Abstract
Cell-cell communication through gap junctions is aberrant or absent in a majority of human cancer cells, compared to cells in corresponding normal tissues. This and other evidence has led to the hypothesis that gap junction channels, comprised of connexin proteins, are important in growth control and cancer progression. The major goal of this ongoing study was to identify bioactive compounds that specifically upregulate gap junction channel-mediated cell-cell communication as potential anti-tumor therapies. Control of cell-cell communication is linked to growth regulatory intracellular signaling pathways; we therefore further aimed to identify signaling pathways modulated by these compounds in order to assess their potential as targeted anti-tumor therapies. Compounds were screened for their ability to upregulate gap junction-mediated cell-cell communication by using a fluorescent dye transfer assay to measure cell-cell communication between tumor promoter-treated astroglial cells or ras-transformed epithelial cells. Western blotting using connexin-specific and phosphorylation site-specific antibodies was used to monitor phosphorylation changes in signaling pathway proteins. Our results identified three compounds that upregulate gap junction-mediated cell-cell communication in our screening assays, chaetoglobosin K(ChK), 4-phenyl-3-butenoic acid (PBA) and the methyl ester of PBA (PBA-Me). Further analyses demonstrated that in tumorigenic cells, ChK downregulates phosphorylation of Akt kinase, an enzyme in the PI3-kinase signaling pathway that is found to be upregulated in a number of human cancers, on a key activation site. However, ChK did not inhibit PI-3 kinase in vitro as did the classic PI-3 kinase inhibitor, Wortmannin. PBA and PBA-Me were found to upregulate phosphorylation of p38 MAPK on a key activation site in tumorigenic cells, which is downregulated in several human cancer cell types. ChK and PBA also decreased activation of SAPK/JNK, another kinase found to be upregulated in a number of human cancers. These studies highlight the potential of monitoring gap junction intercellular communication for identifying experimental anti-tumor compounds.
Keywords: Gap junction, p38 MAPK, JNK, Akt kinase
INTRODUCTION
Poor drug selectivity between normal and cancer cells is a major challenge in creating effective therapies to inhibit the growth of cancer cells. Unlike normal cells, most tumor cells lack the ability to communicate cellular signals with each other and with surrounding cells through gap junction membrane channels [1]. Most neoplastic cells have fewer and smaller gap junctions, express lower levels of connexins and have reduced cell-cell communication compared to their non-neoplastic counterparts [2,3,4,5]. While the reduced cell-cell coupling may be a result of and not the cause of enhanced tumor growth, gap junction-mediated cell-cell communication is thought to be an important mechanism involved in growth regulation and neoplastic cell transformation that functions in tandem with other mechanisms such as cell cycle control, signal transduction pathways, and growth factor response [6,7]. Thus, it is critical to understand the intercellular signaling pathways that are dysfunctional, and how new anti-tumor compounds are able to increase gap junction-mediated cell-cell communication between tumor cells and suppress their tumorigenic characteristics. Chaetoglobosin K (ChK), a bioactive natural product [8], has been shown to suppress the tumorigenic phenotype in ras-transformed epithelial cells, to prevent tumor promoter-induced inhibition of intercellular communication, and to modulate the PI-3/Akt kinase pathway [9]. 4-phenyl-3-butenoic acid (PBA) is an experimental compound that has been shown to up-regulate cell-cell communication, reverse the transformed phenotype [10], and preferentially modulate the p38 MAPK pathway [11]. In this study, we compare the effects of ChK and PBA on upregulation of gap junction cell-cell communication, growth inhibition, and intracellular signaling pathways that regulate growth, providing insights into their biochemical mechanisms. Taken together, our results highlight the utility of using cell-cell communication as a marker for identifying potential new targeted tumor therapies. This work extends numerous previous studies that underscore the importance of monitoring cell-cell communication to control neoplastic growth in drug discovery [12,13,14,15].
MATERIALS AND METHODS
Materials
Alpha-MEM, RPMI-1640 culture media and L-glutamine were purchased from Mediatech (Herndon, VA). Lucifer Yellow-CH, PMSF, G418 and PBS were purchased from Sigma-Aldrich (St. Louis, MO). Dieldrin and lindane were purchased from Accustandard (New Haven, CT). Reagents for gel electrophoresis including pre-cast gels were purchased from BioRad Laboratories (Hercules, CA). Fetal bovine serum (FBS) was from Invitrogen (Carlsbad, CA). Chemical solvents, PBS and other buffer components were purchased from Fisher Scientific (Pittsburgh, PA). Chaetoglobosin K was purified from Diplodia macrospora [8] and was determined to have greater than 97% purity. PBA was obtained from Sigma-Aldrich (St. Louis, MO) and re-purified by re-crystallization before use in experiments. PBA-Me was synthesized as previously described [11]. p38 MAP kinase polyclonal antibody, Phospho-p38 MAP kinase (Thr180/Tyr182) polyclonal antibody, JNK polyclonal antibody, phospho-JNK (Thr183/Tyr185) polyclonal antibody, Akt polyclonal antibody, phospho-Akt (Ser473) polyclonal antibody, and anti-rabbit IgG alkaline phosphatase-conjugated antibody were from Cell Signaling Technology (Beverly, MA).
