Abstract
Objective
Patients with non-transfusion-dependent thalassemia (NTDT) often develop iron overload and related complications, and may require iron chelation. However, the risk of over-chelation emerges as patients reach low, near-normal body iron levels and dose adjustments may be needed. In the THALASSA study, the threshold for chelation interruption was LIC <3 mg Fe/g dw (LIC<3); 24 patients receiving deferasirox for up to 2 yr reached this target. A post hoc analysis was performed to characterize the safety profile of deferasirox as these patients approached LIC<3.
Methods
THALASSA was a randomized, double-blind, placebo-controlled study of two deferasirox regimens (5 and 10 mg/kg/d) versus placebo in patients with NTDT. Patients randomized to deferasirox or placebo in the core could enter a 1-yr extension, with all patients receiving deferasirox (extension starting doses based on LIC at end-of-core and prior chelation response). The deferasirox safety profile was assessed between baseline and 6 months before reaching LIC<3 (Period 1), and the 6 months immediately before achieving LIC<3 (Period 2).
Results
Mean ± SD deferasirox treatment duration up to reaching LIC<3 was 476 ± 207 d, and deferasirox dose was 9.7 ± 3.0 mg/kg/d. The exposure-adjusted AE incidence regardless of causality was similar in periods 1 (1.026) and 2 (1.012). There were no clinically relevant differences in renal and hepatic laboratory parameters measured close to the time of LIC<3 compared with measurements near the previous LIC assessment.
Conclusions
The deferasirox safety profile remained consistent as patients approached the chelation interruption target, indicating that, with appropriate monitoring and dose adjustments in relation to iron load, low iron burdens may be reached with deferasirox with minimal risk of over-chelation.
Keywords: iron chelation, iron overload, thalassemia
Patients with non-transfusion-dependent thalassemias (NTDT) are at risk of iron overload, primarily due to increased gastrointestinal absorption secondary to ineffective erythropoiesis 1,2. Iron overload in this population can have important clinical consequences, such as hepatic dysfunction and endocrine morbidities 3–6. Although there is extensive clinical experience with iron overload and its treatment strategies in patients with transfusion-dependent anemias, data are only now emerging on appropriate approaches for patients with NTDT.
Chelation therapy aims to remove excess body iron and then to prevent any further iron accumulation by controlling body iron at near-normal levels. THALASSA (assessment of Exjade® in non-transfusion-dependent THALASSemiA patients) was the first randomized, double-blind, placebo-controlled trial to assess iron chelation therapy in iron-overloaded patients with NTDT (NCT00873041). In the core THALASSA study, a significant reduction in iron burden and a clinically manageable safety profile were observed with up to 1 yr of treatment with deferasirox 7. In THALASSA, liver iron concentration (LIC) <3 mg Fe/g dry weight (dw) (hereafter referred to as LIC <3) was the chelation interruption threshold as it represents a low or near-normal iron burden. This threshold is now recommended for interruption of chelation and subsequent monitoring for recurrence of iron overload in patients with NTDT 8–10. A 1-yr extension of the study showed continued efficacy in patients receiving deferasirox for up to 2 yr 11. At study initiation, patients had significant iron overload with a mean ± standard deviation (SD) LIC of 14.5 ± 8.8 mg Fe/g dw; as such, 3.6% (6/166) of patients had reached the target of LIC <3 after 1 yr in the core study 7. With continued treatment in the extension, 14.5% (24/166) patients reached LIC <3 11.
The potential for over-chelation increases as patients reach low or near-normal body iron levels, although other factors may influence this risk. Such factors include the chelator(s) used, the rate of change in body iron burden, the rate of body of iron loading, and the dose of chelator in relation to these variables. It is therefore important to establish how to manage a defined chelation regime in defined patient populations, to achieve the optimal therapeutic benefit without reducing iron stores too rapidly and inducing toxicity from over-chelation. In this post hoc analysis, the safety profile of deferasirox was assessed as patients with NTDT participating in the THALASSA study approached the chelation interruption target of LIC <3.
