Abstract
Background
Many physicians believe that the most effective way to treat chronic urticaria is to take a nonsedating second-generation H1-antihistamine in the morning and a sedating first-generation H1-antihistamine, usually hydroxyzine, at night to enhance sleep. But is this belief well founded?
Objectives
To test this belief by comparing the effectiveness and prevalence of unwanted sedative effects when treating patients with chronic spontaneous urticaria (CSU) with levocetirizine 15 mg daily plus hydroxyzine 50 mg at night (levocetirizine plus hydroxyzine) vs. levocetirizine 20 mg daily (levocetirizine monotherapy).
Methods
In this randomized, double-blind, cross-over study, 24 patients with difficult-to-treat CSU took levocetirizine plus hydroxyzine or levocetirizine monotherapy for periods of 5 days each. At the end of each treatment period, assessments were made of quality of life (Chronic Urticaria Quality of Life Questionnaire, CU-Q2oL), severity of urticaria symptoms (Urticaria Activity Score, UAS), sleep disturbance during the night and daytime somnolence.
Results
Both treatments significantly decreased UAS, night-time sleep disturbances and CU-Q2oL scores (P < 0·001) without significant differences between the two. Compared with baseline, daytime somnolence was significantly reduced by levocetirizine monotherapy (P = 0·006) but not by levocetirizine plus hydroxyzine (P = 0·218). Direct comparison of the two treatment modalities in terms of daytime somnolence favoured levocetirizine monotherapy (P = 0·026).
Conclusions
The widespread belief that sleep is aided by the addition of a sedating first-generation H1-antihistamine, usually hydroxyzine, at night is not supported. These results are in line with the urticaria guidelines, which state that first-line treatment for urticaria should be new-generation, nonsedating H1-antihistamines only.
What’s already known about this topic? —
The EAACI/GA2LEN/EDF/WAO guideline for management of urticaria recommends second-generation ‘nonsedating’ H1-antihistamines as first-line treatment for chronic spontaneous urticaria (CSU).
However, it is common practice to add a sedating H1-antihistamine, such as hydroxyzine, at night in the belief that it will reduce itch and improve the quality of sleep.
What does this study add? —
This study compared 5-day treatment of CSU with the second-generation H1-antihistamine, levocetirizine (20 mg daily), with levocetirizine (15 mg daily) plus hydroxyzine (50 mg nightly).
The treatments were equally effective in decreasing symptoms and night-time sleep disturbances and increasing quality of life, but the addition of night-time hydroxyzine significantly increased daytime somnolence.
The belief that addition of a night-time sedating H1-antihistamine is of benefit in the treatment of CSU is unfounded.
Chronic spontaneous urticaria (CSU) is a relatively common condition, with 0·5–1·0% of the population suffering from it at any single time.1 Of the symptoms of this condition, pruritus is the most bothersome, particularly at night when it causes sleep disturbances.1,2 These disturbances in sleep lead to chronic fatigue, with a direct impact on quality of life (QoL) and physical and emotional well-being, which may be assessed for chronic urticaria using specifically designed questionnaires such as the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL).3 In addition, it has been reported that pruritic skin diseases impair workplace productivity, classroom productivity and daily activity by 39%, 45% and 42%, respectively.4
Because chronic urticaria has such a profound impact on QoL, effective treatment is required. The European Academy of Allergology and Clinical Immunology/Global Allergy and Asthma European Network/European Dermatology Forum/World Allergy Organization guideline for management of urticaria5 recommends second-generation ‘nonsedating’ H1-antihistamines as first-line treatment. It also states that if standard dosing is not effective, increasing the dosage up to fourfold is recommended. This recommendation is reiterated in other, newer international guidelines.6,7