Cell cultures
WB-neo and WB-ras cells were derived from WB-F344 rat liver epithelial cells [De Feijter et al., 1990], and RG-2, a rat astroglial cell line derived from embryonic rat cerebral cortex, were a gift from Dr. James Trosko at Michigan State University. H2009 human lung carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA). Human lung carcinoma cells (H2009) were grown in RPMI-1640 media supplemented with 2mM L-glutamine and 10% fetal bovine serum. Ras-transformed liver epithelial cells (WB-ras1), plasmid control WB-neo3 cells were grown in α-MEM medium supplemented with 2 mM L-glutamine, 5% fetal bovine serum and 300 μM G418. Rat glial cells (RG-2) were grown in α-MEM medium supplemented with 2 mM L-glutamine and 5% fetal bovine serum. All cells were incubated at 37°C in a 5% CO2, 95% air atmosphere.
Treatment of Cells
RG-2, H2009, WB-ras1 or WB-neo3 cells were grown to 50-75% confluence. Flasks (25cm2) or dishes (35mm) were treated with the indicated concentrations of PBA or matched vehicle (diH20), or ChK, lindane, dieldrin, ChK and lindane, or ChK and dieldrin or matched vehicle (DMSO). Cells were incubated for the indicated times before total protein extraction or gap junction-mediated communication analyses. In experiments in which ChK was pre-incubated with cells, the pre-incubation time was 15 min. For drug screening experiments, WB-ras1 cells were incubated with test compounds (11 compounds screened) at varying concentrations for 1h to 72 h and gap junction-mediated cellular communication (described below) compared with that of vehicle controls; alternatively, for anti-tumor promoter activity screening (8 compounds screened), RG-2 cells were pretreated with the test compound for 15 min at varying concentrations, followed by incubation with 10 μM dieldrin and compared with cells treated with 10 μM dieldrin only and vehicle only.
Dye Transfer Assay of Gap Junction-mediated Cellular Communication
This method was slightly modified from a previously published method [9]. Briefly, cells grown in 35 mm dishes were washed once with PBS containing 0.5 mM CaCl2, 0.5 mM MgCl2 (Ca2+/Mg2+ PBS, to prevent cell detachment) and twice in PBS. One milliliter of Lucifer Yellow solution (0.5 mg/ml in PBS) was added to each dish and several areas of the cell monolayer immediately scored with a surgical blade to allow entry of Lucifer Yellow. After 90 s incubation in the dark, dye was removed and cells washed once with Ca2+/Mg2+ PBS followed by two washes with PBS and fixed with 4% formaldehyde in PBS. Fluorescence was observed using a Leitz inverted microscope using a 10-X objective lens. At least 5 areas of each test plate were selected for uniform cell density in white light mode, then digitally photographed in both white light and fluorescent mode and the number of fluorescent cells counted in a defined area containing approximately 50 cells. The average number of cells in separate fully inhibited negative control plates (30 min incubation with 10 μM dieldrin) was subtracted from the total number of cells counted in the various treated plates to provide a more accurate estimate of the number of communication-positive cells, since cells directly along the score line were fluorescent due to mechanical disruption of the membranes. For drug screening experiments. WB-ras1 cells were incubated with compounds at varying concentrations for 4 h to 72 h and cell-cell communication levels compared with vehicle controls; alternatively, RG-2 cells were pretreated with the test agent at varying concentrations for 15 min, followed by incubation with 10 μM dieldrin for 30 min and cell-cell communication levels compared with cells treated with 10 μM dieldrin only and vehicle only. Statistical analyses were performed using Statistix 8 for Windows. Data were analyzed by ANOVA, followed by Tukey’s post-hoc test and p<0.05 indicated a statistically significant difference between treatment groups.
Cell Growth Assay
The effect of each drug on cell growth was determined by trypsinizing and counting cells on a hemocytometer. Cells were plated at 5-10% confluence onto 35mm dishes, culture medium (lacking G418) was added to 2 ml, and allowed to attach overnight. Cells were then treated with vehicle or drug for the duration of the experiment. Following incubation, media was removed; cells were washed once with PBS, and treated with 0.5 ml of trypsin until cells no longer adhered to the dish. Following trypsinization, 1.5 or 3 mL of PBS containing 0.1 mM CaCl2, 0.1 mM MgCl2 was added to each dish, dependent on the cell density, and cells in suspension were counted using a hemocytometer to determine the number of cells per milliliter in each dish.
