Abstract
Bordered pits play an important role in permitting water flow among adjacent tracheary elements in flowering plants. Variation in the bordered pit structure is suggested to be adaptive in optimally balancing the conflict between hydraulic efficiency (conductivity) and safety from air entry at the pit membrane (air seeding). The possible function of vestured pits, which are bordered pits with protuberances from the secondary cell wall of the pit chamber, could be increased hydraulic resistance or minimized vulnerability to air seeding. These functional hypotheses have to be harmonized with the notion that the vestured or nonvestured nature of pits contains strong phylogenetic signals (i.e., often characterize large species-rich clades with broad ecological ranges). A literature survey of 11,843 species covering 6,428 genera from diverse climates indicates that the incidence of vestured pits considerably decreases from tropics to tundra. The highest frequencies of vestured pits occur in deserts and tropical seasonal woodlands. Moreover, a distinctly developed network of branched vestures is mainly restricted to warm habitats in both mesic and dry (sub)tropical lowlands, whereas vestures in woody plants from cold and boreal arctic environments are usually minute and simple. A similar survey of the frequency of exclusively scalariform perforation plates illustrates that the major ecological trend of this feature is opposite that of vestured pits. These findings provide previously undescribed insights suggesting that vessels with vestured pits and simple perforation plates function as an efficient hydraulic system in plants growing in warm environments with periodical or continuous drought stress.
Botanists have long speculated on the mutual relationship between the structure and function of different wood anatomical features and how ecological conditions correlate with xylem features (1–4). The evolution of xylem vessels has been considered a key adaptation marking the pinnacle of hydraulic efficiency in flowering plants, because plants with vessels generally have higher hydraulic conductivities than plants that rely solely on tracheids for water transport (5). The traditional view is that evolutionary trends of vessel elements have been regarded as reliable tools in the study of angiosperm phylogeny, because vessel characters were considered conservative traits containing a wealth of potentially significant systematic information (2, 6, 7). Although parallel development of vessel characters is generally accepted, recent phylogenetic analyses of angiosperms suggest there have also been some reversals in the Baileyan transformation series (8, 9). Therefore, parallel evolution and reversibility in vessel characters imply a strong adaptive significance of xylem hydraulic architecture.
The structure of bordered intervessel pits, which are small openings where the secondary cell wall was not deposited over the primary wall (Figs. 1 and 2 A), permits water flow between adjacent vessels, but pits are also a weak link in protecting the vessel network against air leakage into the transpiration stream (10–14). The thin compound middle lamella and adjacent primary cell walls are modified to form a relatively porous pit membrane. The secondary walls overarching the pit chamber form a pit aperture between the inner vessel wall and the roof of the pit chamber. Among woody angiosperms, there is a great structural variety associated with vessel pits, especially with respect to pit size, shape, depth of the pit chamber, pit-field arrangement, pit membrane, and presence or absence of vestures. Vestured pits are defined as bordered pits with the pit chamber or outer pit aperture wholly or partly lined with projections from the secondary cell wall (Figs. 1 and 2B) (15, 16). Although vestures had already been observed by wood anatomists in the 19th century, the feature was correctly interpreted for the first time by Bailey in 1933 (16). The systematic distribution of vestured pits in angiosperms leads us to the conclusion that this character represents a conservative feature that characterizes several monophyletic groups (e.g., Myrtales, Gentianales) and contains strong phylogenetic signals at relatively high taxonomic levels (17). Despite the long period of time that wood anatomists have known about vestured pits, it is still unclear how vestures may affect pit function.
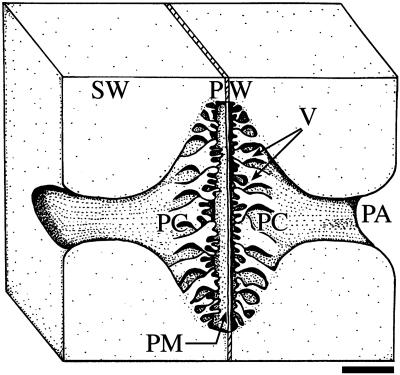
Fig. 1.