These guidelines also recommend against the use of older, sedating first-generation H1-antihistamines in patients with urticaria unless there is a special indication.5–7 This is because first-generation H1-antihistamines have pronounced unwanted effects, including anticholinergic effects and sedative actions on the central nervous system. At night, first-generation H1-antihistamines increase the latency to the onset of rapid eye movement (REM) sleep and reduce the duration of REM sleep. Furthermore, residual effects, or hangover, are still present the next morning. Such effects include impairment in divided attention, vigilance, working memory and sensory–motor performance.8 For commonly used drugs, the incidence of subjectively reported somnolence has been documented to vary from 40% with chlorpheniramine or brompheniramine to 80% with hydroxyzine.9 Disturbingly, lack of subjective drowsiness does not mean that an individual is able to drive a vehicle without impairment because subjective somnolence and impairment of the ability to perform tasks are not necessarily correlated.10
Despite the potential of first-generation H1-antihistamines to cause unwanted effects, many physicians believe that the most effective treatment for chronic urticaria is a nonsedating second-generation H1-antihistamine in the morning and a sedating first-generation H1-antihistamine, usually hydroxyzine or chlorpheniramine, at night to reduce night-time itch and enhance sleep. The rationale for this is that the sedative component makes people sleep better in spite of the pruritus. This is supported by some guidelines, particularly older ones.11–13
But is this belief well founded? To date, there are no back-to-back comparisons of therapy of CSU with a nonsedating H1-antihistamine during the day plus a sedating first-generation sedating H1-antihistamine at night vs. monotherapy with a nonsedating H1-antihistamine. Consequently, we have compared the effectiveness of therapy and the prevalence of unwanted effects when treating patients with severe CSU with levocetirizine 15 mg daily plus hydroxyzine 50 mg at night (levocetirizine plus hydroxyzine) vs. levocetirizine 20 mg daily (levocetirizine monotherapy).
Patients and methods
This was a prospective, randomized, double-blind, cross-over study in patients with CSU in whom the efficacy and adverse effects of treatment with levocetirizine 20 mg daily were compared with those of levocetirizine 15 mg daily plus hydroxyzine 50 mg at night before sleep. In total, 25 patients with a minimum of 6 weeks’ documented history of chronic urticaria treated with systemic steroids were recruited from the Clinic of Allergy and Asthma in Sofia. One woman withdrew from the study for personal reasons during the in-hospital assessment period. The demographics of the 24 patients who completed the study are shown in Table 1. The group size was estimated from a previous study of ours14 using a power of 80% (t-test) with a two-sided significance level of 5% and a medium effect of 1·2 SD.
Table 1.
Demographic characteristics of patients
| Characteristic | Number |
|---|---|
| Number of patients | 24 |
| Age (years) | |
| Mean ± SEM | 45·4 ± 2·8 |
| Median (range) | 44·5 (19–68) |
| Sex | 16 female, 8 male |
| Duration of urticaria (months), median (range) | 7·5 (2–51)a |
| Clinical features (no. patients) | |
| Chronic spontaneous urticaria | 24 |
| Symptomatic dermographisma | 11 |
| Delayed-pressure urticariaa | 17 |
| Angio-oedemaa | 22 |
| NSAID intolerancea | 6 |
| H1-antihistamines taken in the last month (no. patients) | |
| Bilastine 20 mg daily | 1 |
| Cetirizine 10 mg daily | 3 |
| Chloropyramine 20 mg daily | 1 |
| Cinnarizine 25 mg daily | 4 |
| Desloratadine 5 mg daily | 5 |
| Fexofenadine 180 mg daily | 12 |
| Hydroxyzine 50 mg daily | 4 |
| Levocetirizine 5 mg daily | 6 |
| Patients taking prednisolone | 24 |
| Daily dose prednisolone (mg), median (range) | 10 (8–30) |
NSAID, nonsteroidal anti-inflammatory drug.
The presence of concomitant conditions was obtained from patient histories and was not confirmed objectively.
The study was approved by the ethics committee of the Medical University in Sofia (approval no. 3443/18.10.10), and was conducted in accordance with the general principles of Good Clinical Practice and the Declaration of Helsinki as amended in Edinburgh in 2000; its clinicaltrials.gov identifier number is NCT 01250652. Recruitment began in December 2010 and the study was completed in December 2012. All participants gave signed informed consent at the beginning of the study.
