Abstract
Periodontitis (PD) is known to be one of most prevalent worldwide chronic inflammatory diseases. There are several treatments including antibiotics for PD; however, since drug resistance is an increasing problem, new drugs particularly derived from plants with fewer side effects are required. The effects of trans-anethole on IL-1 β and TNF-α level in a rat model of PD were investigated and compared to ketoprofen.
Eschericia coli lipopolysaccharide (LPS, 30 µg) was injected bilaterally into the palatal gingiva (3 µL/site) between the upper first and second molars every two days for 10 days in anesthetized rats. Administration of either trans-anethole (10 or 50 mg/Kg, i.p.) or ketoprofen (10 mg/Kg, i.p.) was started 20 minute before LPS injection and continued for 10 days. Then, IL-1β and TNF-α levels were measured in blood samples by ELISA at day 0 (control) and at day 10.
Anethole at both concentrations significantly suppressed IL-1β and TNF-α production when compared to LPS-treated rats. The suppressive effects of anethole on LPS-induced pro-inflammatory cytokines were almost similar as seen with ketoprofen.
In conclusion, the present results suggest that anethole may have a potent inhibitory effect on PD through suppression of pro-inflammatory molecules; therefore it could be a novel therapeutic strategy for PD.
Key Words: Trans-anethole, LPS-induced periodontitis, Ketoprofen, Anti-inflammatory, IL-1β, TNF-α
Introduction
Periodontitis (PD) is one of most prevalent worldwide chronic inflammatory diseases characterized by the host-mediated destruction of soft and hard periodontal tissue (1). PD, which is known to be a risk factor for several systemic diseases(2, 3, 4) including cardiovascular diseases, primarily initiated by a number of putative pathogenic bacterial infections. It has also been reported that pro-inflammatory cytokines and chemokines play an important role in the pathogenesis of PD (1, 5, 6, 7). Among cytokines, IL-1β and TNF-α are essential in the development and progression of periodontitis, as it has been shown that their antagonists inhibit the inflammatory response in experimental PD (8).
There is a close relationship between the high incidence of oral diseases and microorganisms and because of growing antibiotic bacterial resistance, toxic and harmful side effects associate with use of some common antibacterial agents; there is a need for alternative treatment options and therapies that are effective and safe and affordable such as herbal therapies (9, 10, 11).Therefor, in this study, the anti-inflammatory effect of anethole in an animal model of periodontitis induced by lipopolysaccharide (LPS) was evaluated.
Phytotherapic compounds particularly those that are containing terpene have been suggested to have anti-inflammatory effects because they can inhibit TNF-α (12, 13) and IL-1β production (14). Anethole is a monoterpene position isomer and it is the main constituent of essential oils from aromatic plants including anise, star-anise, and fennel (12, 15). Anethole is used in food and pharmaceutical industries and the United States Food and Drug Administration (FDA-US) has issued its safety certification (12). It has also experimentally shown that anethole has no toxicity at low doses (16) and it is considered non-genotoxic and non-carcinogenic and, therefore, quite safe (17, 18).
Trans-anethole exerts anti-metastatic activity (19), anti-oxidative (17), antimicrobial and antiviral (20), anti-inflammatory (12, 21) properties. It has also been shown that trans-anethole can modify Ca2+ and Ca2+-activated K+ channels function (22).
Based on the above findings, in the present study an attempt was made to investigate the inflammatory potential of trans-anethole in a rat model of periodontitis induced by lipopolysaccharide (LPS). LPS is a potent immune stimulator that induces the release of pro-inflammatory cytokines (e.g. IL-1β and TNF-α) and thereby causes acute inflammatory responses (23, 24). IL-1β and TNF-α are both important inflammation markers in the blood serum. After LPS injection, the circulating TNF reaches to its peak within 90 min before of IL-1 (25). LPS has also been shown to induce osteonecrosis (26) Anethole has been shown to block both early and late cellular responses to TNF (27).