Diagram of a vestured pit pair in Flabellaria paniculata Cav. (Malpighiaceae) with overarching secondary cell wall (SW), vestures (V), pit chamber (PC), pit aperture (PA), pit membrane (PM), and primary cell wall + middle lamella (PW). (Bar = 2 μm.)
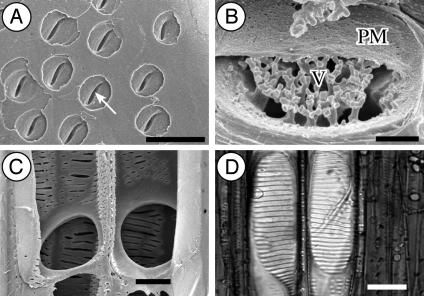
Fig. 2.
Scanning electron micrographs (A–C) and light microscopic picture (D). (A) Nonvestured vessel pits with slit-like outer pit apertures (arrow) in Quiina indigofera Sandwith (Quiinaceae). (B) Detail of a pit chamber with branched vestures (V) pointing toward the pit membrane (PM) in F. paniculata Cav. (Malpighiaceae). (C) Two simple perforation plates in Parathesis chiapensis Fernald (Myrsinaceae). (D) Two scalariform perforation plates with numerous bars in Abelia spathulata Siebold & Zucc. (Linnaeaceae). [Bars = 5 μm(A), 1 μm(B), and 20 μm(C and D).]
Unlike pits, vessel perforation plates are openings in the end wall of a vessel element where the primary cell walls and middle lamella have been hydrolyzed, allowing water flow between neighboring vessel elements. Simple perforation plates have a single opening (Fig. 2C), whereas scalariform perforation plates contain several elongated perforations with ladder-like bars between them (Fig. 2D). The long-held view that scalariform perforations are more primitive than simple perforation plates is supported by recent angiosperm phylogenies, although homoplasy suggests their adaptive function in some environments (8, 18). Woody floras from the cool alpine to arctic regions show a very high incidence of scalariform vessel perforations, whereas dry and/or warm climates with a seasonal or permanent demand for greater hydraulic efficiency are characterized by a predominance of exclusively simple perforations (2, 3, 19, 20). Micromorphological variation in vessel pits may also be the result of ecological adaptations, constrained by phylogeny, because differences in pit characteristics are suggested to be adaptive in optimally balancing trade-offs among conductive efficiency, mechanical strength, and resistance to cavitation (2). However, little attention has been paid to the ecological and functional significance of pit characters (21). As a consequence, there is abundant evidence of ecological trends in vessel perforation plates, but major ecological trends in vestured pits are unclear.
This paper aims to explore critically to what extent an adaptive interpretation is applicable to vestured pits in woody floras. The ecological distribution of vestured pits is examined by comparing their frequency in woody genera and species from diverse macroclimatic regions of the world. In addition, data on the frequency of genera with exclusively scalariform perforation plates for similar regional or floristic areas allow us to investigate the relationship between both features in detail.
Materials and Methods
We surveyed the literature to determine the incidence of vestured pits in a total number of 11,843 woody species and 6,428 genera. A list of all floras examined is published as supporting information on the PNAS web site. Trees, shrubs, dwarf shrubs, and woody climbers were classified into broad ecological categories according to seven macroclimatic conditions. Vesselless genera, woody monocots, and herbs were not included. We recognized the following macroclimatic categories: (i) seasonal (deciduous) tropical woodlands (savannas); (ii) warm and cold deserts; (iii) everwet tropical lowlands (rainforests); (iv) subtropical to warm temperate regions (everwet subtropical rainforests, subtropical savannas with a wet summer, and subtropical areas with a wet winter); (v) tropical, submontane, and montane areas with altitudinal ranges from 1,100 to 2,000 m and above 2,000 m, respectively; (vi) cool temperate areas with cold, wet, or mild winters; and (vii) boreal-arctic localities with severe long winters. We are aware that these categories intergrade. However, it was sometimes not possible to split a national or regional flora into different categories, because many individual species and certainly genera occurred over a wide ecological range, including two or more of the macroclimatic categories distinguished here. Also, difficulties in analyzing floristic accounts of specific vegetation types or regional floras could be caused by inaccurate literature data, misidentified taxa, changed names, or anatomically unknown species. Because the percentages of genera and species in a particular geographic or ecological area could conceivably be influenced by certain families having much higher rates of speciation than other families in specific regions, the surveys of regional and floristic checklists were based on as many woody taxa as possible, but shrubs and lianas were probably underrecorded. The total number of genera and species that were surveyed for vestured pits was as follows: (i) 1,089 genera and 1,003 species from tropical seasonal woodlands; (ii) 458 genera and 784 species from deserts; (iii) 2,850 genera and 7,068 species from tropical lowlands (rainforest); (iv) 599 species and 569 genera from subtropical warm temperate areas; (v) 503 genera and 671 species from tropical (sub)montane regions; (vi) 752 genera and 1,340 species from cool-temperate climates; and (vii) 177 genera and 399 species from boreal arctic areas.
