Abstract
Background
The current study was conducted to develop a multifactorial statistical model to predict the specific head and neck (H&N) tumor site origin in cases of squamous cell carcinoma confined to the cervical lymph nodes (“unknown primaries”).
Methods
The Surveillance, Epidemiology, and End Results (SEER) database was analyzed for patients with an H&N tumor site who were diagnosed between 2004 and 2011. The SEER patients were identified according to their H&N primary tumor site and clinically positive cervical lymph node levels at the time of presentation. The SEER patient data set was randomly divided into 2 data sets for the purposes of internal split-sample validation. The effects of cervical lymph node levels, age, race, and sex on H&N primary tumor site were examined using univariate and multivariate analyses. Multivariate logistic regression models and an associated set of nomograms were developed based on relevant factors to provide probabilities of tumor site origin.
Results
Analysis of the SEER database identified 20,011 patients with H&N disease with both site-level and lymph node-level data. Sex, race, age, and lymph node levels were associated with primary H&N tumor site (nasopharynx, hypopharynx, oropharynx, and larynx) in the multivariate models. Internal validation techniques affirmed the accuracy of these models on separate data.
Conclusions
The incorporation of epidemiologic and lymph node data into a predictive model has the potential to provide valuable guidance to clinicians in the treatment of patients with squamous cell carcinoma confined to the cervical lymph nodes.
Keywords: unknown primary, Surveillance, Epidemiology, End Results (SEER), cervical lymph nodes, predictive model, radiation
Introduction
Squamous cell carcinoma (SCC) confined to cervical lymph nodes, also known as head and neck (H&N) cancer of an unknown primary (CUP), refers to a clinical scenario in which a patient presents with biopsy-proven cervical adenopathy yet appropriate workup reveals no evidence of the primary H&N malignancy. SCC from an unknown primary tumor represents approximately 2% to 4% of all H&N malignancies.1,2 The standard workup in a patient presenting with a histologically confirmed H&N SCC cervical mass without an obvious primary tumor is careful history and physical examination, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT), and then evaluation under anesthesia with appropriate directed (“blind”) biopsies and elective tonsillectomies.3 The use of combination 18F-FDG-PET/CT has become the standard of care for the noninvasive component of the diagnostic evaluation of a CUP.4 A recent meta-analysis found that the diagnostic detection rate of 18F-FDG-PET/CT was 37%, with a sensitivity of 84% and a specificity of 84%.5 Cianchetti et al found that panendoscopy with directed biopsies successfully identified the primary H&N tumor site in 29.2% of patients with H&N cancer with a CUP.6 In addition, the use of an ipsilateral tonsillectomy has been shown to have a higher diagnostic yield than a simple deep tonsil biopsy.7–10 There has also been recent enthusiasm for the incorporation of transoral robotic biopsy for evaluation of CUP.11–13 Although the identification rate for the unknown primary site may increase to 77.3%,12 which is certainly promising, the question of morbidity after elective lingual tonsillectomy, either acute or in terms of swallowing dysfunction after radiotherapy (or chemoradiation), has, to our knowledge, not yet been well addressed to date. As with any many new techniques in oncology, “the good news always comes first.”14
Among patients with an unknown primary site who were treated with surgery alone, the primary lesion appeared after treatment in only 20% of patients (with the hypopharynx and oropharynx being the most common sites). It is important to know that this low rate of delayed appearance of the primary tumor was demonstrated in an article written in 1973, well before both contemporary imaging and flexible endoscopy were available. The combination of surgery and radiotherapy improved the ipsilateral neck control from 76% to 86% and the addition of radiation improved the contralateral neck control from 84% to 100%.15
For CUP, physicians in clinical practice correlate the involved cervical lymph node level(s) at the time of presentation with the most likely corresponding H&N site of primary malignancy and treat the patient accordingly.16 This intuitive correlation is based on work originally published by Lindberg in 1972.17 Although much has changed since that time, including quantum leaps in imaging technology (PET, magnetic resonance imaging, CT, etc) and state-of-the-art diagnostic and surgical techniques, the fundamentals of that work remain to this day.17
If a CUP workup fails to identify a primary H&N site for SCC confined to the cervical lymph nodes, patients are often treated with neck dissection followed by definitive external beam radiotherapy to a wide field. The traditional “usual suspects” have led radiation oncologists to electively treat the nasopharynx, oropharynx, and hypopharynx/larynx.3,18,19 The target selection is based partly on the accessibility of the oral cavity and the low probability of an unknown primary tumor being located there as well as the relative difficulty in evaluating the nasopharynx and the hypopharynx, especially during the pre-fiber optic period. Given the significant acute and late toxicities associated with comprehensive mucosal irradiation and the low incidence of larynx/hypopharynx primary malignancies, some institutions are routinely only irradiating the oropharynx ± the nasopharynx for unknown H&N primary malignancies.20–23
Therefore, the purpose of the current study was to present a multifactorial logistical regression model incorporating the involved cervical lymph node level at the time of presentation and epidemiologic data to calculate the probability of an H&N tumor site of origin for patients with SCC confined to the cervical lymph nodes.