Patient selection
At the screening visit, information about concomitant disease and previous medication use was collected, and the patients were subjected to a general physical examination, laboratory blood analyses and an electrocardiogram. The primary inclusion criterion was a minimum of 6 weeks’ documented history of chronic spontaneous urticaria, with or without concomitant inducible urticaria or angio-oedema, treated with systemic corticosteroids. The exclusion criteria were a documented or suspected history of allergic disease; any acute or chronic disease; symptoms of a clinically significant illness, especially liver or kidney disease; a history of hypersensitivity to the study drug(s) or formulation ingredients; epilepsy or other seizure conditions; hereditary galactose intolerance, lactase deficiency or glucose–galactose malabsorption; drug abuse or excessive use of alcohol or tobacco. Pregnant or nursing women were also excluded. Oral H1- and H2-antihistamines, antidepressants, antipsychotics, corticosteroids, aluminium- and magnesium-containing antacids, ketoconazole and erythromycin, as well as topically applied H1 antihistamines, corticosteroids or mast-cell stabilizers, were forbidden for 2 weeks prior to testing.
Outcome measures
Quality of life
Patients completed a self-administered urticaria-specific QoL questionnaire (CU-Q2oL). The original Italian CU-Q2oL, supplied to us by Professor Canonica (University of Genoa, Italy), was translated into Bulgarian, back-translated into Italian and validated by administration to patients with urticaria of differing severity.
The questionnaire consisted of 23 questions structured into three areas: symptoms (four questions), activities (six questions) and social aspects (13 questions).3 Each question had five possible grades, from 0 = ‘none’ to 4 = ‘maximal impact’. Global summary scores were computed (maximum score = 92 indicating worst possible impact on QoL), reflecting the overall impact of the disease on the health-related QoL of patients. While the accepted recall period for CU-Q2oL in clinical practice is 2 weeks, a period of only 5 days was used in this study.
Urticaria Activity Score
The Urticaria Activity Score (UAS) was calculated using a standard operating procedure as recommended by the 2009 guidelines on the diagnosis of urticaria.15 The investigators, who were blinded to the treatment groups, calculated weal scores as follows: 0, none; 1, mild (< 20 weals); 2, moderate (20–50 weals) or 3, intense (> 50 weals or large confluent areas of weals). The severity of pruritus was recorded as 0, absent; 1, mild (present but not annoying or troublesome); 2, moderate (troublesome but does not interfere with normal daily activity or sleep) or 3, intense (severe pruritus, which is sufficiently troublesome to interfere with normal daily activity or sleep).
Night-time sleep disturbance
Sleep disturbance during the previous night was judged by patients marking a 100-mm visual analogue scale (VAS), marked ‘none’ on one end and ‘worst possible’ on the other. The distance in millimetres between the ‘none’ end and the patient’s mark was used further in the analyses.
Daytime sedation
Daytime somnolence was also recorded by patients in the morning using a similar VAS scale to that above. All patient assessments were made by patients between 07·00 h and 10·00 h. CU-Q2oL and UAS scores referred to the previous 24 h, sleep disturbance to the previous night and daytime somnolence to the present time.
Study design
Day 0
After signing informed consent, the patients’ demographics were documented and they were subjected to thorough clinical evaluation by the physician in charge. Baseline assessments of CU-Q2oL (5 days), UAS, night-time sleep disturbance and daytime somnolence were also made.
Days 1–6
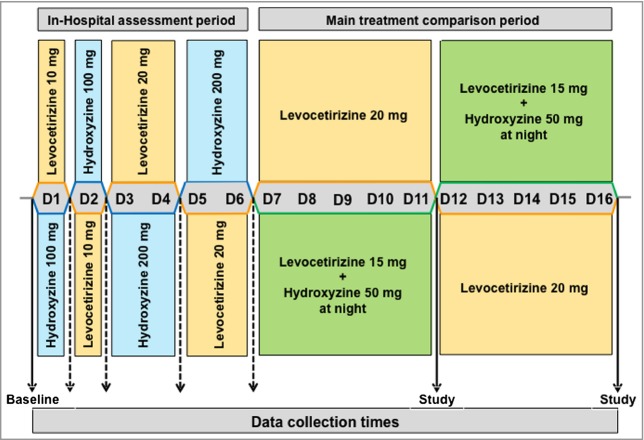
Patients were randomized alternately into one or other of the two treatment groups depending on the sequence of their recruitment. Randomization was made pairwise at a specialized website, http://www.randomizer.org/, to ensure a balanced number of cases in the two treatment arms. Patients underwent an in-hospital assessment of the effectiveness and tolerability of levocetirizine 10 and 20 mg vs. hydroxyzine 100 and 200 mg. This was done in a double-blind fashion on alternate-day regimens (Fig. 1). Medication was given morning and evening in opaque gelatine capsules that were prepared by a technician who was not aware of the clinical work. Patients swallowed the capsules in front of the caregiver with 50 mL plain water. Physician assessments were carried out about noon the same day, and UAS was taken on the mornings after.
