Abstract
Although there is no spontaneous regeneration of mammalian spinal axons after injury, they can be enticed to grow if cAMP is elevated in the neuronal cell bodies before the spinal axons are cut. Prophylactic injection of cAMP, however, is useless as therapy for spinal injuries. We now show that the phosphodiesterase 4 (PDE4) inhibitor rolipram (which readily crosses the blood–brain barrier) overcomes inhibitors of regeneration in myelin in culture and promotes regeneration in vivo. Two weeks after a hemisection lesion at C3/4, with embryonic spinal tissue implanted immediately at the lesion site, a 10-day delivery of rolipram results in considerable axon regrowth into the transplant and a significant improvement in motor function. Surprisingly, in rolipram-treated animals, there was also an attenuation of reactive gliosis. Hence, because rolipram promotes axon regeneration, attenuates the formation of the glial scar, and significantly enhances functional recovery, and because it is effective when delivered s.c., as well as post-injury, it is a strong candidate as a useful therapy subsequent to spinal cord injury.
The adult mammalian CNS does not spontaneously regenerate after injury (1). A major factor in preventing regrowth is the presence of an inhibitory environment, comprised of inhibitors of regeneration in both damaged myelin (2) and up-regulated by astrocytes (3) after injury. Regeneration of spinal, dorsal column axons occurs, however, when an analogue of cAMP, dibutryl cAMP, is injected directly into the cell bodies, the dorsal root ganglia (DRG), either 2 days or 1 week before the spinal axons are cut (4, 5).
To date, three inhibitors of axonal regeneration have been identified in myelin: Nogo, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp) (2, 6). MAG and OMgp, as well as one inhibitory domain on Nogo, exert their inhibitory effects by interacting with the same receptor complex (7–10). Consistent with a common signaling pathway for all three inhibitors, we and others have found that, in culture, manipulations of signaling that overcome inhibition by MAG also overcome inhibition by myelin in general and most important promote regeneration in vivo (4, 5, 11).
Rather than using analogues, an alternative approach to elevate cAMP is to inhibit the enzyme that degrades it, phosphodiesterase (PDE). Rolipram, which readily crosses the blood–brain barrier (12), is a specific inhibitor of the PDE4 subfamily of PDEs, which represent ≈70–80% of PDEs in neural tissue (13). Therefore, rolipram will reach the nervous system when delivered orally or s.c. and has little effect on tissue with a proportionately low PDE4 content, such as heart and ovary (13–15). Here, we show that rolipram in culture can overcome inhibition by MAG and myelin in general and that neurons from animals treated with rolipram for various periods of time are not inhibited by MAG and myelin when cultured. Importantly, when rolipram is delivered 2 weeks after a hemisection lesion, along with embryonic spinal cord tissue implanted at the injury site at the time of lesion, there is not only a significant increase in axon growth and in functional recovery, but also an attenuation of the glial scar.
Materials and Methods
Neurite Outgrowth. Thirty-day-old Long Evan Hooded rats (Harlan Breeders, Indianapolis) were delivered rolipram (0.4, 0.5, or 0.7 μmol/kg per hr) in saline:DMSO, 50:50, or vehicle s.c. by means of osmotic mini pumps (Alzet, Palo Alto, CA) for 24, 48, or 72 hr, as indicated, or killed without delivery of anything as indicated. DRGs were removed and dissociated, and the neurite outgrowth assay was performed as described (16, 17). Briefly, 5 × 104 or 2 × 104 neurons were added to a myelin substrate or to monolayers of transfected Chinese hamster ovary (CHO) cells, respectively. Where indicated, rolipram was added directly to the cultures at 0.1, 0.25, 0.5, 1.0, and 2.0 μM, or neurons were first plated onto poly-l-lysine and primed overnight with the same concentrations of rolipram or brain-derived neurotrophic factor (BDNF) (200 ng/ml) (16, 17) before being transferred to myelin or the monolayers. After overnight incubation, cultures were immunostained for GAP43 as described. The length of the longest neurite of randomly selected 180–200 neurons was measured by using an Oncor image analysis program. Neurite lengths were compared between groups by using the Student t test.