Experimental
Animal
Male Wistar rats (180-220 g) were used in the present experiments. The animals were housed under controlled temperature of 23 ± 2 oC and a 12 h light/dark cycle, with free access to tap water and rat chow. All procedures were approved by the Ethical Committee for Animal experimentations of Shahid Beheshti University of Medical Sciences. Thirty rats were randomly divided into the following experimental groups (5 rats per group): (1) control; (2) LPS; (3) LPS + dimethyl sulfoxide (DMSO, sham operated group); (4) LPS + trans-anethole (10 mg/Kg, Sigma); (5) LPS + trans-anethole (50 mg/Kg); (6) LPS +Ketoprofen.
Model of periodontitis
The model of periodontitis was done as described by Guimarães and colleagues in 2012 (34). Briefly, rats were anesthetized with ketamine hydrochloride (50 mg/Kg, i.p.) and xylazine hydrochloride (5 mg/Kg, i.p.), then were fixed on his back. Eschericia coli LPS (30 µg, Sigma, UK) diluted in PBS was injected bilaterally into the palatal gingiva (3 µL/site) using a 10 µL Hamilton micro-syringe between the upper first and second molars every two days for 10 days (a total 5 injections and 150 µg of LPS in each site). Intra-peritoneal administration of either trans-anethole, DMSO (as a vehicle) or ketoprofen (10 mg/Kg) started 20 minute before LPS injections.
Blood sampling and cytokines measurement
After 10 days, blood samples were collected from the retro-orbital sinus of rats under ketamine/xylasine anesthesia into heparin-coated micro-capillaries, and then animals were sacrificed at the end of the experiment (28). The samples were centrifuged and stored at -70 oC. All blood samplings were performed simultaneously for each group (8:00 to 8:15 AM). IL-1β and TNF-α in serum samples were measured with a rat standard ELISA kit (Abcam, UK) at day 0 (control) and at day 10. All plates were pre-coated with either IL-1β or TNF-α antibody, standards, controls and experimental samples were added. All procedures including washing, adding of antibodies, substrate and stop solutions and analyses were done according to the manufacturer's instructions. The plates were read with a micro-plate reader set to 450 nm.
Statistical analysis
The data were analysed with one way ANOVA followed by the post-hoc Tukey's test. Results are expressed as means ± SEM of five rats. A P value of 0.05 was considered as the limit for statistical significance.
Results
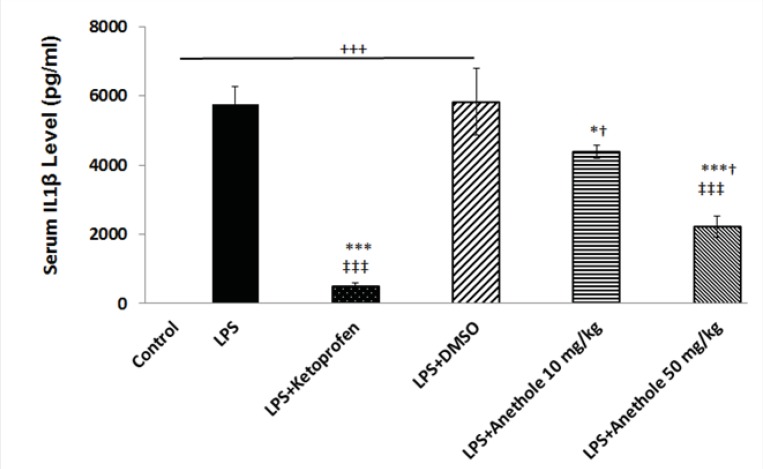
In the present study, it was determined whether trans-anethole, the chief constituent of several essential oils, suppresses cytokine production in a rat model of periodontitis induced by LPS. Therefore, plasma levels of IL-1β and TNF-α were measured in rats receiving either LPS alone or in combination with intra-peritoneal injection of trans-anethole. Then, the results were compared with those that received LPS plus ketoprofen (i.p.), as an anti-inflammatory agent. Administration of LPS into the gingiva between the upper first and second molars significantly increased IL-1β and TNF-α levels (Figures 1 and 2). The plasma level of IL-1β was significantly lower in PD rats receiving concomitant anethole 10 mg/Kg compared to LPS-injected alone group. Although, injection of anethole at 50 mg/Kg led to a further decrease in the IL-1β level, it was still significantly higher than those treated with ketoprofen (Figure 1). Intra-peritoneal injection of 10% DMSO, as the vehicle of anethole, in LPS-treated rats had no effect on theIL1-β level when compared to LPS-treated alone rats (Figure 1).