Figure 1.
Study design. The study consisted of two separate double-blind phases. Phase one was an in-hospital assessment of the effectiveness and tolerability of levocetirizine and hydroxyzine at two doses. Phase two was a comparison of levocetirizine monotherapy vs. levocetirizine plus hydroxyzine at night. Complete data were collected at baseline and at the end of each study period. Broken lines indicate collection of urticaria activity scores during the assessment period.
Days 7–16
Day 7 marked the beginning of the main outpatient part of the study, which involved two 5-day periods comparing treatment with levocetirizine plus hydroxyzine (levocetirizine 15 mg daily, plus hydroxyzine 50 mg at night before sleep) and levocetirizine monotherapy (levocetirizine 20 mg daily). Patients started with their initial medication on day 7 and were crossed over to the opposite medication on day 12. There was no washout period between treatments. Patients were instructed to take two different opaque gelatine capsules each day: a grey one containing two 5-mg levocetirizine tablets in the morning and a blue one containing either two 5-mg levocetirizine tablets, or one 5-mg levocetirizine tablet and two 25-mg hydroxyzine tablets, in the evening before going to sleep. All capsules were placed in coded boxes in accordance with the study period and the treatment arm. The coding was generated by a technical assistant who did not have contact with study participants. Assessments of CU-Q2oL (5 day), UAS, sleep disturbance and daytime somnolence were made on day 12 and day 16.
Statistics
Analysis of results by the Kolmogorov–Smirnov test for normality showed that elements of all outcome measures were not distributed normally. Consequently, all results are expressed as medians with 25% and 75% limits, and differences between groups were tested using Wilcoxon’s nonparametric test for paired data. The minimum level of statistical significance was accepted to be P < 0·05.
Results
Prestudy assessment
In the in-hospital assessment of study drug effectiveness and tolerability, the UAS was reduced from a baseline of 5·5 (3·75–6·00; median with 25% and 75% limits) to 3·5 (1·75–5·00) or 2 (0·75–5·25) following 1 day’s dosage with levocetirizine 10 mg or hydroxyzine 100 mg, respectively. Following 2 days of therapy with levocetirizine 20 mg or hydroxyzine 200 mg, the UAS was 2 (0·75–4·00) or 2 (0·75–4·00), respectively. No patient reported adverse responses that prevented their taking part in the subsequent part of the study. During this period one woman withdrew from the study for personal reasons. She was the only dropout in the whole study.
Main study of levocetirizine plus hydroxyzine vs. levocetirizine monotherapy
Quality of life
The effect of 5 days’ treatment of patients with levocetirizine 15 mg daily plus hydroxyzine 50 mg at night before sleep (levocetirizine plus hydroxyzine) vs. levocetirizine alone 20 mg daily (levocetirizine monotherapy) is shown in Figure 2. The median CU-Q2oL scores (25–75% limits) were reduced from a baseline of 41 (28·25–52·00) to 17·5 (9·75–27·75) (P < 0·001) and 13·5 (6·75–33·25) (P < 0·001) with levocetirizine plus hydroxyzine, and levocetirizine monotherapy, respectively. There was no significant difference between the two treatments (P = 0·426).
Figure 2.