Cell Protein Extraction and Gel Electrophoresis
Cell proteins were extracted using a 2% SDS total protein extraction method [9]. Protein in each sample was quantified using the Bio-Rad DC protein assay. Equal amounts of protein for each sample were loaded onto 12% polyacrylamide gels, and gel electrophoresis was performed.
Western Blot Analysis
Western blot analysis was performed to assess the degree of total Akt, phosphorylated Akt, total SAPK/JNK, phosphorylated SAPK/JNK, total p38 MAPK, and phosphorylated p38 MAPK. After gel electrophoresis on 12% polyacrylamide gels, proteins were transferred to PVDF membranes. Membranes were blocked using a non-fat dry milk based blocking buffer and incubated with primary antibody for 20-24 h at 4°C, followed by incubation with secondary antibodies linked to alkaline phosphatase (AP). Blots were developed using a Bio-Rad AP conjugate development kit. Blots were scanned on an HPscanjet 4400C scanner and band densities quantified using UN-SCAN-IT software (version 5.1) from Silk Scientific, Inc. (Orem, UT). At least two independent blots were performed for each experiment.
PI-3Kinase Assay
An in vitro activity/inhibitor kit for class I PI-3 kinase (Millipore) was utilized according to the manufacturer’s instructions. ChK was used at concentrations of 0.1μM, 1μM, and 5μM. Wortmannin (0.1μM) was used as a positive control.
RESULTS
ChK and PBA upregulate cell-cell communication
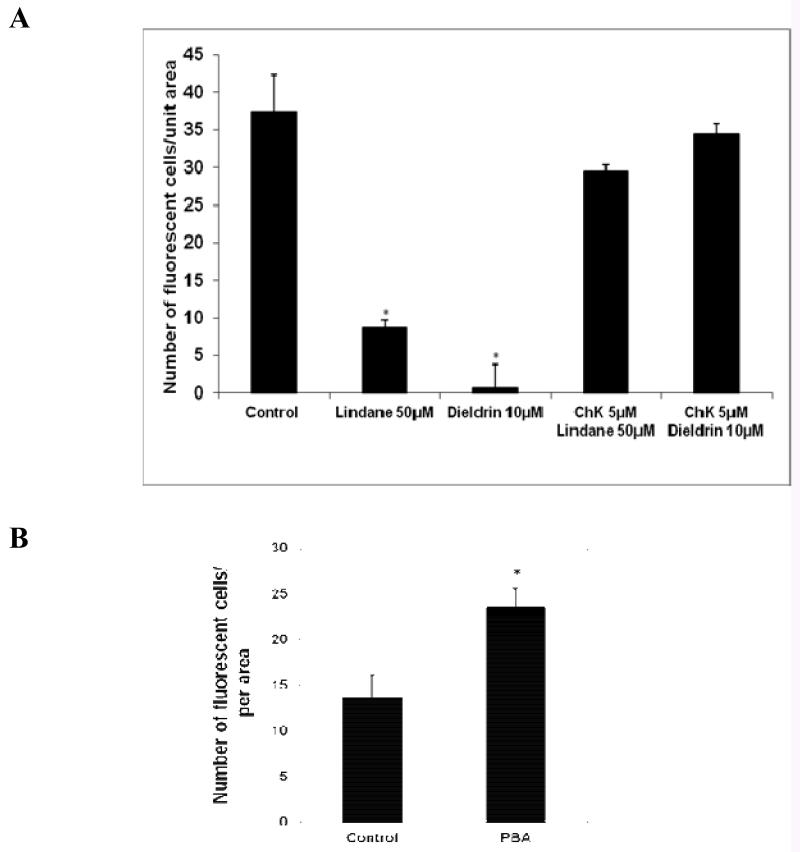
ChK prevents tumor promoter-induced inhibition of cell-cell communication in non-transformed cells, as previously reported [16]. Figure 1A shows that preincubation of cells with 5 μM ChK, followed by 30 min incubation with 10 μM dieldrin or 50 μM lindane, resulted in a greater number of dye-transfer fluorescent cells than treatment with dieldrin or lindane alone (p<0.05). Incubation with 5 μM ChK alone for 45 min showed no effect on dye-transfer compared to vehicle controls (data not shown). Figure 1B shows that PBA up-regulates gap junction-mediated cell-cell communication in ras-transformed cells, as has been previously reported [10]. WB-ras1 cells were treated with vehicle (control) or 0.1 mg/ml PBA. The fluorescence dye transfer assay was performed at 48 hours. There was an approximately 1.7-fold increase in the number of communicating cells in the PBA-treated group compared to the vehicle control group (p < 0.01). Longer treatment times produced greater increases in cell-cell communication compared to vehicle-treated controls, approximately a 5-fold increase at 5 days treatment. [10]. The methyl ester of PBA, PBA-Me, upregulated cell-cell communication in WB-ras1 cells at a 5-fold lower concentration than PBA was previously demonstrated [10].
