Abstract
Beyond its well-documented role in reproduction, embryogenesis and maintenance of body tissues, vitamin A has attracted considerable attention due to its immunomodulatory effects on both the innate and the adaptive immune responses. In infectious diseases, vitamin A has been shown to have a host-protective effect in infections of bacterial, viral or protozoan origin. Nevertheless, its impact in fungal infections remains unknown. Meanwhile, the frequency of invasive mycoses keeps on growing, with Candida albicans being the major opportunistic fungal pathogen and associated with high mortality. In the present work, we explored the impact of all-trans retinoic acid (atRA), the most active metabolite of vitamin A, on the innate immune response against C. albicans in human monocytes. Our results show a strong immunomodulatory role for atRA, leading to a significant down-regulation of the fungi-induced expression and secretion of the pro-inflammatory cytokines TNFα, IL6 and IL12. Moreover, atRA significantly suppressed the expression of Dectin-1, a major fungal pattern recognition receptor, as well as the Dectin-1-dependent cytokine production. Both RAR-dependent and RAR-independent mechanisms seem to play a role in the atRA-mediated immunomodulation. Our findings open a new direction to elucidate the role of vitamin A on the immune function during fungal infections.
Electronic supplementary material
The online version of this article (doi:10.1007/s00430-014-0351-4) contains supplementary material, which is available to authorized users.
Keywords: Vitamin A, Retinoic acid, Candida albicans, Dectin-1
Introduction
Vitamin A is an essential nutrient obtained through the diet either as provitamin-A (carotenoids) or as preformed vitamin A (retinol and retinyl esters) [1, 2]. In its esterified form, it can be stored in the liver, where it is continually hydrolyzed to retinol and deployed into circulation [3, 4]. Once in its target tissues, two dehydrogenases are able to convert the retinol into retinoic acid, the biologically active metabolite of vitamin A [5]. In this form, vitamin A is known to play an essential role in multiple biological processes, including reproduction, embryogenesis, maintenance of body tissues and augmentation of the immune system [6–8]. Over the last decades, an increasing effort has been devoted to better define its involvement in the regulation of the immune response, since vitamin A deficiency (VAD) has been associated with an increased susceptibility to severe infectious diseases [9].
Several in vitro studies have shed light into the role of vitamin A not only as an important factor for normal immune system development, but also as a modulator of both the innate and the adaptive immune responses [4, 10]. Vitamin A has shown to regulate the development of B-lymphocytes and its immunoglobulin production [11–13]. In T-cells, retinoic acid is able to attenuate the Th1-associated gene expression and skew the immune response toward a Th2 profile [14]. Retinoic acid is also able to modulate the LPS-induced cytokine and/or chemokine production in further innate immune cells, including monocytes [15], macrophages and dendritic cells [16]. Moreover, the role of vitamin A as immunomodulator has been reinforced by interventional studies with infants and children with VAD. Here, vitamin A supplementation could reduce the morbidity and/or mortality from measles, malaria and certain forms of diarrhea [17]. These findings are also supported by several in vitro and animal studies, where a host-protective effect of vitamin A could be described for infections involving a bacterial, viral or protozoan origin [18]. Nevertheless, the ability of vitamin A to modulate the immune response against fungal infections is still unknown.
The frequency of invasive mycoses due to opportunistic fungal pathogens has been significantly growing in intensive care units over the last decades [19, 20]. Among invasive fungal infections in humans, Candida albicans remains the most important cause and is associated with high morbidity and mortality [21]. As shown for Candida-induced sepsis in mice models, the fatal host damage results from an exaggerated immune response rather than from the pathogen itself [22]. Therefore, modulation of the immune response might be an interesting strategy to reduce C. albicans-associated immunopathology.
The orchestration of the antifungal response starts with the recognition of the pathogen by immune cells provided with fungal pattern recognition receptors (PRR) [23]. Monocytes express most of the fungal PRRs and have shown to play a particularly important role in the early recognition of the pathogen in invasive candidiasis [23, 24]. Moreover, monocytes are the most effective mononuclear cell-type at killing C. albicans, while their cytokine secretion is important for subsequent innate and adaptive immune activation [23, 25]. However, inflammatory monocytes have also been linked to dysregulated immune responses in invasive candidiasis [22]. Thus, modulation of the immune response in monocytes might be of particular importance for the course of infection. Among the PRRs expressed on these innate cells, Dectin-1 has shown to play a prominent role in the immune response against C. albicans, not only mediating phagocytosis but also triggering the oxidative burst and the production of several pro-inflammatory cytokines [26]. Hence, in the present study, we analyzed the immunomodulatory role of vitamin A on the innate immune response against C. albicans with a main focus on the Dectin-1-mediated response. For this purpose, we employed β-1,3-glucan beads which were designed to serve as “fungal-like particles” eliciting a dominant Dectin-1 response [27, 28].