Methods
Patients and study design
Full study design and patient inclusion/exclusion criteria for the THALASSA study (NCT00873041) have been described previously 7. In brief, a prospective, randomized, placebo-controlled, double-blind, 1-yr trial was performed to evaluate deferasirox (starting dose 5 or 10 mg/kg/d) or placebo in patients with NTDT aged ≥10 yr with iron overload (LIC ≥5 mg Fe/g dw and serum ferritin >300 ng/mL). This was followed by a preplanned 1-yr extension in which all eligible patients, irrespective of initial treatment received, were treated with deferasirox. The starting dose in the extension phase was based on absolute LIC and LIC decrease by the end of the core study (Fig.1). For this post hoc analysis, patients reaching low iron burden (LIC <3; the predefined target for interruption of chelation) in the core or extension phases of the trial were included. Adverse events (AEs) and safety laboratory parameters were monitored throughout the study and the extension period. Auditory and ocular examinations were performed at baseline and at the end of the core and extension phases. Safety parameters during the period 6 months before reaching the LIC <3 target (Period 2) were compared with those during the period from baseline up to 6 months before reaching LIC <3 (Period 1; Fig.2).
Figure 1.
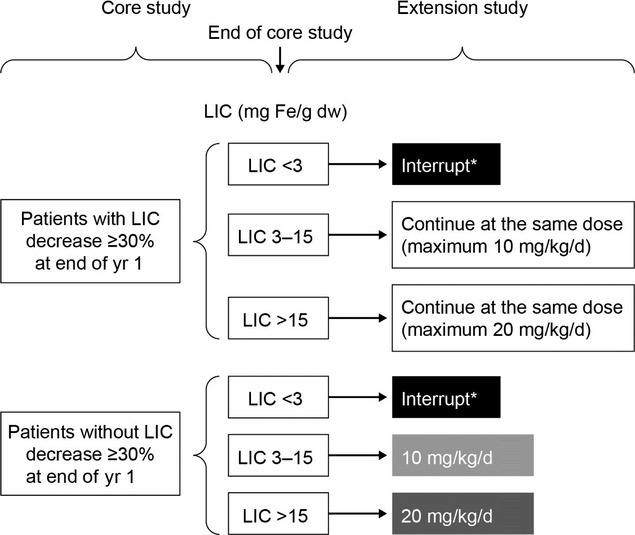
Starting doses in the THALASSA extension study *Wait until LIC>5 mg Fe/g dw and then restart at 5 mg/kg/d if 5 mg/kg/d was effective before interruption or at 10 mg/kg/d if 10–20 mg/kg/d was effective before interruption. With kind permission from Springer Science+Business Media: Ann Hematol, Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassemia (NTDT) patients: 1-yr extension results from the THALASSA study, 92, 2013, AT Taher, JB Porter, V Viprakasit, A Kattamis, S Chuncharunee, P Sutcharitchan, N Siritanaratkul, R Galanello, Z Karakas, T Lawniczek, D Habr, J Ros, Z Zhu, and MD Cappellini, 1485–1493, Figure 1.
Figure 2.
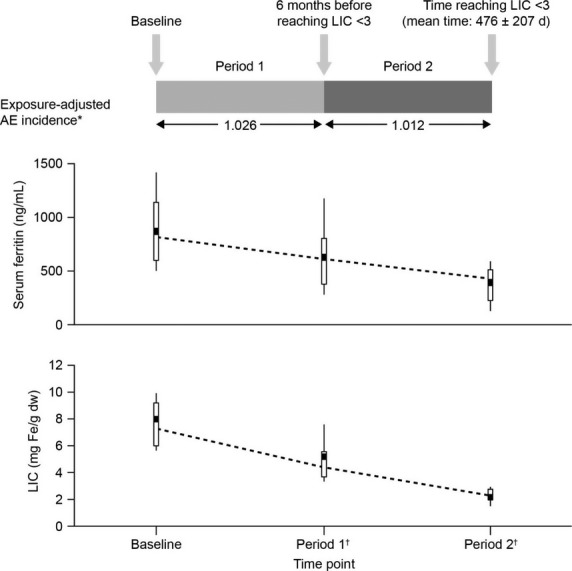
Exposure-adjusted incidence of AEs, serum ferritin, and LIC over periods 1 and 2 prior to reaching the LIC <3 target *Exposure-adjusted AE incidence is the number of patients with new/worsened AE during the period/total number of days that the patient was on treatment summed for all patients/365.25 d. †Nearest serum ferritin measurement to LIC assessment.