To compare data on vestured pits with the incidence of the type of perforation plate, we determined the frequency of exclusively scalariform perforation plates in a total number of 4,370 genera from all macroclimatic categories recognized. The genera surveyed for quantifying percentages of scalariform perforation plates were nearly always similar to the genera analyzed for the incidence of vestured pits. The frequency of scalariform perforation plates in tropical everwet rainforests, however, was based on a much lower number of woody genera (n = 918) than the number of woody genera incorporated for determining the incidence of vestured pits (n = 2,850). Also, we did not investigate the frequency of scalariform perforation plates at the species level.
Although not more than 30% of the taxa investigated were wood anatomically documented in the literature, we did not examine the wood anatomy of each species or genus but confidently could assume the presence or absence of these two wood anatomical features based on literature. In most genera and many families studied extensively, the presence or absence of vestured pits and exclusively scalariform perforation plates is highly diagnostic and constant in all species belonging to these higher taxonomic categories. With respect to vestured pits, we followed the occurrence as summarized in a previous paper (17). The distribution of exclusively scalariform perforation plates was based on literature surveys (2, 19, 22). We did not include percentages of genera with mixed perforation plates, because scalariform plates can be rare, abundant, or absent in different species within a genus that is recorded to have both simple and scalariform perforations.
Results
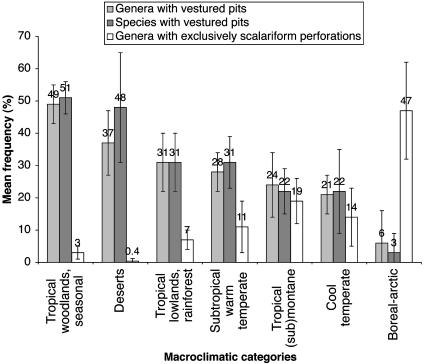
The distribution of species and genera with vestured pits and exclusively scalariform perforation plates in different vegetation types is summarized in Fig. 3. The average percentage of vestured pits for all 6,428 genera and 11,834 species analyzed is 32%. The highest percentages of vestured pits are found in warm areas, especially in deserts and seasonal woodlands of the tropics. The data suggest that there is a considerably higher incidence of vestured pits in tropical lowland areas that are seasonal woodlands (≈50%) compared to everwet rainforests (31%). Also, variance in the frequency of vestured pits seems to be wider in deserts and tropical rainforests than in tropical seasonal low-lands, where vestured pits appear consistently common in ≈50% of all plant species and genera. Mean percentages of vestured pits in tropical rainforests are similar to the frequencies found for subtropical warm temperate areas.
Fig. 3.
The frequency of vestured pits and exclusively scalariform perforation plates in woody angiosperms as extrapolated from literature surveys; bars represent mean percentages (±SD) of woody genera and species with vestured pits and woody genera with exclusively scalariform perforation plates according to different macroclimatic categories.
Relatively low percentages of vestured pits are found in tropical submontane to montane areas. The average vestured pit frequencies in these areas (species, 22%; genera, 24%) are close to the mean percentages of cool, temperate environments (species, 22%; genera, 21%). In addition, there is a clear reduction of vestured pits with increasing altitude in tropical mountains. For instance, of all woody species from two (sub-) montane rain forests in Malawi (23), 19% (n = 93) is assumed to show vestured pits in the lowest rainforest (1,370–2,290 m), whereas 6% (n = 46) is found in the forest with higher altitudinal ranges (1,675–2,590 m). Similar differences are found for bush-veld (320 m) and afromontane (1,400 m) species from Swaziland (24), where respectively 46% (n = 23) and 25% (n = 11) of all woody species have vestured pits.
