Abstract
Summary
Background
Unwanted submental fat (SMF) is aesthetically unappealing, but methods of reduction are either invasive or lack evidence for their use. An injectable approach with ATX-101 (deoxycholic acid) is under investigation.
Objectives
To evaluate the efficacy and safety of ATX-101 for the reduction of unwanted SMF.
Methods
In this double-blind, placebo-controlled, phase III study, 363 patients with moderate/severe SMF were randomized to receive ATX-101 (1 or 2 mg cm−2) or placebo injections into their SMF at up to four treatment sessions ∽28 days apart, with a 12-week follow-up. The co-primary efficacy endpoints were the proportions of treatment responders [patients with ≥ 1-point improvement in SMF on the 5-point Clinician-Reported Submental Fat Rating Scale (CR-SMFRS)] and patients satisfied with their face and chin appearance on the Subject Self-Rating Scale (SSRS). Secondary endpoints included skin laxity, calliper measurements and patient-reported outcomes. Adverse events were monitored.
Results
Significantly more ATX-101 recipients met the primary endpoint criteria vs. placebo: on the clinician scale, 59·2% and 65·3% of patients treated with ATX-101 1 and 2 mg cm−2, respectively, were treatment responders vs. 23·0% for placebo (CR-SMFRS;P < 0·001); on the patient scale, 53·3% and 66·1%, respectively, vs. 28·7%, were satisfied with their face/chin appearance (SSRS;P < 0·001). Calliper measurements showed a significant reduction in SMF (P < 0·001), skin laxity was not worsened and patients reported improvements in the severity and psychological impact of SMF with ATX-101 vs. placebo. Most adverse events were transient and associated with the treatment area.
Conclusions
ATX-101 was effective and well tolerated for nonsurgical SMF reduction.
What's already known about this topic?
Unwanted submental fat (SMF) is considered aesthetically unappealing.
Liposuction and face-lift are effective treatments for SMF reduction but are invasive and not suitable for all patients, whereas nonsurgical alternatives lack robust clinical evidence related to their safety and efficacy.
ATX-101, a proprietary formulation of synthetically derived deoxycholic acid, is the first nonsurgical treatment for SMF reduction to be investigated in randomized, placebo-controlled clinical studies.
What does this study add?
This study provides the first data from a large-scale, randomized, placebo-controlled, phase III study of an injectable therapy for SMF reduction in a field currently lacking a sound evidence base.
ATX-101 was superior to placebo for the clinician- and patient-evaluated reduction of unwanted SMF and led to improved patient perception about their appearance.
ATX-101 was well tolerated; treatment-associated adverse events were mainly transient and localized injection-site reactions.
Loss of definition in the submental area and an obtuse cervicomental angle may result from unwanted submental fat (SMF), which is considered aesthetically unappealing.1,2 Undesirable SMF can have a psychological impact, particularly because it is suggestive of ageing or obesity.1–3 This SMF may derive from a genetic predisposition,4 ageing5 and/or lifestyle and diet.4,5
Unwanted SMF can be addressed surgically as part of a platysmaplasty (with or without face-lift) or liposuction of the neck and chin.1,6 These procedures are effective but may not be suitable for all patients,5 and the need for anaesthesia, an operating room and qualified staff substantially increases costs and risks.1 Liposuction, with or without an accompanying energy device,7,8 is the most commonly used intervention and, although less invasive than platysmaplasty or face-lift, may result in postoperative complications and an unfavourable result, such as prominent anterior platysmal banding.6,9 Furthermore, invasive procedures may require significant recovery times, occasionally up to 1 year.10 Nonsurgical energy devices exist,7 but clinical evidence supporting their efficacy is limited. An efficient, evidence-based, minimally invasive treatment approach may be an appropriate option for reducing unwanted SMF.
Unregulated injectables for localized fat reduction, often based on a combination of phosphatidylcholine and deoxycholate, have been available for some time. Deoxycholate, and not phosphatidylcholine, has been shown to be the active lytic agent in these preparations.11 ATX-101, the subject of this study, is an injectable proprietary formulation of synthetically derived deoxycholic acid (DCA), which disrupts adipocyte membranes leading to irreversible cell breakdown (adipocytolysis).12 After adipocytolysis, a mild inflammatory response is induced, with macrophage recruitment and phagocytosis,12–14 through which cellular debris is cleared over time. Plasma lipid levels show no meaningful increase over time after ATX-101 treatment.13 DCA does not accumulate in adipose tissue, owing to rapid clearance facilitated by protein binding, which also attenuates the action of DCA in nonlipid-rich tissues such as muscle and bone.12
ATX-101 demonstrated efficacy in the reduction of SMF with appropriate tolerability in phase I and II studies,15–18 and is the first pharmacological adipocytolytic formulation to be investigated in large-scale randomized, controlled clinical studies. Results from the first European phase III study of ATX-101 for the reduction of unwanted SMF are presented here.