Materials and Methods
Data Source
Involved cervical lymph node levels, age, race, and sex were included in the model used in the current study. These factors were all obtained from the Surveillance, Epidemiology, and End Results (SEER) 17 registry, which was released in April 2013 and includes tumors diagnosed between 2004 and 2011.24 This database was accessed using SEER*Stat (version 7.1.0). The inclusion criteria for the case listing session required that all cases have a known age, race, sex, cervical lymph node level, and primary H&N tumor site. Cases included only a patient’s first diagnosis of cancer and those registered at the time of autopsy or death certificate only were excluded from the analysis. Lymph node-level data were parsed from Collaborative Stage site-specific factors 3 and 4 of the SEER data for H&N tumor site, which included information regarding lymph node involvement for levels 1, 2, and 3 and levels 4, 5, and retropharyngeal lymph nodes, respectively. Race was categorized into white (non-Hispanic), Hispanic, black, and Asian (Asian or Pacific Islander) using SEER race recode variables. Age at the time of diagnosis was used for patient age.
Statistical Analysis
Descriptive statistics were generated for the SEER data, along with an analysis of race, sex, age, and lymph node level by primary tumor site using either chi-square tests or analysis of variance where appropriate.25 A split-sample internal validation procedure was performed to establish that the models worked sufficiently among patients other than those whose data generated the model. Observations were assigned a random number between 0 and 1, and those observations whose random number fell between 0 and 0.5 were placed in the test set. Observations whose random number fell between 0.5 and 1 were placed in the validation (or training) set. Univariate and multivariate logistic regression models were fit for each primary tumor site separately for the test set. For example, for the larynx model, the outcome was either larynx or not larynx. As a result, we determined the effect of race, sex, age, and each cervical lymph node on the presence of each primary site while adjusting for the other covariates. Each primary site multivariate model contained the same covariates for consistency. Graphical nomograms, derived from the multivariate logistic regression model, were created for each site using the R statistical package rms (R Foundation for Statistical Computing, Vienna, Austria). Plots of observed versus predicted estimates at each decile for the validation set were produced, and the root mean square error (RMSE) was calculated for both the test set and validation set using the model generated from the test set. RMSE is the square root of the difference between the observed and predicted values squared divided by the number of observations, and is a measure of accuracy. Similar RMSE values between the test and validation sets indicate adequate model validation. Logistic regression model fit also was assessed using the c-statistic. Assumptions for chi-square tests, analysis of variance, and logistic regression were checked and verified. The descriptive statistics, comparisons, and logistic regression models were produced using SAS statistical software (version 9.3; SAS Institute Inc, Cary, NC), and significance was set at α = .05.26–28
Results
In the analysis of the SEER data, a total of 20,011 cases were identified with complete data regarding the primary tumor site, cervical lymph node level, age, sex, and race (Table 1). The data set consisted of 16,212 males (81.0%) and the mean age was 59 years. The majority of cases (64.1%) were located in the oropharynx (Table 1). As seen in Table 2, it was found that race, sex, and age were all significantly associated with H&N primary sites (P < .001), thus indicating that these variables would likely be useful in differentiating between H&N primary tumor sites in an unknown primary predictive model. In addition, in Table 2, it is shown that all cervical lymph node levels were found to be significantly associated with H&N primary sites (P < .001). This finding indicates that the level of cervical lymph node involvement at the time of presentation of an H&N unknown primary malignancy would also be useful in differentiating between H&N primary sites in an unknown primary predictive model.