Spinal Cord Lesion. All animals were adults at the time of surgery (180–200 g). The surgical techniques have been described (19, 20). Briefly, iridectomy scissors were used to create spinal cord hemisection lesions at C3/4 in all animals. This lesion destroys the right side of the cord plus the dorsal columns bilaterally. After the hemisection, the transplant was placed into the lesion cavity in direct apposition to the rostral and caudal ends of the lesion cavity. Two weeks later, either rolipram, (0.40 or 0.8 μmol/kg per hr in a solution of saline and 16% DMSO) or vehicle solution was administered for 10 days at 10 μl/hr by means of mini osmotic pumps. Pumps were removed at the end of the 10 days. All animals survived 4–6 weeks after the rolipram or vehicle treatment (6–8 weeks after the spinal cord lesion). A total of 12 animals met all criteria for inclusion in the final data analysis (lesion size and location and transplant apposition; rolipram, n = 7; vehicle, n = 5). After killing, the lesion site from each of the animals was sectioned serially in cross section, and the extent of lesion and the apposition of the transplant were documented. All of the animals included in the behavioral and anatomical analysis below had lesions that interrupted the dorsal columns bilaterally and the lateral funiculus unilaterally and transplants that were apposed to the host spinal cord.
Behavioral Analysis. Individuals unaware of the treatment group of the animals did all behavioral testing. With respect to rearing (vertical exploration), animals were placed in a clear, open-top plastic cylinder measuring 26.5 cm in height by 17.5 cm in diameter. Normal animals spontaneously rear onto their hind limbs and vertically explore the walls with their forelimbs (18). Cervical hemisection abolished ipsilateral forelimb use in rearing. Animals were tested 4 weeks after rolipram or vehicle administration, and rearing was tested on 3 consecutive days. Behavior was videotaped, and the number of rears, forelimb use in rearing, and paw placement were analyzed from slow-motion of the videotapes by individuals unaware of the treatment group.
Immunocytochemistry. Antibodies against serotonin, 5-hydroxytryptamine (5-HT), were used to visualize raphe-spinal projections within the host cord and transplant by using techniques from procedures described in detail previously (21)
Image Analysis and Statistics. Bright-field images of three nonadjacent sections representing the best central area (33,000 μm2 at ×40) of the transplant were taken with a Zeiss Axiophot microscope with axiovision software. By using sigmascan pro 4,4.01, positive 5-HT fibers were shaded by defining a threshold of intensity to shade the fibers of interest. The number of pixels was measured in the image to find total pixels, and an average of total pixels of three images for each animal was calculated. For sections stained for glial fibrillary acidic protein (GFAP), bright-field images of three nonadjacent sections representing the best central area of the transplant encompassing an area of 133,250 μm2 at ×20 were taken. The purpose of these images was to quantify the amount of GFAP expression in astrocytes. The number of pixels was measured in the image to find total pixels, and an average of total pixels of three images for each animal was calculated.
The average total pixels for either 5-HT fibers or GFAP-positive astrocytes were calculated in each group, and a one-way ANOVA was performed with prism 3.02 (GraphPad, San Diego) and Tukey's multiple comparison test, post hoc.
Results
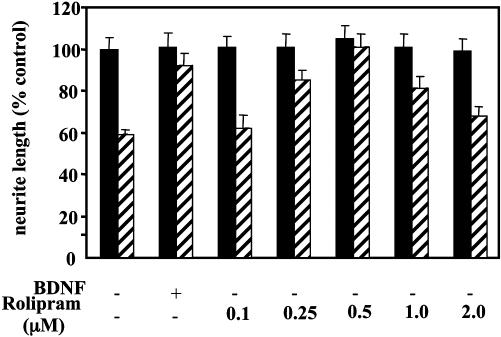
We first tested the ability of rolipram to overcome inhibition by MAG and myelin in culture. Initially, we added a range of concentrations of rolipram directly to DRG neurons growing on a monolayer of MAG-expressing CHO cells, control CHO cells, or a substrate of purified myelin. Rolipram blocked inhibition by both MAG and myelin in a dose-dependent manner, but the block was never complete (results not shown). Previously, we showed that prior exposure of neurons to neurotrophins, such as BDNF, did completely overcome inhibition by MAG and myelin whereas adding BDNF directly to the cultures, without priming, had no effect (17). We therefore tested whether rolipram was more effective if neurons were primed with the drug before being transferred to the inhibitory substrates. Fig. 1 shows that, when DRG neurons are dissociated and exposed to various concentrations of rolipram for 18 hr before being transferred to MAG-expressing or control CHO cells, inhibition is overcome in a dose-dependent manner. At 0.5 μM, rolipram blocked inhibition by MAG completely (Fig. 1). Similar results were obtained when myelin was used as a substrate (data not shown).