Methods
Study design
This multicentre, phase III, randomized, double-blind, placebo-controlled, parallel-group study evaluated the efficacy and safety of ATX-101 administered at fixed doses of 1 and 2 mg cm−2. The study was divided into a screening period (week –8 to baseline; visit 1), followed by a 12-week treatment period (visits 2–5) and a 12-week efficacy and safety follow-up period (visits 6 and 7). Patients were recruited at 28 centres in Belgium, France, Germany, Spain and the United Kingdom. Written informed consent was obtained from all patients in accordance with the ethical principles established in the Declaration of Helsinki and the International Conference on Harmonisation guideline E6: Good Clinical Practice, and all local legal and regulatory requirements were followed.
Inclusion and exclusion criteria
Men and women aged 18–65 years were eligible to participate if they presented with moderate or severe SMF [grade 2 or 3 on the 5-point Clinician-Reported Submental Fat Rating Scale (CR-SMFRS)] and expressed dissatisfaction with the appearance of their submental area [Subject Self-Rating Scale (SSRS) score 0–3] at visit 2. Patients agreed to undergo clinical evaluations and laboratory tests and to maintain stable body weight, diet and exercise practices during the study. Women of reproductive age were required not to be pregnant or lactating and to practise birth control during the study. The principal exclusion criteria were: previous intervention to treat SMF; anatomical features or previous trauma liable to interfere with SMF evaluation or result in an aesthetically unacceptable outcome after treatment; evidence of any cause of submental enlargement other than SMF; and any medical condition likely to affect safety or efficacy assessments or the patient's ability to undergo study procedures or provide informed consent. Patients with a body mass index (BMI) > 30 kg m−2, those undergoing or considering a weight-reduction programme, and patients with a history of sensitivity to any components of the study material or topical or local anaesthetics were also excluded.
Randomization
Patients were randomized (1 : 1 : 1) at visit 2, after completion of baseline evaluations, through allocation of a unique randomization number using a computerized web/voice-response system. ATX-101 and placebo treatment kits had an identical appearance and carried a blinded label with a random kit number; these were assigned to patients using the computerized system.
Interventions
All randomized patients were to have at least one treatment session, with a maximum of four sessions, separated by 28 ± 5 day intervals (visits 2–5). Patients received up to 10 mL of the study drug per treatment session, and the number of sessions was dependent on the amount of remaining SMF and each patient's satisfaction with the appearance of their face and chin. Injections were administered subcutaneously directly into the pre-platysmal SMF at a volume of 0·2 mL per injection and spaced approximately 1·0 cm apart using a grid to provide even coverage. Topical anaesthesia was provided, if needed. Premature treatment discontinuation could occur owing to adverse events (AEs), early therapeutic success or at the patient's request. The treatment area was evaluated 7 ± 3 days after each treatment and concomitant medication use was reported. Patients attended two follow-up visits (visits 6 and 7) 4 weeks (± 5 days) and 12 weeks (± 7 days) after the final treatment session.
Efficacy outcome measures
Efficacy endpoints (Table 1) were evaluated at visit 7 (12 weeks after final treatment). There were two co-primary efficacy endpoints: the proportion of treatment responders, i.e. with a reduction in SMF of ≥ 1 point on the 5-point CR-SMFRS relative to baseline, and the proportion of patients satisfied with their appearance in association with their face and chin (i.e. with a score of ≥ 4 on the 7-point SSRS rating scale). To confirm the primary endpoint results, sensitivity analyses were conducted on the secondary parameters of change from baseline in CR-SMFRS and SSRS scores, and changes in calliper measurements of SMF thickness, by treatment session. The effect of treatment on skin laxity (Skin Laxity Rating Scale, SLRS) was evaluated, and patient-reported outcomes were assessed using the Patient-Reported Submental Fat Rating Scale (PR-SMFRS) and Patient-Reported Submental Fat Impact Scale (PR-SMFIS). Additional patient-reported instruments were also used but outcomes are not discussed here.
Table 1.
Efficacy parameters and scales used in the study
| Efficacy outcome | Evaluator | Outcome measure | Method of evaluation | Rating scale (range) |
|---|---|---|---|---|
| Primary | Physician | SMF severity (submental convexity and amount of SMF) | Clinician-Reported SMF Rating Scale (CR-SMFRS) | 0 (absent) to 4 (extreme) |
| Primary | Patient | Satisfaction with appearance in association with the face and chin | Subject Self-Rating Scale (SSRS) | 0 (extremely dissatisfied) to 6 (extremely satisfied) |
| Secondary | Physician | Skin laxity | Skin Laxity Rating Scale (SLRS) | 1 (no laxity) to 4 (very lax) |
| Secondary | Patient | Perceived SMF severity | Patient-Reported SMF Rating Scale (PR-SMFRS) | 1 (absent) to 5 (very large amount) |
| Secondary | Patient | Psychological impact of SMF appearance on feelings and perceived visual self-image | Patient-Reported SMF Impact Scale (PR-SMFIS) | 0 (not at all) to 10 (extremely) |
| Secondary | Objective | SMF thickness | Calliper measurement | Measured continuously (mm) |
SMF, submental fat.