Materials and methods
Material
Beta-1,3-glucan beads were prepared as previously described [27]. atRA was purchased from Sigma-Aldrich (Germany) and dissolved in absolute ethanol. The RARα-agonist BMS753, the RARγ-agonist BMS961, as well as the RARα antagonist BMS195614 and the RARγ antagonist MM11253 were purchased from Tocris Bioscience (UK). Monoclonal mouse anti-human Dectin-1 MAB1859 (clone #259931) antibody was purchased from R&D Systems (Germany). Mouse IgG2B isotype control antibody was purchased from eBioscience (UK). APC-conjugated polyclonal goat anti-mouse antibody and APC-conjugated monoclonal mouse anti-human CD14 antibody were purchased from BD Biosciences (Germany). Polyclonal rabbit anti-Galectin-3 SC-20157 antibody was purchased from Santa Cruz (USA) and polyclonal rabbit anti-Actin (20–33) antibody was purchased from Sigma-Aldrich (Germany). HRP conjugated goat anti-rabbit IgG (H+L) antibody was purchased from Dianova (Germany).
Candida albicans isolate
Overnight fungal cultures of the virulent wild-type strain SC5314 [29] were grown in YPD medium, washed three times and resuspended in PBS at a concentration of 108 yeasts/ml. To avoid overbalanced growth of C. albicans and monocyte-killing due to hyphae formation, we inactivated the fungal yeasts. UV inactivation of the cells was performed on a UVC-500-Crosslinker (Amersham, UK) using two doses of 100,000 μj/cm2 immediately before cell stimulation.
Monocyte isolation
Human monocytes were isolated from buffy coats kindly provided by Dagmar Barz (Institute of Transfusional Medicine of the Jena University Hospital). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation following manufacturer’s instructions. Briefly, blood diluted 1:1 with PBS was layered onto an equal volume of Ficoll-Paque Plus (GE-Healthcare, Germany) and centrifuged in Leukosep Falcon tubes at 800 × g for 15 min. After centrifugation, the leukocyte band was collected, washed with cold NaCl 0.45 % and subjected to erythrocyte lysis using a hypotonic buffer. Cells were then washed twice in cold PBS and counted on a hemocytometer. Cell viability was assessed by trypan blue and propidium iodide/AnnexinV staining. To further isolate the monocytes, we used the monocyte isolation kit II (Miltenyi, UK) which couples negative selection with a cocktail of biotin-conjugated monoclonal antibodies and magnetic cell sorting using the quadro-MACS (Miltenyi, UK). Purity of the obtained monocytes was >92 % as assessed by CD14-labeling and flow cytometric analysis.
Stimulation assays
After monocyte isolation, cells were resuspended at 4 × 106 cells/ml in RPMI GlutaMax-Medium (Invitrogen, UK) supplemented with 1 % Penicillin/Streptomycin (Invitrogen, UK), plated on 6-well plates (VWR International, Germany) and allowed to equilibrate at 37 °C for 2 h. Monocytes were then pre-incubated with 1 μM of atRA or the specific RAR agonists for 30 min, followed by addition of the previously prepared C. albicans yeast at a fungus-monocyte ratio of 1:1. This ratio was predetermined in pilot experiments to preserve cell viability while yielding a suitable host gene response. When RAR antagonists were used, these were added 30 min before atRA, at a concentration of 1 μM. In the stimulation assay using β-1,3-glucan beads as specific ligands of Dectin-1, a 5:1 ratio was used. The cells were then incubated for 5 or 16 h at 37 °C and 5 % CO2. Viability of the monocytes was >90 %, as assessed by trypan blue and propidium iodide-staining. Additionally, AnnexinV staining was used to exclude an increase in apoptotic events. After incubation, the monocytes were harvested for RNA isolation and the culture supernatants were collected and stored at −80 °C.
RT-PCR and quantitative PCR
To analyze the gene expression of the target genes, total RNA was isolated from 8 × 106 monocytes using the Qiagen RNeasy mini kit. An additional step was included to remove the residual genomic DNA using DNaseI (Qiagen, Germany). A NanoDrop D-1000 Spectrophotometer (Thermo-Fisher Scientific, Germany) was then used to assess the amount and quality of the RNA. Complementary DNA (cDNA) was synthesized from 1,5 μg of RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, UK) following manufacturer’s instructions. For PCR-analysis, specific primers for each target gene were designed using the online primer-BLAST tool of the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/tools/primer-blast/). In order to improve the PCR efficiency, possible secondary structures of the amplicons were taken into account by characterizing their nucleotide sequence using the Mfold algorithm [30]. The sequences of all primers used for amplification are listed in Table 1.
Table 1.
