished aeration of alveoli in lungs from CHT–/– mice affected their buoyancy such that whereas CHT+/+ or +/– lungs floated, the CHT–/– lungs sank when placed in aqueous solutions (data not shown). These findings support the hypothesis that CHT–/– mice die as a result of hypoxia arising from a failure of neurotransmission at the NMJs of the diaphragm and intercostal muscles that support respiration. In our analysis of hematoxylin- and eosin-stained sections of CHT–/– embryos and pups we did not observe any other differences in gross organ development or morphology. For example, unlike the ChAT–/– mice (10), the CHT knockouts did not exhibit herniation of the diaphragm.
Fig. 1.
CHT mediated choline uptake is essential for postnatal viability. (A) Although the two pups are equal in size, the CHT–/– pup is visibly cyanotic compared with its CHT+/+ littermate (photo taken ≈30 min after birth). (B) Consistent with the breathing defect in CHT–/– mice, lungs from these animals contain fewer inflated alveoli than their +/+ littermates. (C) HC-3-sensitive choline uptake (inhibited by 1 μM HC-3) is lost in synaptosomes from CHT–/– mice compared with healthy littermates (mean ± SEM of measurements made in triplicate). The HC-3-sensitive choline uptake for three CHT–/– mice (from separate litters) was compared with eight healthy littermates (six CHT+/– and two CHT+/+). The remaining HC-3-sensitive uptake in the CHT–/– mice was not significantly different from 0 (t test, P = 0.2). In contrast, uptake of [3H]GABA was unimpaired in synaptosomes from the CHT–/– mice. (D) No significant difference (ANOVA, P > 0.05) in whole-brain ChAT activity was observed between CHT genotypes (mean ± SEM of assays performed in triplicate on samples from 8 +/+,16 +/–, and 11–/– mice). (E) ACh levels as detected by LC/MS were not different (ANOVA, P > 0.05) in either forebrain or hindbrain extracts for any of the CHT genotypes (mean ± SEM, n = 4 +/+, 5+/–, and 5–/–). (F) HC-3-sensitive synthesis of [3H]ACh from [3H]choline is detected in CHT+/+ brain slices (E19 or newborn, n = 3, *, P < 0.05, Student's t test). (G) The HC-3-senstive component of ACh synthesis is absent from CHT–/– brain slices (n = 3 mice for each genotype, *, P < 0.05, Student's t test).
We attempted to rescue the CHT knockouts by enriching the diets of the pregnant dams throughout gestation with choline-supplemented chow (4 g/kg or 10 g/kg versus the normal 1 g/kg, Testdiet, Richmond, IN) alone or in combination with choline-supplemented water (25 mM or 125 mM plus 50 mM saccharin). Similar choline supplementation of diets during pregnancy has been reported to have an impact on the developing cholinergic system (34, 35). However, these dietary manipulations failed to enhance the survival of the CHT-knockout pups (data not shown). We also attempted to rescue newborn CHT–/– pups by limiting the degradation of ACh. Newborn mice were injected sequentially with physostigmine (500 μg/kg i.p.) and neostigmine (5 mg/kg intramuscular). Signs of cholinergic hyperactivity (splay posture and urination) were evident in the control littermates (n = 4) but not in the CHT–/– mice (n = 3), yet these treatments failed to noticeably prolong survival of the knockouts (data not shown).
Loss of HC-3-Sensitive Choline Uptake and ACh Synthesis in CHT–/– CNS. To test whether CHT is solely responsible for HC-3-sensitive HACU in the brain, we measured rates of [3H]choline uptake into synaptosomes prepared from whole newborn mouse brains. As shown in Fig. 1C, CHT–/– synaptosomes failed to accumulate [3H]choline in a HC-3-sensitive manner, but they were otherwise normal, as they did not differ from samples prepared from +/+ littermates with respect to the uptake of [3H]GABA (Fig. 1C). The loss of CHT expression also did not appear to affect the viability of cholinergic neurons as revealed by assays of forebrain ChAT activity (Fig. 1D). Surprisingly, measurements of bulk tissue ACh levels, in either hindbrain or forebrain extracts from E19 mice, did not reveal a difference between any of the three genotypes (Fig. 1E). We hypothesized that CHT–/– mice may acquire these ACh stores under conditions of low demand on the cholinergic system during development but might not be able to rapidly synthesize ACh via synaptic pathways overseen by CHT. To monitor de novo synthesis and storage of ACh, we tested the ability of CHT–/– brain slices to synthesize [3H]ACh from [3H]choline in vitro. We observed an HC-3-sensitive pool of ACh synthesis in the CHT+/+ samples (Fig. 1F) but not in the slices from the CHT–/– mice (Fig. 1G). These results reveal a specific deficit in HC-3-sensitive ACh synthesis in CHT–/– mice.