Assessments and statistical analysis
LIC was measured at screening and every 6 months thereafter, and read centrally using a validated R2 magnetic resonance imaging (MRI) technique (FerriScan®; Resonance Health Ltd, Claremont, WA, Australia) 12. Serum ferritin was measured at a central laboratory at screening and subsequently on a monthly (in patients receiving deferasirox) or quarterly basis (in patients no longer receiving deferasirox, having reached LIC <3).
AEs were adjusted for exposure [exposure-adjusted AEs = number of patients with new or worsened AEs during period/(total number of days patient was on treatment summed for all patients/365.25 d)]. Safety parameters observed or measured during Period 2 were compared with those during Period 1 by descriptive statistical analysis.
Results
Patients in the THALASSA study achieving LIC <3
Patients enrolled in the THALASSA study were heavily iron overloaded at baseline. In patients treated with deferasirox for 2 yr, LIC decreased from 13.8 ± 7.6 mg Fe/g dw at baseline to 7.5 ± 6.2 mg Fe/g dw at the end of the extension 11. During the core or extension phases, 24/166 (14.5%) patients reached an interruption target of LIC <3 (range 1.2–2.9 mg Fe/g dw). Among these patients (β-thalassemia intermedia, n = 12; α-thalassemia, n = 6; HbE/β-thalassemia, n = 6), baseline characteristics were generally similar to the overall THALASSA study population, although iron burden was slightly lower 7. Mean age ± SD was 34.3 ± 16.3 yr; 54.2% of patients were male; 70.8% were Caucasian and 29.2% Asian; 41.7% had undergone splenectomy; 79.2% had prior transfusions; and 25% had prior chelation therapy.
Among the 24 patients reaching the LIC <3 target over the study observation period, baseline mean LIC ± SD was 8.1 ± 3.2 mg Fe/g dw, and by the end of Period 2 had declined to 2.2 ± 0.5 mg Fe/g dw (range 1.2–2.9; Fig.2). Baseline median serum ferritin was 825 ng/mL [range 393–2169], and by the end of Period 2 had declined to 427 ng/mL (range 102–1010; Fig.2). Of the 24 patients reaching LIC <3, 18 received deferasirox in both core and extension phases (mean LIC decrease of −5.9 ± 2.8 mg Fe/g dw), and six switched from placebo to deferasirox at the start of the extension phase (mean LIC decrease of −5.7 ± 5.0 mg Fe/g dw). None of the patients included in this analysis were receiving placebo only.
Mean ± SD deferasirox treatment duration up to the point of achieving LIC <3 was 476 ± 207 d (median 449 d [range 162–779 d]), and mean ± SD actual deferasirox dose was 9.7 ± 3.0 mg/kg/d.
Deferasirox safety profile when approaching LIC <3
Among patients reaching the LIC <3 target, the overall incidence of AEs, regardless of drug causality, was 87.5% (periods 1 and 2). Incidence was 83.3% (n = 20) between baseline and the time point 6 months before reaching LIC <3 (Period 1), and 50.0% (n = 12) during the 6 months immediately prior to achieving LIC <3 (Period 2). Furthermore, when adjusted for deferasirox exposure, this incidence was comparable between periods 1 and 2 (1.026 and 1.012, respectively; Fig.2). Exposure-adjusted incidence was also the same for patients with LIC ≥3 mg Fe/g dw at any point postbaseline (112 patients with any AE/190.2 total time on treatment [years] = 0.59) as for patients with LIC <3 at least once postbaseline [19/24 patients with any AE/32.3 total time on treatment (years) = 0.59].