Table 1.
SEER Data Descriptive Statistics
| Variable | Level | N = 20,011 | % |
|---|---|---|---|
| Sex | Female | 3799 | 19.0 |
| Male | 16,212 | 81.0 | |
| Primary tumor site | Oropharynx | 12,829 | 64.1 |
| Nasopharynx | 1650 | 8.2 | |
| Hypopharynx | 1854 | 9.3 | |
| Larynx | 3678 | 18.4 | |
| Race | Asian | 1307 | 6.5 |
| Black | 2458 | 12.3 | |
| Hispanic | 1289 | 6.4 | |
| White | 14,957 | 74.7 | |
| Level 1 lymph nodes | Not involved | 15,294 | 76.4 |
| Involved | 4717 | 23.6 | |
| Level 2 lymph nodes | Not involved | 5612 | 28.0 |
| Involved | 14,399 | 72.0 | |
| Level 3 lymph nodes | Not involved | 12,723 | 63.6 |
| Involved | 7288 | 36.4 | |
| Level 4 lymph nodes | Not involved | 16,587 | 82.9 |
| Involved | 3424 | 17.1 | |
| Level 5 lymph nodes | Not involved | 17,210 | 86.0 |
| Involved | 2801 | 14.0 | |
| Retropharyngeal lymph nodes | Not involved | 19,456 | 97.2 |
| Involved | 555 | 2.8 | |
| Age, y | Mean | 59.37 | — |
| Median | 59 | — | |
| Minimum | 1 | — | |
| Maximum | 100 | — | |
| SD | 11.34 | — |
Abbreviation: SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results.
Table 2.
Relationship of Head and Neck Primary Tumor Site With SEER Variables
| Covariate | Statistics | Level | Oropharynx N=12,829 | Nasopharynx N=1650 | Hypopharynx N=1854 | Larynx N=3678 | Parametric Pa |
|---|---|---|---|---|---|---|---|
| Sex | No. (row %) | Female | 2108 (55.49) | 460 (12.11) | 332 (8.74) | 899 (23.66) | <.001 |
| No. (row %) | Male | 10,721 (66.13) | 1190 (7.34) | 1522 (9.39) | 2779 (17.14) | ||
| Race | No. (row %) | Asian | 382 (29.23) | 682 (52.18) | 116 (8.88) | 127 (9.72) | <.001 |
| No. (row %) | Black | 1240 (50.45) | 210 (8.54) | 319 (12.98) | 689 (28.03) | ||
| No. (row %) | Hispanic | 758 (58.81) | 141 (10.94) | 129 (10.01) | 261 (20.25) | ||
| No. (row %) | White | 10,449 (69.86) | 617 (4.13) | 1290 (8.62) | 2601 (17.39) | ||
| Level 1 lymph nodes | No. (row %) | Not involved | 9680 (63.29) | 1240 (8.11) | 1471 (9.62) | 2903 (18.98) | <.001 |
| No. (row %) | Involved | 3149 (66.76) | 410 (8.69) | 383 (8.12) | 775 (16.43) | ||
| Level 2 lymph nodes | No. (row %) | Not involved | 3230 (57.56) | 510 (9.09) | 650 (11.58) | 1222 (21.77) | <.001 |
| No. (row %) | Involved | 9599 (66.66) | 1140 (7.92) | 1204 (8.36) | 2456 (17.06) | ||
| Level 3 lymph nodes | No. (row %) | Not involved | 8534 (67.08) | 1107 (8.7) | 1021 (8.02) | 2061 (16.2) | <.001 |
| No. (row %) | Involved | 4295 (58.93) | 543 (7.45) | 833 (11.43) | 1617 (22.19) | ||
| Level 4 lymph nodes | No. (row %) | Not involved | 10,934 (65.92) | 1309 (7.89) | 1436 (8.66) | 2908 (17.53) | <.001 |
| No. (row %) | Involved | 1895 (55.34) | 341 (9.96) | 418 (12.21) | 770 (22.49) | ||
| Level 5 lymph nodes | No. (row %) | Not involved | 11,396 (66.22) | 1112 (6.46) | 1539 (8.94) | 3163 (18.38) | <.001 |
| No. (row %) | Involved | 1433 (51.16) | 538 (19.21) | 315 (11.25) | 515 (18.39) | ||
| Retropharyngeal lymph nodes | No. (row %) | Not involved | 12,586 (64.69) | 1475 (7.58) | 1784 (9.17) | 3611 (18.56) | <.001 |
| No. (row %) | Involved | 243 (43.78) | 175 (31.53) | 70 (12.61) | 67 (12.07) | ||
| Age, y | No. | 12,829 | 1650 | 1854 | 3678 | <.001 | |
| Mean | 59.1 | 52.23 | 63.29 | 61.52 | |||
| Median | 58 | 53 | 62 | 61 |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
The parametric P value was calculated using the analysis of variance for numerical covariates and the chi-square test for categorical covariates.