Fig. 1.
Rolipram overcomes inhibition by MAG in culture in a dose-dependent manner. Dissociated DRG neurons from P6–10 rat pups were first cultured overnight on poly l-lysine with or without rolipram at 0.1–2 μM as indicated, or with 200 ng/ml of BDNF before being trypsinized and transferred to a monolayer of either MAG-expressing CHO cells (striped bars) or control CHO cells (filled bars) for further overnight culture before being fixed and immunostained for GAP43. Results show the mean length of the longest neurite per neuron (± SEM) for 180–200 for each experiment and represent the mean of at least three times. Results are standardized to percentage of control, taken as neurite length from neurons cultured overnight without rolipram or BDNF and then grown on control CHO cells.
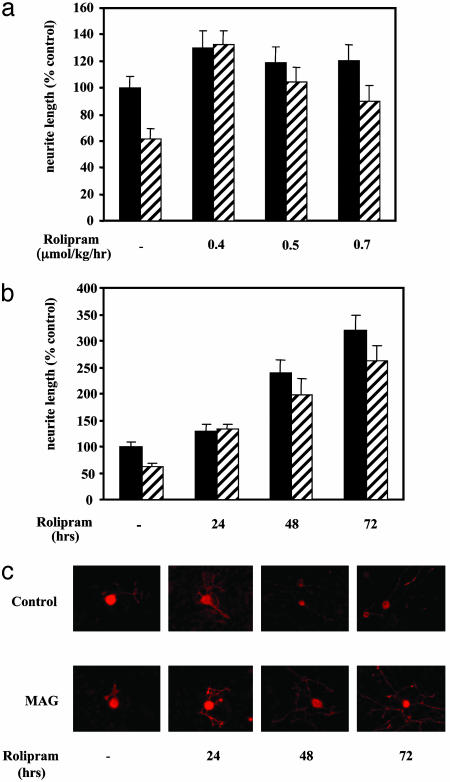
Because rolipram readily crosses the blood–brain barrier, we tested its effects on 30-day-old rats, by s.c. delivery using osmotic minipumps. After continuous overnight rolipram delivery, the DRG neurons were removed and assessed for their ability to grow on MAG and myelin. Fig. 2a shows that, after s.c. delivery of various doses of rolipram, DRG neurons are no longer inhibited by MAG after they are removed and cultured. Interestingly, rolipram is most effective and overcomes inhibition completely when delivered at a dose of 0.4 μmol/kg per hr whereas, at higher doses, the effect is lost and neurons are once again inhibited by MAG. Using this effective dose, we next delivered rolipram for 24, 48, or 72 hr, before removing the DRG neurons and culturing them on MAG or myelin. After 24 hr, inhibition by MAG is completely blocked (Fig. 2 b and c), consistent with what is shown in Fig. 2a. However, after 48 hr of continuous delivery, not only is inhibition by MAG completely blocked, but growth on the control CHO cells is also improved. This effect is even more pronounced for DRG neurons from animals treated for 72 hr with the same dose of rolipram (Fig. 2 b and c). Under all of these conditions, the same effects of rolipram were found when myelin was used as a substrate (results not shown). These results suggest that rolipram not only overcomes inhibition by MAG and myelin, but also generally improves growth.
Fig. 2.