The types of AEs reported in patients reaching LIC <3 did not differ from those observed for the overall THALASSA population 7. The most common AEs (≥2 patients overall), regardless of drug causality, in Period 1 were upper abdominal pain, pyrexia, nasopharyngitis, nausea (all n = 4, 16.7%), diarrhea, upper respiratory tract infection, gastroenteritis, oropharyngeal pain, cough, rash (all n = 3, 12.5%), pharyngitis, back pain, and syncope (all n = 2, 8.3%). In Period 2, the most common AEs were upper respiratory tract infection (n = 3, 12.5%), nausea, pyrexia, and cough (all n = 2, 8.3%). Serious AEs (SAEs), regardless of drug causality, were experienced by 20.8% (n = 5) patients. These SAEs were hemolysis, gastroenteritis, road traffic accident, upper limb fracture and syncope (Period 1), and anemia and atrial fibrillation (Period 2). No SAEs in these patients were suspected by the investigator to be related to study drug.
AEs suspected to be causally related to study drug were reported in 29.2% (n = 7) of patients achieving LIC <3. AEs in Period 1 were nausea (n = 3); rash (n = 2); upper abdominal pain (n = 2); and decreased appetite, lethargy, somnolence, blood creatinine increase, pain, and diarrhea (all n = 1). The blood creatinine increase occurred for 18 d beginning during Period 1 (Day 313–330) in a patient who subsequently reached LIC <3 at Day 501; further information on the management of this patient is provided below. In Period 2, nausea, decreased appetite, vomiting, and abdominal pain (all n = 1) were reported. One patient presented with a clinically significant auditory abnormality which was present both at screening for study entry and at study completion following deferasirox treatment. At study entry, this was reported as a low frequency, mild, sensorineural hearing loss, and at the end of the core phase as hearing impairment. No clinically significant ocular abnormalities were reported.
There were no clinically relevant differences in mean serum creatinine, creatinine clearance or alanine aminotransferase levels measured close to the time of LIC <3, and near the time of the previous LIC assessment (Table1).
Table 1.
Laboratory parameters at baseline and prior to reaching LIC <3
| Parameter, mean ± SD | Baseline | End of period 1 | End of period 2 |
|---|---|---|---|
| Creatinine, μmol/L | 51.8 ± 14.6 | 62.0 ± 21.9 | 61.0 ± 19.9 |
| Creatinine clearance, mL/min | 144.8 ± 42.3 | 129.8 ± 53.9 | 129.5 ± 52.3 |
| Alanine aminotransferase, U/L | 31.4 ± 20.4 | 16.9 ± 7.4 | 16.4 ± 6.8 |
| Urinary protein/creatinine ratio, mg/mg | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.1 |
Last available assessment.
Three patients who reached LIC <3 were reported to have >33% increase in serum creatinine from baseline and above the upper limit of normal on two consecutive visits. In two of these three patients (Patient 1: male, 48 yr old, α-thalassemia; Patient 2: female, 27 yr old, β-thalassemia intermedia), serum creatinine increases were observed during Period 2 close to the time that LIC <3 was reached; increases were reversible on dose interruption and levels returned to baseline by study completion. In Patient 3, where the increase was also reported as an AE (male, 69 yr old, β-thalassemia intermedia), serum creatinine returned to baseline levels on dose interruption in Period 1, but was subsequently elevated in Period 2 and the remainder of the study after deferasirox was resumed at the same dose.