Sequence of forward and reverse primers of indicated target genes and the size expected for each PCR product
| Human gene | Symbol | Forward primer | Reverse primer | Size (bp) |
|---|---|---|---|---|
| Peptidylpropyl isomerase B | PPIB | ATGTAGGCCGGGTGATCTTT | TGAAGTTCTCATCGGGGAAG | 219 |
| Hypoxanthine phosphoribosyltransferase 1 | HPRT1 | GACCAGTCAACAGGGGACAT | AACACTTCGTGGGGTCCTTTTC | 195 |
| Tumor necrosis factor alpha | TNFα | TTCTCCTTCCTGATCGTGGC | ACTCGGGGTTCGAGAAGATG | 150 |
| Interleukin 6 | IL6 | GAGGAGACTTGCCTGGTGAA | TGGGTCAGGGGTGGTTATTG | 186 |
| Interleukin 10 | IL10 | GCTGAGAACCAAGACCCAGA | GCATTCTTCACCTGCTCCAC | 143 |
| Interleukin 12 subunit beta | IL12B | ACAACATCTGTTTCAGGGCCA | GGTCCAAGGTCCAGGTGATA | 239 |
| Dectin-1 | CLEC7A | ACACTTCGACTCTCAAAGCA | TACAGCAATGAGGCGCCAA | 91 |
| Toll-like receptor 2 | TLR2 | TGCATTCCCAAGACACTGGA | AGGGAGGCATCTGGTAGAGT | 131 |
| Galectin-3 | LGALS3 | CCCATCTTCTGGACAGCCAA | CTTCACCGTGCCCAGAATTG | 151 |
| Retinoic acid receptor alpha | RARA | CCACATGTTCCCCAAGATGC | GCCCTCTGAGTTCTCCAACA | 145 |
| Retinoic acid receptor beta | RARB | TCGTCTGCCAGGACAAATCA | TTGGCATCGATTCCTGGTGA | 158 |
| Retinoic acid receptor gamma | RARG | CAAGGTCAGCAAAGCCCATC | ACTTGGTAGCCAGCTCACTG | 137 |
PCR of the cDNA was carried out on a S1000™ Thermal Cycler (BioRad, UK) in a 25 μl reaction volume containing 0.2 μM primers, 1 U Taq DNA polymerase (5-Prime, UK) and 200 μM dNTPs. Thermal conditions included an initial 95 °C denaturation step for 3 min, and then 35 cycles of 10 s at 94 °C, 30 s at 60 °C and 30 s at 72 °C. The resulting PCR products were separated on an ethidium bromide stained agarose gel and visualized under a UV-transiluminator to confirm the expected amplicon size.
To quantify the relative expression of each gene, a Corbett Rotor-Gene 6000 (Qiagen, Germany) was used as Real-Time qPCR apparatus. Each sample was analyzed in duplicate in a total reaction volume of 20 μl containing 10 μl of 2 × SensiMix SYBR Master Mix (Bioline, UK) and 0.2 μM of each primer. All qPCRs were set up using a CAS-1200 pipetting robot (Qiagen, Germany). The cycling conditions were 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s, 60 °C for 20 s and 72 °C for 20 s. For each experiment, an RT-negative sample was included as control. Specificity of the qPCRs was assessed by melting curve analysis and size verification by electrophoresis. The relative expression of the target genes was analyzed using a modified Pfaffl method [31, 32]. To determine significant differences in the mRNA expression between different experimental conditions, the relative quantity (RQ) for each sample was calculated using the formula 1/ECt, where E is the efficiency and Ct the threshold cycle. The RQ was then normalized to the geometric mean of two housekeeping genes: hypoxanthine phosphoribosyltransferase1 (HPRT1) and peptidylpropyl isomerase B (PPIB). The stability of the housekeeping genes was assessed using the BestKeeper algorithm [33]. The normalized RQ (NRQ) values were log2-transformed for further statistical analysis with GraphPad PRISM v5.0.
Flow cytometry
To analyze the expression of Dectin-1 on the cell surface, monocytes were washed with PBS containing 10 % FBS and stained with anti-Dectin-1 antibody (1 µg/ml, 30 min) and with APC-conjugated goat anti-mouse IgG (2,5 µg/ml, 30 min). A mouse-IgG2B-antibody was used as isotype control. Samples were measured on a FACSAria II apparatus (BD Biosciences; Germany) and data were analyzed using the FLOWJO 7.6.4 software. The resulting mean fluorescence intensities (MFIs) were normalized to those of unstained cells in each case.
Cytokine measurements
TNFα, IL6, IL12 and IL10 secretion by the monocytes was analyzed using commercially available ELISA kits (Human TNFα Elisa Kit, Thermo Scientific; Human IL12 Elisa Kit, Hölzel Diagnostika; Human IL6 Elisa Kit, eBioscience; Human IL10 Elisa Kit, eBioscience) according to the manufacturer’s instructions. An Infinite M200 reader (Tecan, UK) was used to measure the optical density (OD), and the concentration of each cytokine was calculated from the respective standard curve by five-parameter logistic analysis using the Magellan v.6 software (Tecan, UK).
Statistical analysis
Statistical analyses were performed using GraphPad PRISM v5.0 software (San Diego, USA). Two-sided pairwise t test or repeated measures ANOVA with Dunnett’s post hoc test were used to determine statistical significance. In all cases, the level of significance was set at p ≤ 0.05.
Results
Retinoic acid modulates the cytokine production induced by C. albicans and β-1,3-glucan in human monocytes
We assessed the impact of vitamin A on the immune response to C. albicans by challenging monocytes with UV-killed yeasts in the absence or presence of 1 μM atRA. In a first approach, we analyzed the expression of TNFα, IL6, IL12b and IL10 at transcriptional level by Real-Time qPCR. For accurate normalization purposes, we tested the stability of the housekeeping genes among the experimental conditions using the BestKeeper algorithm [33]. Since both PPIB (standard deviation (SD) of the Ct = 0,48; coefficient of variance (CV) of the Ct = 2,60) and HPRT1 (SD(Ct) = 0,49; CV(Ct) = 2,22) showed a highly stable expression, the geometric mean of both genes was used for further relative expression calculations.