Figure 1.
(A) Preventative effect of ChK on lindane and dieldrin inhibition of gap junctional communication. RG-2 cells were grown to 90-100% confluence on 35 mm2 dishes. Cells were preincubated with 5 μM ChK for 15 min followed by treatment with 50 μM lindane or 10 μM dieldrin for 30 min. Control dishes were treated with an equal volume of vehicle. Fluorescent dye transfer assay was performed as described in “Methods.” Values from separate dieldrin dishes were subtracted as background fluorescent cells for each group. Data are presented as the mean ± SD for each group (n=5). *Statistically significant (p<0.05) compared to control. (B) Fluorescent dye transfer assay of gap junction-mediated cell-cell communication between vehicle-treated or PBA-treated WB-ras1 cells. Values are the mean + S.E.M., n=11 (control) or 13 (PBA) determinations taken from 2 separate dishes for each treatment. *Statistically significant (p<0.05) compared to control.
ChK and PBA inhibit growth of tumorigenic cells
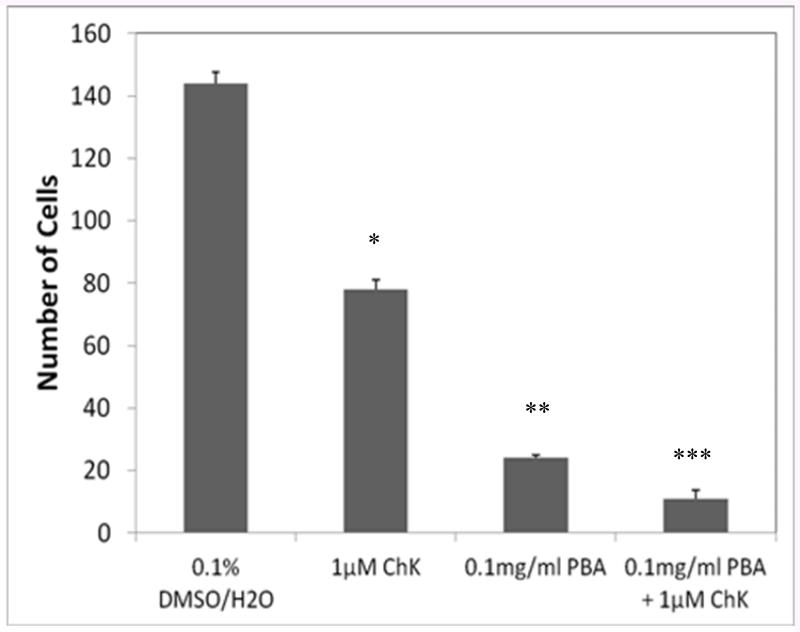
Figure 2 shows that ChK and PBA alone inhibited growth of WB-ras1 cells treated for 2 days at non-cytotoxic concentrations, as has been previously published for each agent at varying times and concentrations [9,10]. Combined treatment of the two compounds resulted in an approximately additive effect of the two compounds at the indicated concentrations (Figure 2). ChK and PBA administered alone also inhibited growth of H2009 human lung carcinoma cells [11,17].
Figure 2.
Effect of ChK and PBA alone and in combination on growth of WB-ras1 cells. Cells were plated on 35mm2 culture dishes and treated the following day with vehicle, 0.1 mg/ml PBA, 1 μM ChK or both and counted using a hemocytometer on days 5. Values are the mean ± S.D. (all treatment groups are significantly different from each other, p< 0.01).