Thirteen of the 24 patients achieved LIC <3 prior to the end of the extension phase and stopped chelation for approximately 6 months or more, allowing assessment of serum creatinine over this follow-up period. Four of these 13 patients had a single, unconfirmed serum creatinine increase >33% from baseline levels at the last assessment before treatment cessation. In the follow-up period up until study completion, serum creatinine levels in these four patients normalized and were comparable to those at baseline. This is consistent with previous data demonstrating that deferasirox produces a mild effect on renal hemodynamics that is reversible after drug interruption over the short and long term 13.
Discussion
Among patients with NTDT participating in the THALASSA study, the exposure-adjusted AE incidence in the immediate 6 months prior to reaching LIC <3 did not differ from that for the period from baseline to 6 months before reaching LIC <3. Furthermore, the exposure-adjusted AE incidence in the patients who did not achieve LIC <3 during the observation period did not differ from those reaching LIC <3. Notable increases in serum creatinine (>33% increase from baseline and above the upper limit of normal on two consecutive occasions) observed in three patients receiving deferasirox treatment were reversible on dose interruption. Limitations of these data include the small population size and the post hoc nature of the analysis.
The benefit of achieving low body iron burden has been shown in patients with transfusion-dependent thalassemia treated with a different iron chelator, for whom cardiac and endocrine complications were frequently prevented or reversed 14. Optimizing outcomes in terms of both efficacy and safety during iron chelation therapy targeting low body iron levels requires close attention to the patient population, dose, type of chelator used, and importantly, the rate of iron loading and the rate of decrease in iron load. With regard to monitoring the rate of decrease in iron burden, MRI-assessed LIC is particularly important as iron burden approaches low levels. Although serum ferritin assessments are useful to guide chelation management, recent data show that at low iron burdens, serum ferritin levels can be less reliable to accurately assess LIC 15.
Here, we show that the safety profile of deferasirox remained consistent as patients with NTDT approached the lower, near-normal iron burden and chelation interruption target of LIC <3. This was achieved using doses lower than those typically used for patients on regular transfusion and with a dosing algorithm that took into account both the absolute level of LIC and the rate of change in LIC. These results suggest that, with appropriate monitoring and dose adjustments, the target of LIC <3 may be reached during deferasirox treatment with minimal risk of over-chelation in patients with NTDT.
Acknowledgments
This study was funded by Novartis Pharma AG. We thank Bethan Hahn, PhD, for medical editorial assistance. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship contributions
AT Taher, JB Porter, V Viprakasit, A Kattamis, S Chuncharunee, P Sutcharitchan, N Siritanaratkul, R Origa, Z Karakas, and MD Cappellini served as investigators on this trial, enrolling patients. They contributed to data interpretation, reviewed and provided their comments on this manuscript. AT Taher, JB Porter, V Viprakasit, A Kattamis, and MD Cappellini served as Study Steering Committee members overseeing the conduct of the trial, from study design to analysis plan and data interpretation. D Habr assisted in developing the trial protocol; coordinated the execution of the trial; and contributed to the analysis, interpretation, and reporting of the trial data. Z Zhu served as the trial statistician. All authors approved the final manuscript.
Conflict of interests
AT Taher reports receiving research funding and honoraria from Novartis Pharmaceuticals; JB Porter reports consultancy, receiving research grant funding and honoraria from Novartis Pharmaceuticals, consultancy and receiving research grant funding from Shire, and consultancy for Celgene; V Viprakasit received research grant support and lecture fees from Novartis Pharmaceuticals and research grant support from GPO-L-ONE, Thailand, FerroKin Biosciences and National Research University (NRU), Thailand; A Kattamis received research funding from Novartis Pharmaceuticals and participated in a Novartis speakers’ bureau; S Chuncharunee, P Sutcharitchan, and N Siritanaratkul received research funding from Novartis Pharmaceuticals; R Origa received a speaker’s honorarium from Novartis Pharmaceuticals; Z Karakas received research grants and speaker’s honoraria from Novartis Pharmaceuticals; D Habr and Z Zhu are full-time employees of Novartis Pharmaceuticals; MD Cappellini reports participating in a Novartis Pharmaceuticals speakers’ bureau, and receiving honoraria from Novartis Pharmaceuticals and from Genzyme.
