Abstract
Background:
Literature describing the application of modern segmental instrumentation to thoracic and lumbar fracture dislocation injuries is limited and the ideal surgical strategy for this severe trauma remains controversial. The purpose of this article was to investigate the feasibility and efficacy of single-stage posterior reduction with segmental instrumentation and interbody fusion to treat this type of injury.
Materials and Methods:
A retrospective review of 30 patients who had sustained fracture dislocation of the spine and underwent single stage posterior surgery between January 2007 and December 2011 was performed. All the patients underwent single stage posterior pedicle screw fixation, decompression and interbody fusion. Demographic data, medical records and radiographic images were reviewed thoroughly.
Results:
Ten females and 20 males with a mean age of 39.5 years were included in this study. Based on the AO classification, 13 cases were Type B1, 4 cases were B2, 4 were C1, 6 were C2 and 3 cases were C3. The average time of the surgical procedure was 220 min and the average blood loss was 550 mL. All of the patients were followed up for at least 2 years, with an average of 38 months. The mean preoperative kyphosis was 14.4° and reduced to -1.1° postoperatively. At the final followup, the mean kyphosis was 0.2°. The loss of correction was small (1.3°) with no significant difference compared to postoperative kyphotic angle (P = 0.069). Twenty seven patients (90%) achieved definitive bone fusion on X-ray or computed tomography imaging within 1 year followup. The other three patients were suspected possible pseudarthrosis. They remained asymptomatic without hardware failure or local pain at the last followup.
Conclusion:
Single stage posterior reduction using segmental pedicle screw instrumentation, combined with decompression and interbody fusion for the treatment of thoracic or lumbar fracture-dislocations is a safe, less traumatic and reliable technique. This procedure can achieve effective reduction, sagittal angle correction and solid fusion.
Keywords: Fracture dislocation, posterior interbody fusion, segmental instrumentation, spine trauma, thoracolumbar spine
MeSH terms: Spine, spinal fractures, dislocations, spinal fusion, instrumentation
INTRODUCTION
The primary distinguishing feature of spinal fracture-dislocation is a failure of all three columns under compression, tension and rotation, resulting in the vertebral fracture and subluxation or dislocation.1 Based on the AO thoracic and lumbar fracture classification, fracture dislocation injuries could be B1.2, B2.3, B3.3, and C types.2 This kind of trauma represents a relatively small, but significant subset of injuries often resulting from high energy trauma, accompanied with canal compromise and neurological deficits. Denis has reported 412 thoracolumbar spinal injuries; with fracture-dislocations representing 16% of the injuries.1 This is the same rate as in another multicenter study including 1019 consecutive patients.3
The treatment goals for thoracic or lumbar fracture dislocations are to achieve reduction, immediate stabilization with spine fusion, neural element decompression and early rehabilitation. The literature about segmental instrumentation of thoracolumbar dislocation injuries is extremely limited due to the low incidence of this type of trauma and the ideal surgical strategy remains controversial.
There are several operative approaches available: Anterior, posterior and a combination of both. The anterior only approach is seldom used in this unstable injury owning to an intrinsic drawback in failure to reduce the fracture dislocation.4,5 In general, the posterior approach is a conventional, less traumatic and relatively classic method.6,7,8 However, reliable bone grafting and solid fusion has not occurred in the long term followup if only a posterolateral bone grafting is done.9 The pseudarthrosis rate is as high as 11-53%,10 which leads to a loss of reduction, loss of restored height and progressive sagittal imbalance over time.9,11 McCormack et al. proposed a load sharing classification to select spinal fractures for a combined posterior and anterior reconstruction using strut graft and short pedicle screw fixation.12 However, thoracic and lumbar fracture dislocations are commonly associated with other chest or abdominal visceral injuries, which may complicate the anterior approach.13
Recently, a posterior approach with interbody fusion in the thoracic spine has gained acceptability for reconstructing the anterior and middle columns with spinal deformity and trauma.14 We have adopted this posterior approach by single stage segmental instrumentation and interbody bone graft to achieve reduction, decompression and reconstruction, while minimizing surgical trauma.