ChK and PBA decrease activation of SAPK/JNK
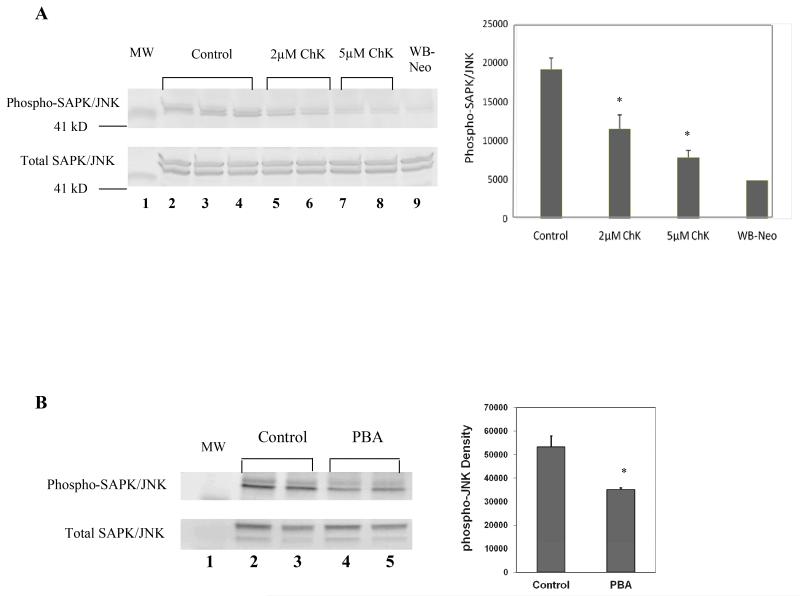
We have previously reported that ChK and PBA decrease phosphorylation of SAPK/JNK at the thr183/tyr185 activation sites [11, 17]. Figure 3A shows that WB-ras1 cells treated for 4 h with 2 or 5μM ChK showed decreased phosphorylation at this site site (lanes 5-8 compared to vehicle control lanes 2-4), and that the level of SAPK/JNK phosphorylation at 5μM ChK in these cells was similar to the level of SAPK/JNK phosphorylation in untreated non-transformed WB-neo3 cells (lane 9, and quantification in graph to the right). Total SAPK/JNK levels were unaltered by ChK treatment (bottom panel). Figure 3B shows that WB-ras1 cells treated with 0.1 mg/mL PBA for 4 h also showed decreased phosphorylation of SAPK/JNK at the thr183/tyr185 activation site (lanes 5-8 compared to vehicle control lanes 2-4). Total SAPK/JNK levels were not decreased compared to controls (bottom panel). We previously reported that phospho-SAPK/JNK levels were decreased in WB-ras1 cells following a longer (48 h) treatment with PBA [11].
Figure 3.
Effect of ChK (A) or PBA (B) on SAPK/JNK activation in WB-ras1 cells. In A, cells were treated with vehicle, 2 or 5 μM ChK for 4 h then extracted for Western blotting. In B, cells were treated with vehicle or 0.1 mg/ml PBA for 4 h then extracted for Western blotting. Antibodies used are specific for phospho-SAPK/JNK at thr183/tyr185 or total SAPK/JNK. (MW= 41 kD pre-stained molecular mass marker). Quantification of blots is shown in graph to the right. *Statistically significant (p<0.05) compared to control.
ChK decreases activation of Akt kinase
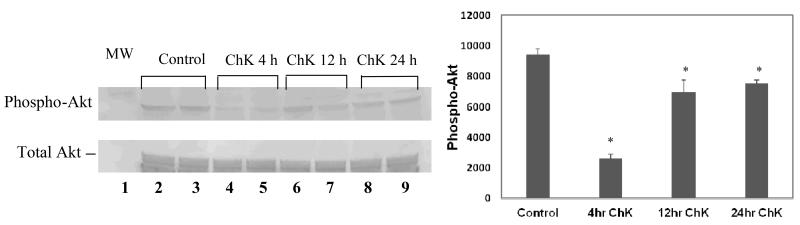
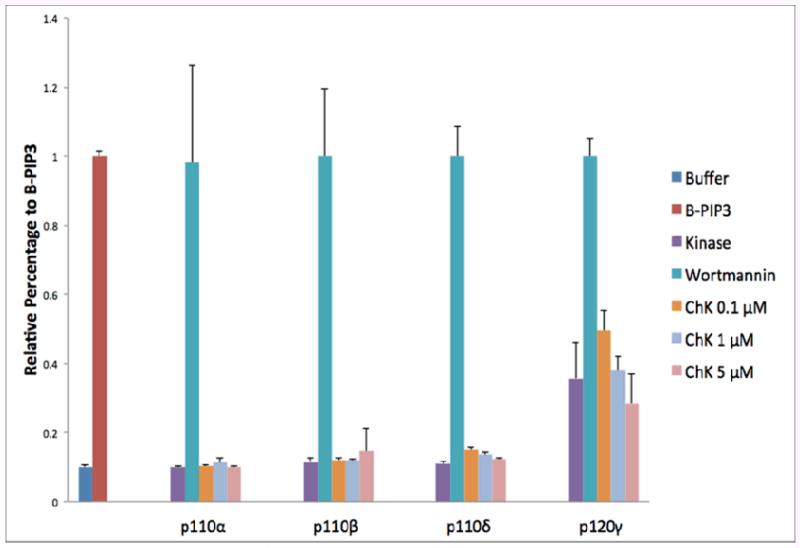
ChK has been shown to decrease Akt kinase phosphorylation at the ser473 activation site in WB-ras1 epithelial cells [9]. Figure 4 shows decreased phosphorylation of Akt kinase on ser473 following treatment of H2009 human carcinoma cells with 5μM ChK at 4, 12 and 24 h (lanes 4-9 and quantification in graph to the right), compared to vehicle-treated controls (lanes 2 and 3). An analysis of the effect ChK on PI-3 kinase activity in vitro, however, shows that ChK does not inhibit class I PI-3 kinase isoforms, unlike the classic PI-3 kinase inhibitor, Wortmannin (Figure 5). Further, we have previously shown that ChK inhibits both Akt and SAPK/JNK, but Wortmannin and another PI3K inhibitor, LY294002, inhibit only Akt [17].