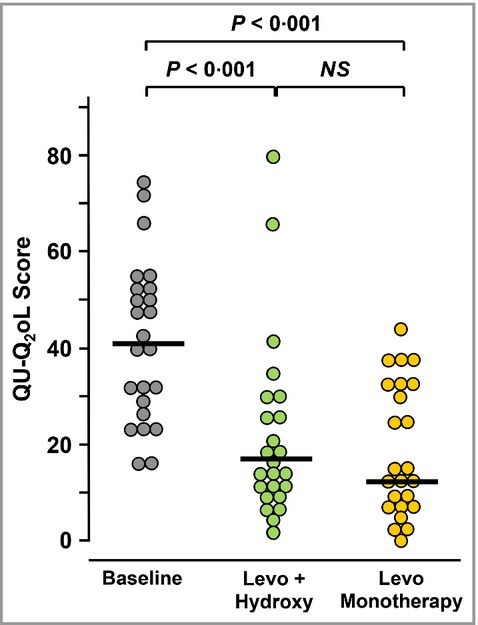
Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) scores at the start of the study (baseline) and after 5 days of treatment with levocetirizine 15 mg daily + hydroxyzine 50 mg at night (Levo + Hydroxy) or levocetirizine 20 mg daily alone (Levo Monotherapy). The maximum possible score for CU-Q2o Lis 92. Horizontal bars indicate median values. Significance of differences between treatments was calculated by Wilcoxon’s nonparametric test for paired data. NS, not significant.
Urticaria Activity Score
The individual UASs are shown in Figure 3. The median scores were reduced from a baseline of 5·5 (3·75–6·00) to 2 (0–3) (P = 0·002) or 1 (0–4) (P < 0·001) following 5 days’ treatment with levocetirizine plus hydroxyzine or levocetirizine monotherapy, respectively. There was no significant difference between the two treatments (P = 0·182).
Figure 3.
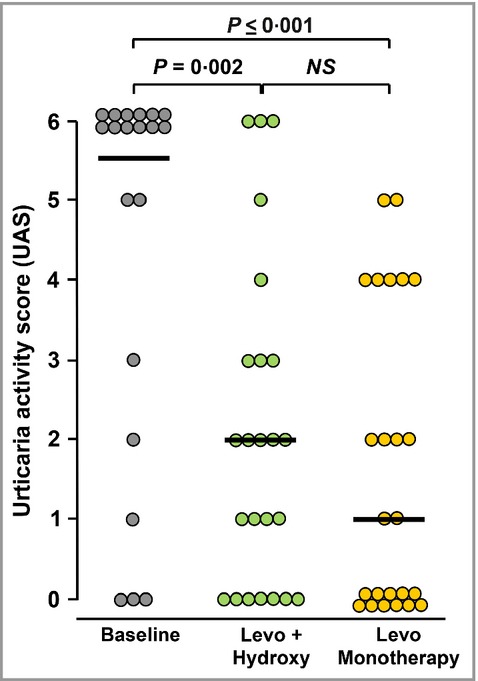
Urticaria Activity Scores at the start of the study (baseline) and after 5 days of treatment with levocetirizine 15 mg daily + hydroxyzine 50 mg at night (Levo + Hydroxy) or levocetirizine 20 mg daily alone (Levo Monotherapy). Horizontal bars indicate median values. Significant differences between treatments were calculated by Wilcoxon’s nonparametric test for paired data. NS, not significant.
As the UAS combines objective and subjective elements, i.e. weals and pruritus, these were also analysed separately. Weal scores were significantly reduced by levocetirizine plus hydroxyzine (P = 0·005) and by levocetirizine monotherapy (P = 0·003). There was no significant difference between treatments (P = 0·314). Pruritus scores were also significantly reduced by levocetirizine plus hydroxyzine (P = 0·001) and by levocetirizine monotherapy (P < 0·001). Again, there was no significant difference between treatments (P = 0·141).
Night-time sleep disturbance
There were highly significant reductions in night-time sleep disturbances (Fig. 4a), with the median baseline VAS of 71 (21·75–78·5) being reduced to 10 (0–25·25) (P = 0·001) by levocetirizine plus hydroxyzine and 17 (0–24·75) (P = 0·002) by levocetirizine monotherapy. There was no significant difference between treatments (P = 0·868).
Figure 4.