CHT–/– NMJs Cannot Sustain ACh Release. The lethal phenotype in CHT–/– is consistent with a deficit in ACh release at NMJs as a result of the elimination of presynaptic choline uptake. To directly test this hypothesis, cholinergic neurotransmission was assessed by using in vitro preparations of the sternomastoid muscle of newly born CHT–/– mice and their CHT+/+ littermates. Although comparable evoked and spontaneous responses were initially detected by intracellular recordings from muscle fibers in the two genotypes, the ACh release in the CHT–/– mice was not sustainable (Fig. 2). After 2–3 h of perfusion, failures predominated in the CHT–/– tissue, whereas the amplitude of evoked EPPs remained robust and reliable in the CHT+/+ tissue (see Fig. 6, which is published as supporting information on the PNAS web site, for quantitative analysis of the electrophysiological results). By 4 h, evoked responses were no longer obtained in the CHT–/– muscle fibers, although the resting membrane potential remained stable at –52 mV (Fig. 2 A). This decline in evoked responses between 1 and 4 h of perfusion is paralleled by a decrease in the amplitude and frequency of miniature EPPs from a level that is initially indistinguishable from the CHT+/+ control (Fig. 2B). The loss of this functional measure of ACh release over time parallels the decline in movement and progressive cyanosis seen in the CHT–/– pups after birth. Both of these findings indicate the presence of an initial limited supply of choline and ACh that becomes depleted in the absence of CHT.
Fig. 2.
Electrophysiological characterization of ACh release at CHT–/– NMJs. (A) Examples of evoked EPP traces (arrows) from CHT+/+ (left) and CHT–/– (right) sternomastoid NMJs 1 or 4 h after birth. Note the absence of evoked EPPs after 4 h in the CHT–/– muscles. Stimulation ranged from 1 to 15 V at 1 Hz with 1-ms pulse duration. (B) Examples of spontaneous miniature EPPs recorded in the absence of d-tubocurarine from CHT+/+ and CHT–/– muscle fibers 1 and 4 h after birth.
Alterations in NMJ Morphology Are Consistent with Diminished ACh Release. ACh plays a role in utero in the development and maturation of both presynaptic and postsynaptic aspects of the NMJ (36). ChAT–/– mice cannot synthesize ACh, and the resulting lack of neurotransmission impairs proper development of neuromuscular junctions (9, 10). Therefore, we examined several aspects of NMJ morphology to investigate whether the loss of CHT function significantly diminished ACh availability during NMJ development. In ChAT–/– mice, the loss of ACh synthesis during development resulted in an abnormally broad band of nicotinic ACh receptor (nAChR) clusters along intramuscular nerve trunks innervating the diaphragm (9, 10). We observed a similar phenotype in the CHT–/– mice at E19 (Fig. 3 B versus E). To quantify this difference, measurements were made at 30-μm intervals to assess the maximum diameter (perpendicular to axis of band) occupied by nAChR clusters. The mean band diameter was 124 ± 22 μm compared with 194 ± 24 μm for CHT+/+ and –/– samples, respectively (data not shown). These results represent the average of measurements made from multiple fields of Alexa-488-α-bungarotoxin-labeled whole-mount diaphragms from five pairs of E19 littermates (640 μm × 640 μm fields, 20 measurements per field, >3 fields per animal, P < 0.005, paired t test). Increases in endplate area were reported in the ChAT mutants (10), and we also observed that individual nAChR clusters are on average larger in the CHT–/– mice compared with wild-type littermates (261 ± 17 μm2 versus 202 ± 9 μm2). These data represent the average area measured from a total of 4,621 CHT+/+ and 4,209 CHT–/– individual Alexa-488-α-bungarotoxin-labeled nAChR clusters on whole-mount diaphragm preparations analyzed for five pairs of CHT+/+ and –/– littermates (E19, P < 0.005, paired t test).