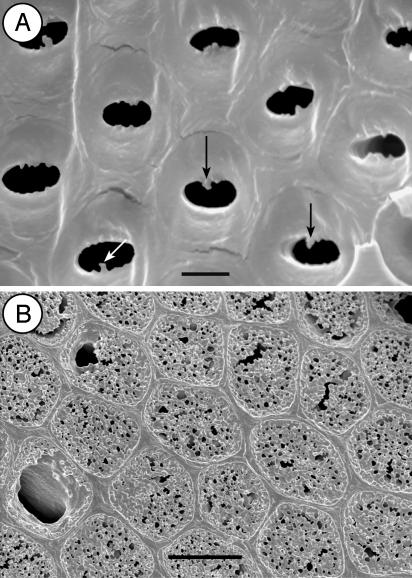
The lowest frequencies of vestured pits are found in boreal and arctic regions. Vestured pits are almost entirely lacking in the relatively low number of woody plants from these areas. A relatively high incidence of vestured pits (species, 18%; genera, 27%) is found in the woody plants from Fireland (the southern end of Argentina) because of the occurrence of single species of Myrteola (Myrtaceae), Tepualia (Myrtaceae), Fuchsia (Onagraceae), and Ulex (Fabaceae) (25). Besides these genera and a few other woody Fabaceae, vestured pits in cold areas with a severe and long period of frost are mainly restricted to Elaeagnus (Elaeagnaceae), Shepherdia (Elaeagnaceae), some Thymelaeaceae, and some species of Olea (Oleaceae) and Spiraea (Rosaceae). Except for Myrtaceae and Fabaceae, the vestures in representatives of Elaeagnaceae, Oleaceae, Rosaceae, and Thymelaeaceae are poorly developed (Fig. 4A). They may even be restricted to the bordered pits of tracheids or narrow vessel elements, whereas the wide vessel elements show nonvestured pits (17, 26, 27). In contrast, vestures in tropical plant families are consistently present in pits of all vessel elements and frequently form a large complex network of branched vestures occluding the pit chambers (Fig. 4B).
Fig. 4.
SEM images of vestured vessel pits. (A) Vessel pits in Fraxinus americana L. (Oleaceae) showing weakly developed vestures (arrows) near the outer pit aperture of a narrow vessel element. (B) Vessel pits in Pisonia cuspidata Heimerl (Nyctaginaceae) showing a complex network of branched vestures, almost completely occluding the outer pit aperture. [Bars = 2 μm(A) and 5 μm(B).]
Of 505 woody species from Europe (20), 125 species show vestured pits. The absolute percentages of vestured pits for each major macroclimatic category are: 0% in boreal species, 8% in montane species, 21% in temperate species, and up to 31% in Mediterranean species (including Madeira). Moreover, 75% of all European species with vestured pits are from the Mediterranean area, 21% from temperate areas, and only 4% occur in montane localities. According to moisture availability, 67% of all 125 species with vestured pits occur in dry areas, 26% in intermediate (normal) sites, and 6% is found in relatively mesic localities. Of all 505 woody species studied, percentages of species with vestured pits represent 34% in dry environments, 21% in intermediate sites, and 8% in mesic areas.
In most of the macroclimatic categories distinguished, the vestured pit frequencies obtained for genera correspond very well with the percentages of species with vestured pits. Although in tropical warm to cool temperate environments the averages of vestured pits appear lower for genera than for species, the reverse seems to hold true for tropical (sub)montane floras and especially boreal, arctic areas. A paired t test, however, shows that the differences between genera and species are not statistically significant (P = 0.262).