After 5 h of incubation with C. albicans yeasts, we could observe a clear increase in the mRNA expression levels of all four cytokines analyzed (Fig. 1a). However, in the presence of atRA in the cell culture medium, the up-regulation of the pro-inflammatory cytokines was significantly suppressed. As shown in Fig. 1a, the C. albicans-mediated expression of TNFα could be dropped from a 78-fold expression (in the absence of atRA) to a 21-fold expression when atRA was present. In a similar way, we could observe a significant reduction of 83 and 96 % in the gene expression levels of IL6 and IL12b, respectively (Fig. 1a). This modulation by retinoic acid occurs in a dose-dependent manner, and an inhibitory effect of atRA on all three cytokines could already be observed with concentrations as low as 0,01 μM (Suppl. Fig. 1). On the other hand, we could not observe any effect of atRA on the C. albicans-induced IL10 expression (Fig. 1a), even if in the absence of fungal challenge, the addition of atRA led to an up-regulation of this anti-inflammatory cytokine (data not shown).
Fig. 1.

Relative mRNA expression levels of TNFα, IL6 and IL12b measured by qPCR. Monocytes were stimulated with either a UV-treated C. albicans yeasts (UV-Ca) or b β-1,3-glucan beads (βG-b) for 5 h in the presence or absence of 1 μM atRA. Both the β-1,3-glucan-induced and the C. albicans-mediated up-regulation of pro-inflammatory cytokine expression was significantly attenuated by atRA. Data were obtained from five independent experiments, each performed with cells from different donors. Results are presented as mean ± SEM of the fold change relative to the control (unstimulated cells). *** p ≤ 0.001
Comparable results were obtained when the monocytes were stimulated with β-1,3-glucan beads to specifically address the Dectin-1-response (Fig. 1b). As shown in Fig. 1b, the activation of this early fungal-recognition receptor, leading to an up-regulation of all three pro-inflammatory cytokines, was down-regulated by atRA at transcriptional level. In contrast, the expression of the anti-inflammatory cytokine IL10 was rather potentiated by atRA, although not in a statistically significant manner.
To further confirm our findings at protein level, we analyzed the culture supernatants for the presence of TNFα, IL6, IL12 and IL10 after 16 h of stimulation with either C. albicans or β-glucan beads. As shown in Fig. 2, pro-inflammatory cytokine secretion upon fungal stimulation was severely affected by atRA. Co-stimulation with atRA decreased the amount of secreted TNFα, IL6 and IL12 in a significant manner, whereas no effect could be observed on the IL10 release. (Fig. 2a). Similar results were observed when the monocytes were stimulated with the β-glucan beads. In this case, atRA showed an inhibitory effect on the release of all pro-inflammatory cytokines, whereas the secretion of IL10 was rather potentiated (Fig. 2b). These observations resemble the results obtained at transcriptional level and suggest a strong anti-inflammatory role of vitamin A in fungal infections.
Fig. 2.

Cytokine measurement in the culture supernatants. Monocytes were stimulated with either a UV-treated C. albicans yeasts (UV-Ca) or b β-1,3-glucan beads (βG-b) in the presence or absence of 1 μM atRA. After 16 h culture supernatants were analyzed using specific ELISAs for TNFα, IL6, IL12 and IL10. In the presence of atRA, the secretion of all pro-inflammatory cytokines was significantly inhibited. Columns and error bars represent the mean ± SEM of five independent experiments. * p ≤ 0.05; ** p ≤ 0.01
Retinoic acid modulates the expression of Dectin-1
Since we could observe an impact of vitamin A on the function of Dectin-1 in terms of the receptor-mediated cytokine production, we next wanted to investigate whether vitamin A had an effect on the expression of this PRR. Under the experimental settings of our stimulation assay, the expression of Dectin-1 was measured at transcriptional level after 5 and 16 h of incubation with C. albicans. At both time points, atRA supplementation led to a decreased expression of Dectin-1 mRNA. Moreover, the inhibitory effect of atRA seemed to increase over time, reaching a fivefold down-regulation of Dectin-1 mRNA after 16 h of incubation with C. albicans (Fig. 3a). Similar down-regulation was also observed when cells were stimulated with atRA alone, in the absence of fungal challenge (Suppl. Fig. 2). Interestingly, C. albicans itself was also able to slightly dampen the expression of Dectin-1 at transcriptional level (Fig. 3a).
Fig. 3.

Modulation of the Dectin-1-expression by retinoic acid upon C. albicans infection. Monocytes were stimulated with UV-treated C. albicans (CaUV) yeasts in the presence or absence of 1 μM atRA. a Dectin-1-mRNA expression was measured after 5 and 16 h. b Representative flow cytometry plot of Dectin-1-APC after 24 h of stimulation. c Relative Dectin-1 MFI over time. The relative MFI is defined as the MFI of the cells challenged with C. albicans in the presence of atRA divided by the MFI of the monocytes challenged with the fungi in the absence of atRA. d mRNA expression of Dectin-1 co-receptors TLR2 and Galectin-3 after 5 and 16 h of stimulation. Data for the transcriptional analysis were obtained from five independent experiments, each performed with cells from different donors. Results are presented as mean ± SEM of the fold change relative to the control (unstimulated cells). For flow cytometry analysis, data from four independent experiments were collected. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
Next, we explored the expression of Dectin-1 on the cell surface of the monocytes. As shown in Fig. 3b, our findings at transcriptional level could be reproduced at protein level by flow cytometry. A shift in the MFI became apparent after C. albicans stimulation and reached its maximum drop in the presence of atRA (Fig. 3b). To further assess the impact of atRA on Dectin-1 over time, relative MFI values were calculated for each time point. In agreement with the observations at transcriptional level, the atRA-mediated down-regulation of Dectin-1 increased over time as assessed by a trend test (Page’s L test; p < 0.001; Fig. 3c). These results suggest a sustained modulation of the anti-fungal response by vitamin A. Moreover, when we analyzed the influence of atRA on the expression pattern of two known co-receptors of Dectin-1, TLR2 and Galectin-3, a highly significant down-regulation could be observed in both cases (Fig. 3d). While C. albicans increased the mRNA expression of both TLR2 and Galectin-3, this up-regulation was almost completely abrogated in the presence of atRA.