Figure 4.
Effect of ChK on Akt phosphorylation in H2009 human carcinoma cells. Cells were treated with vehicle (DMSO) or 5 μM ChK for 4, 12, or 24 h and extracted for Western blot analysis of phospho-Akt at ser473 or total Akt. Quantification is shown in graph to the right. *Statistically significant (p<0.05) compared to control.
Figure 5.
Effect of ChK on class I PI-3 kinase p110 isoforms (α,β,γ,δ). The activity kit detects biotinylated-PIP3 (B-PIP3) using a streptavidin-HRP colorimetric readout (OD 450). PIP3 generated by the kinase reduced the signal from the maximal B-PIP3 level. A lower B-PIP3 signal indicates a higher PI-3 kinase enzyme activity. B-PIP3 was set as 100%, and the kinase reactions were normalized to the B-PIP3 signal to determine the relative percentage. Relative percentages >1 were considered 100%. Values represent the mean ±S.D. with n=4 for each group. All ChK-treated samples were significantly different from Wortmannin (p<0.01), but not significantly different from kinase only samples (p>0.05).
PBA upregulates p38 MAPK activation
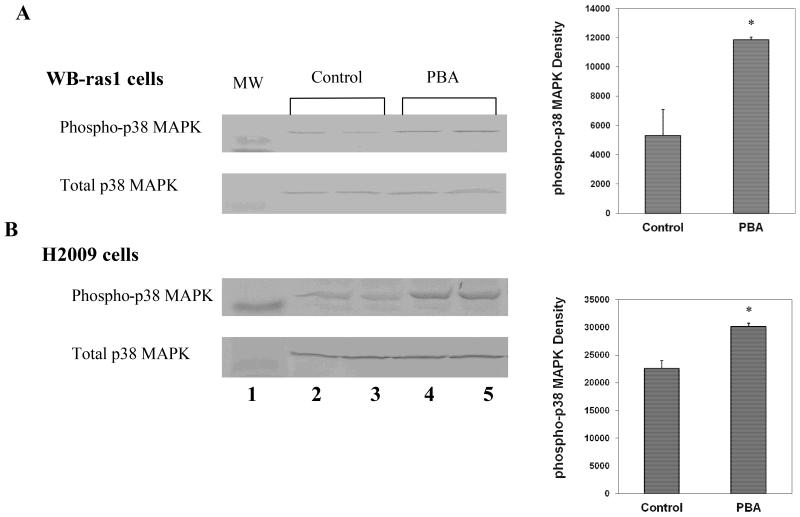
Figure 6A demostrates that 0.1 mg/mL PBA increases phosphorylation of p38 MAPK at the thr180/tyr182 activation site after 48 h treatment in WB-ras1 cells, as has been previously reported [11]. We also reported PBA increased phosphorylation at this site in H2009 cells after 48 h, but now we show that PBA increases phosphorylation at this site in H2009 cells as early as 4 h post treatment (Figure 6B). PBA-Me was previously shown to increase phosphorylation of p38 MAPK at a 5 fold lower concentration than PBA [11].
Figure 6.
Effect of PBA on phospho-p38 MAPK (thr180/tyr182 phosphorylation) in (A) WB-ras1 or (B) H2009 human carcinoma cells. Cells were treated with 0.1 mg/ml PBA for 48 h (A) or 4 h (B) and extracted for total protein for Western blot analysis as described in Methods. Samples from vehicle control (lanes 2 and 3) or PBA-treated (lanes 4 and 5) replicate cultures are shown on each blot. Identical blots were incubated with phospho-p38 MAPK (upper panels) or p38 MAPK (lower panels) antibodies. (MW=41 kD pre-stained molecular mass marker). Quantification is shown in graph. *Statistically significant (p<0.05) compared to control.
