Summary
Cereblon (CRBN), the molecular target of lenalidomide and pomalidomide, is a substrate receptor of the cullin ring E3 ubiquitin ligase complex, CRL4CRBN. T cell co-stimulation by lenalidomide or pomalidomide is cereblon dependent: however, the CRL4CRBN substrates responsible for T cell co-stimulation have yet to be identified. Here we demonstrate that interaction of the transcription factors Ikaros (IKZF1, encoded by the IKZF1 gene) and Aiolos (IKZF3, encoded by the IKZF3 gene) with CRL4CRBN is induced by lenalidomide or pomalidomide. Each agent promotes Aiolos and Ikaros binding to CRL4CRBN with enhanced ubiquitination leading to cereblon-dependent proteosomal degradation in T lymphocytes. We confirm that Aiolos and Ikaros are transcriptional repressors of interleukin-2 expression. The findings link lenalidomide- or pomalidomide-induced degradation of these transcriptional suppressors to well documented T cell activation. Importantly, Aiolos could serve as a proximal pharmacodynamic marker for lenalidomide and pomalidomide, as healthy human subjects administered lenalidomide demonstrated Aiolos degradation in their peripheral T cells. In conclusion, we present a molecular model in which drug binding to cereblon results in the interaction of Ikaros and Aiolos to CRL4CRBN, leading to their ubiquitination, subsequent proteasomal degradation and T cell activation.
Keywords: lenalidomide, pomalidomide, Cereblon, Ikaros, Aiolos
The immunomodulatory drugs (IMiDs®) lenalidomide and pomalidomide are analogues of thalidomide and are orally active agents used for the treatment of multiple myeloma (MM), myelodysplastic syndromes associated with a deletion (del)5q abnormality and mantle cell lymphoma. As the nomenclature suggests, one of the first known properties of IMiD compounds was their immunomodulatory capacity, including cytokine modulation and T cell co-stimulation (Corral et al, 1999; Schafer et al, 2003). Subsequently, these compounds were shown to have pleiotropic effects on a wide range of immune cells, such as natural killer (NK) cell activation and B cell and monocyte inhibition (Corral et al, 1999; Wu et al, 2008; Gandhi et al, 2013). The modulation of T cells has been well documented, with T cell co-stimulation resulting in increased interleukin (IL)-2 production in both CD4+ and CD8+ T cells (Schafer et al, 2003), the shift of T helper (Th) responses from Th2 to Th1, inhibition of T regulatory cells (Tregs), and improvement of immunological synapse function in follicular lymphoma and chronic lymphocytic leukaemia (CLL; Dredge et al, 2002; Galustian et al, 2008; Ramsay et al, 2008). In a recent study of patients with KRAS-mutant metastatic colorectal cancer, lenalidomide induced a significant decrease in the percentage of CD45RA+ naïve T cells while increasing the percentage of HLA-DR+ activated T helper cells and the percentage of total CD45RO+ CD8+ memory T cytotoxic cells (Gandhi et al, 2013). With the identification of Cereblon (encoded by gene CRBN) as a target of thalidomide, lenalidomide and pomalidomide (Ito et al, 2010; Zhu et al, 2011; Lopez-Girona et al, 2012), the molecular mechanisms for the pleiotropic activities of the agents may be more readily elucidated.
Cereblon (encoded by the CRBN gene) is the substrate receptor of the cullin 4 ring E3 ubiquitin ligase complex (CRL4CRBN), which is required for the teratogenic effects by thalidomide in zebrafish and chick embryos and for the anti-proliferative activity of lenalidomide and pomalidomide in MM cells. Cereblon also mediates the T cell co-stimulation by lenalidomide and pomalidomide because knockdown of Cereblon expression in primary human T cells abrogates the drug-induced IL2 expression (Ito et al, 2010; Lopez-Girona et al, 2012).
Up to this point, the substrates regulated by CRL4CRBN have been unknown. Here we report that members of the Ikaros family of transcription factors, specifically Ikaros and Aiolos (encoded by the genes IKZF1 and IKZF3 respectively), are recruited as protein substrates for CRL4CRBN in T cells in response to lenalidomide or pomalidomide treatment. Degradation of these substrates by lenalidomide and pomalidomide results in enhanced production of IL2 and other cytokines known to regulate T cell function. Ikaros and Aiolos are biologically relevant substrates because they are known negative regulators of IL2 expression in T cells (Bandyopadhyay et al, 2007; Gandhi et al, 2010; Quintana et al, 2012). Furthermore, the demonstration of decreased Aiolos levels in peripheral blood upon lenalidomide administration to healthy volunteers provide one of the first clinical pharmacodynamic markers for the immunomodulatory class of drug. These measurements will enable better dosing and scheduling for the emerging next generation of compounds.