RAR-dependent and RAR-independent mechanisms mediate the atRA-induced anti-inflammatory effect in monocytes
To determine the involvement of specific nuclear receptors in our findings, we first characterized the expression profiles of all possible RARs in our monocytes by RT-PCR. We could not detect any expression of RARβ, but obtained a clear signal for the expression of both RARα and RARγ mRNA in all five monocyte samples used in this study (Fig. 4a). To define the relevance of these two receptors in the atRA-mediated anti-inflammatory effect, specific agonists and antagonists of each RAR were used in our experimental setting.
Fig. 4.
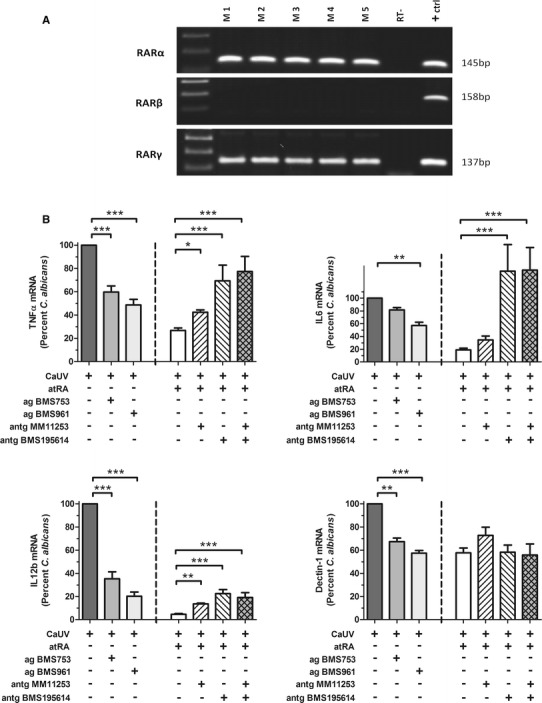
Expression profile and immunomodulatory activity of retinoic acid receptors in human monocytes. a Transcriptional expression of RARα, RARβ and RARγ in monocytes (samples M1-M5) as discriminated by agarose gel electrophoresis of PCR products. HEK293 cells (RARα), A549 cells (RARβ) or NHBE cells (RARγ) served as positive controls in each case. (RT- = reverse transcription negative control) b Immunomodulatory effect of specific RAR-agonists BMS753 and BMS961 on the expression of TNFα, IL6, IL12b and Dectin-1 upon C. albicans infection. Also shown is the inhibitory power of the specific RAR antagonists MM11253 and BMS195614 on the atRA-mediated modulation of these genes. Monocytes were pre-incubated for 30 min with either RAR-specific agonists or atRA (in the presence or absence of RAR antagonists) and then challenged with UV-treated C. albicans yeasts (CaUV). Columns represent the percentage of the C. albicans-induced mRNA expression in each case. Data were collected from five independent experiments. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001
When the monocytes were pre-incubated with the specific RAR agonists, both RARα agonist BMS753 and RARγ agonist BMS961 were able to mimic the repressive effect of atRA, although only partially in most cases (Fig. 4b). These results suggest that both RAR receptors are capable to mediate the observed down-regulation of the inflammatory response against C. albicans. To further prove their involvement in our particular experimental setting, specific antagonists of both RARs were used to test whether the expression levels of each gene could be restored in their presence. For both TNFα and IL6, the C. albicans-induced expression levels could be fully restored in the presence of the RAR antagonists in a significant manner (Fig. 4b). Also the expression of IL12b could be restored significantly, but only partially. In contrast, the atRA-mediated down-regulation of Dectin-1 mRNA was not affected by the addition of the RAR antagonists (Fig. 4b). Taken together, these results suggest a RAR-dependent mechanism for the atRA-mediated modulation of the pro-inflammatory cytokines, whereas an additional RAR-independent mechanism seems to play a major role in the transcriptional regulation of Dectin-1.
Discussion
Despite the increasing interest in the immunomodulatory role of vitamin A, no evidence has been reported addressing the impact of vitamin A on the immune response to fungi. In the present study, we have characterized the effect of atRA on the C. albicans-induced immune response in human monocytes. Our results show a strong immunomodulatory role for atRA, leading to a highly significant suppression of the fungi-induced expression of TNFα, IL6 and IL12. This down-regulation could be assessed at both transcriptional and post-translational level.