Fig. 3.
Alterations in nAChR distribution at CHT–/– NMJs. (A) CHT immunoreactivity is found in the axons and presynaptic terminals of motor neurons at CHT+/+ NMJs of the diaphragm (age = E19; scale bar = 80 μm). (B) Alexa-488-α-bungarotoxin labels clusters of postsynaptic nAChR clusters that form a band across the diaphragm. (C) The CHT-positive presynaptic terminals overlap the nAChRs on the muscle. Alexa-660-phalloidin labeling of actin reveals a regular pattern of muscle fibers. (D) CHT immunoreactivity is absent from motor neuron axons and terminals of the CHT–/– littermate. (E) The central band of nAChR clusters is wider in the CHT–/– diaphragm. (F) The loss of CHT–/– expression does not alter the morphology of the muscle fibers.
The morphology of the motor neuron axons at the diaphragm was examined by labeling for neurofilament immunoreactivity (Fig. 7A, which is published as supporting information on the PNAS web site). There is a close overlap between CHT+/+ motor axons and underlying nAChR clusters, whereas motor axons in the CHT–/– mice frequently extend processes beyond areas occupied by nAChRs. Finally, we have observed reduced acetylcholinesterase activity at individual synapses in the CHT knockouts relative to +/+ littermates (Fig. 7B). These alterations in NMJ morphology parallel, but are perhaps less severe than, those reported in the ChAT-knockout mice that are incapable of ACh synthesis (9, 10).
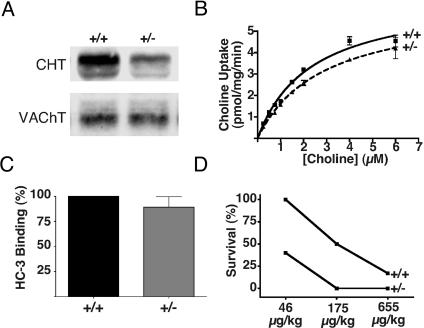
Posttranslational Compensation Maintains Cholinergic Function in CHT+/– Mice. As evident from the successful generation of CHT–/– mice, heterozygotes are viable and fertile, yet they exhibit only half the wild-type levels of CHT protein (Fig. 4A). Interestingly, although CHT protein levels are diminished, the rate of choline uptake into synaptosomes from CHT+/– mice was equivalent to that of their +/+ littermates (Fig. 4B). Analysis of saturation kinetics revealed no difference in either the Kd or the Vmax of whole-brain synaptosomal [3H]choline transport. Furthermore, in additional choline uptake experiments in whole-brain synaptosome preparations that used a single concentration of [3H]choline (50 or 100 nM) no significant difference (t test, P > 0.05) was detected between CHT+/+ and +/– littermates (CHT–/– = 107 ± 11% of wild-type choline uptake, mean ± SEM, n = 8 pairs of male littermates, mean age = 12 weeks, range 5–37 weeks). Consistent with these findings, choline clearance rates monitored with ceramic-based choline-sensitive electrodes in the striatum of CHT+/– mice were also indistinguishable from clearance dynamics observed in the striatum of +/+ littermates (Subbu Apparsundaram, personal communication). Notably, when we measured [3H]HC-3 binding to whole-brain membrane fractions, binding levels exceeded the 50% predicted by genotype and total CHT protein levels and were not significantly different (t test, P > 0.05) from the wild-type littermates (Fig. 4C). The complete compensation of CHT activity and HC-3 binding site density in the CNS of CHT+/– mice suggests that cholinergic neurons can monitor CHT availability and mobilize reserves of CHT (25) through posttranslational regulatory mechanisms to control the rate of presynaptic choline uptake.