MATERIALS AND METHODS
Ethical approval was taken from our Institution's Institutional Review Board. We searched the digital medical record system in our hospital using “thoracic,” “thoracolumbar,” “lumbar,” and “fracture-dislocation” as keywords. Inclusion criteria was (1) spine trauma following violent force (2) complete disruption of three columns of the spine (3) Geographically located in the thoracic, thoracolumbar or lumbar segments (4) acute fracture with injury occurring within 1 month (5) followup time more than 2 years. Thirty patients between January 2007 and December 2011 who had sustained fracture dislocation of the thoracolumbar spine and received single stage posterior surgery were enrolled in this retrospective study. Demographic data, medical records and radiographic images from PACS workstation (Picture archiving and communication system) were reviewed thoroughly.
Preoperative radiographic assessment was done using posterior anterior and lateral plain radiographs of the region, three dimensional computed tomography (CT) and magnetic resonance imaging. There were anterior translation (n = 17) patients [Figure 1], lateral displacement (n = 7) and a combination of both (n = 6) [Figure 2]. In all patients, the ruptured disc fragments at the injured segments were found scattered and displaced both anteriorly and posteriorly entering the spinal canal and causing compression of the neural elements [Figure 1b and c].
Figure 1.
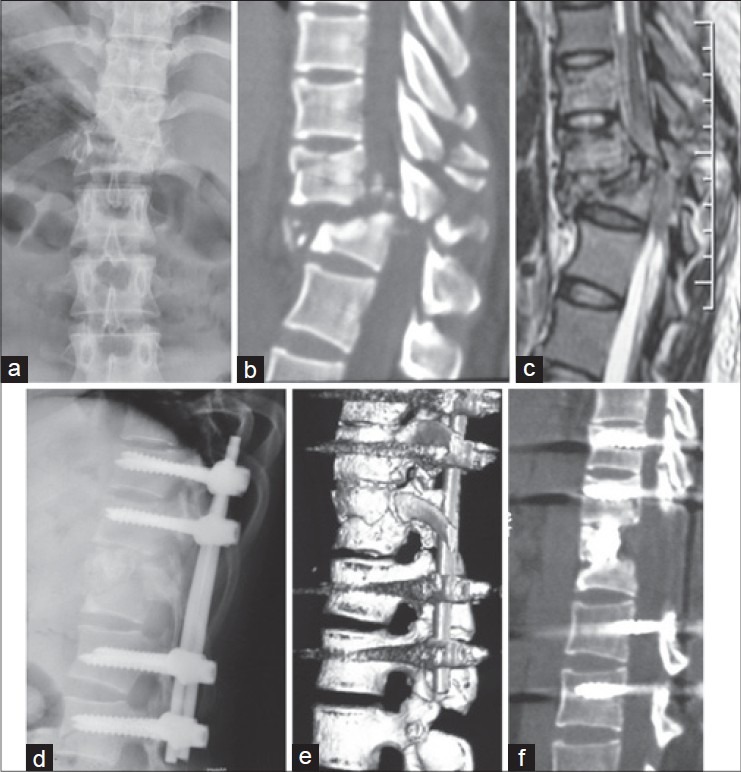
X-ray dorsolumbar spine anteroposterior view (a) showing a burst fracture of T12 (AO Type B1.2), T11 also had an impact fracture. Computed tomography (CT) (b) and magnetic resonance imaging (c) demonstrated posterior elements fracture and spinal cord injury. (d) X-ray dorsolumbar spine lateral view showing a single stage posterior segmental instrumentation, reduction and interbody fusion (e, f) Three dimensional CT scan at 1 year followup showing anatomical alignment and solid bony fusion in the T11-T12 disc space
Figure 2.