DISCUSSION
Using gap junction-mediated cell-cell communication screening assays, we identified two novel compounds that upregulate cell-cell communication, chaetoglobosin K (ChK) and 4-phenyl-3-butenoic acid (PBA). In addition, we previously reported [10,11] that the methyl ester of PBA (PBA-Me) similarly upregulates cell-cell communication at an approximately five-fold lower concentration than PBA. All three of these compounds inhibit growth of tumorigenic cells in vitro at non-cytotoxic concentrations and modulate key signaling pathways involved in tumorigenesis [9,10,11]. ChK inhibits both SAPK/JNK and Akt kinase activation [17], which would be predicted to inhibit tumor growth, as both of these kinase pathways have been reported to be upregulated in numerous human tumor types [18,19,20,21,22]. PBA also inhibits SAPK/JNK activation while concomitantly upregulating activation of p38 MAPK [11], again favoring tumor growth inhibition for tumor types with reduced p38 MAPK activation [23] and/or increased SAPK/JNK activation. The present study reveals the additive effect on cell growth of a combination treatment with these two compounds (Figure 2). While this effect does not appear to be synergistic, it suggests these compounds may act on similar pathways to reduce tumor cell growth. Our data showing that both componds decrease activation of the SAPK/JNK pathway support this idea. This study also provides evidence that ChK does not inhibit PI-3 kinase isoforms in vitro, in contrast to Wortmannin (Figure 5) and is therefore not likely a classic PI-3 kinase inhibitor, and further suggests that ChK modulates Akt phosphorylation downstream from a membrane receptor and PI-3 kinase.
Persistent dysregulation of oncogenic signaling pathways in cells results in disruption of normal growth control and can lead to neoplastic transformation. Targeted tumor therapy is based on the concept that modulation of these constitutively turned on or off key signaling pathways can control tumor cell growth by altering the phenotype of the tumor cells, with minimal effects on cells in which these dysregulated signaling pathways are not dominant. Numerous tumor cell models exist in which inhibition of oncogenic signaling pathways such as ras, neu, myc, and src impairs cell growth, decreases tumorigenicity of treated cells, and at least partially reverses the transformed phenotype [10, 24, 25, 26, 27, 28, 29]. In some cases, even brief pharmacological inactivation of an oncogene caused sustained reversal of the transformed phenotype [27].
Our results demonstrate that compounds that upregulate gap junction-mediated cell-cell communication may target major growth regulatory pathways such as the PI-3/Akt kinase, p38 MAPK, and SAPK/JNK pathways, in tumorigenic cells in vitro. One of the compounds, ChK, has recently been shown to downregulate Akt in ovarian cancer cells and inhibit angiogenesis in an in vivo model [30]. Additional studies of the ability of these compounds to suppress tumor growth in animal models is the next step in determining their potential as targeted tumor therapies. Taken together, our results provide further evidence that gap junction channels, comprised of connexin proteins, are important in cellular growth control and neoplastic development and provide a useful marker for identifying potential cancer therapies that target key growth regulatory signaling pathways.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health, 1RO1 CA096973 and 1R15CA135415. Contributions to the paper are as follows: D. Matesic, designed the research/study, analyzed data, prepared figures and wrote the paper, A. Ali, T. Sidorova, and T. Burns collected, data, analyzed data and prepared figures.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest concerning this report.
REFERENCES
- [1].Trosko JE, Chang CC. Potential role of intercellular communication in the rate-limiting step in carcinogenesis. J. Am. Coll. Toxicol. 1983;2:5–22. [Google Scholar]
- [2].Yamasaki H. Gap junctional intercellular communication and carcinogenesis. Carcinogenesis. 1990;11:1051–1058. doi: 10.1093/carcin/11.7.1051. [DOI] [PubMed] [Google Scholar]
- [3].Loewenstein WR, Rose B. The cell-cell channel in the control of growth. Semin. Cell Biol. 1992;3:59–79. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- [4].Holder JW, Elmore E, Barrett JC. Gap Junction Function and Cancer. Cancer Res. 1993;53:3475–3485. [PubMed] [Google Scholar]
- [5].Chipman JK, Mally A, Edwards GO. Disruption of Gap Junctions in Toxicity and Carcinogenicity. Toxicol. Sci. 2003;71:146–153. doi: 10.1093/toxsci/71.2.146. [DOI] [PubMed] [Google Scholar]
- [6].Yamasaki H, Naus CC. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199–1213. doi: 10.1093/carcin/17.6.1199. [DOI] [PubMed] [Google Scholar]