Data on the distribution of exclusively scalariform perforation plates confirm that the incidence of this feature gradually increases from deserts to tropical, subtropical, cool temperate, and boreal-arctic climates (Fig. 3). The feature is almost completely lacking in deserts but very common in boreal-arctic areas. Relatively high percentages of exclusively scalariform perforation plates are characteristic of woody plants in tropical montane regions (19%), indicating that the distribution of this feature increases with increasing altitude.
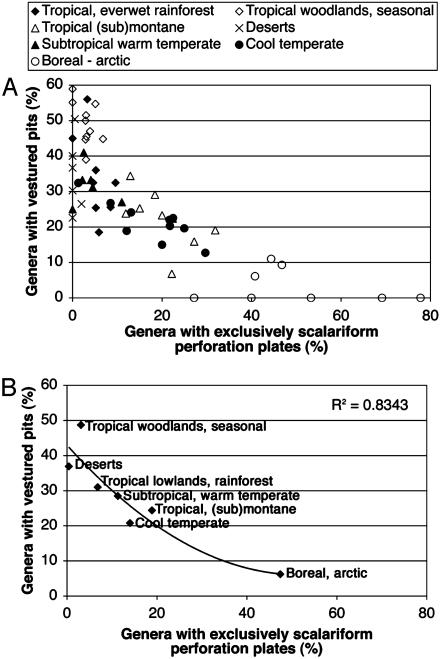
There is a strong negative correlation between vestured pits and exclusively scalariform perforation plates (Fig. 5 A and B). The r2 value of the second-order polynomial trendline is 0.69 for all floristic and regional checklists surveyed (trendline not shown in Fig. 5A). A stronger correlation is found when plotting the mean values of vestured pits and exclusively scalariform perforation plates for the seven macroclimatic areas recognized, with r2 = 0.83 for a second-order polynomial trendline (Fig. 5B).
Fig. 5.
The correlation between vestured pits and exclusively scalariform perforation plates. (A) Scatter plot of all floristic or regional checklists examined according to the frequency of genera with exclusively scalariform perforation plates and vestured pits; each print represents a floristic or regional checklist of woody angiosperms. (B) Scatter plot of the mean percentages of exclusively scalariform perforation plates and vestured pits for the seven macroclimatic areas recognized, with a second-order polynomial trendline.
Discussion
Our results indicate that the mean percentages of vestured pits show a significant decrease from tropical seasonal woodlands and deserts to tropical rainforests, subtropical areas, tropical mountains, cool temperate zones, and boreal-arctic regions. Despite their conservative nature and strong phylogenetic significance (17), vestured pits appear more common in warm environments with continuous or periodical drought (deserts and tropical seasonal woodlands) than in mesic areas and cold climates. There is a strongly negative correlation between vestured pits and exclusively scalariform vessel perforation plates, which indicates that the major ecological trend of vestured pits is opposite that of exclusively scalariform perforation plates. Nevertheless, we found considerable variation for each biome, and the percentages summarized in Fig. 1 should be interpreted with caution. For some localities, the variation in vestured pit frequencies could be explained by the low number of taxa on which some percentages are based. Because there are more species in temperate areas than in boreal regions, low numbers of woody genera are obvious for the boreal-arctic category, but it is clear that the presence of vestured pits is restricted to a few genera in this biome. The taxonomic impact may also play a certain role when calculations are based on a low number of taxa. Hence, high rates of speciation in Rubiaceae, Melastomataceae, Myrtaceae, and Fabaceae, which are all characterized by vestured pits, could exaggerate differences among various localities. The same may also explain differences between genera and species as found, for instance, in woody plants from the Sahara and Sahel (species, 56%; genera, 40%) (28), a savanna area in Belize (species, 38%; genera, 30%) (29) and the moist deciduous forests of Dangs in India (species, 46%; genera, 39%) (30). Because percentages of species frequently appear higher than percentages of genera, this may indicate that clades with vestured pits are more successful in terms of speciation than nonvestured pits. This would especially apply to tropical warm areas, whereas the reverse appears to hold true for colder floras. However, despite a clear trend supporting this hypothesis, no statistically significant differences can be provided based on our data.