Fig. 4.
Posttranslational compensation of CHT function in adult CHT+/– mice. (A) CHT protein levels are diminished in whole-brain extracts of CHT+/– mice compared with samples from +/+ littermates, whereas vesicular ACh transporter (VAChT) protein levels are unchanged. (B) In whole-brain synaptosome preparations, saturation kinetics analysis of HC-3-sensitive choline uptake demonstrates no significant difference in either Kd or Vmax between CHT+/+ mice and their +/– littermates. From three independent experiments (7- to 37-week-old mice) the mean Kd values were 2.9 ± 0.3 μM and 3.3 ± 0.3 μM and the mean Vmax values were 4.2 ± 1.2 and 3.9 ± 1.2 pmol per mg of protein per min for CHT+/+ and +/–, respectively (mean ± SEM). (C) In parallel with the compensations in HC-3-sensitive choline uptake, [3H]HC-3 binding to whole-brain membrane preparations was not different between CHT+/+ and +/– mice (n = 3, mean ± SEM of assays performed in triplicate, t test P > 0.05). (D) CHT+/– mice are more sensitive to the lethal effects of HC-3 (i.p. injection) than their +/+ littermates: 100% (n = 9), 50% (n = 6), and 17% (n = 6) survival were observed in CHT+/+ mice with increasing HC-3 doses, whereas the same HC-3 doses resulted in lower survival rates, 40% (n = 10), 0% (n = 6), and 0% (n = 9) in CHT+/– littermates.
Intraperitoneal injection of HC-3 results in hypocholinergic function and death (28). Furthermore, inhibition of choline uptake into synaptosomes with HC-3 evokes a subsequent increase in choline uptake after the inhibitor is washed away, suggesting that the CHT reserve can be recruited by inhibiting choline uptake (37). If the CHT-heterozygous mice have already functionally compensated for diminished CHT levels by depleting a portion of their vesicular reserve, then they might exhibit greater sensitivity to the lethal in vivo effects of HC-3. Therefore, we next tested the HC-3 sensitivity of adult male CHT+/– mice (Fig. 4D). The behavior of the mice after HC-3 administration was similar to that in the original reports of the toxicity of this drug and included labored breathing and convulsions (28). At a HC-3 dose of 46 μg/kg, wild-type mice displayed labored breathing but then recovered normal function with 100% survival (n = 9), whereas the majority (60%) of the CHT+/– mice did not survive. Likewise, at a dose of 175 μg/kg, all CHT+/– mice died (n = 6) but 50% of wild-type mice still survived (n = 6). At the highest dose examined (655 μg/kg) we observed no survival for the CHT+/– animals (n = 9), whereas only 1 of 6 CHT+/+ mice survived. In comparing the animals of the two genotypes that did not survive the 655 μg/kg HC-3 challenge, the time required for death to occur was significantly less for the CHT+/– mice compared with their +/+ littermates (7.6 ± 0.8 min versus 12 ± 1.2 min, P < 0.05, Student's t test).
Discussion
Previous work has identified the HC-3-sensitive, NaCl-dependent choline uptake mechanism as a major regulator of ACh synthesis (16, 18, 27). The lethal effects and spectrum of cholinergic deficits arising from in vivo HC-3 administration support the physiological importance of this mechanism (28). Nonetheless, work in cultured cells of neuroendocrine origin has demonstrated the feasibility of ACh synthesis and vesicular release (38) in the absence of detectable CHT expression (22, 25). Studies in neuroblastoma cells have also indicated pathways for the release of choline from membrane phosphatidylcholine by phospholipase D (14) and low-affinity choline uptake mechanisms as sources of choline for ACh synthesis (39). Even in neuronal preparations where CHT is present, such as the perfused superior cervical ganglia and striatal synaptosome preparations, there are reports of a small fraction (≈20%) of ACh synthesis that is independent of HC-3-sensitive choline uptake (15, 18).