Bold values indicate statistical significance less than 0.05.
A univariate logistic regression was performed (see online supporting information). All variables were statistically significant for the oropharynx. However, for the nasopharynx, involvement of level 1 lymph nodes did not statistically predict for involvement of the nasopharynx, whereas all other variables were found to be statistically significant. Likewise, for the larynx, involvement of level 5 lymph nodes was not statistically as useful as the other variables. In the hypopharynx, the epidemiological factors of sex and race were not found to be statistically significant, but all the cervical lymph node levels did reach statistical significance.
Multivariate logistic regression models were derived using the SEER patient data set with the inclusion of sex, race, age, and cervical lymph node involvement as independent variables (Table 3). As seen in Table 3, for the oropharynx, all variables considered were statistically significant. However, only the epidemiologic variables (sex, age, and race) and level 3, 5, and retropharyngeal lymph nodes were significant for the nasopharynx site. In the larynx model, all variables were significant except for the level 5 lymph nodes, whereas in the hypopharynx model, all variables were significant expect for sex and the retropharyngeal lymph nodes.
Table 3.
Multivariate Analysis of SEER Variables
| Oropharynx | Nasopharynx | Hypopharynx | Larynx | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Level | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Sex | Male | 1.54 (1.39-1.71) | <.001 | 0.51 (0.42-0.61) | <.001 | 1.15 (0.96-1.38) | .126 | 0.72 (0.63-0.81) | <.001 |
| Female | — | — | — | — | — | — | — | — | |
| Race | Asian | 0.16 (0.13-0.19) | <.001 | 27.6 (22.4-33.9) | <.001 | 1.00 (0.74-1.34) | .996 | 0.48 (0.37-0.64) | <.001 |
| Black | 0.44 (0.38-0.49) | <.001 | 2.09 (1.66-2.64) | <.001 | 1.60 (1.32-1.93) | <.001 | 1.86 (1.61-2.15) | <.001 | |
| Hispanic | 0.61 (0.52-0.72) | <.001 | 2.74 (2.08-3.61) | <.001 | 1.29 (0.99-1.68) | .056 | 1.15 (0.94-1.41) | .175 | |
| White | — | — | — | — | — | — | — | — | |
| Age | 0.99 (0.99-0.99) | <.001 | 0.95 (0.94-0.96) | <.001 | 1.04 (1.03-1.04) | <.001 | 1.02 (1.02-1.03) | <.001 | |
| Level 1 lymph nodes | Involved | 1.34 (1.20-1.49) | <.001 | 0.96 (0.79-1.17) | .687 | 0.79 (0.66-0.94) | .008 | 0.77 (0.68-0.88) | <.001 |
| Level 2 lymph nodes | Involved | 1.46 (1.32-1.61) | <.001 | 0.97 (0.80-1.18) | .771 | 0.71 (0.61-0.83) | <.001 | 0.74 (0.66-0.83) | <.001 |
| Level 3 lymph nodes | Involved | 0.77 (0.70-0.84) | <.001 | 0.71 (0.59-0.86) | <.001 | 1.37 (1.19-1.59) | <.001 | 1.38 (1.24-1.54) | <.001 |
| Level 4 lymph nodes | Involved | 0.79 (0.70-0.89) | <.001 | 1.07 (0.86-1.33) | .567 | 1.22 (1.03-1.45) | .024 | 1.24 (1.08-1.42) | .002 |
| Level 5 lymph nodes | Involved | 0.71 (0.63-0.80) | <.001 | 2.42 (1.98-2.96) | <.001 | 1.22 (1.01-1.47) | .040 | 0.92 (0.79-1.07) | .281 |
| Retropharyngeal lymph nodes | Involved | 0.59 (0.46-0.76) | <.001 | 3.46 (2.50-4.81) | <.001 | 1.20 (0.81-1.78) | .352 | 0.60 (0.41-0.87) | .008 |
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results.