It should be emphasized that major differences in vestured pits occur within the tropics. The vestured pit percentages are ≈20% higher in seasonal woodlands (≈50%) than in everwet tropical rainforests (31%). Among the 78 tree species recorded in two forest communities of the cerrado region of central Brazil (31), 45 (58%) possess vestured pits. These 45 species account for 53% of the total importance value (=relative frequency + relative density + relative dominance) of the two forest communities. Hence, seasonality in tropical lowlands seems to result in a higher proportion of taxa with vestured pits as well as a reduction of scalariform perforation plates. On the other hand, relatively low proportions of plants with vestured pits occur in tropical montane areas, with percentages of vestured pits in these areas relatively close to those for temperate areas. This trend also parallels the trend in exclusively scalariform perforation plates and may reflect similar average temperatures in these two areas.
The results of this study illustrate that plants with distinctly developed vestured pits are mainly restricted to warm frost-free habitats, such as deserts, seasonal lowlands (savannas), lowland rainforests, or subtropical habitats (e.g., Mediterranean areas) (Fig. 3). Plants in these environments are likely to be subject to high transpiration rates and high negative xylem pressures (32, 33). Drought-induced cavitation has been related to the porosity of pit membranes. When an embolized vessel lies adjacent to a functional vessel under pressure, a substantial pressure difference can develop across a pit membrane that connects these vessels. Gas will penetrate the pit membrane at a critical pressure difference (i.e., the air-seeding pressure), which may lead to cavitation of the previously water-filled vessel (34). Vestures have been suggested to play a functional role in preventing pit membrane rupture by providing mechanical support of the pit membrane and could thereby increase resistance to drought-induced embolism. By affecting pit membrane porosity due to deflection and stretching, vestures may act to reduce the probability of air seeding and decrease the vulnerability to water-stress-induced embolism by preventing or minimizing pit membrane deformation (35–37). Likewise, vestures would permit a greater air-seeding pressure for the same membrane conductivity vs. a nonvestured pit and could prevent “cavitation fatigue,” i.e., a considerable reduction in cavitation resistance after a cavitation-refilling cycle (38). Besides mechanical properties of the pit membrane itself, the degree of stretching and deflection that a pit membrane undergoes will also depend on the depth of the pit chamber and the radius of the outer pit aperture (37, 39, 40). However, these ideas require experimental evidence, because the properties of the pit membrane in vestured and nonvestured pits remain largely unknown. The hypotheses that vestures increase hydraulic resistance or aid in the dissolution of air bubbles, which may result in restoration of vessels to a functional state, remain attractive but also need to be tested experimentally (21, 36, 41).
The observation that vestures in cold areas are best developed in the narrowest tracheary elements parallels the distribution of other vessel characteristics such as helical thickenings and tori. Although a pit membrane with a torus, i.e., a central thickening of the pit membrane, is very rare in angiosperms, this feature is more common in narrow vessel elements and/or tracheids than in wide earlywood vessels (42–44). Hence, the similar distribution of vestures, helical thickenings, and tori in narrow tracheary elements could indicate that these structures contribute to a high safety factor in elements that mainly function as a reserve conducting system, whereas wide vessels allow more efficient water transport but at the same time are more vulnerable to cavitation.
Concerning functional explanations for the adaptive nature of vessel perforation plates, it has been suggested that seasonality in tropical and subtropical areas drives the loss of bars in scalariform perforation plates to provide a more efficient hydraulic system during the wet seasons (2, 3, 19). Simple perforations are generally thought to provide less resistance to water flow than scalariform perforations. For the few species investigated, the additional resistance to water flow offered by perforation plates is higher for scalariform perforation plates (from 2% to 20% of the total resistance) than for simple perforation plates (from 2% to 5%) (45–47). It is possible that perforation plates with a large number (>50) of closely spaced bars and with steeply inclined end walls would offer still greater resistance to flow, but this needs to be tested experimentally. An alternative explanation is that scalariform vessel perforations may be instrumental in catching air bubbles during the thawing of frozen water (34). That idea could be adaptive in regions with frost, such as arctic and cool temperate regions, but may not explain the high incidence of scalariform perforation plates in tropical montane regions that, although cool, usually are frost free at 1,500–2,000 m. According to Sperry (12, 13), a more universal advantage of scalariform perforation plates may be that this type of perforation plate facilitates vessel refilling, presuming that water is wicked across the closely spaced bars and divides up the previously continuous gas-filled vessel into smaller bubbles. A small gas bubble in a vessel element with scalariform perforation plates may dissolve more rapidly than the fewer but larger bubbles in vessel elements with simple perforation plates.