A Lethal Phenotype Arising from Loss of CHT Expression. At the outset of our current studies, we envisioned several possible major phenotypes for CHT–/– mice. Because ACh synthesis and release are absolutely required for postnatal survival but are not necessary for viability in utero (9, 10), one prediction was that the loss of CHT-mediated presynaptic choline uptake might result in neonatal lethality. Alternatively, given the evidence for the existence of CHT-independent sources of choline for ACh synthesis, there was a possibility that these mechanisms could be up-regulated during development to compensate for the lack of CHT. A third possibility was that CHT could have as-yet-unknown functions during development that could cause embryonic lethality for the CHT–/– mice.
In this study, we show that the CHT–/– mice are born in expected Mendelian ratios from the mating of heterozygous parents. However, they fail to survive their first hours of life, and their phenotype indicates an impaired ability to sustain the synthesis of adequate releasable pools of ACh. Unlike what is observed in the ChAT–/– mice (9, 10), the normal posture and capacity for movement observed in CHT–/– mice at birth indicate the presence of limited stores of ACh produced in the absence of CHT. Indeed, our electrophysiological studies of cholinergic neurotransmission indicate the presence of a releasable pool of ACh that becomes depleted over time unless CHT is available to recapture choline back into the presynaptic terminal. Similarly, although we confirmed that CHT is uniquely responsible for both HC-3-sensitive HACU and HC-3-sensitive ACh synthesis, we also found evidence for HC-3-insensitive ACh synthesis in brain slices from the –/– mice. Overall, these results confirm a unique role for CHT in sustaining cholinergic neurotransmission and indicate that although other pathways can supply choline that contributes to ACh synthesis, they are not sufficiently robust to compensate for the loss of the CHT-mediated transport activity in active cholinergic terminals.
It seems reasonable to conclude that the phenotypes of the CHT-null mice arise from a defect in ACh synthesis at the NMJ, because the only known function of CHT is to transport choline into cholinergic neurons, and cholinergic neurotransmission at the NMJs of the diaphragm and intercostal muscles controls breathing. Although the presence of limited movement and early time point recordings from CHT–/– NMJs indicate that some ACh is synthesized and released in the absence of CHT, the CHT–/– NMJs exhibit morphological changes that are similar to, but less severe than, the effects reported for ChAT–/– mice that completely lack ACh synthesis (9, 10). As ChAT and CHT are proposed to act sequentially in ACh synthesis and the two knockouts yield similar phenotypes, the most direct explanation is that the diminished cholinergic function at the NMJ of the CHT–/– mice is sufficiently severe to reproduce some of the developmental effects previously attributed to total ACh loss. Because the CHT–/– mice recapitulate some, but not all, of the phenotypes of ChAT–/– animals, we propose that these findings reveal different quantitative thresholds for cholinergic signaling, particularly during development.
CHT Sustains Synaptically Releasable Pools of ACh. The similar total ACh levels in the brains of newborn CHT–/– compared with CHT+/+ mice (Fig. 2D) suggest that low levels of neuronal activity and ACh turnover in the newborn brain might not place a large demand on ACh synthesized from CHT-independent supplies of choline. At birth, the CNS cholinergic system is not well developed, particularly in the forebrain (40). However, ACh levels greatly exceed expectations based on the relative abundance of ChAT activity at this age (40). It is possible that developing cholinergic neurons possess abundant CHT-independent mechanisms for choline uptake to support phosphatidylcholine synthesis that could also contribute to ACh production. Indeed, in the newborn brain, we measured a sizable pool of HC-3-insensitive ACh synthesis (Fig. 2F). Nonetheless, both electrophysiological and morphological analysis of CHT–/– NMJs demonstrate a defect in the availability of releasable ACh, indicating that the CHT-dependent choline supply is intimately linked to the generation of synaptically releasable pools of ACh.