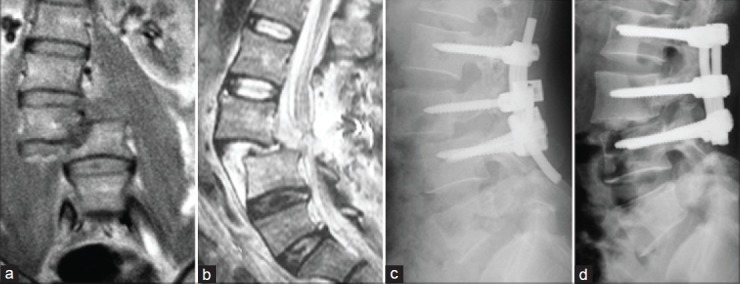
(a and b) MRI T2W I coronal and midsagittal images showing AO Type C3.1 injury at L3-L4 segment. (c) X-ray lumbar spine lateral view of a patient who underwent emergency surgery in another hospital, but reduction was not achieved owning to shallow screw depth in L3 and L2, which resulted in insufficient pulling force. (d) Revision surgery was performed and complete reduction was achieved. Interbody fusion was also accomplished via posterior approach
Operative procedure
The patient was put in the prone position on a Jackson table. A standard posterior midline approach with subperiosteal dissection of the paraspinal musculature was done over the involved levels, exposing the spine out to the transverse processes. Anatomical structures at the fracture-dislocation site should be careful identified, so as not to inadvertently injure the possibly exposed dural elements. Exposure was done at least one level above and below the fracture dislocated segment. Once the standard bony landmarks were identified, pedicle screws were inserted at the cephalad level, the screws were inserted purposefully flush to the laminar element, while the caudal screws were slightly proud. This offset would facilitate spinal reduction in the sagittal plane. The number of screws used varied according to the severity of fracture dislocation and the number of involved segments. Three to five segments are fixed with 6-10 pedicle-screws. Later, the jumped or impacted facets were released by resecting the superior and/or inferior articular processes. Precontoured rods were placed and fixed to the distal pedicle-screws. Before tightening the proximal screw nuts, distraction was applied using instrumentation setting. This maneuver can help to reduce the dislocated spine both in the sagittal and the coronal planes. In some cases, complete reduction was hard to achieve at first; thus, additional maneuvers were needed such as adjusting the depth of the pedicle screws, reshaping the rod contour to a more lordotic curve, and/or in situ contouring.
Decompression of the spinal canal was performed in each patient because of the protruding fracture fragments and the accompanying neurological deficit. Laminectomy (and at least one sided facetectomy) was performed to expose the dura and the lateral parts of the disc, without stretching the neural structures. Teared dura sac, if seen, was stitched to control cerebrospinal fluid (CSF) leak. The ruptured disc and bone fragments in the spinal canal were removed through the posterolateral approach, similar to the transforaminal lumbar interbody fusion (TLIF) technique. Intervertebral disc and endplates were also removed. Once the bone graft bed was prepared, autologous bone harvest from the resected posterior arch was implanted and packed tightly into the gap. Transverse connector was used in all Type C and most Type B injuries. Hemostasis was performed with absorbable gelatin sponge and hemostatic agents. In addition, posterolateral fusion was routinely performed before drainage and wound closure.
Rehabilitation was allowed in an TLSO orthotic 5-10 days after surgery depending on the patients’ general condition and comorbidity. Radiography was carried out at 3 and 6 months, and then every year postoperatively to assess the fusion of bone graft, loss of correction and implant failure. No patient was lost to followup. Sagittal kyphosis was measured from the superior endplate of the cephalic intact vertebra to the inferior endplate of the caudal intact vertebra. Methods recommended by Lee et al.15 were used to evaluate the fusion status. Definitive fusion was confirmed by bony trabecular bridging across the graft/host interface. CT was done when there was uncertainty on an X-ray. Neurological evaluation was also documented at each followup to assess recovery. Surgery was considered a failure if (1) the implant was loose or was broken before bony fusion occurred (2) an increase of sagittal kyphosis by 10° or more compared to immediate postoperative imaging (3) dislocation or subluxation recurred (4) refractory local pain related to body position change.13
Statistic analysis
The preoperative ASIA grade and the ASIA grade at the last followup were compared using the Wilcoxon signed rank test. Repeated measure analysis of variance was used for comparison of kyphosis angle before and after surgery and at the last followup. Results were considered significant when P < 0.05.