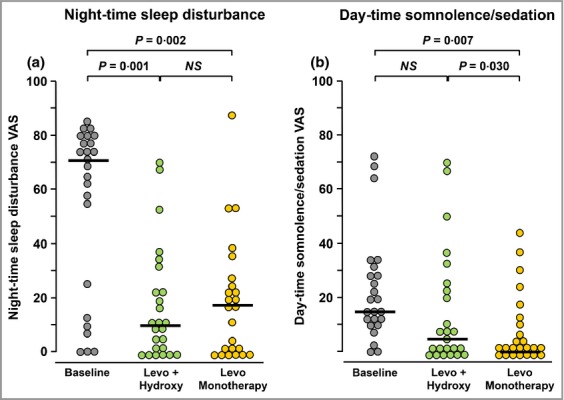
Visual analogue scale (VAS) scores for (a) night-time sleep disturbance and (b) daytime sedation at the start of the study (baseline) and after 5 days of treatment with levocetirizine 15 mg daily + hydroxyzine 50 mg at night (Levo + Hydroxy) or levocetirizine daily 20 mg alone (Levo Monotherapy). Horizontal bars indicate median values. Significant differences between treatments were calculated by Wilcoxon’s nonparametric test for paired data. NS, not significant.
Daytime sedation
Daytime sedation (Fig. 4b) was significantly (P = 0·007) reduced by levocetirizine monotherapy from a median baseline VAS of 14 (11–28·5) to 0 (0–11). In contrast, the median sedation VAS following levocetirizine plus hydroxyzine of 5 (0–23·5) was not significantly different from baseline (P = 0·218). Furthermore, patients treated with levocetirizine monotherapy were significantly less sedated than those receiving levocetirizine plus hydroxyzine (P = 0·030).
Discussion
The primary result of this study was that both levocetirizine monotherapy and therapy with levocetirizine plus hydroxyzine were similarly effective in reducing urticarial symptoms, reducing night-time sleep disturbances and improving QoL. In contrast, daytime sedation was significantly less with levocetirizine monotherapy in comparison with levocetirizine plus hydroxyzine.
The effectiveness of levocetirizine in relieving the symptoms of CSU and improving QoL confirms previous findings.14,16–18 It is of particular relevance in this study that the subjective element of the UAS, the severity of pruritus, was similarly reduced by both treatment regimens, suggesting that the central effects of hydroxyzine do not contribute to the overall antipruritic effect, which is agreement with the hypothesis that histamine is involved primarily in the peripheral genesis of itch.19 Furthermore, both treatment regimens having a similar effect in reducing night-time sleep disturbances again indicates that it is the peripheral antipruritic effect of the nonsedating second-generation H1-antihistamine that is of prime importance rather than any central sedative effects.
What is very clear from this study is that patients receiving levocetirizine monotherapy were significantly less sedated during the day than they were before treatment at the beginning of the study, confirming the findings of Staevska et al.14 In contrast, when treated with levocetirizine plus hydroxyzine, patients experienced a similar level of sedation to those who were not treated. Furthermore, patients receiving levocetirizine monotherapy experienced less daytime sedation than those receiving levocetirizine plus hydroxyzine. This strongly suggests that the detrimental sedative effects of hydroxyzine were due to its prolonged central nervous system effects. With a terminal half life of 20–25 h,20 it is not surprising that hydroxyzine has hangover effects into the next day.
We believe that this study has two weaknesses. Firstly, we did not perform objective assessments of alertness or productivity. Even so, our study would support the conclusion of Murota et al.21 that pruritic diseases negatively impact on daily activity and impair productivity, and that this is improved in patients taking second-generation nonsedative, but not first-generation sedative, H1-antihistamines, and that the improvements correlated with the alleviation of itch and improved QoL. The second weakness is that the length of the study, 10 days, is less than that of many similar studies in urticaria. This was because the study was performed at a tertiary referral clinic with patients from all over Bulgaria and we wanted to ensure a sustainable follow-up of patients, particularly given compliance issues.
In conclusion, the results of this study do not support the widely held belief that the most effective treatment for chronic urticaria is a nonsedating second-generation H1-antihistamine in the morning and a sedating first-generation H1-antihistamine, usually hydroxyzine, at night to enhance sleep. In view of the potential detrimental effects of residual daytime sedation on school performance and study, impaired productivity at work and, possibly more importantly, driving motor vehicles,8 it is clear that it is better not to offer a sedating antihistamine at night for the treatment of CSU.