Besides being the mark of phylogenetic traits, vestured pits appear to represent a successful adaptive strategy for survival and competition of plants that are subject to continuous or periodical drought stress in warm (tropical) climates. This finding underscores the need to conduct critical functional tests by comparative wood anatomists, xylem physiologists, and biophysicists to understand the adaptive role of micromorphological characters in the hydraulic architecture of woody plants.
Supplementary Material
Acknowledgments
We thank Miss Anja Vandeperre (Katholieke Universiteit Leuven) for assistance with the preparation of the figures. Steven Jansen is a postdoctoral fellow of the Fund for Scientific Research–Flanders (Belgium) (F.W.O.–Vlaanderen). Research at the Laboratory of Plant Systematics is supported by grants from the Research Council of the Katholieke Universiteit Leuven (OT/01/25) and the Fund for Scientific Research–Flanders (Belgium) (F.W.O.–Vlaanderen) (G.0104.01, 1.5.069.02, 1.5.061.03, G.0268.04).
References
- 1.Carlquist, S. (1975) Ecological Strategies of Xylem Evolution (Univ. of California Press, Berkeley).
- 2.Carlquist, S. (2001) Comparative Wood Anatomy. Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood (Springer, Berlin), 2nd Ed.
- 3.Baas, P. (1986) in On the Economy of Plant Form and Function, ed. Givnish, J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 327–353.
- 4.Hacke, U. G. & Sperry, J. S. (2001) Perspect. Plant Ecol. Evol. Syst. 4, 97–115. [Google Scholar]
- 5.Tyree, M. T. & Ewers, F. W. (1991) New Phytol. 119, 345–360. [Google Scholar]
- 6.Bailey, I. W. & Tupper, W. W. (1918) Proc. Am. Acad. Arts Sci. 54, 149–204. [Google Scholar]
- 7.Dickison, W. C. (1975) Ann. Mo. Bot. Gard. 62, 590–620. [Google Scholar]
- 8.Baas, P. & Wheeler, E. A. (1996) Int. Assoc. Wood Anat. J. 17, 351–364. [Google Scholar]
- 9.Feild, T. S., Brodribb, T. & Holbrook, N. M. (2002) Evolution (Lawrence, Kans.) 56, 464–478. [DOI] [PubMed] [Google Scholar]
- 10.Cochard, H., Cruizal, P. & Tyree, M. T. (1992) Plant Physiol. 100, 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarbeau, J. A., Ewers, F. W. & Davis, S. D. (1995) Plant Cell Environ. 18, 189–196. [Google Scholar]
- 12.Sperry, J. S. (1986) Plant Physiol. 80, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperry, J. S. (2003) Int. J. Plant Sci. 164, Suppl. 3, 115–127. [Google Scholar]
- 14.Tyree, M. T. & Sperry, J. S. (1989) Annu. Rev. Plant Phys. Mol. Biol. 40, 19–38. [Google Scholar]
- 15.Wardrop, A. B., Ingle, H. D., Davies, G. W. (1963) Nature 197, 202–203. [Google Scholar]
- 16.Jansen, S., Smets, E. & Baas, P. (1998) Int. Assoc. Wood Anat. J. 19, 347–382. [Google Scholar]
- 17.Jansen, S., Baas, P. & Smets, E. (2001) Taxon 50, 135–167. [Google Scholar]
- 18.Baas, P., Wheeler, E. A. & Chase, M. W. (2000) Bot. J. Linn. Soc. 134, 3–17. [Google Scholar]
- 19.Baas, P. (1976) in Wood Structure in Biological and Technological Research, eds. Baas, P., Bolton, A. J. & Catling, D. M. (Leiden Univ. Press, Leiden, The Netherlands), pp. 157–181.