Figure 1.
Variation of Serum IL1β level in different study groups
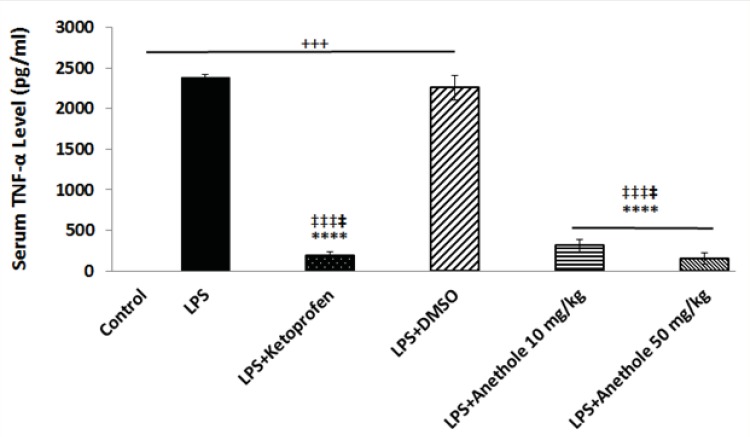
Figure 2.
Changes in serum TNF-α level in different study group in a rat model of LPS-induced periodontitis
Injection of LPS into the gingival between the upper first and second molar induced a significant increase in the plasma level of IL-1β. DMSO, as a vehicle, did not affect the increased level of IL-1β due to LPS treatment. Treatment with ketoprofen, as an antiinflamatory drug and anethole both attenuated the inflammatory response induced by LPS as evidenced by a significant reduction in the IL-1β concentration.
+, *,†, ‡, represents a statistically significant differences compared to the control, LPS, LPS+Keto, LPS+DMSO groups, respectively (by one way ANOVA followed by Tukey's post hoc).
The plasma level of TNF-α was also measured to assess whether trans-anethole treatment was associated with reduce inflammation in LPS-induced periodontitis. LPS injection alone resulted in a significant increase in the concentration of TNF-α (Figure 2). Anethole treatment, at both doses, caused a significant decrease in the TNF-α level and suppressed the inflammatory response induced by LPS, although this effect was more pronounced at dose 50 mg/Kg. The anti-inflammatory effect of anethole was the same as the effect of ketoprofen (Figure 2). Treatment with DMSO did not attenuate the LPS-induced increase in the plasma level of TNF-α.
A significant increase in serum TNF-α level was observed after LPS administration alone into the gingival of the upper first and second molars, when compare to control rats; whereas anethole treatment, both at lower and higher concentrations, reduced significantly the TNF-α level in the plasma of rats receiving also LPS, which was identical to the effect of ketoprofen.
Discussion
Tissue destruction in periodontitis results from the inflammatory response to the microbial products and cytokines including IL-1β and TNF- α. Lipopolysaccharide, a component of the bacterial cell wall is involved in the pathogenesis of several inflammatory diseases as well as periodontitis (29). There are several treatment strategies in order to eliminate or control PD. These include mechanical plaque control, the use of chemicals and herbal drugs (30). The mechanical method is unpleasant and painful and the chemical method, which is based on the systemic antibiotics therapy, is associated with the risk of bacterial resistance. However, natural plant compounds' ability to modulate immune inflammatory response has evoked interest as alternates for many diseases, including periodontitis.