In fungal infections, the immune response is initiated after recognition of the pathogen by specific PRRs, such as Dectin-1 [26]. To address the impact of vitamin A on this important receptor in the fungal immune activation, we stimulated the monocytes using β-1,3-glucan beads as specific Dectin-1 ligand. Under these conditions, the modulatory effect of atRA resembled the one observed in experiments performed with C. albicans. Besides the impact of atRA on the functional output of Dectin-1 activation, we investigated the effect of atRA on the expression of the receptor upon C. albicans challenge. Interestingly, C. albicans alone was able to down-regulate the expression of Dectin-1, which has not been described previously in the literature. This observation might suggest a new mechanism to be included in the growing record of immune-escaping strategies described for this fungus [34, 35]. When atRA was added in this experimental setting, an even stronger inhibitory impact on the expression of Dectin-1 could be observed. The atRA-mediated down-regulation of the receptor could be assessed at transcriptional level and by flow cytometry. The inhibitory effect of atRA was not challenge-dependent, since a similar effect could be observed in the absence of C. albicans (Suppl. Fig. 2). This effect seems also not to be related to a process of atRA-induced differentiation, since a comparable down-regulation of Dectin-1 could be observed when terminally differentiated dendritic cells were stimulated with atRA (data not shown). Moreover, we could demonstrate that the suppressive effect of atRA increased over time, which suggests that the immunomodulatory impact of vitamin A might be sustained over a prolonged period. In addition, the known Dectin-1 co-receptors TLR2 [36] and Galectin-3 [37], which have been shown to enhance the Dectin-1-dependent immune response, were also down-regulated by atRA.
Our results raised the question whether the observed drop in pro-inflammatory cytokine production could be explained by the atRA-mediated down-regulation of Dectin-1 and its co-receptors. The effect of atRA on the cytokine expression could be observed at transcriptional level after 5 h of incubation. Interestingly, at this stage, the atRA-mediated Dectin-1 down-regulation at the cell surface was already apparent, although only in a very incipient manner (Suppl. Fig. 3). Therefore, we cannot exclude that the down-regulation of the receptor might contribute, at least in part, to the observed drop in cytokine levels. Nevertheless, it is likely that other direct mechanisms play a prominent role in the early phase of the atRA-mediated immunomodulation. This is supported by our observation that short pre-incubation periods with atRA led to a stronger down-regulation of the fungi-induced cytokine expression than pre-incubation periods of 24 h, when the Dectin-1-down-regulation on the cell surface reached a maximum (Suppl. Fig. 4). This is also in agreement with the observation that atRA has been described to modulate the immune response to LPS, a Dectin-1-independent immunological challenge, at least in other cell types such as dendritic cells and macrophages [16].
In monocytes, the immunomodulatory role of atRA on the immune response against LPS has been reported by Oeth et al. [38] and Wang et al. [39]. While Oeth et al. could not detect any atRA-mediated changes in the TNFα secretion after stimulation with LPS, Wang et al. observed only a very slight impact of atRA (<twofold change) on the LPS-induced TNFα and IL12 mRNA expression levels. In our study, the addition of atRA led to an almost 100 % abrogation of the Dectin-1-mediated expression and secretion of TNFα, IL6 and IL12 in human monocytes. It would be interesting to investigate whether the higher impact of atRA observed in our study could be related to the nature of the immunological challenge. The signal transduction of the LPS/TLR4 axis differs consistently from that of the Dectin-1-activation [26]. It is tempting to speculate that different PRR-signaling pathways might display different sensitivities to atRA-modulation. Further studies are required to comprehensively elucidate the mechanisms of atRA-mediated modulation of the immune response.
Most of the biological actions of atRA are exerted through binding its specific nuclear receptors: RARα, RARβ and/or RARγ [40]. We investigated the expression of all three RARs in monocytes and could detect the expression of both RARα and RARγ mRNA, which is in agreement with previous studies [41]. The use of specific agonists and antagonists for each RAR allowed us to verify that both receptors are involved in the suppressive effects observed on TNFα, IL6 and IL12. On the other hand, the vitamin A modulation of the Dectin-1 expression seemed to occur mainly through a RAR-independent mechanism.
The impact of vitamins on immunity and inflammation has been widely investigated in allergy and bacterial infections [42], but to a much lesser extent in fungal infections. Nevertheless, vitamin D has already been described to possess significant anti-inflammatory properties in Candida infections [43]. Meanwhile, the role of vitamin A in invasive candidiasis has remained unknown. Moreover, only very few studies have been conducted to investigate the vitamin A-status in septic patients, were C. albicans is a common nosocomial pathogen [44, 45]. Indeed, 33–55 % of all episodes of candidemia have been shown to occur in intensive care units (ICU) [46]. Such ICU-acquired candidemia in critically ill patients is associated with a particular high mortality of >50 % [47]. The search for anti-inflammatory immunomodulators is of particular importance in invasive candidiasis, where the dysregulation of the immune response rather than the pathogen has been shown to be responsible for the fatal host damage [22, 43]. Interestingly, a recent study reported an important inadequacy of retinol and β-carotene in a cohort of sepsis patients [45]. Thus, monitoring the serum levels of vitamin A and its adequate supplementation in individuals admitted in ICUs might have far-reaching prophylactic implications.