- 20.Baas, P. & Schweingruber, F. H. (1987) Int. Assoc. Wood Anat. Bull. 8, 245–274. [Google Scholar]
- 21.Jansen, S., Baas, P., Gasson, P. & Smets, E. (2003) Int. J. Plant Sci. 164, 405–413. [Google Scholar]
- 22.Metcalfe, C. R. & Chalk, L. (1983) Anatomy of the Dicotyledons (Clarendon Press, Oxford), Vol. 2.
- 23.Chapman, J. D. & White, F. (1970) The Evergreen Forest of Malawi (Commonwealth Forestry Institute, Univ. of Oxford, Oxford).
- 24.Prior, J. A. B. & Gasson, P. E. (1990) Int. Assoc. Wood Anat. Bull. 11, 319–336. [Google Scholar]
- 25.Moore, D. M. (1983) Flora of Tierra del Fuego (Nelson, Oswestry, U.K.).
- 26.Baas, P., Esser, P. M., van der Westen, M. E. T. & Zandee, M. (1988) Int. Assoc. Wood Anat. Bull. 9, 103–182. [Google Scholar]
- 27.Jansen, S., Piesschaert, F. & Smets, E. (2000) Am. J. Bot. 87, 20–28. [PubMed] [Google Scholar]
- 28.Neumann, K., Schoch, W., Détienne, P. & Schweingruber, F. H. (2001) Woods of the Sahara and the Sahel, An Anatomical Atlas (Haupt, Bern, Switzerland).
- 29.Laughin, D. C. (2002) Carib. J. Sci. 38, 151–155. [Google Scholar]
- 30.Nair, M. N. B. & Mohan Ram, H. Y. (1989) Bot. J. Linn. Soc. 100, 323–336. [Google Scholar]
- 31.Haridasan, M. & de Araújo, G. M. (1988) For. Ecol. Manage. 24, 15–26. [Google Scholar]
- 32.Holbrook, M. N., Burns, M. J. & Field, C. B. (1995) Science 270, 1193–1194. [Google Scholar]
- 33.Pockman, W. T., Sperry, J. S. & O'Leary, J. W. (1995) Nature 378, 715–716. [Google Scholar]
- 34.Tyree, M. T. & Zimmermann, M. H. (2002) Xylem Structure and the Ascent of Sap (Springer, Berlin).
- 35.Zweypfenning, R. C. V. J. (1978) Int. Assoc. Wood Anat. Bull. 1978/1, 13–15. [Google Scholar]
- 36.Carlquist, S. (1982) Ann. Mo. Bot. Gard. 69, 755–769. [Google Scholar]
- 37.Choat, B., Jansen, S., Zwieniecki, M. A. & Smets, E. (2004) J. Exp. Bot. 55, in press. [DOI] [PubMed]
- 38.Hacke, U. G., Stiller, V., Sperry, J. S., Pitterman, J. & McCulloh, K. A. (2001) Plant Physiol. 125, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperry, J. S. & Hacke, U. G. (2004) Am. J. Bot. 91, 369–385. [DOI] [PubMed] [Google Scholar]
- 40.Hacke, U. G., Sperry, J. S. & Pittermann, J. (2004) Am. J. Bot. 91, 386–400. [DOI] [PubMed] [Google Scholar]
- 41.Zwieniecki, M. A. & Holbrook, N. M. (2000) Plant Physiol. 123, 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dute, R. R., Freeman, J. D., Henning, F. & Barnard, L. D. (1996) Int. Assoc. Wood Anat. J. 17, 161–181. [Google Scholar]
- 43.Wheeler, E. A. (1983) Int. Assoc. Wood Anat. Bull. 4, 79–88. [Google Scholar]
- 44.Jansen, S., Choat, B., Vinckier, S., Lens, F., Schols, P. & Smets, E. (2004) New Phytol. 163, in press. [DOI] [PubMed]
- 45.Schulte, P. J. & Castle, A. L. (1993) J. Exp. Bot. 44, 1135–1142. [Google Scholar]
- 46.Ellerby, D. J. & Ennos, A. R. (1998) J. Exp. Bot. 49, 979–985. [Google Scholar]
- 47.Schulte, P. J. (1999) J. Exp. Bot. 50, 1179–1187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.