Safety outcome measures
AEs were evaluated at each visit and approximately 7 days after each treatment session, and were characterized descriptively by the day on which they started and stopped, and by severity and intensity. Treatment-emergent AEs were defined as those with onset or exacerbation after the first treatment dose. Changes from baseline in clinical laboratory parameters and other tests, and variations in vital signs, body temperature and body weight, were measured.
Statistical methodology
Using 80% of the effect size observed in two previous placebo-controlled phase II studies (NCT00618722 and NCT00618618) and a 10% dropout rate per treatment group with a Pearson chi-square test for two proportions (two-sided test with α = 0·025), we determined a conservatively rounded sample size of 120 patients per treatment group to guarantee a power of 90%.
The efficacy analyses were based on the intention-to-treat population (all randomized patients who had at least one efficacy assessment at baseline), with missing values at visit 7 imputed using last observation carried forward. The null hypothesis for the treatment comparisons was that there was no difference between each dose of ATX-101 and placebo for the two co-primary efficacy endpoints. Comparisons of the two ATX-101 dose groups with placebo were made via odds ratios from binary logistic regression. An adjustment for multiplicity was performed. Because there were two co-primary endpoints, the null hypotheses for both variables had to be rejected at the same significance level (α = 0·05). This was accounted for by using the larger of the two P-values in the Bonferroni–Holm procedure.
For the secondary endpoints, changes from baseline in CR-SMFRS and calliper scores were analysed using a mixed-models repeated-measures analysis. Changes from baseline in SSRS scores were analysed by an analysis of variance (ANOVA). PR-SMFRS improvements of ≥ 1 point were analysed by binary logistic regression. Changes from baseline in SLRS scores were recorded in frequency tables and Pearson's chi-square test was applied to compare each ATX-101 group with placebo. Descriptive statistics were calculated for PR-SMFIS scores, with the change from baseline analysed by a post hoc Fisher's least-square difference test for continuous variables, only if ANOVA showed an overall treatment effect. Statistical summaries for AEs were based on treatment-emergent AEs in the safety population (all patients who received at least one treatment with study drug). The numbers of AEs and patients presenting with each AE were categorized according to association with treatment, study withdrawal, death, severity, intensity, system organ class and preferred term.
Results
Baseline characteristics
Between December 2010 and January 2012, 363 patients successfully completed screening and baseline evaluations and were randomized to receive ATX-101 1 mg cm−2 (n = 120), ATX-101 2 mg cm−2 (n = 121) or placebo (n = 122) (Fig.1). One patient randomized to the lower ATX-101 dose did not receive treatment; therefore, 362 patients were treated (safety population). Baseline characteristics were similar between treatment groups (Table 2). Overall, the majority of patients were female (76·5%) and white (94·8%), although other Fitzpatrick skin types were represented. Patients' mean BMI was 25·7 kg m−2, and the mean age was 46·4 years. At baseline, 68% of patients in the intention-to-treat population had a baseline CR-SMFRS score of 2 (moderate submental convexity) and 32% had a score of 3 (severe convexity); 94% of patients had a baseline SSRS score between 0 (extremely dissatisfied) and 2 (slightly dissatisfied). No significant demographic differences were detected between treatment groups, confirming an unbiased randomization.
Figure 1.
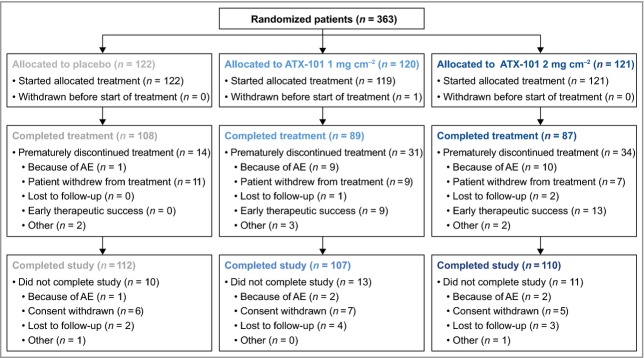
Disposition of randomized subjects. Premature treatment discontinuation: patient still completed study follow-up visits. Did not complete study: patient did not complete follow-up visits. AE, adverse event.
Table 2.
Patient baseline characteristics (safety population)
| Variable | Placebo (n = 122) | ATX-101 | Overall (N = 362) | |
|---|---|---|---|---|
| 1 mg cm−2 (n = 119) | 2 mg cm−2 (n = 121) | |||
| Age (years); mean (SD) | 46·6 (10·17) | 45·9 (10·96) | 46·7 (9·78) | 46·4 (10·29) |
| Sex (%) | ||||
| Female | 71·3 | 79·8 | 78·5 | 76·5 |
| Male | 28·7 | 20·2 | 21·5 | 23·5 |
| Race (%) | ||||
| White | 95·9 | 92·4 | 95·9 | 94·8 |
| Other | 4·1 | 7·6 | 4·1 | 5·2 |
| BMI (kg m−2); mean (SD) | 25·5 (2·76) | 25·9 (2·72) | 25·7 (3·06) | 25·7 (2·85) |
BMI, body mass index.
