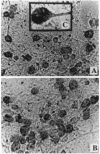
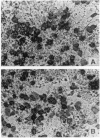
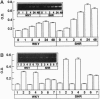
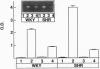
Abstract
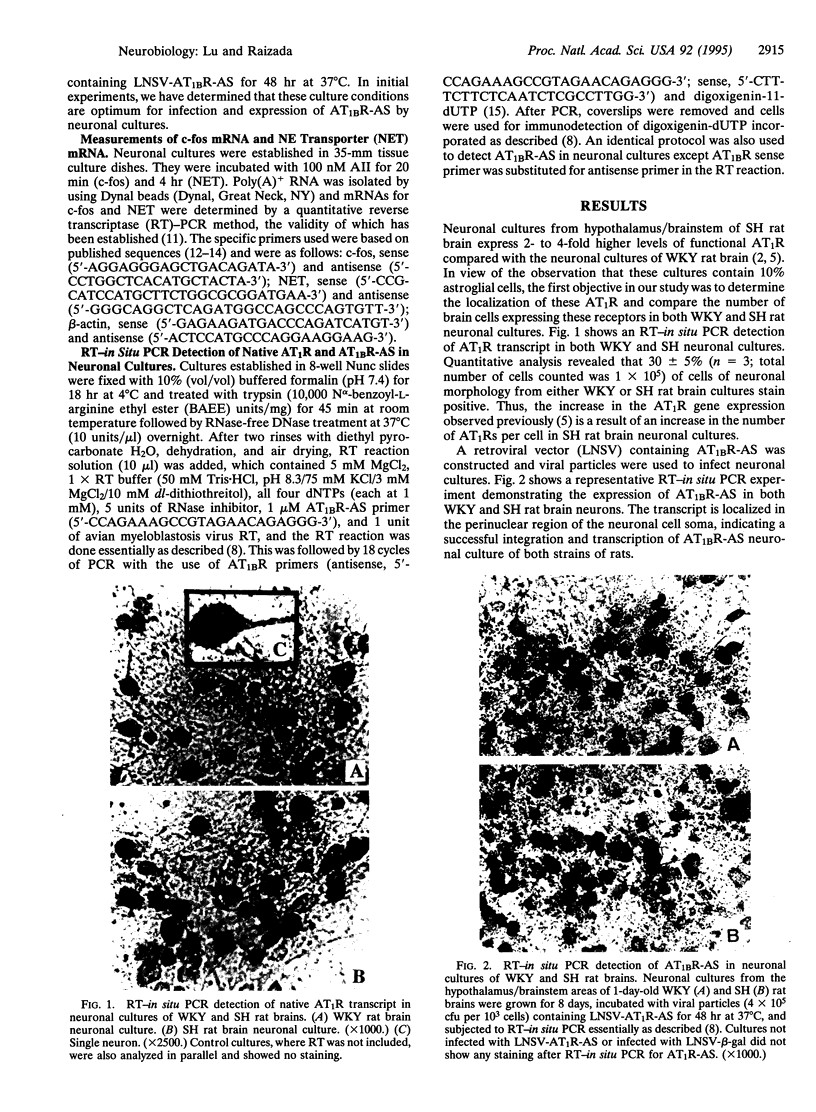
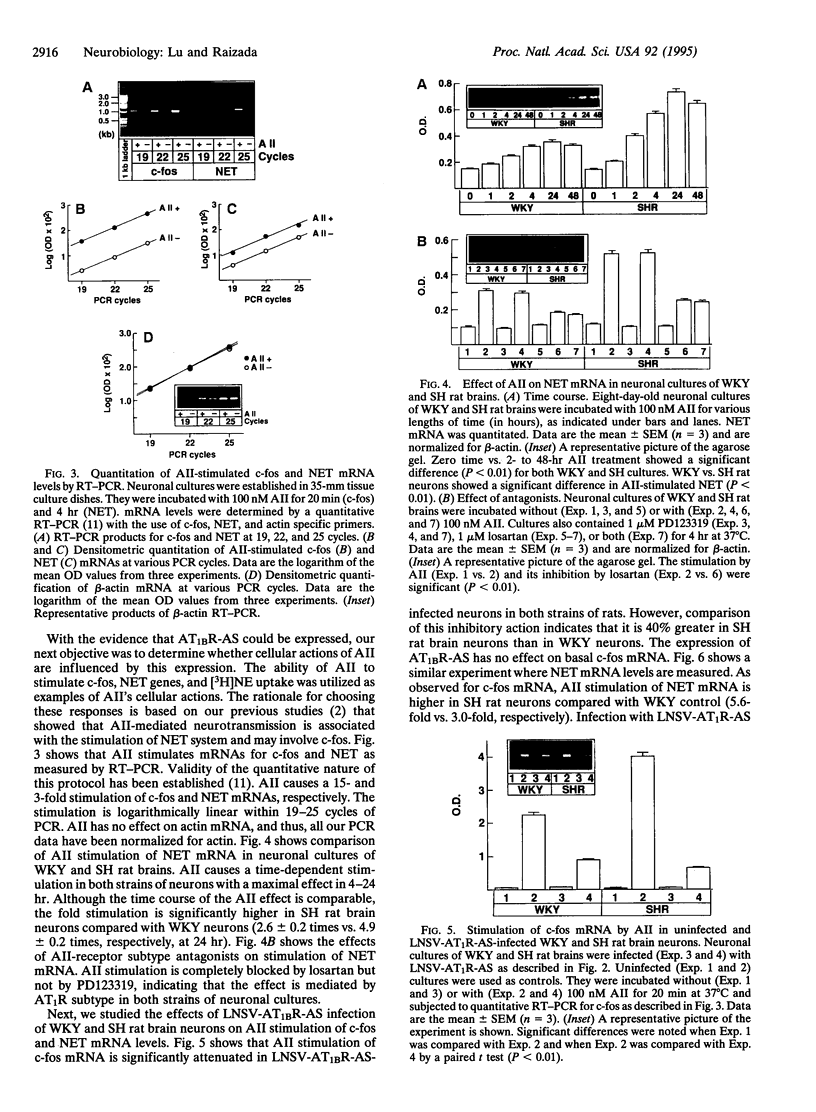
Increased brain angiotensin II (AII) type 1 receptor (AT1R) expression has been implicated in the hyperactive brain angiotensin system and the development and maintenance of hypertension in the genetically spontaneously hypertensive (SH) rat. Neuronal cells in primary culture from the cardioregulatory-relevant brain areas (hypothalamus/brainstem) mimic increased brain AT1R gene expression and AT1R function of the adult SH rat. They have been utilized in the present study to determine whether cellular actions of AII could be regulated by the transfer of AT1R antisense (AT1R-AS) with the use of a retroviral-mediated gene delivery system developed for the central nervous system cultures. AII stimulates norepinephrine (NE) uptake in neuronal cultures of both normotensive (Wistar Kyoto) and SH rat brains. This neuromodulatory action is mediated by the AT1R subtype, is significantly higher in SH neurons, and is associated with a parallel stimulation of mRNAs for c-fos and NE transporter. Infection of neuronal cultures with a retrovirus vector that contains AT1R-AS (LNSV-AT1R-AS) results in an inhibition of AT1R-mediated stimulation of both c-fos and NE transporter mRNA, as well as NE uptake in both strains of rats; however, the inhibition is more pronounced in SH neurons compared with Wistar Kyoto rat brain neurons. The higher sensitivity of the SH rat brain neurons is further supported by our observation that a certain dose of LNSV-AT1R-AS that fails to induce inhibition of cellular actions of AII in WKY neurons causes a significant inhibition of AII actions in SH neurons. These observations show that retrovirally mediated delivery of AT1R-AS could be used to selectively control the actions of AII in primary neuronal cultures from SH rat brain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Sumners C., Raizada M. K. Regulation of angiotensin II type 1 receptor mRNA in neuronal cultures of normotensive and spontaneously hypertensive rat brains by phorbol esters and forskolin. J Neurochem. 1994 Jun;62(6):2079–2084. doi: 10.1046/j.1471-4159.1994.62062079.x. [DOI] [PubMed] [Google Scholar]