In conclusion, in this study, we have demonstrated a strong immunomodulatory role of vitamin A on the innate host response to C. albicans in human monocytes. In addition, we could show that atRA modulates both the function and the expression of Dectin-1. Moreover, the modulation of this PRR seems to increase over time, leading to a sustained regulation of the immune response. The observed effect of atRA on the cytokine production is likely to occur via activation of either or both RARα and RARγ. This study opens a new avenue to explore the role of vitamin A in fungal infections and to elucidate further molecular mechanisms of its immunomodulatory function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Figure 1.- Dose-dependent regulation of the cytokine mRNA expression by retinoic acid. The modulatory effect of increasing concentrations of atRA (0.01 µM – 10 µM) on the C. albicans-induced cytokine expression was measured by qPCR. Shown is the percentage of inhibition as normalized to the C. albicans-induced mRNA expression of each gene. (TIFF 22,313 kb)
Suppl. Figure 2.- Modulatory role of atRA on the Dectin-1 expression in the absence of inflammatory stimuli. We measured the effect of atRA on the expression of Dectin-1 A) at transcriptional level after 5 h of incubation with 1 µM atRA by qPCR, and B) at the cell surface after 24 h of incubation by flow cytometry (data representative of 3 biological replicates). * p ≤ 0.05 (TIFF 67,138 kb)
Suppl. Figure 3.- Analysis of the expression of Dectin-1, TLR2 and Galectin-3 after 4 h of incubation with C. albicans (in the presence or absence of 1 µM atRA). Receptor expression was measured on protein level by flow cytometry for A) Dectin-1 and B) TLR2, and by Western Blot for C) Galectin-3 after 4 h of incubation. Incipient regulation of the Dectin-1 expression is observed after C. albicans challenge, being potentiated in the presence of atRA. The data are representative of 3 independent experiments. (TIFF 59,077 kb)
Suppl. Figure 4.- Regulation of the cytokine mRNA expression by retinoic acid exposure over different time-periods. Monocytes were pre-incubated with 1 µM atRA for either 0,5 or 24 h. Then the cells were challenged with C. albicans for 5 h and the cytokine expression was measured by Real-Time qPCR. Shown is the percentage of inhibition achieved by atRA pre-incubation on the C. albicans-induced cytokine expression. (TIFF 27,642 kb)
Acknowledgments
We want to thank Simone Tänzer for excellent technical assistance. This work was funded by the German Research Foundation (Collaborative Research Center/Transregio 124—Pathogenic fungi and their human host: Networks of Interaction, Project A5), by the German Federal Ministry of Education and Research (BMBF 03Z2JN22 to H.S. and 01EO1002 to A.H. and E.K./Center for Sepsis Control and Care), and the grants NIH/NIAID 1K08AI110655 to M.K.M., 1R01AI092084 to J.M.T. and 1R01AI097519 to J.M.V.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This work complies with the current laws of Germany.
References
- 1.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3(1):63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf G. A history of vitamin A and retinoids. FASEB J. 1996;10(9):1102–1107. doi: 10.1096/fasebj.10.9.8801174. [DOI] [PubMed] [Google Scholar]
- 3.Wolf G. Identification of a membrane receptor for retinol-binding protein functioning in the cellular uptake of retinol. Nutr Rev. 2007;65(8 Pt 1):385–388. doi: 10.1301/nr.2007.aug.385-388. [DOI] [PubMed] [Google Scholar]
- 4.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35(1):13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell Mol Life Sci. 2010;67(9):1423–1445. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3(4):385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96(5):1204s–1206s. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 9.Glasziou PP, DE Mackerras Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ. 1993;306(6874):366–370. doi: 10.1136/bmj.306.6874.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pino-Lagos K, Guo Y, Noelle RJ. Retinoic acid: a key player in immunity. BioFactors. 2010;36(6):430–436. doi: 10.1002/biof.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Israel H, Odziemiec C, Ballow M. The effects of retinoic acid on immunoglobulin synthesis by human cord blood mononuclear cells. Clin Immunol Immunopathol. 1991;59(3):417–425. doi: 10.1016/0090-1229(91)90037-B. [DOI] [PubMed] [Google Scholar]
- 12.Ross AC, Chen Q, Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam Horm. 2011;86:103–126. doi: 10.1016/B978-0-12-386960-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worm M, Krah JM, Manz RA, Henz BM. Retinoic acid inhibits CD40 + interleukin-4-mediated IgE production in vitro. Blood. 1998;92(5):1713–1720. [PubMed] [Google Scholar]
- 14.Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai YC, Chang HW, Chang TT, Lee MS, Chu YT, Hung CH. Effects of all-trans retinoic acid on Th1- and Th2-related chemokines production in monocytes. Inflammation. 2008;31(6):428–433. doi: 10.1007/s10753-008-9095-x. [DOI] [PubMed] [Google Scholar]
- 16.Wojtal KA, Wolfram L, Frey-Wagner I, Lang S, Scharl M, Vavricka SR, Rogler G. The effects of vitamin A on cells of innate immunity in vitro. Toxicol In Vitro. 2013;27(5):1525–1532. doi: 10.1016/j.tiv.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. J Infect Dis. 2000;182(Suppl 1):S122–S133. doi: 10.1086/315921. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58(3):719–727. doi: 10.1017/S0029665199000944. [DOI] [PubMed] [Google Scholar]