Similar to placebo (91·8%), more than 90% of patients treated with ATX-101 completed the study. Premature treatment discontinuation (fewer than four treatment sessions) was more frequent in both ATX-101 groups (25·8% and 28·1%) than with placebo (11·5%) (Fig.1). In total, 284 (78·2%) patients completed all four treatment sessions. Both early treatment success and AEs resulted in a higher rate of treatment discontinuation with ATX-101 compared with placebo (Fig.1).
Primary efficacy outcomes
At 12 weeks after the final treatment, the proportion of treatment responders (≥ 1-point improvement in the 5-point CR-SMFRS) was significantly greater in both ATX-101 groups than in the placebo group, with a trend towards greater efficacy with the higher ATX-101 dose (1 mg cm−2: 59·2%; 2 mg cm−2: 65·3%; placebo: 23·0%; P < 0·001 for both ATX-101 doses vs. placebo). Odds ratios were 4·73 [95% confidence interval (CI) 2·70–8·28] for ATX-101 1 mg cm−2 and 6·21 (95% CI 3·52–10·94) for ATX-101 2 mg cm−2 (Fig.2). The proportion of patients satisfied with the appearance of their face and chin after treatment (score ≥ 4 on the 7-point SSRS) was significantly greater in both ATX-101 groups compared with placebo (53·3% and 66·1%, respectively, vs. 28·7%; P < 0·001 for both ATX-101 doses vs. placebo) (Fig.3). Odds ratios were 2·99 (95% CI 1·74–5·14) for ATX-101 1 mg cm−2 and 5·22 (95% CI 2·99–9·11) for ATX-101 2 mg cm−2, respectively, vs. placebo. According to the predefined confirmatory testing procedure, statistically significant efficacy was achieved for both ATX-101 dose groups (P < 0·001).
Figure 2.
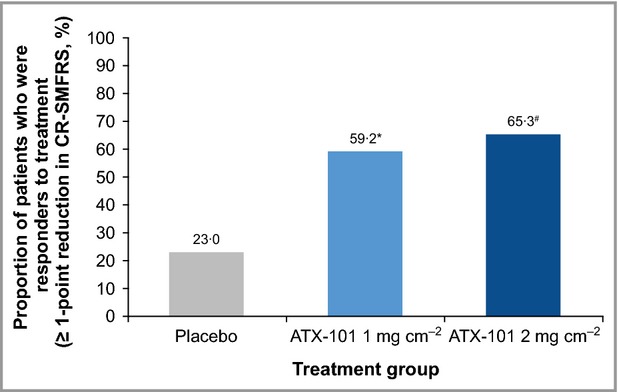
Proportion of treatment respondersa at visit 7b (12 weeks after the final treatment). *OR = 4·73 (95% CI 2·70–8·28); P < 0·001. #OR = 6·21 (95% CI 3·52–10·94); P < 0·001. a≥ 1-point reduction in submental fat on the 5-point Clinician-Reported Submental Fat Rating Scale (CR-SMFRS); blast observation carried forward. Intention-to-treat population. P-values calculated using binary logistic regression. CI, confidence interval; OR, odds ratio.
Figure 3.
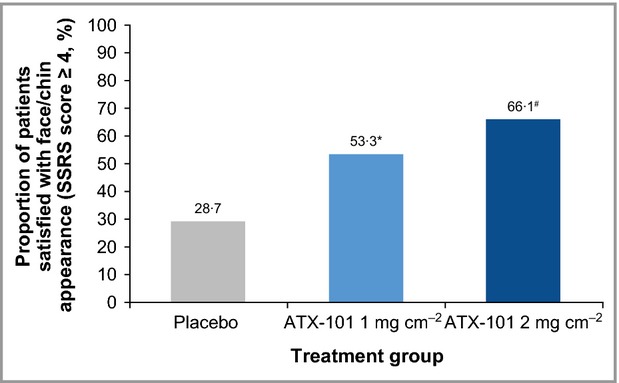
Proportion of patients satisfied with their appearance in association with their face and china at visit 7b (12 weeks after the final treatment). *OR = 2·99 (95% CI 1·74–5·14); P < 0·001. #OR = 5·22 (95% CI 2·99–9·11); P < 0·001. aScore ≥ 4 out of a maximum of 6 on the Subject Self-Rating Scale (SSRS); blast observation carried forward. Intention-to-treat population. P-values calculated using binary logistic regression. CI, confidence interval; OR, odds ratio.
Photographs of representative patients treated with ATX-101 2 mg cm−2 are shown in Figure4.
Figure 4.