Posttranslational Mechanisms Control CHT Function. The adult CHT heterozygous mice represent a novel model for the identification of mechanisms of presynaptic cholinergic plasticity. Our findings of wild-type levels of HC-3-sensitive HACU in whole-brain synaptosomes from CHT+/– mice indicate an unrecognized capacity of cholinergic terminals to compensate for significant reductions in CHT protein levels. Our recent identification of a large pool of CHTs residing on a subset of cholinergic synaptic vesicles (25) pointed to the presence of a substantial intracellular CHT reserve in wild-type animals. The functional compensation that we observed in [3H]choline uptake and [3H]HC-3 binding is consistent with a redistribution of this vesicular pool of CHTs in CHT+/– neurons. In support of this speculation, the coordinated up-regulation of choline uptake capacity and HC-3 binding site density in response to stimulation of ACh release is well documented (20, 41, 42)
The concept that CHT+/– mice have compensated by depleting their CHT reserves led us to predict that they might be sensitized to stresses that interfere with choline transport or enhance ACh turnover. If cholinergic neurons actively control CHT localization or activity to regulate ACh synthesis and homeostasis, then in response to sublethal in vivo concentrations of HC-3 there should be compensatory recruitment of CHTs (37) that may no longer be sustainable in CHT+/– mice. Indeed, we observed lethality in CHT+/– mice at concentrations of HC-3 where all WT mice survived (Fig. 4D). These results are functionally consistent with our hypothesis regarding CHT redistribution but may also indicate other sensitizing responses to a lifelong loss of CHT reserve capacity. With respect to functional compensation and in vivo antagonist sensitivity in the CHT heterozygotes, we note parallels to the serotonin transporter (SERT)+/– mice. SERT+/– exhibit wild-type rates of serotonin transport in synaptosome preparations but are more sensitive than the SERT+/+ mice with respect to their locomotor response to 3,4-methylenedioxymethamphetamine (43).
Summary. The generation and initial characterization of CHT-knockout mice reported here demonstrates that choline transported by CHT contributes to ACh synthesis and is essential for sustaining cholinergic neurotransmission at levels required to support life. The recent report of a relatively common, nonsynonymous, single nucleotide polymorphism (SNP) in the coding region of human CHT that results in diminished choline transport (44), as well as the identification of nonsynonymous ChAT SNPs with clinical phenotypes (45, 46), predicts that variations in absolute levels of cholinergic capacity might be relatively common across human populations and set risk thresholds for a variety of disorders or their onset/severity. In addition to the well recognized deficits in cholinergic function in myasthenic syndromes, defects in the capacity for ACh synthesis in the CNS may contribute to cognitive dysfunction and dementia and may place demands on the regulatory mechanisms revealed by our studies that control CHT function. Further characterization of CHT+/– mice, especially with respect to their responses to behavioral or pharmacological challenges, should identify distinct functions that are sensitive to diminished CHT reserve.
Supplementary Material
Acknowledgments
We greatly appreciate the helpful consultations with Allan Levey and Laura Volpicelli-Daley (Emory University, Atlanta), Joshua Sanes and Jeffrey Lichtman (Washington University), and Subbu Apparsundaram (University of Kentucky, Lexington). This work was supported by a predoctoral fellowship from the Vanderbilt Brain Institute (to S.M.F.) as well as National Institutes of Health Grants MH58921 and HL56693 (to R.D.B.) and NS34448 (to J. Sanes and J. Lichtman). We gratefully acknowledge the support of the Vanderbilt Transgenic Mouse/Embryonic Stem Cell Shared Resource (supported by National Institutes of Health Grants CA68485, DK20593, and HD15052), the Vanderbilt University Medical Center Cell Imaging Core Resource (supported by National Institutes of Health Grants CA68485 and DK20593), and the Neurochemistry, Neurogenomics, and Neural Histology and Imaging Cores of the Center for Molecular Neuroscience (Vanderbilt University Medical Center).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACh, acetylcholine; ChAT, choline acetyltransferase; CHT, choline transporter; E19, embryonic day 19; EPP, endplate potential; GABA, γ-aminobutyric acid; HACU, high-affinity choline uptake; HC-3, hemicholinium-3; nAChR, nicotinic ACh receptor; NMJ, neuromuscular junction.