Rolipram delivered in vivo overcomes inhibition by MAG in culture. Rolipram or vehicle was delivered to P30 rats by means of miniosmotic pumps inserted s.c. for various lengths of time before the animals were killed and the DRG neurons dissociated and assessed for inhibition by MAG. (a) Rolipram at 0.4, 0.5, or 0.7 μmol/kg per hr was delivered for 24 h, or (b and c) rolipram at 0.4 μmol/kg per hr was delivered for 24, 36, or 72 hr before being plated directly onto MAG-expressing (striped bars) or control (filled bars) CHO cells and cultured overnight before being stained for GAP43. Results show the mean length of the longest neurite per neuron (± SEM) for 180–200 for each experiment and for each condition and represent the mean of at least three times. Results are standardized to percentage of control, taken as neurite length from neurons from control animal-delivered vehicle and cultured overnight without rolipram on control CHO cells.
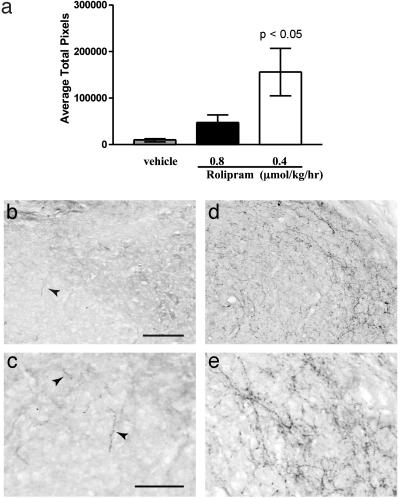
To assess the effects of rolipram on spinal axon regeneration in vivo, a hemisection lesion was created at C3/C4, and embryonic spinal cord tissue (embryonic day 14) was immediately implanted into the lesion site (19, 20). Two weeks later, delivery of rolipram at a dose of 0.4 or 0.8 μmol/kg per hr was commenced and continued for a further 10 days. Animals were assessed for functional recovery before being killed at 4 weeks post-lesion. Because there is little growth of supraspinal axons into the transplant after spinal cord injury in untreated animals, this is an excellent model for demonstrating, unequivocally, improved regrowth as a consequence of a particular treatment. If the treatment does induce axon growth into the transplant after spinal cord hemisection, it can represent both sprouting of undamaged axons and regrowth of damaged axons. The tissue at the lesion site was immunostained for serotonergic axons that had regrown into the graft and also for reactive astrocytes by immunostaining for GFAP. After spinal cord injury, in untreated adult rats, as reported (20, 21), host axons extend short processes into the transplant but are restricted to the host/transplant border. In the rolipram-treated animals, there are numerous 5-HT-positive processes in the grafted tissue (Fig. 3 d and e), compared with very, very few in the untreated or vehicle-treated control animals (Fig. 3 b and c); all of the 5-HT fibers seen in the graft are from the host because embryonic-day-14 embryonic spinal cord tissue has no serotonergic neurons. Consistent with this qualitative assessment of axonal growth, when the 5-HT processes in the graft are quantified by image analysis to measure the area fraction within the transplant occupied by serotonergic fibers, the axon projection within the transplant is >100-fold higher in the rolipram-treated animals compared with the vehicle-treated control (Fig. 3a).
Fig. 3.
Treatment with rolipram after spinal cord injury and transplant results in an increase of serotonergic fibers in the transplant. (a) The central area of the transplant shows significantly more 5-HT fibers in animals treated with 0.4 μmol/kg per hr rolipram (n = 7, P < 0.05) compared with vehicle (n = 5). Animals treated with 0.8 μmol/kg per hr rolipram (n = 3) showed no significant difference. Representative images of transplant from a rat treated with rolipram (d and e) show more fibers compared with vehicle (b and c). Arrows in b and c identify small 5-HT fibers. (Scale bars = 100 μmin b and 50 μmin c.)