- 19.Lamagni TL, Evans BG, Shigematsu M, Johnson EM. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–9) Epidemiol Infect. 2001;126(3):397–414. doi: 10.1017/S0950268801005507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis RE. Overview of the changing epidemiology of candidemia. Curr Med Res Opin. 2009;25(7):1732–1740. doi: 10.1185/03007990902990817. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Muller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8(7):e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6(1):67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 24.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209(1):109–119. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netea MG, Gijzen K, Coolen N, Verschueren I, Figdor C, Van der Meer JW, Torensma R, Kullberg BJ. Human dendritic cells are less potent at killing Candida albicans than both monocytes and macrophages. Microbes Infect. 2004;6(11):985–989. doi: 10.1016/j.micinf.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 2011;14(4):392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Tam JM, Mansour MK, Khan NS, Yoder NC, Vyas JM. Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr Biol. 2012;4(2):220–227. doi: 10.1039/c2ib00089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour MK, Tam JM, Khan NS, Seward M, Davids PJ, Puranam S, Sokolovska A, Sykes DB, Dagher Z, Becker C, Tanne A, Reedy JL, Stuart LM, Vyas JM. Dectin-1 activation controls maturation of beta-1,3-glucan-containing phagosomes. J Biol Chem. 2013;288(22):16043–16054. doi: 10.1074/jbc.M113.473223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(1):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 30.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieu I, Powers SJ. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell. 2009;21(4):1031–1033. doi: 10.1105/tpc.109.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 34.Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun. 2012;80(4):1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zipfel PF, Skerka C, Kupka D, Luo S. Immune escape of the human facultative pathogenic yeast Candida albicans: the many faces of the Candida Pra1 protein. Int J Med Microbiol. 2011;301(5):423–430. doi: 10.1016/j.ijmm.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10(10):2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 37.Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci USA. 2011;108(34):14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oeth P, Yao J, Fan ST, Mackman N. Retinoic acid selectively inhibits lipopolysaccharide induction of tissue factor gene expression in human monocytes. Blood. 1998;91(8):2857–2865. [PubMed] [Google Scholar]
- 39.Wang X, Allen C, Ballow M. Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol. 2007;27(2):193–200. doi: 10.1007/s10875-006-9068-5. [DOI] [PubMed] [Google Scholar]
- 40.Laudet V, Gronemeyer H. The nuclear receptor facts book. San Diego: Academic; 2002. [Google Scholar]
- 41.Fritsche J, Stonehouse TJ, Katz DR, Andreesen R, Kreutz M. Expression of retinoid receptors during human monocyte differentiation in vitro. Biochem Biophys Res Commun. 2000;270(1):17–22. doi: 10.1006/bbrc.2000.2371. [DOI] [PubMed] [Google Scholar]
- 42.Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Caraffa A, Antinolfi P, Tete S, Tripodi D, Conti F, Cianchetti E, Toniato E, Rosati M, Speranza L, Pantalone A, Saggini R, Tei M, Speziali A, Conti P, Theoharides TC, Pandolfi F. Role of vitamins D, E and C in immunity and inflammation. J Biol Regul Homeost Agents. 2013;27(2):291–295. [PubMed] [Google Scholar]
- 43.Khoo AL, Chai LY, Koenen HJ, Kullberg BJ, Joosten I, van der Ven AJ, Netea MG. 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: impact of seasonal variation of immune responses. J Infect Dis. 2011;203(1):122–130. doi: 10.1093/infdis/jiq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, Bauer M, Riedemann NC. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15(4):R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro Nogueira C, Ramalho A, Lameu E, Da Silva Franca CA, David C, Accioly E. Serum concentrations of vitamin A and oxidative stress in critically ill patients with sepsis. Nutr Hosp. 2009;24(3):312–317. [PubMed] [Google Scholar]
- 46.Bouza E, Munoz P. Epidemiology of candidemia in intensive care units. Int J Antimicrob Agents. 2008;32(Suppl 2):S87–S91. doi: 10.1016/S0924-8579(08)70006-2. [DOI] [PubMed] [Google Scholar]
- 47.de Gonzalez Molina FJ, Leon C, Ruiz-Santana S, Saavedra P. Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care. 2012;16(3):R105. doi: 10.1186/cc11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1.- Dose-dependent regulation of the cytokine mRNA expression by retinoic acid. The modulatory effect of increasing concentrations of atRA (0.01 µM – 10 µM) on the C. albicans-induced cytokine expression was measured by qPCR. Shown is the percentage of inhibition as normalized to the C. albicans-induced mRNA expression of each gene. (TIFF 22,313 kb)
Suppl. Figure 2.- Modulatory role of atRA on the Dectin-1 expression in the absence of inflammatory stimuli. We measured the effect of atRA on the expression of Dectin-1 A) at transcriptional level after 5 h of incubation with 1 µM atRA by qPCR, and B) at the cell surface after 24 h of incubation by flow cytometry (data representative of 3 biological replicates). * p ≤ 0.05 (TIFF 67,138 kb)
Suppl. Figure 3.- Analysis of the expression of Dectin-1, TLR2 and Galectin-3 after 4 h of incubation with C. albicans (in the presence or absence of 1 µM atRA). Receptor expression was measured on protein level by flow cytometry for A) Dectin-1 and B) TLR2, and by Western Blot for C) Galectin-3 after 4 h of incubation. Incipient regulation of the Dectin-1 expression is observed after C. albicans challenge, being potentiated in the presence of atRA. The data are representative of 3 independent experiments. (TIFF 59,077 kb)
Suppl. Figure 4.- Regulation of the cytokine mRNA expression by retinoic acid exposure over different time-periods. Monocytes were pre-incubated with 1 µM atRA for either 0,5 or 24 h. Then the cells were challenged with C. albicans for 5 h and the cytokine expression was measured by Real-Time qPCR. Shown is the percentage of inhibition achieved by atRA pre-incubation on the C. albicans-induced cytokine expression. (TIFF 27,642 kb)