References
- 1.Wess, J. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 423–450. [DOI] [PubMed] [Google Scholar]
- 2.Winkler, J., Suhr, S. T., Gage, F. H., Thal, L. J. & Fisher, L. J. (1995) Nature 375, 484–487. [DOI] [PubMed] [Google Scholar]
- 3.Dani, J. A. (2001) Biol. Psychiatry 49, 166–174. [DOI] [PubMed] [Google Scholar]
- 4.Laviolette, S. R. & Van Der Kooy, D. (2004) Nat. Rev. Neurosci. 5, 55–65. [DOI] [PubMed] [Google Scholar]
- 5.Hardman, J. G. & Limbird, L. E. (2001) The Pharmacological Basis of Therapeutics (McGraw–Hill, New York).
- 6.Engel, A. G., Ohno, K. & Sine, S. M. (2003) Nat. Rev. Neurosci. 4, 339–352. [DOI] [PubMed] [Google Scholar]
- 7.Whitehouse, P. J., Price, D. L., Struble, R. G., Clark, A. W., Coyle, J. T. & Delong, M. R. (1982) Science 215, 1237–1239. [DOI] [PubMed] [Google Scholar]
- 8.Doody, R. S. (2003) J. Clin. Psychiatry 64, Suppl. 9, 11–17. [PubMed] [Google Scholar]
- 9.Brandon, E. P., Lin, W., D'Amour, K. A., Pizzo, D. P., Dominguez, B., Sugiura, Y., Thode, S., Ko, C. P., Thal, L. J., Gage, F. H. & Lee, K. F. (2003) J. Neurosci. 23, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misgeld, T., Burgess, R. W., Lewis, R. M., Cunningham, J. M., Lichtman, J. W. & Sanes, J. R. (2002) Neuron 36, 635–648. [DOI] [PubMed] [Google Scholar]
- 11.Tucek, S. (1985) J. Neurochem. 44, 11–24. [DOI] [PubMed] [Google Scholar]
- 12.Jope, R. S. (1979) Brain Res. 180, 313–344. [DOI] [PubMed] [Google Scholar]
- 13.Blusztajn, J. K. & Wurtman, R. J. (1981) Nature 290, 417–418. [DOI] [PubMed] [Google Scholar]
- 14.Lee, H. C., Fellenz-Maloney, M. P., Liscovitch, M. & Blusztajn, J. K. (1993) Proc. Natl. Acad. Sci. USA 90, 10086–10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birks, R. I. & MacIntosh, F. C. (1961) Can. J. Biochem. Physiol. 39, 787–827. [Google Scholar]
- 16.Haga, T. (1971) J. Neurochem. 18, 781–798. [DOI] [PubMed] [Google Scholar]
- 17.Yamamura, H. I. & Snyder, S. H. (1972) Science 178, 626–628. [DOI] [PubMed] [Google Scholar]
- 18.Guyenet, P., Lefresne, P., Rossier, J., Beaujouan, J. C. & Glowinski, J. (1973) Mol. Pharmacol. 9, 630–639. [PubMed] [Google Scholar]
- 19.Kuhar, M. J. & Murrin, L. C. (1978) J. Neurochem. 30, 15–21. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson, S. M. & Blakely, R. D. (2004) Mol. Interv. 4, 22–37. [DOI] [PubMed] [Google Scholar]
- 21.Okuda, T., Haga, T., Kanai, Y., Endou, H., Ishihara, T. & Katsura, I. (2000) Nat. Neurosci. 3, 120–125. [DOI] [PubMed] [Google Scholar]
- 22.Apparsundaram, S., Ferguson, S. M. & Blakely, R. D. (2001) Biochem. Soc. Trans. 29, 711–716. [DOI] [PubMed] [Google Scholar]
- 23.Apparsundaram, S., Ferguson, S. M., George, A. L., Jr., & Blakely, R. D. (2000) Biochem. Biophys. Res. Commun. 276, 862–867. [DOI] [PubMed] [Google Scholar]
