Abstract
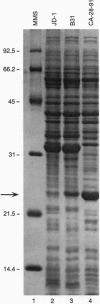
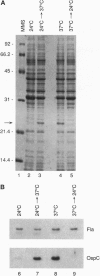
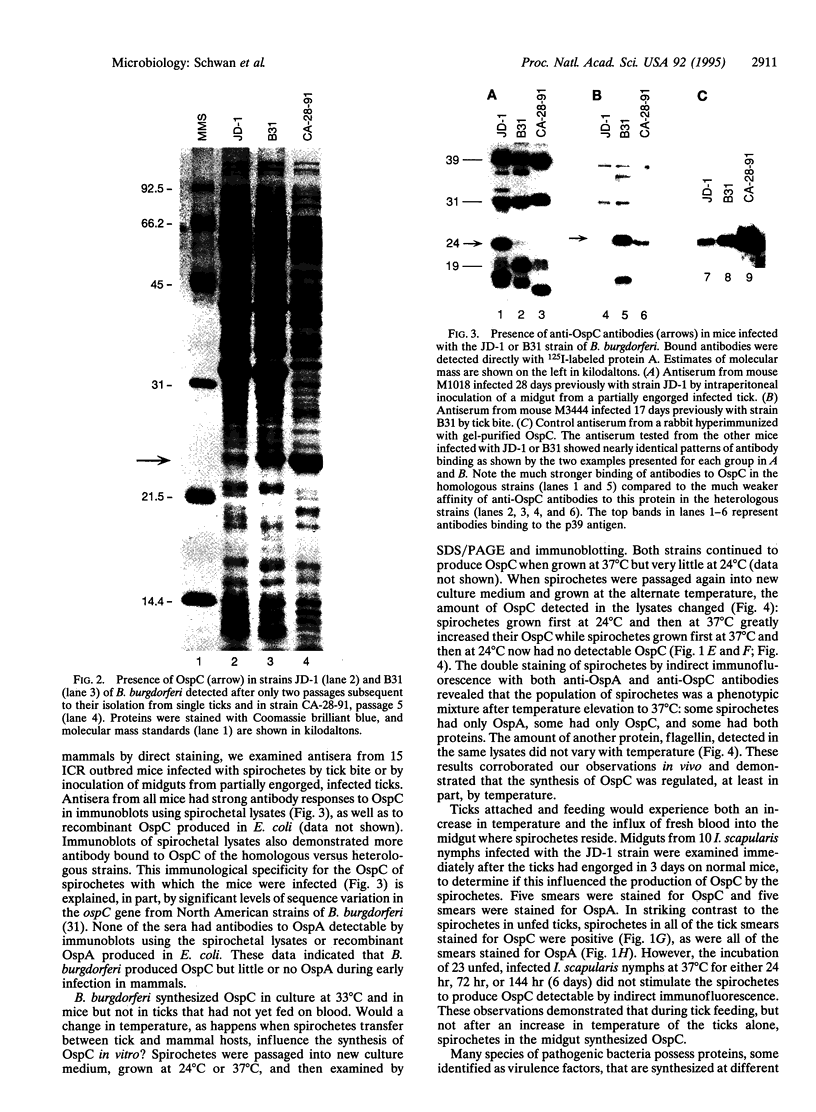
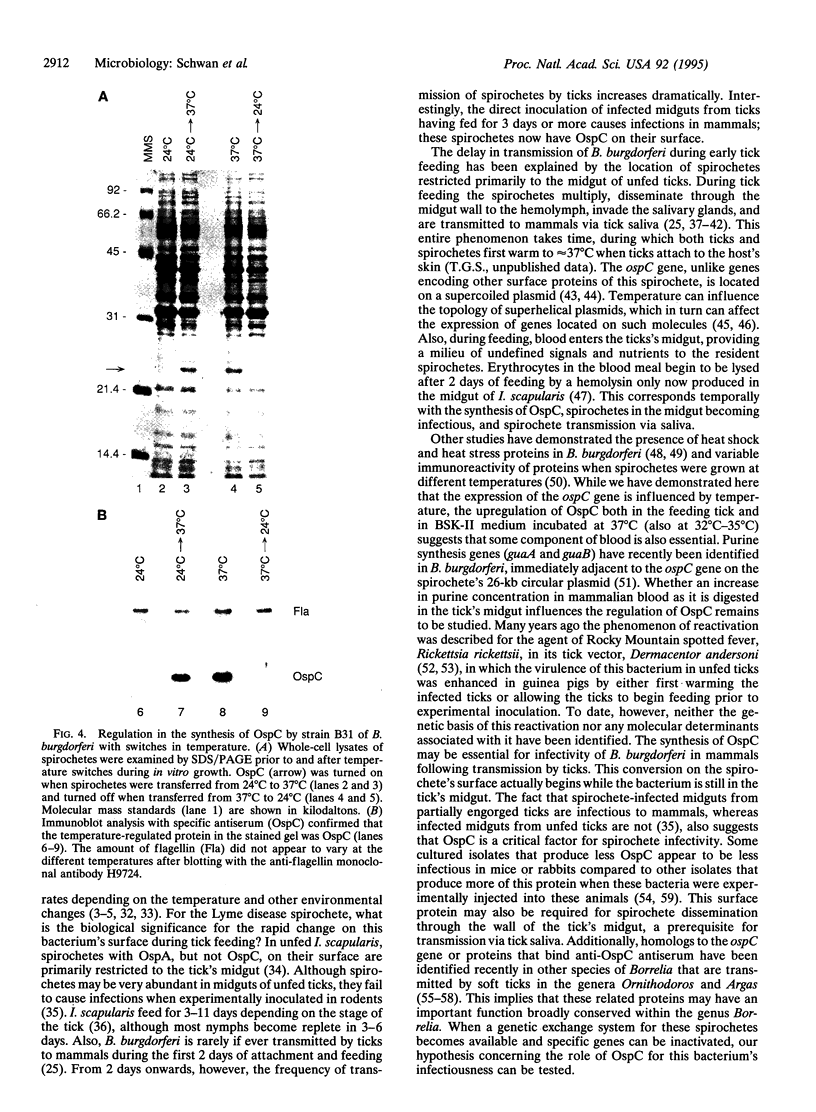
Lyme disease spirochetes, Borrelia burgdorferi sensu lato, are maintained in zoonotic cycles involving ticks and small mammals. In unfed ticks, the spirochetes produce one outer surface protein, OspA, but not OspC. During infection in mammals, immunological data suggest that the spirochetes have changed their surface, now expressing OspC but little or no OspA. We find by in vitro growth experiments that this change is regulated in part by temperature; OspC is produced by spirochetes at 32-37 degrees C but not at 24 degrees C. Furthermore, spirochetes in the midgut of ticks that have fully engorged on mice now have OspC on their surface. Thus two environmental cues, an increase in temperature and tick feeding, trigger a major alteration of the spirochetal outer membrane. This rapid synthesis of OspC by spirochetes during tick feeding may play an essential role in the capacity of these bacteria to successfully infect mammalian hosts, including humans, when transmitted by ticks.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Skinner R. A., Bosler E. M. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J Infect Dis. 1987 Jun;155(6):1300–1306. doi: 10.1093/infdis/155.6.1300. [DOI] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Hayes S. F., Benach J. L. Development of Borrelia burgdorferi in ixodid tick vectors. Ann N Y Acad Sci. 1988;539:172–179. doi: 10.1111/j.1749-6632.1988.tb31851.x. [DOI] [PubMed] [Google Scholar]
- Carreiro M. M., Laux D. C., Nelson D. R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990 Jul;58(7):2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. J., Bergström S., Norris S. J., Barbour A. G. A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect Immun. 1994 Jul;62(7):2792–2799. doi: 10.1128/iai.62.7.2792-2799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluss R. G., Boothby J. T. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect Immun. 1990 Apr;58(4):1038–1042. doi: 10.1128/iai.58.4.1038-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J. DNA supercoiling and environmental regulation of gene expression in pathogenic bacteria. Infect Immun. 1991 Mar;59(3):745–749. doi: 10.1128/iai.59.3.745-749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. P., McHugh G. L., Leong J. M., Steere A. C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994 Aug;62(8):3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde W. T., Kappel K. J., Dequesne G., Feron C., Plainchamp D., Capiau C., Lobet Y. Tick transmission of Borrelia burgdorferi to inbred strains of mice induces an antibody response to P39 but not to outer surface protein A. Infect Immun. 