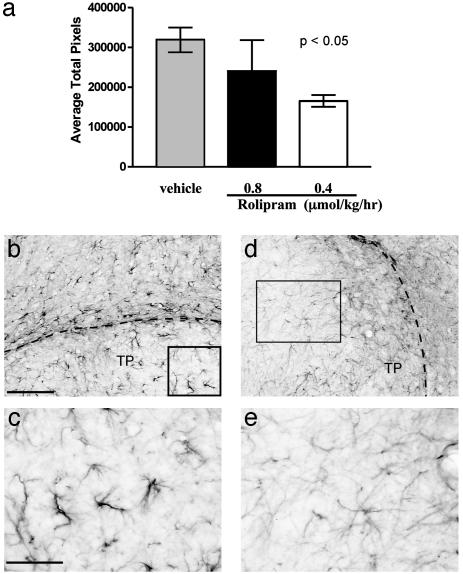
When similar sections were stained for GFAP, in vehicle-treated animals, the staining is very dark both within and surrounding the grafted tissue, indicative of astrogliosis (Fig. 4 b and c). In sharp contrast, the GFAP staining in the rolipram-treated animals was dramatically reduced (Fig. 4 d and e). When the density of GFAP was quantitated, there was more than one-third less GFAP in the rolipram-treated animals, compared with controls. It is of note that, for animals treated with a higher dose of rolipram (0.8 μmol/kg per hr), there was no significant difference in either serotonergic staining or GFAP staining compared with the vehicle-treated control (Figs. 3a and 4a). This finding is consistent with the results presented in Fig. 2, which suggest that higher doses of rolipram are ineffective.
Fig. 4.
Treatment with rolipram after spinal cord injury and transplant results in a decrease in GFAP expression. (a) The central area of the transplant shows significantly less GFAP staining only in animals treated with 0.4 μmol/kg per hr rolipram (P < 0.05) compared with vehicle. Animals treated with 0.8 μmol/kg per hr rolipram (n = 3) showed no significant difference. Representative images of transplant and host tissue from a rat treated with rolipram (d and e) show less GFAP staining compared with vehicle (b and c). (Scale bars = 100 μmin b and 50 μmin c.) Boxed areas in b and d are magnified in c and e, respectively.
Finally, before being killed, the animals were subjected to a functional test that measures automatic use of both forelimbs for support in rearing and also the correct palm placement of the paw in an exploratory motion on the side of a clear plastic cylinder (18). In this behavioral test, control uninjured animals rear in the tube and explore the sides with both paws, always placing the palm (ventral side) of their paw on the wall. After hemisection, animals in all groups, regardless of treatment, will still rear, and, although there is use of the injured forelimb, they will preferentially use the uninjured forelimb to balance themselves and explore the tube. However, when they do use the injured forelimb to explore, the untreated or vehicle-treated animals place the dorsal (wrong) side of their paw against the wall of the tube in 75–80% of tries (Fig. 5). This finding indicates a lack of distal control of the forelimb. In sharp contrast, when the rolipram-treated animals place the paw of their injured forelimb, they make a mistake and place the dorsal side of their paw against the wall only 35% of the time, representing a significant (>40%) decrease in abnormal dorsal placement (Fig. 5). In addition to this improved distal control, the rolipram-treated animals also have greater proximal forelimb control because they raise the injured limb more frequently above the horizontal (shoulder flexion >90°; 76% for rolipram, 56% for vehicle). Consistent with the effects of rolipram on both regeneration and astrogliosis described above, only treatment with the lower dose of rolipram resulted in improved functional recovery; at the higher rolipram dose, there was no improvement relative to untreated animals (results not shown). Together, these results represent a substantial and significant improvement in functional recovery compared with the control animals.
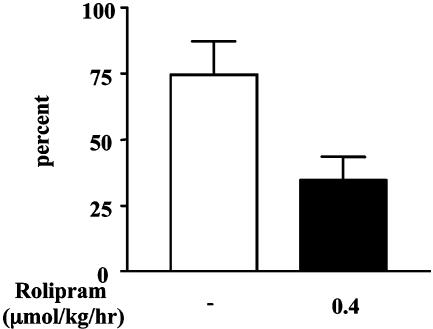
Fig. 5.
Rolipram improves functional recovery. Paw placement after spinal cord injury, transplant, and treatment with rolipram improves in the impaired forelimb. The number of right contacts that were dorsal only taken as a percentage of total right contacts significantly decreased in rats treated with rolipram (35%, n = 7, P < 0.05) compared with vehicle (75%, n = 5).