Bold values indicate statistical significance less than 0.05.
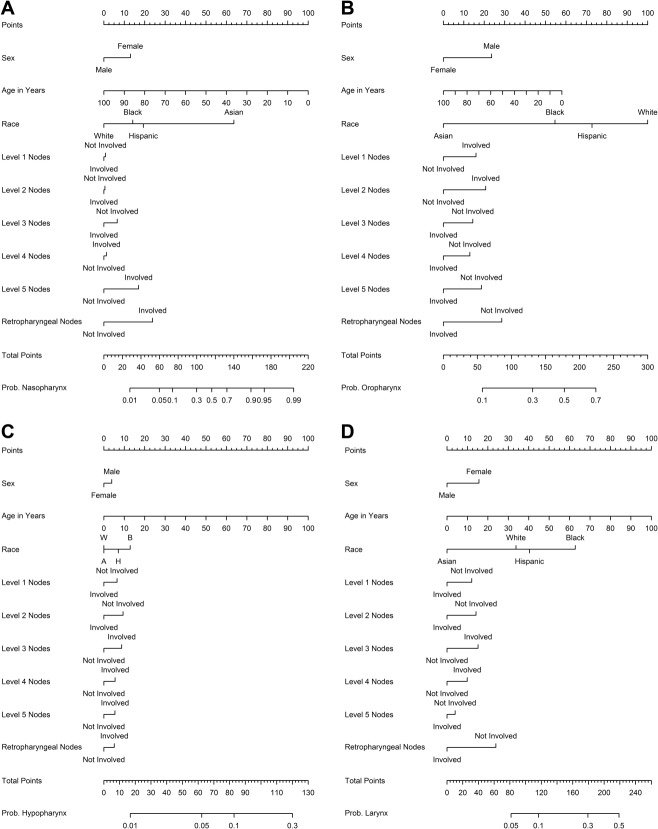
The resulting coefficients from the multiple logistic regression models (see online supporting information) were then used to create nomograms for each of the H&N tumor sites, which yield the probability of involvement of that site. For example, in comparing Table 3 with Figure 1, the higher magnitude odds ratio variables correlate with the largest point allocation on each of the H&N site nomograms. An interesting observation is the robust strength of Asian race as a predictor for nasopharyngeal involvement. Likewise, young age, male sex, and level 1 or 2 lymph node involvement would appear to indicate a strong probability of the oropharynx as the site of origin.
Figure 1.
Predictive nomograms are shown for (A) nasopharynx primary site, (B) oropharynx primary site, (C) hypopharynx primary site, and (D) larynx primary site. Prob indicates probability.
After the creation of the multivariate logistic regression models from the SEER patient data set, model validation was performed using split-sample internal validation with separate training and validation data sets. As seen in Figure 2, there is excellent agreement between predicted and observed probabilities for all deciles of each of the 4 H&N tumor sites. RMSE values for both the training and validation sets were calculated and are reported in Table 4. In all 4 models, the RMSE value calculated for the validation set was close to the value generated from the training set using the training set model estimates (differences ranging from 0.4%-1.3%). This indicates a high level of model validation and accuracy. C-statistic values for the oropharynx, nasopharynx, hypopharynx, and larynx models were 0.67, 0.81, 0.65, and 0.64, respectively, indicating adequate model fit, particularly for the nasopharynx model.
Figure 2.