Therefore, natural agents having antimicrobial and anti-inflammatory activities might be able to control the inflammatory diseases such as periodontitis. The flavonoid anethole has been shown to suppress inflammation by inhibiting the cellular responses induced by TNF (27). Anethole has been reported to exert local anesthetic (31), sedative (32), oestrogenic, anti-genotoxic and anti-tumor activities (33) with no or little toxic side effect. As mentioned above, herbal medicine principally has been used as traditional treatments for many human diseases, including infectious diseases (e.g. periodontitis). The natural plant-derived medicines used in folk medicine have been proven to be efficient in treating infections (34). Very recently has been reported that resveratrol, a polyphenolic compound found in several plants, may exert immunomodulatory effects on the host response and decreases periodontal breakdown and modulates local level of cytokines in an animal model of periodontitis (35). It has also been demonstrated that curcumin, a component of tumeric which is used as a dietary spice, exerts a potent anti-inflammatory activity against LPS-induced periodontal disease (36).
In the present the anti-inflammatory effect of trans-anethole was evaluated on a rat model of LPS-induced periodontitis. Findings indicated that anethole particularly at 50 mg/Kg suppressed significantly both IL-1β and TNF-α when compared to LPS-treated rats. The suppressive effects of trans-anethole on LPS-induced pro-inflammatory cytokines (IL-1β and TNF-α) were almost similar as seen with ketoprofen, a nonsteroidal anti-inflammatory drug. It has already been reported that anethole is a potent inhibitor of TNF-α (Chainy et al., 2000). In agreement with our findings, Ponte and colleagues (2012) documented the inhibitory activity of anethole on paw edema induced by TNF-α (12).
Although in the present work the mechanism responsible for the suppressive action of trans- anethole on IL-1β or TNF-α production was not assessed, it has been previously reported that trans- anethole modifies possibly the voltage-gated L-type Ca2+ channel and KCa2+ channels function (22, 37). The blockade of L-type Ca2+ channels has been shown to inhibit the LPS-induced inflammatory molecules production (38). L-type Ca2+ channels are members of voltage gated membrane ion channels which mediate Ca2+ entry in many excitable and non-excitable cells, including immune cells (39). On the other hand, it has been experimentally demonstrated that KCa2+ channel opener and Kv1.3 channel blockers exert anti-inflammatory effects (40).
Besides the effects of anethole in suppressing the inflammatory responses possibly through modulation of ion channel functions, it has been shown that different participation of substance P, bradykinin, histamine and serotonin might be also involved in the anti-inflammatory effect of anethole (27). However, further studies are needed to elucidate the exact mechanism through which anethole exerts its anti-inflammatory effects in periodontitis.
In conclusion, the results of the present investigation suggest that anethole may have a potent inhibitory effect on periodontitis through suppression of pro-inflammatory molecules (i.e. IL-1β and TNF-α); therefore it could be a novel therapeutic strategy for periodontitis. However, further studies are needed using morphometric analysis to define the effect of anethole on bone loss and molecular techniques to investigate the exact mechanism through which anethole exerts its anti-inflammatory actions.
Acknowledgment
The authors would like to thank for the financial support of the research deputy of Hamadan University of Medical Sciences.