- [7].Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front Biosci. 1998;3:208–236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- [8].Cutler HG, Crumley F, Cox R. Chaetoglobosin K: a new plant growth inhibitor and toxin from Diplodia macrospora. J. Agric. Food Chem. 1980;28:139–142. doi: 10.1021/jf60227a011. [DOI] [PubMed] [Google Scholar]
- [9].Matesic DF, Villio KN, Folse SL, Garcia EL, Cutler SJ, Cutler HG. Inhibition of cytokinesis and Akt phosphorylation by Chaetoglobosin K in ras-transformed epithelial cells. Cancer Chemother. Pharmacol. 2006;57:708–718. doi: 10.1007/s00280-005-0113-5. [DOI] [PubMed] [Google Scholar]
- [10].Sunman JA, Foster MS, Folse SL, May SW, Matesic DF. Reversal of the transformed phenotype and inhibition of peptidylglycine alpha-monooxygenase in Ras-transformed cells by 4-phenyl-3-butenoic acid. Mol. Carcinog. 2004;41:231–246. doi: 10.1002/mc.20060. [DOI] [PubMed] [Google Scholar]
- [11].Matesic DF, Sidorova TS, Burns TJ, Bell AM, Tran PL, Ruch RJ, May SW. p38 MAPK activation, JNK inhibition, neoplastic growth inhibition, and increased gap junction communication in human lung carcinoma and ras-transformed cells by 4-phenyl-3-buteonic acid. J. Cell. Biochem. 2012;113:269–281. doi: 10.1002/jcb.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr. Drug Targets. 2002;18:465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- [13].Salameh A, Dhein S. Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim. Biophys. Acta, Biomembr. 2005;1719:36–58. doi: 10.1016/j.bbamem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- [14].Kandouz M, Batist G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin. Ther. Targets. 2010;14:681–692. doi: 10.1517/14728222.2010.487866. [DOI] [PubMed] [Google Scholar]
- [15].Bertram JS, Vine AL. Cancer prevention by retinoids and cartenoids: Independent action on a common target. Biochim. Biophys. Acta. 2005;1740:170–178. doi: 10.1016/j.bbadis.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [16].Sidorova TS, Matesic DF. Protective effect of the natural product, Chaetoglobosin K, on lindane- and dieldrin-induced changes in astroglia: identification of activated signaling pathways. Pharm. Res. 2008;25:1297–1308. doi: 10.1007/s11095-007-9487-x. [DOI] [PubMed] [Google Scholar]
- [17].Ali A, Sidorova TS, Matesic DM. Dual modulation of JNK and Akt signaling pathways by chaetoglobosin K in human lung carcinoma in ras-transformed epithelial cells. Investigational New Drugs. 2012;31:525–534. doi: 10.1007/s10637-012-9883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, Cheng JQ. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am. J. Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin. Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- [20].Antonyak MA, Kenyon LC, Godwin AK, James DC, Emlet DR, Okamoto I, Tnani M, Holgado-Madruga M, Moscatello DK, Wong AJ. Elevated JNK activation contributes to the pathogenesis of human brain tumors. Oncogene. 2002;21:5038–5046. doi: 10.1038/sj.onc.1205593. [DOI] [PubMed] [Google Scholar]
- [21].Vivanco I, Sawyers CI. The phosphatidylinositol 3-kinase-Akt pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- [22].Khatlani TS, Wislez M, Sun M, Srinivas H, Iwanaga K, Ma L, Hanna AE, Liu D, Girard L, Kim YH, Pollack JR, Minna JD, Wistuba II, Kurie JM. c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene. 2007;26:2658–2666. doi: 10.1038/sj.onc.1210050. [DOI] [PubMed] [Google Scholar]
- [23].Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5:824–828. doi: 10.4161/cc.5.8.2685. [DOI] [PubMed] [Google Scholar]
- [24].Murakami Y, Mizuno S, Hori M, Uehara Y. Reversal of Transformed Phenotypes by Herbimycin A in src Oncogene Expressed Rat Fibroblasts. Cancer Res. 1988;48:1587–1590. [PubMed] [Google Scholar]
- [25].Ruch RJ, Madhukar BV, Trosko JE, Klaunig JE. Reversal of ras-induced inhibition of gap-junctional intercellular communication, transformation, and tumorigenesis by lovastatin. Mol. Carcinog. 1993;7:50–59. doi: 10.1002/mc.2940070109. [DOI] [PubMed] [Google Scholar]
- [26].Jou Y-S, Layhe B, Matesic DF, Chang CC, de Feijter AW, Lockwood L, Welsh CW, Klaunig JE, Trosko JE. Inhibition of gap junctional intercellular communication and malignant transformation of rat liver epithelial cells by neu oncogene. Carcinogenesis. 1995;16:311–317. doi: 10.1093/carcin/16.2.311. [DOI] [PubMed] [Google Scholar]
- [27].Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- [28].Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J. Natl. Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- [29].Timofeev O, Lee TY, Bulavin DV. A subtle change in p38 MAPK activity is sufficient to suppress in vivo tumorigenesis. Cell Cycle. 2005;4:118–120. doi: 10.4161/cc.4.1.1342. [DOI] [PubMed] [Google Scholar]
- [30].Luo H, Li B, Li Z, Cutlet SJ, Rankin GO, Chen YC. Chaetoglobosin K inhibits tumor angiogenesis through downregulation of vascular epithelial growth factor-binding hypoxia-inducible factir 1α. Anti-Cancer Drugs. 2013;24:715–724. doi: 10.1097/CAD.0b013e3283627a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]