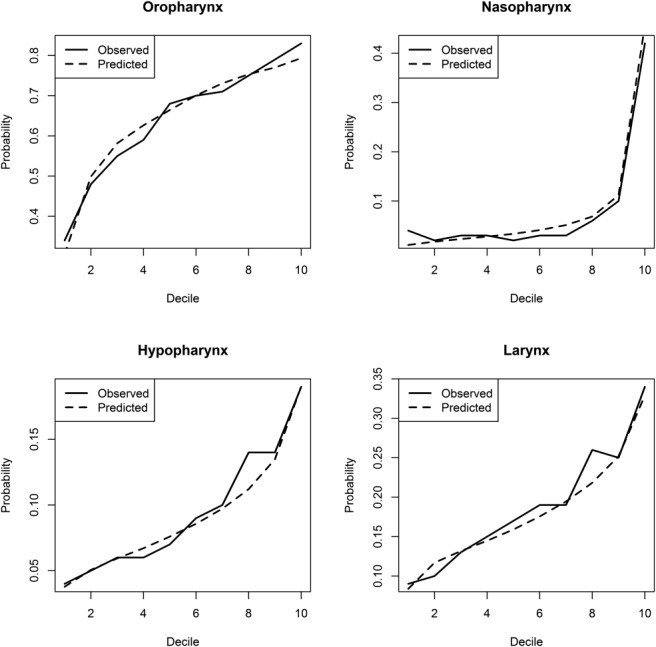
Validation plots for assessing the predictive ability of each primary site model through split-sample internal validation are shown.
Table 4.
RMSE Estimates for Validation and Training Sets Using Validation Model Estimates
| Site | Validation RMSE | Training RMSE | Absolute Difference | % Difference |
|---|---|---|---|---|
| Nasopharynx | 0.235 | 0.233 | 0.002 | 0.9% |
| Oropharynx | 0.457 | 0.459 | 0.002 | 0.4% |
| Hypopharynx | 0.285 | 0.287 | 0.002 | 0.7% |
| Larynx | 0.378 | 0.383 | 0.005 | 1.3% |
Abbreviation: RMSE, root mean square error.
Discussion
The data used to construct the model in the current study were derived from the SEER database and compare favorably with independent data sources and prospective trials. For example, as seen in Table 2, there was a significant difference in age between the nasopharyngeal group (median, 53 years) and the rest of the sites (median age range, 58 years-62 years). This compares well with both the Intergroup 0099 study (nasopharyngeal median age range, 50 years-52 years)29 and the more recent phase 3 cetuximab H&N trial by Bonner et al (oropharynx, larynx, and hypopharynx median age, 56 years-58 years).30 Likewise, the cervical lymph node level correlation with primary tumor site compares well with prior published data. For example, in Online Supporting Information Table 5, the hypopharynx had the highest percentage (23%) of involved level 4 cervical lymph nodes out of all the considered sites. Chao et al similarly listed the hypopharynx as the site with the highest incidence of level 4 cervical lymph nodes.31 In addition, the oropharynx appeared most likely to metastasize to the level 2 or 3 cervical lymph nodes (75% and 33%, respectively) (see online supporting information). Again, this compares favorably with the study by Chao et al, which also listed the cervical level 2 and 3 lymph nodes as the most likely levels of metastasis from a clinically lymph node-positive oropharynx primary tumor (tonsil, 74% and 31%, respectively).31 Likewise, Lindberg listed the upper, middle, and lower jugular lymph nodes as the most involved cervical metastatic sites from primary tumors of the soft palate, tonsil, base of tongue, and oropharyngeal wall.17
Although the SEER database has the advantage of large patient numbers and a favorable comparison with prior published data, a potential limitation of a model derived from the SEER database would be the finding that the model would primarily be applicable only to patients in the United States because other parts of the world are likely to have different epidemiologic percentages. In addition, the model does not include information regarding viral biomarkers or social risk factors such as smoking, alcohol and marijuana use, and number of oral sexual partners. SEER is currently tracking human papillomavirus (HPV) status, but to our knowledge has not yet publicly released those data. When these data are available, it is anticipated that the current model will be easily updated with that additional variable. The inclusion of HPV will likely be most relevant to the oropharynx site. Prior studies have shown a strong correlation between HPV status and oropharyngeal primary tumors.32–35 Thus, the inclusion of the HPV variable and a positive HPV result would likely increase the calculated probability of the oropharyngeal site to the exclusion of the other H&N sites compared with calculated probabilities in the current model. Likewise, Epstein-Barr virus has been shown to be correlated primarily with the nasopharyngeal site and inclusion of that variable would similarly affect the calculated probabilities of the nasopharynx site.36
A minor limitation to the current study that must also be noted is the finding that the other 3 primary tumor sites provide the reference level for a given primary site’s logistic regression model. For example, the combined totals of oropharynx, nasopharynx, and hypopharynx serve as the reference level for the larynx model. As a result, the sum of the predicted probabilities for the 4 models will be approximately 1 for a given set of patient characteristics, but not exactly 1.