Discussion
These results show that rolipram delivered 2 weeks after a spinal cord lesion promotes axonal growth into the transplant, attenuates astrogliosis, and, importantly, improves functional recovery. It is highly likely that rolipram's ability to overcome inhibitors in myelin, as well as the general improvement in growth it induces and its effect on scar formation, all contribute to the observed functional recovery. Rolipram has also been shown to be antiinflammatory, but it is unlikely that this property is influencing recovery under the conditions used here for two reasons. First, the dose of rolipram most effective as an antiinflammatory agent is 3- to 6-fold higher than the optimal dose we report here for functional recovery after spinal cord injury (22–24). Second, methylprednisolone, a known antiinflammatory already used as a therapy in humans, prevents some secondary cell loss in animal models of spinal cord injury but does not produce any significant improvement in functional recovery (25–27) Regardless, of the mechanism of action, rolipram is effective and should be considered as a therapy for spinal cord injury. It is of note that rolipram is effective at lower but not at high doses, for both the culture and the in vivo assays. We do not know the reason for this result, but it is essential that this observation be taken into consideration in the design of future experiments.
Because rolipram is a PDE inhibitor and so allows cAMP to accumulate by blocking its degradation, we predicted that rolipram would indeed promote regeneration when we and others showed earlier that elevation of cAMP was sufficient to promote regeneration of spinal axons (4, 5). What was surprising about the findings reported here was, first, that rolipram had an effect when delivered post-lesion. In the previous studies, cAMP was elevated before lesioning the spinal cord in the belief that the axons needed to be “primed” before being asked to grow through an inhibitory environment. The hypothesis was that elevation of cAMP triggered a cascade of events that took some time to reach a point where the axons were ready to grow through inhibitors of myelin. If they were lesioned before the cascade reached this point, the glial scar would have matured before the axons were ready to grow and so, rather than just having to overcome the inhibitors in myelin, which seem to be the major impediments to growth immediately after injury (28), they would then also encounter the glial scar and so be locked in (2, 17). With rolipram treatment, however, that model is no longer supported. Part of the explanation for the apparent discrepancy may be that astrogliosis is attenuated, the second surprising observation we made in the rolipram-treated animals. This effect could result from preventing or reversing reactive gliosis. That is to say, by 2 weeks post-injury, the time when delivery of rolipram was begun, in untreated animals without transplant, the scar would be quite mature. It is possible that the presence of the embryonic tissue alone delays scar formation and rolipram then prevents its maturation. Alternatively, the embryonic tissue may have had no effect on scar formation, in which case rolipram, delivered at 2 weeks post-injury must have reversed astrogliosis. Although we show here that rolipram attenuates astrogliosis both within the transplanted tissue and in the surrounding host tissue, the question remains whether the transplant is necessary for rolipram to have this effect. This question can be answered by delivering rolipram in the absence of transplanted tissue. Furthermore, it is likely that the hospitable environment of the implanted embryonic tissue also favored regrowth without priming.
To quantitate the extent of axonal growth after rolipram treatment, we chose spinal cord lesion with transplant as an assay. We used a transplant because the high cervical hemisections (C3/4) always lead to a number of unlesioned axons, the presence of which makes it impossible to distinguish axons that have regrown from those left intact. The presence of a transplant enabled easy identification and measurement of regenerated host axons. Furthermore, there is very little background ingrowth of host axons with only transplant, making any increase in growth as a result of rolipram treatment very easy to detect.
In the rolipram-treated rats, the axonal growth is a proof of principle that regeneration occurs. However, although both increased axonal plasticity and functional recovery coincide after rolipram treatment, we cannot confirm a causal relationship between the two. The pathways that regenerated, underwent plasticity, or rearranged to account for the functional recovery observed are not known. Indeed, rearing and vertical exploration are complex behaviors, and many pathways contribute to their control in normal animals. The precise circuitry responsible for the paw-placing behavior in intact animals is not clear.
Rolipram has been shown to have an effect on a number of processes that involve the cAMP pathway. It has been shown to improve memory in a hippocampal-dependent memory task (29), and also to attenuate age-related defects in spatial memory (30). More recently, through a mechanism dependent on the transcription factor cAMP response element binding protein (CREB), it has been shown to enhance memory in an animal model of the human disease Rubinstein-Taybi syndrome (31). Also, we have found that activation of CREB is necessary and sufficient to overcome inhibitors of regeneration in myelin (unpublished results). Taken together, these observations suggest similarities in the mechanisms whereby rolipram promotes regeneration and enhances memory.