- Lu D., Yu K., Raizada M. K. Retrovirus-mediated transfer of an angiotensin type I receptor (AT1-R) antisense sequence decreases AT1-Rs and angiotensin II action in astroglial and neuronal cells in primary cultures from the brain. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1162–1166. doi: 10.1073/pnas.92.4.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukoyama M., Nakajima M., Horiuchi M., Sasamura H., Pratt R. E., Dzau V. J. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993 Nov 25;268(33):24539–24542. [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., Forde A., MacConnell P., Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993 Jul;143(1):40–48. [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Boyd C. J. Expressing antisense P0 RNA in Schwann cells perturbs myelination. Development. 1991 Jun;112(2):639–649. doi: 10.1242/dev.112.2.639. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Lu D., Tang W., Kurian P., Sumners C. Increased angiotensin II type-1 receptor gene expression in neuronal cultures from spontaneously hypertensive rats. Endocrinology. 1993 Apr;132(4):1715–1722. doi: 10.1210/endo.132.4.8462471. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Muther T. F., Sumners C. Increased angiotensin II receptors in neuronal cultures from hypertensive rat brain. Am J Physiol. 1984 Nov;247(5 Pt 1):C364–C372. doi: 10.1152/ajpcell.1984.247.5.C364. [DOI] [PubMed] [Google Scholar]
- Raizada M. K., Sumners C., Lu D. Angiotensin II type 1 receptor mRNA levels in the brains of normotensive and spontaneously hypertensive rats. J Neurochem. 1993 May;60(5):1949–1952. doi: 10.1111/j.1471-4159.1993.tb13426.x. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M. Brain and pituitary angiotensin. Endocr Rev. 1992 May;13(2):329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- Sumners C., Muther T. F., Raizada M. K. Altered norepinephrine uptake in neuronal cultures from spontaneously hypertensive rat brain. Am J Physiol. 1985 May;248(5 Pt 1):C488–C497. doi: 10.1152/ajpcell.1985.248.5.C488. [DOI] [PubMed] [Google Scholar]
- Sumners C., Tang W., Zelezna B., Raizada M. K. Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7567–7571. doi: 10.1073/pnas.88.17.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Verity A. N., Bredesen D., Vonderscher C., Handley V. W., Campagnoni A. T. Expression of myelin protein genes and other myelin components in an oligodendrocytic cell line conditionally immortalized with a temperature-sensitive retrovirus. J Neurochem. 1993 Feb;60(2):577–587. doi: 10.1111/j.1471-4159.1993.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]