References
- 1.Graves DT, Liu R, Alikhani M, Al-Mashat H, Trackman PC. Diabetes-enhanced inflammation and apoptosis impact on periodontal pathology. J. Dent. Res. 2006;85:15–21. doi: 10.1177/154405910608500103. [DOI] [PubMed] [Google Scholar]
- 2.Shrihari TG. Potential correlation between periodontitis and coronary heart disease-an overview. Gen. Dent. 2012;60:20–24. [PubMed] [Google Scholar]
- 3.Carallo C, De Franceschi MS, Tripolino C, Figliuzzi M, Irace C, Fortunato L, Gnasso A. Common carotid and brachial artery hemodynamic alterations in periodontal disease. J. Clin. Periodontol. 2013;40:431–436. doi: 10.1111/jcpe.12099. [DOI] [PubMed] [Google Scholar]
- 4.Kebschull M, Haupt M, Jepsen S, Deschner J, Nickenig G, Werner N. Mobilization of endothelial progenitors by recurrent bacteremias with a periodontal pathogen. PLoS One. 2013;8:54860. doi: 10.1371/journal.pone.0054860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa I. Host responses in periodontal diseases: a preview. Periodontol. 2007;43:9–13. doi: 10.1111/j.1600-0757.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 6.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee H M, Golub LM, Brown DL, Mäntylä P. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 2006;38:306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 7.Mahanonda R, Pichyangkul S. Toll-like receptors and their role in periodontal health and disease. Periodontol. 2007;43:41–55. doi: 10.1111/j.1600-0757.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 8.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 9.Prabu GR, Gnanamani A, Sadulla S. Guaijaverin-a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006;101:487–495. doi: 10.1111/j.1365-2672.2006.02912.x. [DOI] [PubMed] [Google Scholar]
- 10.Rishton GM. Natural products as a robust source of new drugs and drug leads: past successes and present day issues. Am. J. Cardiol. 2008;101:43–49. doi: 10.1016/j.amjcard.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid-Based Compl. Alt. 2011;2011:15. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponte EL, Sousa PL, Rocha MV, Soares PM, Coelho-de-Souza AN, Leal-Cardoso JH, Assreuy AM. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol. Rep. 2012;64:984–990. doi: 10.1016/s1734-1140(12)70895-2. [DOI] [PubMed] [Google Scholar]
- 13.De Las Heras B, Hortelano S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy Drug Targets. 2009;8:28–39. doi: 10.2174/187152809787582534. [DOI] [PubMed] [Google Scholar]
- 14.Juergens UR, StöberM , Vetter H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in-vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur. J. Med.Res. 1998;3:539–545. [PubMed] [Google Scholar]
- 15.Huxley A. The New RHS Dictionary of Gardening. 4nd ed. United Kingdom: 1992. p. 3000. Paper and slipcase edition. [Google Scholar]
- 16.Smith R L, Adams TB, Doull J, Feron VJ, Goodman JI, Marnett LJ, Portoghese PS, Waddell WJ, Wagner BM, Rogers AE, Caldwell J, Sipes IG. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances – methyl eugenol and estragole. Food Chem. Toxicol. 2002;40:851–870. doi: 10.1016/s0278-6915(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 17.Freire RS, Morais SM, Catunda-Junior FEA, Pinheiro DCN. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorg. Med. Chem. 2005;13:4353–4358. doi: 10.1016/j.bmc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 18.Yea SS, Jeong HS, Choi CY, Park KR, Oh S, Shin JG, Yun CH. Inhibitory effect of anethole on T-lymphocyte proliferation and interleukin-2 production through down-regulation of the NF-AT and AP-1. Toxicol. In-vitro. 2006;20:1098–1105. doi: 10.1016/j.tiv.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Choo EJ, RheeY H, Jeong SJ, Lee HJ, Kim HS, Ko HS, Kim JH, Kwon TR, Jung JH, Kim JH, Lee HJ, Lee EO, Kim DK, Chen CY, Kim SH. Anethole exerts antimetatstaic activity via inhibition of matrix metalloproteinase 2/9 and AKT/mitogen-activated kinase/nuclear factor kappa B signaling pathways. Biol.Pharm. Bull. 2011;34:41–46. doi: 10.1248/bpb.34.41. [DOI] [PubMed] [Google Scholar]
- 20.Astani A, Reichling R, Schnitzler P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Compl. Alternat Med. 2011;2011:253643. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domiciano TP, DalalioM M, Silva EL, Ritter AM, Estevão-Silva CF, RamosFS , Caparroz-Assef SM, Cuman RK, Bersani-Amado CA. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:331–338. doi: 10.1007/s00210-012-0820-5. [DOI] [PubMed] [Google Scholar]