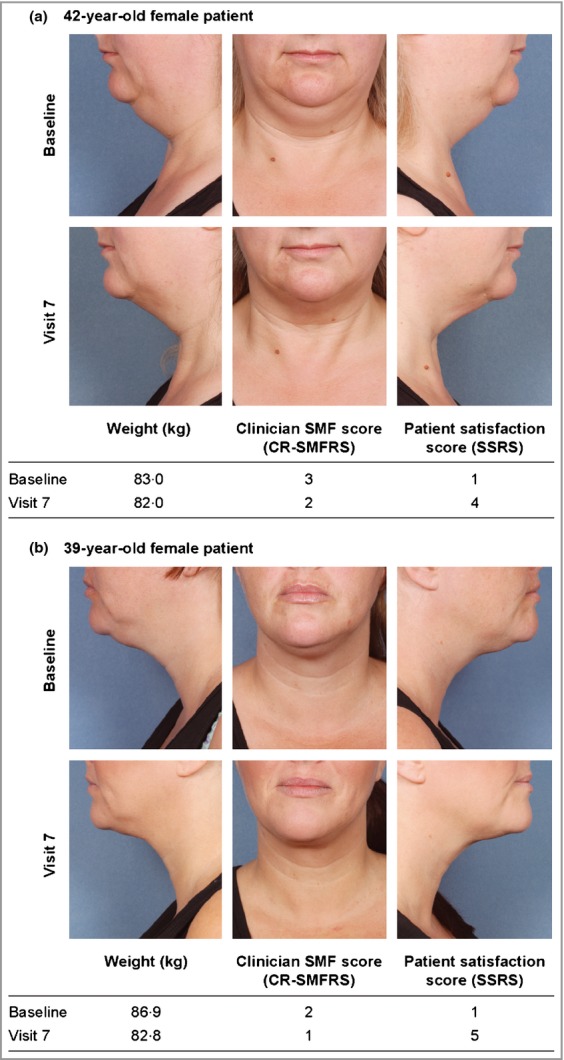
Representative images of the aspect of the submental fat of patients before treatment (baseline) and after treatment (12 weeks after the final treatment) with ATX-101 2 mg cm−2. Note: the patients shown here have provided written, informed consent for their images to be published. CR-SMFRS, Clinician-Reported Submental Fat Rating Scale; SMF, submental fat; SSRS, Subject Self-Rating Scale (satisfaction in association with the appearance of the face and chin).
Secondary efficacy outcomes
Mean changes from baseline at visit 7 in CR-SMFRS score were statistically greater with ATX-101 than placebo (–0·7 points and –0·9 points for ATX-101 1 and 2 mg cm−2, respectively, vs. –0·2 points for placebo; P < 0·001 for both ATX-101 doses vs. placebo). Reductions in the amount of SMF were more pronounced after the first two or three ATX-101 treatments but continued throughout the treatment period (Fig.5). For some ATX-101 recipients (7·5% and 10·7%, respectively), but no placebo recipients, fewer than four treatment sessions were required owing to early therapeutic success. SMF reduction was further confirmed by mean changes from baseline in SMF thickness (measured using callipers), which were significantly greater with ATX-101 than placebo (–3·8 and –4·2 mm, respectively, vs. –1·7 mm; P < 0·001 for both ATX-101 doses vs. placebo). Most patients treated with ATX-101 and placebo showed either no change (61·3% and 66·4%, respectively, vs. 75·9%), or an improvement (30·2% and 21·8%, respectively, vs. 13·4%) in skin laxity (SLRS).
Figure 5.
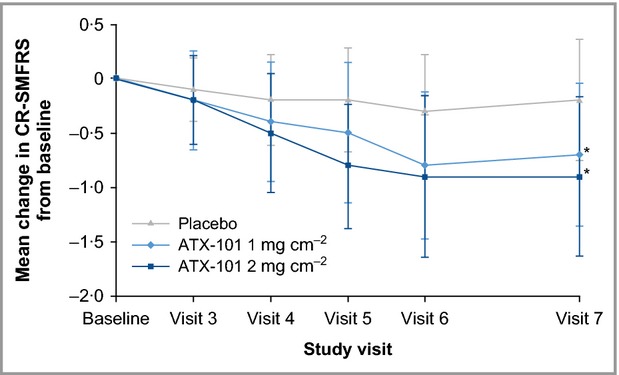
Change (mean ± SD) in CR-SMFRS from baseline by study visit. *P < 0·001 vs. placebo. Intention-to-treat population. Changes from baseline analysed using an overall analysis of variance (ANOVA). CR-SMFRS, Clinician-Reported Submental Fat Rating Scale.
Significantly more patients treated with ATX-101 than placebo reported an improvement of ≥ 1 point in PR-SMFRS score vs. baseline at visit 7 (67·0% and 73·6% for ATX-101 1 and 2 mg cm−2, respectively, and 32·4% for placebo; P < 0·001 for both ATX-101 doses vs. placebo). ATX-101 recipients (vs. placebo) also reported statistically significant improvements in the psychological impact of their SMF regarding the PR-SMFIS criteria of feeling happier, less bothered, less self-conscious, less embarrassed, looking less old and looking less overweight (P < 0·001 overall and for each criterion; Fig.6). Consistent with the primary efficacy measures, this improvement was more pronounced for the ATX-101 2 mg cm−2 group.
Figure 6.