1994 Jun;62(6):2625–2627. doi: 10.1128/iai.62.6.2625-2627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe N. P., Minion F. C., Goguen J. D. Temperature sensing in Yersinia pestis: regulation of yopE transcription by lcrF. J Bacteriol. 1992 Jul;174(13):4275–4286. doi: 10.1128/jb.174.13.4275-4286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromockyj A. E., Tucker S. C., Maurelli A. T. Temperature regulation of Shigella virulence: identification of the repressor gene virR, an analogue of hns, and partial complementation by tyrosyl transfer RNA (tRNA1(Tyr)). Mol Microbiol. 1992 Aug;6(15):2113–2124. doi: 10.1111/j.1365-2958.1992.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Kalish R. A., Leong J. M., Steere A. C. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993 Jul;61(7):2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky W. L. Development of the tick Ixodes dammini (Acarina: Ixodidae) in the laboratory. J Med Entomol. 1979 Nov 7;16(4):354–355. doi: 10.1093/jmedent/16.4.354. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam T. T., Nguyen T. P., Montgomery R. R., Kantor F. S., Fikrig E., Flavell R. A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994 Jan;62(1):290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. S., Brown R. N., Piesman J., Peavey C. A. Vector competence of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) for various isolates of Lyme disease spirochetes. J Med Entomol. 1994 May;31(3):417–424. doi: 10.1093/jmedent/31.3.417. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Piesman J., Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- Marconi R. T., Samuels D. S., Garon C. F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993 Feb;175(4):926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Samuels D. S., Schwan T. G., Garon C. F. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993 Oct;31(10):2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis N., Hogan D., Cieplak W., Jr, Schwan T. G., Rosa P. A. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994 May 27;143(1):105–110. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]
- Margolis N., Hogan D., Cieplak W., Jr, Schwan T. G., Rosa P. A. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994 May 27;143(1):105–110. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]
- Margolis N., Hogan D., Tilly K., Rosa P. A. Plasmid location of Borrelia purine biosynthesis gene homologs. J Bacteriol. 1994 Nov;176(21):6427–6432. doi: 10.1128/jb.176.21.6427-6432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis N., Rosa P. A. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa T., Kurita T., Kawabata H., Yanagihara Y. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol Lett. 1994 Nov 1;123(3):319–324. doi: 10.1111/j.1574-6968.1994.tb07242.x. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Norris S. J., Carter C. J., Howell J. K., Barbour A. G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992 Nov;60(11):4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula S. J., Sampieri A., Dias F., Szczepanski A., Ryan R. W. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993 Dec;61(12):5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993 May;167(5):1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- Piesman J., Mather T. N., Sinsky R. J., Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987 Mar;25(3):557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J., Maupin G. O., Campos E. G., Happ C. M. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J Infect Dis. 1991 Apr;163(4):895–897. doi: 10.1093/infdis/163.4.895. [DOI] [PubMed] [Google Scholar]
- Piesman J., Oliver J. R., Sinsky R. J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg. 1990 Apr;42(4):352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- Public Health Weekly Reports for FEBRUARY 23, 1923. Public Health Rep. 1923 Feb 23;38(8):333–381. [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. M., Mather T. N., Piesman J., Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J Med Entomol. 1987 Mar;24(2):201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M. The midgut hemolysin of Ixodes dammini (Acari:Ixodidae). J Parasitol. 1988 Aug;74(4):532–537. [PubMed] [Google Scholar]
- Rohde J. R., Fox J. M., Minnich S. A. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol Microbiol. 1994 Apr;12(2):187–199. doi: 10.1111/j.1365-2958.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Wilske B., Ferdows M. S., Barbour A. G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993 May;61(5):2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Kime K. K., Schrumpf M. E., Coe J. E., Simpson W. J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect Immun. 1989 Nov;57(11):3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Schrumpf M. E., Karstens R. H., Clover J. R., Wong J., Daugherty M., Struthers M., Rosa P. A. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol. 1993 Dec;31(12):3096–3108. doi: 10.1128/jcm.31.12.3096-3108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J. Factors influencing the antigenic reactivity of Borrelia burgdorferi, the Lyme disease spirochete. Scand J Infect Dis Suppl. 1991;77:94–101. [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Simon M. M., Hofmann H., Moter S. E., Schaible U. E., Kramer M. D. Molecular and immunological characterization of a novel polymorphic lipoprotein of Borrelia burgdorferi. Infect Immun. 1993 Oct;61(10):4158–4166. doi: 10.1128/iai.61.10.4158-4166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Jauris S., Hofmann A., Pradel I., Soutschek E., Schwab E., Will G., Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- Wéry M. Les lents progrès du contrôle de la maladie du sommeil. Ann Parasitol Hum Comp. 1990;65 (Suppl 1):89–93. doi: 10.1051/parasite/1990651089. [DOI] [PubMed] [Google Scholar]