- 22.Ghasemi Z, Hassanpour-Ezatti M, KamalinejadM , Janahmadi M. Functional involvement of Ca(2+) and Ca(2+)-activated K(+) channels in anethol-induced changes in Ca(2+) dependent excitability of F1 neurons in Helix aspersa. Fitoterapia. 2011;82:750–756. doi: 10.1016/j.fitote.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Jonarta AL, Asmara W, Astuti I, Tandelilin RTC. Systemic IL-1β and TNF-α productions of E coli lipopolysaccharide-induced periodontitis model on rats. Indonesian J. Dent. Res. 2010;1:1–6. [Google Scholar]
- 24.Dong XQ, Du Q, Yu WH, Zhang ZY, Zhu Q, Che ZH, Chen F, Wang H, Chen J. Anti-inflammatory Effects of Oxymatrine Through Inhibition of Nuclear Factor-kappa B and Mitogen-activated Protein Kinase Activation in Lipopolysaccharide-induced BV2 Microglia Cells. Iran. J. Pharm. Res. 2013;12:165–174. [PMC free article] [PubMed] [Google Scholar]
- 25.Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G. A reduced mathematical model of the acute inflammatory response II Capturing scenarios of repeated endotoxin administration. J. Theor. Biol. 2006;242:237–256. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Noa M, Más R, Valle M, Mendoza S, Mendoza N. Effect of D-003, a Mixture of High Molecular Weight Aliphatic Acids, on Glucocorticoid- and Lipopolysaccharides (LPS)-Induced Osteonecrosis. Iran. J.Pharm. Res. 2012;11:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- 27.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-κB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol. Scand. Suppl. 1986;554:221–233. [PubMed] [Google Scholar]
- 29.Wendell KJ, Stein SH. Effect of Lipopolysaccharide and Inflammatory Cytokines on Interleukin-6 Production by Healthy Human Gingival Fibroblasts. J. Periodontol. 2001;72:1038–1044. doi: 10.1902/jop.2001.72.8.1038. [DOI] [PubMed] [Google Scholar]
- 30.Bansal S, Rastogi S, Meenakshi Bajpai M. Mechanical, chemical and herbal aspects of periodontitis. IJPSR. 2012;3:1260–1267. [Google Scholar]
- 31.GhelardiniC , Galeotti N, MazzantiG Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta. Med. 2001;67:564–566. doi: 10.1055/s-2001-16475. [DOI] [PubMed] [Google Scholar]
- 32.Dallmeier K, Carlini E. Anesthetic, hypothermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogues. Pharmacol. 1981;22:113–127. doi: 10.1159/000137479. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal BB, Prasad S, Reuter S, Kannappan R, Yadev VR, Park B, Kim JH, Gupta SC, Phromnoi K, Sundaram C, Prasad S, Chaturvedi MM, Sung B. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: reverse pharmacology and bedside to bench approach. Curr. Drug Targets. 2011;12:1595–1653. doi: 10.2174/138945011798109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casati MZ, Algayer C, Cardoso da Cruz G, Ribeiro FV, Casarin RC, Pimentel SP, Cirano FR. Resveratrol decreases periodontal breakdown and modulate local levels of cytokines during periodontitis in rats. J. Periodontol. 2013;84:58–64. doi: 10.1902/jop.2013.120746. [DOI] [PubMed] [Google Scholar]
- 36.Guimarães MR, De Aquino SG, Coimbra LS, Spolidorio LC, Kirkwood KL, Rossa C JR. Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 2012;18:155–163. doi: 10.1177/1753425910392935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares PM, Lima RF, De Freitas Pires A, Souza EP, Assreuy AM, Criddle DN. Effects of anethole and structural analogues on the contractility of rat isolated aorta: Involvement of voltage-dependent Ca2+-channels. Life Sci. 2007;81:1085–1093. doi: 10.1016/j.lfs.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 38.Lin CY, Tsai PS, Hung YC, Huang CJ. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br. J. Anaesth. 2010;104:44–51. doi: 10.1093/bja/aep336. [DOI] [PubMed] [Google Scholar]
- 39.Azenabor AA, Chaudhry AU. Effective macrophage redox defense against Chlamydia pneumoniae depends on L-type Ca2+ channel activation. Med. Microbiol. Immunol. 2003;192:99–106. doi: 10.1007/s00430-002-0164-8. [DOI] [PubMed] [Google Scholar]
- 40.Tanhehco EJ. Potassium channel modulators as anti-inflammatory agents. Expert. Opin. Ther. Patents. 2001;11:1137–1145. [Google Scholar]