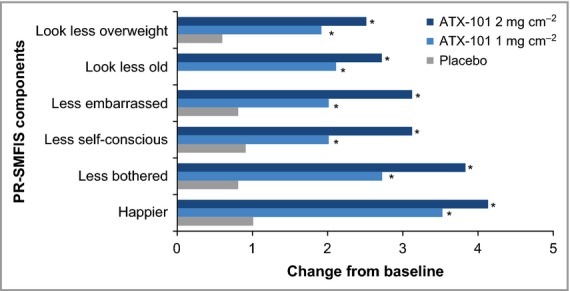
Change in PR-SMFIS single-item scores (assessing psychological impact of SMF appearance on feelings and perceived visual self-image) from baseline to visit 7 (12 weeks after the final treatment). *P < 0·001. Changes from baseline were analysed by an overall analysis of variance (ANOVA) and pairwise Fisher's Least Significant Difference tests for continuous variables. Intention-to-treat population. PR-SMFIS, Patient-Reported Submental Fat Impact Scale.
Safety outcomes
Treatment-emergent AEs related to the study treatment were experienced by 50·8% of patients receiving placebo, compared with 90·8% and 95·0% of patients receiving ATX-101 1 and 2 mg cm−2, respectively. Most treatment-emergent AEs were associated with the treatment area (in 59·0% vs. 93·3% and 95·0% of patients, respectively). Among AEs with the highest incidence, injection-site pain, swelling, numbness, bruising, erythema and induration occurred more frequently in the ATX-101 groups than with placebo (Table 3). These AEs were predominantly transient and commonly resolved within the 28-day interval between treatments. The median duration of pain was 1·0 day for all groups. Swelling had a median duration of 9·0 and 10·0 days for the ATX-101 1 and 2 mg cm−2 groups, and 3·0 days for placebo. Numbness and induration generally resolved within 17–25 days after treatment. Most treatment area-associated events were mild or moderate in intensity in the placebo group (57·4% of patients) and in the ATX-101 1 and 2 mg cm−2 treatment groups (67·2% and 65·2%, respectively). The exception was injection-site pain occurring during and after the injections, with a higher proportion of patients who received ATX-101 reporting moderate (32·8% and 35·5% for ATX-101 1 and 2 mg cm−2, respectively, vs. 10·7% for placebo) to severe intensity (26·1% and 27·3%, respectively, vs. 0·8%).
Table 3.
Treatment-emergent adverse events at the injection site (incidence ≥ 10%)
| Adverse event by injection-site category | Incidence (%) | ||
|---|---|---|---|
| Placebo | ATX-101 1 mg cm−2 | ATX-101 2 mg cm−2 | |
| Pain including burning | 25·4 | 77·3 | 80·2 |
| Swelling including oedema | 30·3 | 65·5 | 66·1 |
| Bruising including bleeding | 41·0 | 55·5 | 53·7 |
| Numbness | 2·5 | 47·9 | 51·2 |
| Erythema | 23·0 | 38·7 | 37·2 |
| Induration including fibrosis | 2·5 | 18·5 | 26·4 |
No deaths occurred, and of the seven serious AEs reported (abdominal adhesions, gastric cancer, spontaneous abortion, depression, chronic tonsillitis, non-Hodgkin lymphoma and sleep apnoea syndrome), all were outside the treatment area and none was related to the study drug. Twenty patients discontinued the study drug owing to treatment-emergent AEs; most received ATX-101 (9 and 10 patients in the ATX-101 1 and 2 mg cm−2 groups, respectively, vs. one patient in the placebo group). In 18 of these patients, an AE that was drug-related and associated with the treatment area was recorded as at least one of the reasons for discontinuation [most commonly injection-site pain (13 patients)]. Clinical laboratory and other parameters generally remained in the normal range, and no clinically relevant differences were observed between the treatment groups.
Discussion
ATX-101 (vs. placebo) resulted in significantly reduced SMF severity and increased patient satisfaction with the appearance of the face and chin, evaluated using primary efficacy outcome measures. Significant efficacy was achieved for both ATX-101 dose groups. Secondary efficacy outcomes, including calliper measurements, demonstrated significant reductions in SMF with ATX-101 compared with placebo. No overall worsening of skin laxity was seen. ATX-101 treatment was associated with improvements in the psychological impact of SMF. Overall, the ATX-101 2 mg cm−2 dose showed a trend towards better efficacy outcomes compared with the 1 mg cm−2 dose but it should be noted that this study was not designed to compare the two ATX-101 doses.
The efficacy measures used in this study (clinician and patient ratings) were found to be appropriate for the assessment of SMF following formal validation studies (data on file). Although a relatively simple tool that is potentially subject to investigator-dependent variability, calliper measurements demonstrated a reduction in SMF with ATX-101 compared with placebo. These results are consistent with observations from a phase II study (currently unpublished), in which the objective measurement tool was magnetic resonance imaging.15 Although magnetic resonance imaging would have been more precise, it is laborious and would not have added greatly to the evidence provided by the clinical efficacy scales.
Both ATX-101 doses were well tolerated. Most AEs occurred in association with the treatment area, were temporally associated with treatment, and were mild or moderate in intensity. Most AEs resolved in the interval between treatments and generally did not lead to treatment discontinuation. Although more treatment area-associated AEs occurred with ATX-101 than with placebo, and it might be expected that patients could infer that they were receiving active treatment based on AEs, the distribution of events among the ATX-101 and placebo groups suggested that the integrity of the blinding was maintained. Furthermore, patients receiving active treatment had no basis for comparison with placebo treatment, and positive treatment responses using patient outcome measures, including satisfaction with appearance of the face and chin, were recorded in all groups.