References
- Pippard MJ, Callender ST, Warner GT, Weatherall DJ. Iron absorption and loading in b-thalassaemia intermedia. Lancet. 1979;2:819–21. doi: 10.1016/s0140-6736(79)92175-5. [DOI] [PubMed] [Google Scholar]
- Pootrakul P, Kitcharoen K, Yansukon P, Wasi P, Fucharoen S, Charoenlarp P, Brittenham G, Pippard MJ, Finch CA. The effect of erythroid hyperplasia on iron balance. Blood. 1988;71:1124–9. [PubMed] [Google Scholar]
- Musallam KM, Cappellini MD, Wood JC, Motta I, Graziadei G, Tamim H, Taher AT. Elevated liver iron concentration is a marker of increased morbidity in patients with b-thalassemia intermedia. Haematologica. 2011;96:1605–12. doi: 10.3324/haematol.2011.047852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taher AT, Musallam KM, El-Beshlawy A, Karimi M, Daar S, Belhoul K, Saned MS, Graziadei G, Cappellini MD. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br J Haematol. 2010;150:486–9. doi: 10.1111/j.1365-2141.2010.08220.x. [DOI] [PubMed] [Google Scholar]
- Mancuso A, Sciarrino E, Renda MC, Maggio A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. 2006;30:119–24. doi: 10.1080/03630260500455565. [DOI] [PubMed] [Google Scholar]
- Musallam KM, Motta I, Salvatori M, Fraquelli M, Marcon A, Taher AT, Cappellini MD. Longitudinal changes in serum ferritin levels correlate with measures of hepatic stiffness in transfusion-independent patients with beta-thalassemia intermedia. Blood Cells Mol Dis. 2012;49:136–9. doi: 10.1016/j.bcmd.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Taher AT, Porter J, Viprakasit V, et al. Deferasirox significantly reduces iron overload in non-transfusion-dependent thalassemia: 1-year results from a prospective, randomized, double-blind, placebo-controlled study. Blood. 2012;120:970–7. doi: 10.1182/blood-2012-02-412692. [DOI] [PubMed] [Google Scholar]
- Taher AT, Viprakasit V, Musallam KM, Cappellini MD. Treating iron overload in patients with non-transfusion-dependent thalassemia. Am J Hematol. 2013;88:409–15. doi: 10.1002/ajh.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals UK Ltd. 2013. Summary of Product Characteristics - EXJADE 125 mg, 250 mg, 500 mg dispersible tablets. Available at: http://www.medicines.org.uk/emc/medicine/18805/SPC/
- Thalassemia International Federation. 2013. Guidelines for the management of non transfusion dependent thalassaemia (NTDT). Available at: http://www.thalassaemia.org.cy/wp-content/uploads/pdf/educational-programmes/Publications/Non-Transfusion%20Dependent%20Thalassaemias%20%282013%29/NTDT%20ENGLISH.pdf.
- Taher AT, Porter JB, Viprakasit V, et al. Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassemia (NTDT) patients: 1-year extension results from the THALASSA study. Ann Hematol. 2013;92:1485–93. doi: 10.1007/s00277-013-1808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- Piga A, Fracchia S, Lai ME, Cappellini MD, Habr D, Wegener M, Bouillaud E, Forni GL. Two-year renal hemodynamic effects of deferasirox in patients with transfusion-dependent β-thalassemia. Blood. 2012;120 doi: 10.1111/bjh.13217. abst 3257. http://abstracts.hematologylibrary.org/cgi/content/abstract/120/21/3257. [DOI] [PubMed] [Google Scholar]
- Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–75. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- Taher A, Porter J, Viprakasit V, et al. Serum ferritin for predicting clinically relevant LIC thresholds to guide management of patients with non-transfusion-dependent thalassemia treated with deferasirox: THALASSA study extension analysis. Haematologica. 2013;98(Suppl 1):486. abst S1171. [Google Scholar]