Rolipram was developed as an antidepressant and was used in clinical trials, but, because of side effects of emesis (nausea) in some patients, the trial was stopped (32–34). It has also been shown to have immunosuppressive and antiinflammatory effects (35). However, treatment of depression requires long-term administration. For spinal cord injuries, rolipram may need to be delivered only for a short period, during which time the side effects may be tolerable. A further attraction of rolipram for treating spinal cord injury is that it readily crosses the blood–brain barrier. Therefore, it can be delivered s.c. to avoid intervention at the site of injury; any treatment that requires surgery at the injury site runs the risk of damaging axons that were spared by the initial trauma. Although here we have shown rolipram to be an effective therapy for spinal cord damage when combined with embryonic tissue grafts, it is highly probable that rolipram treatment alone will also have a marked effect on spinal axon regeneration and possibly functional recovery.
Acknowledgments
We thank Dr. Lloyd Williams for his help with the image analysis and James V. Lynskey for his assistance with the behavior analyses. This work was supported by grants from the New York State Spinal Cord Fund, National Institute of Neurological Disorders and Stroke Grant 37060, the National Multiple Sclerosis Society, and core facility grants from the Research Centers for Minorities Institute–National Institutes of Health (NIH) and Specialized Neuroscience Research Programs–NIH Grant 41073.
Abbreviations: MAG, myelin-associated glycoprotein; PDE, phosphodiesterase; DRG, dorsal root ganglia; CHO, Chinese hamster ovary; BDNF, brain-derived neurotrophic factor; 5-HT, 5-hydroxytryptamine; GFAP, glial fibrillary acidic protein.
References
- 1.Schwab, M. E. & Bartholdi, D. (1996) Physiol. Rev. 76, 319–370. [DOI] [PubMed] [Google Scholar]
- 2.Filbin, M. T. (2003) Nat. Rev. Neurosci. 4, 703–713. [DOI] [PubMed] [Google Scholar]
- 3.Fitch, M. T. & Silver, J. (1999) in CNS Regeneration, eds. Tuszynski, M. H. & Kordower, J. H. (Academic, San Diego), pp. 55–88.
- 4.Neumann, S., Bradke, F., Tessier-Lavigne, M. & Basbaum, A. I. (2002) Neuron 34, 885–893. [DOI] [PubMed] [Google Scholar]
- 5.Qiu, J., Cai, D., Dai, H., McAtee, M., Hoffman, P. N., Bregman, B. S. & Filbin, M. T. (2002) Neuron 34, 895–903. [DOI] [PubMed] [Google Scholar]
- 6.Spencer, T., Domeniconi, M., Cao, Z. & Filbin, M. T. (2003) Curr. Opin. Neurobiol. 13, 133–139. [DOI] [PubMed] [Google Scholar]
- 7.Domeniconi, M., Cao, Z., Spencer, T., Sivasankaran, R., Wang, R. C., Nikulina, E., Kimura, N., Cai, H., Deng, K., Gao, Y., et al. (2002) Neuron 35, 283–290. [DOI] [PubMed] [Google Scholar]
- 8.Fournier, A. E., GrandPre, T. & Strittmatter, S. M. (2001) Nature 409, 341–346. [DOI] [PubMed] [Google Scholar]
- 9.Liu, B. P., Fournier, A., GrandPre, T. & Strittmatter, S. M. (2002) Science 297, 1190–1193. [DOI] [PubMed] [Google Scholar]
- 10.Wang, K. C., Koprivica, V., Kim, J. A., Sivasankaran, R., Guo, Y., Neve, R. L. & He, Z. (2002) Nature 417, 941–944. [DOI] [PubMed] [Google Scholar]
- 11.Cai, D., Deng, K., Mellado, W., Lee, J., Ratan, R. R. & Filbin, M. T. (2002) Neuron, in press. [DOI] [PubMed]
- 12.Krause, W. & Kuhne, G. (1988) Xenobiotica 18, 561–571. [DOI] [PubMed] [Google Scholar]