Of the most common injection-site AEs, localized pain, swelling, bruising and erythema may result partially from a response to the injections, as suggested by the relatively high frequency of these events with placebo. However, the greater number of such events, and also of numbness and induration, in the ATX-101 groups may also relate to the adipocytolytic action of DCA. Adipocytolysis leads to a mild inflammatory response and clearance of the lysed cellular material from the injection site.12–14 Therefore, these AEs may result from a combination of both the mechanism of delivery and action of the agent. It is possible that higher doses of DCA might be associated with more severe AEs affecting nonadipose cell types.19 However, this would be unlikely with the concentrations of DCA used in this study.12
In this study, a substantial placebo effect was observed, exceeding 30% for some outcome measures. The placebo effect is a well-known phenomenon, particularly with aesthetic treatments,20 and should be taken into account when looking at case series data from other interventions for the reduction of SMF.21 Perhaps unsurprisingly, the placebo response was greatest for the patient-reported outcomes (e.g. SSRS, PR-SMFRS). Nevertheless, ATX-101 treatment was associated with significantly better outcomes than placebo across the range of efficacy measures evaluated and, although the CR-SMFRS response for placebo remained fairly constant over the four treatment sessions, a consistent improvement was recorded in the active treatment groups (Fig.5).
ATX-101 is a proprietary formulation of synthetically derived DCA and is the first injectable drug for SMF reduction to undergo comprehensive clinical evaluation. Although injectable lipolytic therapies have existed since the 1950s,22 no previous formulation has been subject to appropriate controlled clinical testing and thorough pharmaceutical development. Small-scale studies of deoxycholate with or without phosphatidylcholine for the reduction of small areas of fat have been reported previously.23–27 Concerns were expressed about the unregulated nature of these formulations and many health authorities banned their use. In contrast, this study and three additional phase III clinical studies of ATX-101 (results to be published) will provide the first true evidence base for adipocytolytic therapy with a specific formulation of DCA alone. Given that surgical procedures, such as face-lift and liposuction,1,6 are invasive and not appropriate for all patients,27 and that energy devices have been subject to minimal clinical study, a state-of-the-art and thoroughly tested nonsurgical alternative is warranted.
In this phase III, randomized clinical study, ATX-101 showed a favourable efficacy profile for the nonsurgical reduction of unwanted SMF and was well tolerated.
Acknowledgments and disclosures
The authors would like to thank all the investigators who contributed to and recruited patients for this study: Joan Vandeputte (Belgium); Jean-Paul Ortonne, Bruno Halioua, Isaac Bodokh, Daniel Interligator, Hugues Cartier (France); Thomas Dirschka, Tatjana Pavicic, Ridwan Weber, George Popp, Gerhard Sattler, Diamant Thaci, Torsten Walker, Klaus Fritz, Marc Heckmann, Tanja Fischer, Andrea Graefe, Maurizio Podda (Germany); Jaume Masia Ayala, Jesús Benito Ruiz, Gerardo Moreno Arias (Spain); Ross Martin, Stephanie Ogden, John Tanqueray, Shahida Ullah (United Kingdom). The authors also wish to acknowledge Stephen Purver, who provided editorial support with funding from Bayer HealthCare.
Plain language summary available online
References
- 1.Co AC, Abad-Casintahan MF, Espinoza-Thaebtharm A. Submental fat reduction by mesotherapy using phosphatidylcholine alone vs. phosphatidylcholine and organic silicium: a pilot study. J Cosmet Dermatol. 2007;6:250–7. doi: 10.1111/j.1473-2165.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 2.Hatef DA, Koshy JC, Sandoval SE, et al. The submental fat compartment of the neck. Semin Plast Surg. 2009;23:288–91. doi: 10.1055/s-0029-1242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rzany B, Carruthers A, Carruthers J, et al. Validated composite assessment scales for the global face. Dermatol Surg. 2012;38:294–308. doi: 10.1111/j.1524-4725.2011.02252.x. [DOI] [PubMed] [Google Scholar]
- 4.Duncan DI, Chubaty R. Clinical safety data and standards of practice for injection lipolysis: a retrospective study. Aesthet Surg J. 2006;26:575–85. doi: 10.1016/j.asj.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Patel BCK. Aesthetic surgery of the aging neck: options and techniques. Orbit. 2006;25:327–56. doi: 10.1080/01676830601034532. [DOI] [PubMed] [Google Scholar]