RESULTS
There were 10 females and 20 males with a mean age of 39.5 years (range 18-58 years). Mechanisms of injury included traffic accidents (n = 10), falls from height (n = 13) and mine collapsing accidents (n = 7). The level of injury included thoracic spine (n = 7), thoracolumbar spine (T10-L2) (n = 17) and lumbar spine (n = 6). The neurological deficit was assessed using the American Spinal Injury Association (ASIA) grade. 20 patients were Grade A, 4 were ASIA Grade B, 3 were Grade C and 3 patients were ASIA Grade D. Based on the AO classification for thoracolumbar fracture and dislocations, 13 cases were Type B1, 4 cases were B2, 4 were C1, 6 were C2 and 3 cases were C3 injury pattern. Four patients had initial surgery in other medical institutions and had revision in our department due to failure of reduction and insufficient neural decompression.
Twenty six patients received surgery at a mean interval of 5.4 days (range 2-12 days) after the injury. The rest four patients had emergency surgery in other medical institutions and received revision surgery within 1-month due to failure of reduction and insufficient neural decompression [Figure 2]. All patients had successful reduction with no neurologic deterioration or any other adverse events. The average time of the surgical procedure was 220 min (range 170-340 min) and the average blood loss was 550 ml (range 300-1200 mL).
Postoperative complications included superficial wound infection in 4 patients, (which healed without surgical intervention). Drain removal was delayed (>7 days) in five patients owing to CSF leak, with the longest drain placement being for 14 days. The average hospital stay was 10.2 days (range 7-14 days).
All of the patients were followed up for at least 2 years, with an average of 38 months (range 2-60 months). Hardware loosening or breakage was not detected during followup in any of the patient. The mean preoperative kyphosis was 14.4° (range 6°-27°). This became -1.1° (range -18°-7°) postoperatively (P = 0.000). At the final followup, the mean kyphosis was 0.2° (range -15°-7°). The loss of correction was small (1.3°) and with no significant difference compared to postoperative kyphotic angle (P = 0.069). Twenty seven patients (90%) achieved definitive bony fusion on X-ray or CT imaging within 1 year of followup [Figure 3]. The other three patients had suspected pseudarthrosis on plain X-rays. They continued to be asymptomatic without hardware failure or local pain at the last followup.
Figure 3.
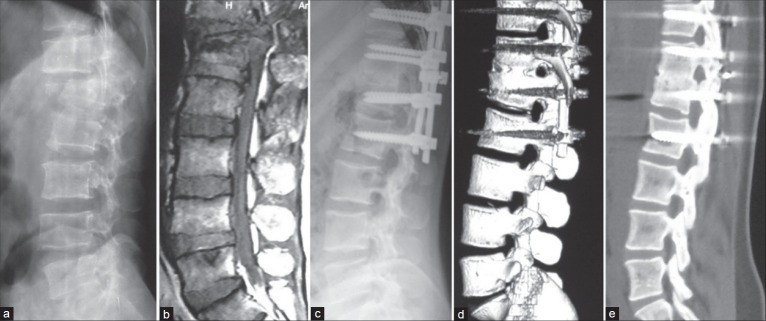
X-ray dorsolumbar spine lateral view (a) and MRI (b) T2W sagittal image dorsolumbar spine showing AO Type B1.2 fracture-dislocation at T12-L1 segment. (c) X-ray dorsolumbar spine lateral view showing posterior instrumentation, decompression and autologous morselized bone graft in the disc space in single posterior approach. (d and e) Computed tomography scan at 1 year followup showing solid fusion and good sagittal alignment
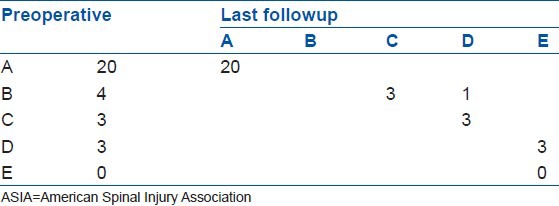
There was no measurable improvement in the neurologic function in 20 patients with complete paraplegia at initial evaluation (ASIA Grade A). At the final followup, three out of four patients in Grade B preoperatively, improved to Grade C. The remaining one patient improved to Grade D. Three patients in Grade D preoperatively recovered to Grade E [Table 1]. The average improvement of ASIA grade was 1.0 for incomplete neurological injury patients, which was significantly better than their preoperative status (P = 0.013).