- 13.Jin, S. L., Richard, F. J., Kuo, W. P., D'Ercole, A. J. & Conti, M. (1999) Proc. Natl. Acad. Sci. USA 96, 11998–12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polson, J. B. & Strada, S. J. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 403–427. [DOI] [PubMed] [Google Scholar]
- 15.Raeburn, D. & Advenier, C. (1995) Int. J. Biochem. Cell Biol. 27, 29–37. [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay, G., Doherty, P., Walsh, F. S., Crocker, P. R. & Filbin, M. T. (1994) Neuron 13, 757–767. [DOI] [PubMed] [Google Scholar]
- 17.Cai, D., Shen, Y., De Bellard, M., Tang, S. & Filbin, M. T. (1999) Neuron 22, 89–101. [DOI] [PubMed] [Google Scholar]
- 18.Jones, T. A. & Schallert, T. (1994) J. Neurosci. 14, 2140–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein-Goral, H. & Bregman, B. S. (1993) Exp. Neurol. 123, 118–132. [DOI] [PubMed] [Google Scholar]
- 20.Bregman, B. S., Kunkel-Bagden, E., Reier, P. J., Dai, H. N., McAtee, M. & Gao, D. (1993) Exp. Neurol. 123, 3–16. [DOI] [PubMed] [Google Scholar]
- 21.Coumans, J. V., Lin, T. T., Dai, H. N., MacArthur, L., McAtee, M., Nash, C. & Bregman, B. S. (2001) J. Neurosci. 21, 9334–9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttini, M., Mir, A., Appel, K., Wiederhold, K. H., Limonta, S., Gebicke-Haerter, P. J. & Boddeke, H. W. (1997) Br. J. Pharmacol. 122, 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francischi, J. N., Yokoro, C. M., Poole, S., Tafuri, W. L., Cunha, F. Q. & Teixeira, M. M. (2000) Eur. J. Pharmacol. 399, 243–249. [DOI] [PubMed] [Google Scholar]
- 24.Laemont, K. D., Schaefer, C. J., Juneau, P. L. & Schrier, D. J. (1999) Int. J. Immunopharmacol. 21, 711–725. [DOI] [PubMed] [Google Scholar]
- 25.Haghighi, S. S., Agrawal, S. K., Surdell, D., Jr., Plambeck, R., Agrawal, S., Johnson, G. C. & Walker, A. (2000) Spinal Cord 38, 733–740. [DOI] [PubMed] [Google Scholar]
- 26.Lankhorst, A. J., ter Laak, M. P., Hamers, F. P. & Gispen, W. H. (2000) Brain Res. 859, 334–340. [DOI] [PubMed] [Google Scholar]
- 27.Takami, T., Oudega, M., Bethea, J. R., Wood, P. M., Kleitman, N. & Bunge, M. B. (2002) J. Neurotrauma 19, 653–666. [DOI] [PubMed] [Google Scholar]
- 28.Huang, D. W., McKerracher, L., Braun, P. E. & David, S. (1999) Neuron 24, 639–647. [DOI] [PubMed] [Google Scholar]
- 29.Barad, M., Bourtchouladze, R., Winder, D. G., Golan, H. & Kandel, E. (1998) Proc. Natl. Acad. Sci. USA 95, 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bach, M. E., Barad, M., Son, H., Zhuo, M., Lu, Y. F., Shih, R., Mansuy, I., Hawkins, R. D. & Kandel, E. R. (1999) Proc. Natl. Acad. Sci. USA 96, 5280–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourtchouladze, R., Lidge, R., Catapano, R., Stanley, J., Gossweiler, S., Romashko, D., Scott, R. & Tully, T. (2003) Proc. Natl. Acad. Sci. USA 100, 10518–10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wachtel, H. (1983) Neuropharmacology 22, 267–272. [DOI] [PubMed] [Google Scholar]
- 33.Wachtel, H. & Schneider, H. H. (1986) Neuropharmacology 25, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 34.Hebenstreit, G. F., Fellerer, K., Fichte, K., Fischer, G., Geyer, N., Meya, U., Sastre-y-Hernandez, M., Schony, W., Schratzer, M., Soukop, W., et al. (1989) Pharmacopsychiatry 22, 156–160. [DOI] [PubMed] [Google Scholar]
- 35.Souness, J. E., Aldous, D. & Sargent, C. (2000) Immunopharmacology 47, 127–162. [DOI] [PubMed] [Google Scholar]