The model presented in the current study delivers a value for each of the 4 H&N sites (nasopharynx, oropharynx, larynx, and hypopharynx) representing the probability that a patient’s unknown H&N primary malignancy arose from that site. This probability value may be graphically derived from the respective nomogram (Fig. 1) or calculated directly from the multiple logistic regression equations (see online supporting information). Such a probability value has the potential for many uses, including more personalized radiation treatment planning and/or further guidance for higher spatial resolution biopsies. In reviewing the nomograms, it can be observed that the resulting probabilities compare favorably with clinical intuition based on prior studies. For example, if a white man aged 62 years presented with a mass primarily centered at approximately the level 3 cervical lymph nodes, there would be a 63.7% probability that the primary H&N site was the oropharynx, a 15.6% probability for the hypopharynx, a 22.4% probability for the larynx, and a 2.0% probability for the nasopharynx. If a radiation oncologist’s personal cutoff is 15% for covering a given H&N primary tumor site, they might choose to omit the nasopharynx in that case. Consider another example in which a 42-year-old Asian woman presents with a level V cervical mass, but no known H&N primary tumor: there would be a 91.2% probability that the primary site was the nasopharynx, a 17.1% probability for the oropharynx, a 6.0% probability for the hypopharynx, and a 8.0% probability for the larynx. This might prompt a more thorough investigation of the nasopharynx with another nasopharyngoscopy, additional biopsies of multiple locations within the nasopharynx by the otolaryngologist, and/or a magnetic resonance image of that region. If still no H&N primary tumors were identified, a radiation oncologist might choose to omit the larynx and hypopharynx from the mucosal field.
The current study reports on the use of the SEER database to create a multivariate logistic regression model that provides predicted probabilities of involvement of H&N tumor sites based on age, race, sex, and cervical lymph node metastasis level. The model has demonstrated a high level of validation and accuracy when subjected to internal split-sample validation with separate training and validation data sets. Although good clinical judgment must remain the foundation of any treatment decision, it is hoped that the probabilities derived from this model may provide clinicians with additional insight into the site of origin of an unknown H&N primary tumor.
Funding Support
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292 (W. Curran). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Disclosures
The authors made no disclosures.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- Waltonen JD, Ozer E, Hall NC, Schuller DE, Agrawal A. Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg. 2009;135:1024–1029. doi: 10.1001/archoto.2009.145. [DOI] [PubMed] [Google Scholar]
- Grau C, Johansen LV, Jakobsen J, Geertsen P, Andersen E, Jensen BB. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother Oncol. 2000;55:121–129. doi: 10.1016/s0167-8140(00)00172-9. [DOI] [PubMed] [Google Scholar]
- Strojan P, Ferlito A, Langendijk JA, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: II. a review of therapeutic options. Head Neck. 2013;35:286–293. doi: 10.1002/hed.21899. [DOI] [PubMed] [Google Scholar]
- Roh JL, Kim JS, Lee JH, et al. Utility of combined (18)F-fluorodeoxyglucose-positron emission tomography and computed tomography in patients with cervical metastases from unknown primary tumors. Oral Oncol. 2009;45:218–224. doi: 10.1016/j.oraloncology.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kwee T, Kwee R. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianchetti M, Mancuso AA, Amdur RJ, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119:2348–2354. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- Waltonen JD, Ozer E, Schuller DE, Agrawal A. Tonsillectomy vs. deep tonsil biopsies in detecting occult tonsil tumors. Laryngoscope. 2009;119:102–106. doi: 10.1002/lary.20017. [DOI] [PubMed] [Google Scholar]
- Righi PD, Sofferman RA. Screening unilateral tonsillectomy in the unknown primary. Laryngoscope. 1995;105:548–550. doi: 10.1288/00005537-199505000-00021. [DOI] [PubMed] [Google Scholar]