- 24.Misawa, H., Nakata, K., Matsuura, J., Nagao, M., Okuda, T. & Haga, T. (2001) Neuroscience 105, 87–98. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson, S. M., Savchenko, V., Apparsundaram, S., Zwick, M., Wright, J., Heilman, C. J., Yi, H., Levey, A. I. & Blakely, R. D. (2003) J. Neurosci. 23, 9697–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kus, L., Borys, E., Ping Chu, Y., Ferguson, S. M., Blakely, R. D., Emborg, M. E., Kordower, J. H., Levey, A. I. & Mufson, E. J. (2003) J. Comp. Neurol. 463, 341–357. [DOI] [PubMed] [Google Scholar]
- 27.Freeman, J. J., Macri, J. R., Choi, R. L. & Jenden, D. J. (1979) J. Pharmacol. Exp. Ther. 210, 91–97. [PubMed] [Google Scholar]
- 28.Schueler, F. W. (1955) J. Pharmacol. Exp. Ther. 115, 127–143. [PubMed] [Google Scholar]
- 29.Sandberg, K. & Coyle, J. T. (1985) Brain Res. 348, 321–330. [DOI] [PubMed] [Google Scholar]
- 30.Frick, K. M., Burlingame, L. A., Delaney, S. S. & Berger-Sweeney, J. (2002) Neurobiol. Aging 23, 145–158. [DOI] [PubMed] [Google Scholar]
- 31.Koc, H., Mar, M. H., Ranasinghe, A., Swenberg, J. A. & Zeisel, S. H. (2002) Anal. Chem. 74, 4734–4740. [DOI] [PubMed] [Google Scholar]
- 32.Bertrand, N., Beley, P. & Beley, A. (1994) J. Neurosci. Methods 53, 81–85. [DOI] [PubMed] [Google Scholar]
- 33.Damsma, G., Westerink, B. H. & Horn, A. S. (1985) J. Neurochem. 45, 1649–1652. [DOI] [PubMed] [Google Scholar]
- 34.Cermak, J. M., Holler, T., Jackson, D. A. & Blusztajn, J. K. (1998) FASEB J. 12, 349–357. [DOI] [PubMed] [Google Scholar]
- 35.Garner, S. C., Mar, M. H. & Zeisel, S. H. (1995) J. Nutr. 125, 2851–2858. [DOI] [PubMed] [Google Scholar]
- 36.Sanes, J. R. & Lichtman, J. W. (1999) Annu. Rev. Neurosci. 22, 389–442. [DOI] [PubMed] [Google Scholar]
- 37.Rylett, R. J., Davis, W. & Walters, S. A. (1993) Brain Res. 626, 184–189. [DOI] [PubMed] [Google Scholar]
- 38.Bauerfeind, R., Regnier-Vigouroux, A., Flatmark, T. & Huttner, W. B. (1993) Neuron 11, 105–121. [DOI] [PubMed] [Google Scholar]
- 39.Richardson, U. I., Liscovitch, M. & Blusztajn, J. K. (1989) Brain Res. 476, 323–331. [DOI] [PubMed] [Google Scholar]
- 40.Coyle, J. T. & Yamamura, H. I. (1976) Brain Res. 118, 429–440. [DOI] [PubMed] [Google Scholar]
- 41.Simon, J. R., Atweh, S. & Kuhar, M. J. (1976) J. Neurochem. 26, 909–922. [DOI] [PubMed] [Google Scholar]
- 42.Lowenstein, P. R. & Coyle, J. T. (1986) Brain Res. 381, 191–194. [DOI] [PubMed] [Google Scholar]
- 43.Bengel, D., Murphy, D. L., Andrews, A. M., Wichems, C. H., Feltner, D., Heils, A., Mossner, R., Westphal, H. & Lesch, K. P. (1998) Mol. Pharmacol. 53, 649–655. [DOI] [PubMed] [Google Scholar]
- 44.Okuda, T., Okamura, M., Kaitsuka, C., Haga, T. & Gurwitz, D. (2002) J. Biol. Chem. 277, 45315–45322. [DOI] [PubMed] [Google Scholar]
- 45.Ohno, K., Tsujino, A., Brengman, J. M., Harper, C. M., Bajzer, Z., Udd, B., Beyring, R., Robb, S., Kirkham, F. J. & Engel, A. G. (2001) Proc. Natl. Acad. Sci. USA 98, 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maselli, R. A., Chen, D., Mo, D., Bowe, C., Fenton, G. & Wollmann, R. L. (2003) Muscle Nerve 27, 180–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.