References
- Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317–30. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Lawlor F, Simpson J, et al. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136:197–201. [PubMed] [Google Scholar]
- Baiardini I, Pasquali M, Braido F, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL) Allergy. 2005;60:1073–8. doi: 10.1111/j.1398-9995.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- Murota H, Kitaba S, Tani M, et al. Effects of nonsedative antihistamines on productivity of patients with pruritic skin diseases. Allergy. 2010;65:929–30. doi: 10.1111/j.1398-9995.2009.02262.x. [DOI] [PubMed] [Google Scholar]
- Zuberbier T, Asero R, Bindslev-Jensen C, et al. EAACI/GA2LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–43. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- Godse KV, Zawar V, Krupashankar D, et al. Consensus statement on the management of urticaria. Indian J Dermatol. 2011;56:485–9. doi: 10.4103/0019-5154.87119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SK. Management of chronic urticaria in Asia: 2010 AADV consensus guidelines. Asia Pac Allergy. 2012;2:149–60. doi: 10.5415/apallergy.2012.2.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MK, Maurer M, Simons FE, et al. Risk of first-generation H1-antihistamines: a GA2LEN position paper. Allergy. 2010;65:459–66. doi: 10.1111/j.1398-9995.2009.02325.x. [DOI] [PubMed] [Google Scholar]
- Druce HM, Thoden WR, Mure P, et al. Brompheniramine, loratadine, and placebo in allergic rhinitis: a placebo-controlled comparative clinical trial. J Clin Pharmacol. 1998;38:382–9. doi: 10.1002/j.1552-4604.1998.tb04439.x. [DOI] [PubMed] [Google Scholar]
- Simons FE, Fraser TG, Reggin JD, Simons KJ. Individual differences in central nervous system response to antihistamines (H1-receptor antagonists) Ann Allergy Asthma Immunol. 1995;75:507–14. [PubMed] [Google Scholar]
- Joint Task Force on Practice Parameters. The diagnosis and management of urticaria: a practice parameter part I: acute urticaria/angioedema; part II: chronic urticaria/angioedema. Ann Allergy Asthma Immunol. 2000;85:521–44. [PubMed] [Google Scholar]
- Grattan CE, Humphreys F British Association of Dermatologists Therapy Group and Audit Subcommittee. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157:1116–23. doi: 10.1111/j.1365-2133.2007.08283.x. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Du Toit GL, Siddique N, et al. BSACI guidelines for the management of chronic urticaria and angio-oedema. Clin Exp Allergy. 2007;37:631–50. doi: 10.1111/j.1365-2222.2007.02678.x. [DOI] [PubMed] [Google Scholar]
- Staevska M, Popov TA, Kralimarkova T, et al. The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol. 2010;125:676–82. doi: 10.1016/j.jaci.2009.11.047. [DOI] [PubMed] [Google Scholar]
- Zuberbier T, Asero R, Bindslev-Jensen C, et al. EAACI/GA2LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417–26. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- Kapp A, Demarteau N. Cost effectiveness of levocetirizine in chronic idiopathic urticaria: a pooled analysis of two randomised controlled trials. Clin Drug Investig. 2006;26:1–11. doi: 10.2165/00044011-200626010-00001. [DOI] [PubMed] [Google Scholar]
- Klimek L. Levocetirizine: from scientific evidence to a potent modern-day treatment of today’s allergic patients. Drugs Today (Barc) 2009;45:213–25. doi: 10.1358/dot.2009.45.3.1339920. [DOI] [PubMed] [Google Scholar]
- Church MK, Maurer M. H1-antihistamines and urticaria: how can we predict the best drug for our patient? Clin Exp Allergy. 2012;42:1423–9. doi: 10.1111/j.1365-2222.2012.03957.x. [DOI] [PubMed] [Google Scholar]
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8–19. doi: 10.1111/j.1365-2222.2011.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons FE, Simons KJ, Frith EM. The pharmacokinetics and antihistaminic of the H1 receptor antagonist hydroxyzine. J Allergy Clin Immunol. 1984;73:69–75. doi: 10.1016/0091-6749(84)90486-x. [DOI] [PubMed] [Google Scholar]
- Murota H, Kitaba S, Tani M, et al. Impact of sedative and non-sedative antihistamines on the impaired productivity and quality of life in patients with pruritic skin diseases. Allergol Int. 2010;59:345–54. doi: 10.2332/allergolint.10-OA-0182. [DOI] [PubMed] [Google Scholar]