- Lapeyre M, Malissard L, Peiffert D, et al. Cervical lymph node metastasis from an unknown primary: is a tonsillectomy necessary? Int J Radiat Oncol Biol Phys. 1997;39:291–296. doi: 10.1016/s0360-3016(97)00321-0. [DOI] [PubMed] [Google Scholar]
- Randall DA, Johnstone PA, Foss RD, Martin PJ. Tonsillectomy in diagnosis of the unknown primary tumor of the head and neck. Otolaryngol Head Neck Surg. 2000;122:52–55. doi: 10.1016/S0194-5998(00)70143-4. [DOI] [PubMed] [Google Scholar]
- Abuzeid WM, Bradford CR, Divi V. Transoral robotic biopsy of the tongue base: a novel paradigm in the evaluation of unknown primary tumors of the head and neck. Head Neck. 2013;35:E126–E130. doi: 10.1002/hed.21968. [DOI] [PubMed] [Google Scholar]
- Durmus K, Rangarajan SV, Old MO, Agrawal A, Teknos TN, Ozer E. Transoral robotic approach to carcinoma of unknown primary. Head Neck. 2014;36:848–852. doi: 10.1002/hed.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V, Johnson P, Tassler A, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123:146–151. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- Hellman S. It’s too soon to know. J Natl Cancer Inst. 1990;82:250–251. doi: 10.1093/jnci/82.4.250. [DOI] [PubMed] [Google Scholar]
- Jesse RH, Perez CA, Fletcher GH. Cervical lymph node metastasis: unknown primary cancer. Cancer. 1973;31:854–859. doi: 10.1002/1097-0142(197304)31:4<854::aid-cncr2820310414>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Werner JA, Dunne AA, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003;25:322–332. doi: 10.1002/hed.10257. [DOI] [PubMed] [Google Scholar]
- Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–1449. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Harper CS, Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR. Cancer in neck nodes with unknown primary site: role of mucosal radiotherapy. Head Neck. 1990;12:463–469. doi: 10.1002/hed.2880120603. [DOI] [PubMed] [Google Scholar]
- Mendenhall WM, Mancuso AA, Amdur RJ, Stringer SP, Villaret DB, Cassisi NJ. Squamous cell carcinoma metastatic to the neck from an unknown head and neck primary site. Am J Otolaryngol. 2001;22:261–267. doi: 10.1053/ajot.2001.24820. [DOI] [PubMed] [Google Scholar]
- Barker CA, Morris CG, Mendenhall WM. Larynx-sparing radiotherapy for squamous cell carcinoma from an unknown head and neck primary site. Am J Clin Oncol. 2005;28:445–448. doi: 10.1097/01.coc.0000162963.69302.12. [DOI] [PubMed] [Google Scholar]
- Lu H, Yao M, Tan H. Unknown primary head and neck cancer treated with intensity-modulated radiation therapy: to what extent the volume should be irradiated. Oral Oncol. 2009;45:474–479. doi: 10.1016/j.oraloncology.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Wallace A, Richards GM, Harari PM, et al. Head and neck squamous cell carcinoma from an unknown primary site. Am J Otolaryngol. 2011;32:286–290. doi: 10.1016/j.amjoto.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Hsing CY, Liu SA, Wang CC. Management of unknown primary head and neck squamous cell carcinoma. Am J Otolaryngol. 2012;33:637–638. doi: 10.1016/j.amjoto.2012.01.003. author reply 638-639. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- Neter J, Kutner M, Wasserman W, Nachtsheim C. Applied Linear Statistical Models. 4. Chicago: McGraw-Hill; 1996. [Google Scholar]
- R Project for Statistical Computing. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- SAS Institute Inc. Base SAS 9.3 Procedures Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons; 2000. [Google Scholar]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Chao KS, Wippold FJ, Ozyigit G, Tran BN, Dempsey JF. Determination and delineation of nodal target volumes for head-and-neck cancer based on patterns of failure in patients receiving definitive and postoperative IMRT. Int J Radiat Oncol Biol Phys. 2002;53:1174–1184. doi: 10.1016/s0360-3016(02)02881-x. [DOI] [PubMed] [Google Scholar]
- Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes. Clin Cancer Res. 2003;9:6469–6475. [PubMed] [Google Scholar]
- Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- El-Mofty S, Zhang M, Davila R. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2:163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MQ, El-Mofty SK, Davila RM. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis. Cancer (Cancer Cytopathol) 2008;114:118–123. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.