Table 1.
Neurologic improvements of all patients according to ASIA grade
DISCUSSION
High energy spinal fracture dislocations which disrupt the entire column, are among the most unstable spinal injuries and have the highest rate of complete neurological injury.2 Both static and dynamic stabilization elements such as the vertebrae, disc, facets, ligaments and muscles are destroyed through a combinations of shear rotation and flexion/extension.16 In such injuries the spine should be stabilized at the earliest possible opportunity for neurologic and musculoskeletal protection. Surgery is generally needed, and treatment goals mainly focus on four points: (1) Reduction of the dislocated spinal column (2) decompression of neural structures (3) establishment of permanent spinal stability (4) early mobilization and rehabilitation.16,17
Anterior approach for fracture dislocation injury may not be applicable as the reduction of the fractures through an anterior approach alone is very difficult and in some cases impossible. Realignment and fixation are best accomplished through a posterior approach, reduction, multilevel instrumentation and fusion. At the beginning of 1980s, Aebi et al.18 used Harrington and Luque system to treat thoracolumbar fractures and fracture dislocations. One third patients required re surgery because of insufficient or failed implants. These technically unsatisfactory results propagated the development of the internal fixator system. Later, Carl et al.6 reported using pedicle screw instrumentation for thoracolumbar burst fractures and fracture dislocations; nine of their 38 patients had bent or broken screws in <2 years followup, but most of these patients were satisfied with the overall surgical results. Some authors preferred long pedicle screw fixation (two levels above and below the lesion) and believed that complementary anterior surgery with anterior decompression is rarely required, particularly incomplete spinal cord injuries.17,19,20,21 In a recent study, Wang and Zhu20 used posterior pedicle screw fixation for the treatment of complete fracture dislocation of thoracolumbar spine, 11 patients obtained satisfactory results at a mean followup of 22.3 months. However, other authors opined that single posterior approach can’t afford enough biomechanical stability for long term followup. Ebelke et al.22 used survivorship analysis for thoracolumbar burst fractures and reported that posterior internal fixation failure rate increased in the followup period.
Circumferential anterior and posterior fusion often plays a role in these severely injured case. Machino et al. have reported posterior/anterior combined surgery with a short segmental fixation for thoracolumbar burst fractures, achieving a high union rate and low instrumentation failure rates.23 Xia et al. advocated this combined surgery for more severe injury of thoracolumbar fracture dislocations in a lateral decubitus position, with a mean of 1200 mL intraoperative blood loss.24 Nonetheless, combined surgery is more aggressive for the patient, especially with multiple rib fractures and lung injury, leading to more intra- and post operative complications.13,25 Yadla et al. have reported five cases of traumatic thoracolumbar junction fracture dislocation with a combined approach; three of five cases had complications, including prolonged intubation and postoperative DVT.26
In recent times, as TLIF has gained popularity in the lumbar region, Machino et al. initially reported the use of this technique in thoracic and thoracolumbar regions to reconstruct the anterior and middle columns through a single posterior approach.14 The authors then compared this TLIF technique with posterior/anterior combined surgery in lower thoracic spine region. They reported that TTIF achieved rigid reconstruction and enables early postoperative ambulation without respiratory problems.27 Schmid et al. have reported satisfactory anterior column reconstruction with monocortical strut grafts through a technique similar to posterior lumbar interbody fusion/TLIF in 100 patients with thoracolumbar fractures. Followup in 82 patients proved that the anterior column was restored satisfactorily.28 In our study, we used this posterior TLIF approach with a single stage pedicle screw fixation and interbody bone graft to achieve reduction, decompression and reconstruction for the treatment of thoracic and lumbar fracture dislocations. This is a safe procedure because working zone can be acquired without retraction on the spinal cord. Fragments of discs and bones located anterior to the dura can be removed through this approach. Furthermore, it is possible to resect the interbody discs and endplate cartilages for reconstruction of the anterior column through interbody bone graft. The mean intraoperative blood loss was 550 mL in our series, which is less than that in combined approach reported by Xia et al. (1200 mL).24 We believe that the advantages of one stage posterior approach are multifold namely; less invasive, anatomical reduction and kyphotic correction, sufficient neural decompression, anterior column fusion and long term correction maintenance.