- 6.Koehler J. Complications of neck liposuction and submentoplasty. Oral Maxillofac Surg Clin North Am. 2009;21:43–52. doi: 10.1016/j.coms.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Wollina U, Goldman A, Berger U, Abdel-Naser MB. Esthetic and cosmetic dermatology. Dermatol Ther. 2008;21:118–30. doi: 10.1111/j.1529-8019.2008.00179.x. [DOI] [PubMed] [Google Scholar]
- 8.Paul M, Mulholland RS. A new approach for adipose tissue treatment and body contouring using radiofrequency-assisted liposuction. Aesthetic Plast Surg. 2009;33:687–94. doi: 10.1007/s00266-009-9342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamer FM, Minoli JJ. Postoperative platysmal band deformity. A pitfall of submental liposuction. Arch Otolaryngol Head Neck Surg. 1993;119:193–6. doi: 10.1001/archotol.1993.01880140079013. [DOI] [PubMed] [Google Scholar]
- 10.Abraham MT, Romo T., III Medscape. Liposuction of the face and neck: treatment and management 2 March 2012 (updated). Available at http://emedicine.medscape.com/article/842367-treatment#a1135 (last accessed on 12 September 2013)
- 11.Rotunda AM, Suzuki H, Moy RL, Kolodney MS. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol Surg. 2004;30:1001–8. doi: 10.1111/j.1524-4725.2004.30305.x. [DOI] [PubMed] [Google Scholar]
- 12.Thuangtong R, Bentow JJ, Knopp K, et al. Tissue-selective effects of injected deoxycholate. Dermatol Surg. 2010;36:899–908. doi: 10.1111/j.1524-4725.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- 13.Yagima OdoME, Cuce LC, Odo LM, Natrielli A. Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol Surg. 2007;33:178–88. doi: 10.1111/j.1524-4725.2006.33036.x. [DOI] [PubMed] [Google Scholar]
- 14.Rotunda AM. Injectable treatments for adipose tissue: terminology, mechanism, and tissue interaction. Lasers Surg Med. 2009;41:714–20. doi: 10.1002/lsm.20807. [DOI] [PubMed] [Google Scholar]
- 15.Dover J, Schlessinger J, Young L, et al. Results from a phase IIb study evaluating the efficacy and safety of ATX-101 for reduction of submental fat using magnetic resonance imaging and investigator and subject assessment. 21st Annual Congress of the European Academy of Dermatology and Venereology. Prague, Czech Republic, 27–30 September 2012; Poster P258.
- 16.Goodman G, Smith K, Lee D, et al. ATX-101, a pharmacological treatment, for the reduction of submental fat: results of two pooled phase IIa studies. 21st Annual Congress of the European Academy of Dermatology and Venereology. Prague, Czech Republic, 27–30 September 2012; Poster P259.
- 17.Smith K, Goodman G, Sapra S, et al. ATX-101 treatment offers long-term durability of submental fat reduction: preliminary follow-up study results of subjects from two phase 2A studies. 70th Annual Meeting of the American Academy of Dermatology. San Diego, CA, U.S.A., 16–20 March 2012; Abstract 4899.
- 18.Walker P, Havlickova B, Lee D. ATX-101 for reduction of submental fat: pharmacokinetic and safety findings from a phase I trial. 8th European Masters in Anti-Aging Medicine. Paris, France, 12–14 October 2012; Poster.
- 19.Gupta A, Lobocki C, Singh S, et al. Actions and comparative efficacy of phosphatidylcholine formulation and isolated sodium deoxycholate for different cell types. Aesthetic Plast Surg. 2009;33:346–52. doi: 10.1007/s00266-008-9301-0. [DOI] [PubMed] [Google Scholar]
- 20.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–90. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 21.Finzi E, Spangler A. Multipass vector (mpave) technique with nonablative radiofrequency to treat facial and neck laxity. Dermatol Surg. 2005;31:916–22. doi: 10.1097/00042728-200508000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465–80. doi: 10.1111/j.1524-4725.2006.32100.x. [DOI] [PubMed] [Google Scholar]
- 23.Rittes PG. The use of phosphatidylcholine for correction of localized fat deposits. Aesthetic Plast Surg. 2003;27:315–18. doi: 10.1007/s00266-003-3033-y. [DOI] [PubMed] [Google Scholar]
- 24.Ablon G, Rotunda AM. Treatment of lower eyelid fat pads using phosphatidylcholine: clinical trial and review. Dermatol Surg. 2004;30:422–7. doi: 10.1111/j.1524-4725.2004.30114.x. [DOI] [PubMed] [Google Scholar]
- 25.Rotunda AM, Ablon G, Kolodney MS. Lipomas treated with subcutaneous deoxycholate injections. J Am Acad Dermatol. 2005;53:973–8. doi: 10.1016/j.jaad.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 26.Kopera D, Horejsi R, Werner S, Moeller R. Injection lipolysis for reduction of saddlebag trochanteric bulges – half-side controlled pilot study. J Dtsch Dermatol Ges. 2008;6:287–90. doi: 10.1111/j.1610-0387.2007.06571.x. [DOI] [PubMed] [Google Scholar]
- 27.Rotunda AM, Weiss SR, Rivkin LS. Randomized double-blind clinical trial of subcutaneously injected deoxycholate versus a phosphatidylcholine–deoxycholate combination for the reduction of submental fat. Dermatol Surg. 2009;35:792–803. doi: 10.1111/j.1524-4725.2009.01130.x. [DOI] [PubMed] [Google Scholar]