Although it could be argued that surgical decompression for complete neurologically deficient patients is not necessary and laminectomy adds more destabilization to the spine; In the authors’ opinion, fracture dislocation always accompanies laminae and facet fractures. Dural tear and CSF leak is also common in this kind of severe injury. Moreover, the dislocated, locked facets is usually a resistant force of reduction.19 Therefore, we did laminectomy and at least one side facetectomy at the injured level. The aim of this procedure, besides neural decompression, was to clean up the intracanal fractured laminae and facets fragments, repair the dural sac to control CSF leak, as well as facilitate normal alignment reduction and help in performing the interbody fusion.
One concern of this procedure is the limited load bearing capacity when small autograft is used for anterior column reconstruction. We achieved a mean kyphosis correction of 15.5° immediately. During an average of 33 months followup, the correction was satisfactorily preserved with a small loss of correction of 1.3°, with no statistical differences. In our study, graft related problems were not seen, with 27 of 30 (90%) patients demonstrated adequate fusion. The other three patients suspected with pseudoarthrosis remained asymptomatic at the final followup.
CONCLUSION
Our study demonstrated that single stage posterior reduction with instrumentation and interbody fusion is a safe, less traumatic and reliable technique to treat thoracic and lumbar fracture dislocations. This procedure achieved effective reduction of sagittal angle, associated with solid fusion rate.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817–31. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 3.Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine (Phila Pa 1976) 1992;17:528–40. doi: 10.1097/00007632-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Liljenqvist U, Halm H, Castro WH, Mommsen U. Thoracic fracture-dislocations without spinal cord injury: A case report and literature review. Eur Spine J. 1995;4:252–6. doi: 10.1007/BF00303421. [DOI] [PubMed] [Google Scholar]
- 5.Sapkas GS, Papagelopoulos PJ, Papadakis SA, Themistocleous GS, Stathakopoulos DP, Efstathiou P, et al. Thoracic spinal injuries: Operative treatments and neurologic outcomes. Am J Orthop (Belle Mead NJ) 2003;32:85–8. [PubMed] [Google Scholar]
- 6.Carl AL, Tromanhauser SG, Roger DJ. Pedicle screw instrumentation for thoracolumbar burst fractures and fracture-dislocations. Spine (Phila Pa 1976) 1992;17:S317–24. doi: 10.1097/00007632-199208001-00018. [DOI] [PubMed] [Google Scholar]
- 7.Francaviglia N, Bragazzi R, Maiello M, Bernucci C. Surgical treatment of fractures of the thoracic and lumbar spine via the transpedicular route. Br J Neurosurg. 1995;9:511–8. doi: 10.1080/02688699550041151. [DOI] [PubMed] [Google Scholar]
- 8.Stambough JL. Posterior instrumentation for thoracolumbar trauma. Clin Orthop Relat Res. 1997;335:73–88. [PubMed] [Google Scholar]
- 9.Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine. A comparative study of three fixation devices in 70 patients. Spine (Phila Pa 1976) 1993;18:450–60. [PubMed] [Google Scholar]
- 10.Yuan HA, Garfin SR, Dickman CA, Mardjetko SM. A Historical Cohort Study of Pedicle Screw Fixation in Thoracic, Lumbar, and Sacral Spinal Fusions. Spine (Phila Pa 1976) 1994;19:2279S–96. doi: 10.1097/00007632-199410151-00005. [DOI] [PubMed] [Google Scholar]
- 11.Moon MS, Choi WT, Moon YW, Kim YS, Moon JL. Stabilisation of fractured thoracic and lumbar spine with Cotrel-Dubousset instrument. J Orthop Surg (Hong Kong) 2003;11:59–66. doi: 10.1177/230949900301100113. [DOI] [PubMed] [Google Scholar]
- 12.McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine (Phila Pa 1976) 1994;19:1741–4. doi: 10.1097/00007632-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Oprel PP, Tuinebreijer WE, Patka P, den Hartog D. Combined anterior-posterior surgery versus posterior surgery for thoracolumbar burst fractures: A systematic review of the literature. Open Orthop J. 2010;4:93–100. doi: 10.2174/1874325001004010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machino M, Yukawa Y, Ito K, Nakashima H, Kato F. A new thoracic reconstruction technique “transforaminal thoracic interbody fusion”: A preliminary report of clinical outcomes. Spine (Phila Pa 1976) 2010;35:E1000–5. doi: 10.1097/BRS.0b013e3181dc9153. [DOI] [PubMed] [Google Scholar]
- 15.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:356–61. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Wood KB, Li W, Lebl DS, Ploumis A. Management of thoracolumbar spine fractures. Spine J. 2014;14:145–64. doi: 10.1016/j.spinee.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Alobaid A, Arlet V, Ouellet J, Reindl R. Surgical technique. Technical notes on reduction of thoracic spine fracture dislocation. Can J Surg. 2006;49:131–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Aebi M, Mohler J, Zäch G, Morscher E. Analysis of 75 operated thoracolumbar fractures and fracture dislocations with and without neurological deficit. Arch Orthop Trauma Surg. 1986;105:100–12. doi: 10.1007/BF00455844. [DOI] [PubMed] [Google Scholar]
- 19.Moore TA, Steinmetz MP, Anderson PA. Novel reduction technique for thoracolumbar fracture-dislocations. J Neurosurg Spine. 2011;15:675–7. doi: 10.3171/2011.8.SPINE1129. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Zhu Y. Treatment of complete fracture-dislocation of thoracolumbar spine. J Spinal Disord Tech. 2013;26:421–6. doi: 10.1097/BSD.0b013e31824e1223. [DOI] [PubMed] [Google Scholar]
- 21.Razak M, Mahmud M, Mokhtar SA, Omar A. Thoracolumbar fracture – Dislocation results of surgical treatment. Med J Malaysia. 2000;55(Suppl C):14–7. [PubMed] [Google Scholar]
- 22.Ebelke DK, Asher MA, Neff JR, Kraker DP. Survivorship analysis of VSP spine instrumentation in the treatment of thoracolumbar and lumbar burst fractures. Spine (Phila Pa 1976) 1991;16:S428–32. [PubMed] [Google Scholar]
- 23.Machino M, Yukawa Y, Ito K, Nakashima H, Kato F. Posterior/anterior combined surgery for thoracolumbar burst fractures - Posterior instrumentation with pedicle screws and laminar hooks, anterior decompression and strut grafting. Spinal Cord. 2011;49:573–9. doi: 10.1038/sc.2010.159. [DOI] [PubMed] [Google Scholar]
- 24.Xia Q, Xu BS, Zhang JD, Miao J, Li JG, Zhang XL, et al. Simultaneous combined anterior and posterior surgery for severe thoracolumbar fracture dislocations. Orthop Surg. 2009;1:28–33. doi: 10.1111/j.1757-7861.2008.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu SW, Fang KF, Tseng IC, Chiu YL, Chen YJ, Chen WJ. Surgical outcomes of short-segment fixation for thoracolumbar fracture dislocation. Chang Gung Med J. 2002;25:253–9. [PubMed] [Google Scholar]
- 26.Yadla S, Lebude B, Tender GC, Sharan AD, Harrop JS, Hilibrand AS, et al. Traumatic spondyloptosis of the thoracolumbar spine. J Neurosurg Spine. 2008;9:145–51. doi: 10.3171/SPI/2008/9/8/145. [DOI] [PubMed] [Google Scholar]
- 27.Machino M, Yukawa Y, Ito K, Nakashima H, Kanbara S, Morita D, et al. “Transforaminal thoracic interbody fusion” in the management of lower thoracic spine fracture dislocations: Technical note. J Spinal Disord Tsssech. 2013;26:E209–14. doi: 10.1097/BSD.0b013e318286ba15. [DOI] [PubMed] [Google Scholar]
- 28.Schmid R, Krappinger D, Seykora P, Blauth M, Kathrein A. PLIF in thoracolumbar trauma: Technique and radiological results. Eur Spine J. 2010;19:1079–86. doi: 10.1007/s00586-010-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