Materials and methods
Cells and plasmids
Primary T cells were isolated from human leukocytes (Blood Center of New Jersey, East Orange, NJ, USA) by centrifugation through Ficoll following the ‘RosetteSep’ protocol (Stem Cell Technologies, Vancouver, BC, Canada). Jurkat cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (Cellgro, Manassas, VA, USA) containing 10% (V/V) heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA) supplemented with 2 mmol/l glutamine. Wild-type (WT) IKZF3 and mutant (Mt) IKZF3 were expressed in pCMV6-entry (Blue Heron Biotechnology, Bothell, WA, USA).
Ubiquitin proteomics assay
Purified human T cells from 11 donors were stimulated with anti-CD3 antibody and each donor was treated with either dimethyl sulfoxide (DMSO) or glutarimide analogue for 6 h. The 11 donors within each treatment group were pooled into two batches with 5 or 6 donors in each batch. Donors 1–6 and donors 7–11 served as biological duplicates. Protein lysates were trypsinized and ubiquitinated peptides were enriched using an anti-di-glycine antibody. Enriched peptides were detected by liquid chromatography tandem mass spectrometry – in duplicate, and quantified into raw intensities of peak height. Raw intensities were then log2 transformed, median-centred. The missing values for each sample were imputed using the minimal intensity of the observed ubiquitin (UBI)–peptides of the sample. For identifying UBI-peptides that are significantly affected by glutarimide analogue, r package LIMMA (Smyth, 2004) was used. Peptides with raw P-value ≤ 0·05 and absolute fold change ≥1·5 were considered significant. All statistical analyses were carried out using r software (http://www.r-project.org/).
Primary T cell drug treatments and protein analysis
Purified T cells were stimulated with anti-CD3 antibody (eBioscience, San Diego, CA, USA) and treated with either lenalidomide or pomalidomide (Celgene Corporation, Summit, NJ, USA) for various times and concentrations. The final DMSO concentration was 0·1%. Cells were pre-treated with 10 μmol/l MG-132 (Calbiochem Biochemicals, Billerica, MA, USA) for 30 min before drug addition. For cycloheximide treatment studies, 2·5 × 106 T cells were plated per well in six-well dishes, incubated with 10 μg/ml cycloheximide (Sigma-Aldrich, St Louis, MO, USA), and treated with either DMSO, 10 μmol/l lenalidomide or 1 μmol/l pomalidomide for 0, 1·5, 3 or 6 h. Cells were harvested, washed in phosphate-buffered saline (PBS), and cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad, Hercules, CA, USA). Membranes were immunoblotted with anti-Aiolos (O-21; Santa Cruz Biotechnology, Dallas, TX, USA), anti-Ikaros (Millipore, Billerica, MA, USA), anti-Cereblon (CRBN65; Celgene), anti-actin (Sigma, St. Louis, MO, USA; or LI-COR Biosciences, Lincoln, NE, USA) and secondary antibodies (LI-COR Biosciences). The blots were analysed on an Odyssey imager (LI-COR Biosciences).
Cereblon immunoprecipitation assay
HEK-293T cells were co-transfected with Flag CRBN WT or YW/AA and HA-Aiolos or HA-Ikaros expression vectors and treated with 10 or 30 μmol/l pomalidomide for 1 h before cell harvest. Cells were harvested 48 h after transfection and lysed using nonidet P-40 (NP-40) lysis buffer [50 mmol/l Tris-HCl (pH 8·0), 150 mmol/l NaCl, 0·5% NP-40]. Extracts were incubated with M2 Flag agarose beads (Sigma, St. Louis, MI, USA). After washing three times with NP-40 lysis buffer, bound proteins were eluted with free Flag peptides. Samples were subjected to SDS-PAGE followed by immunoblotting using anti-Cereblon (CRBN65; Celgene), anti-Aiolos (Santa Cruz Biotechnology), or anti-Ikaros (Abcam, Cambridge, MA, USA).
Jurkat T cell transfection
Jurkat cells were transfected using the Neon Transfection System (Invitrogen, Grand Island, NY, USA) with either empty vector control, Flag-tagged WT Aiolos (WT-IKZF3) or a Flag-tagged Aiolos construct with all lysines mutated to arginines (Mt-IKZF3). Cells were cultured in 10 cm tissue culture dishes at 37°C for 6 h then treated with DMSO or drug and OKT3 (3 μg/ml; eBioscience) for another 24 h. Cells were harvested, and Aiolos and Ikaros protein expression was measured by immunoblot analysis as described above.
CRBN, Aiolos, and Ikaros siRNA transfection and cytokine measurement
Purified T cells were treated with 1 μg/ml phytohaemagglutinin-L (PHA-L; Sigma, St. Louis, MO, USA) at 37°C for 24 h and then electroporated with either small interfering Cereblon (siCRBN; sense sequence CAGCUUAUGUGAAUCCUCAUGGAUA), Ikaros (siIkaros; catalogue s20180), or Aiolos (siAiolos; catalogue s22417; Invitrogen). T cell electroporation conditions used with the Neon Transfection System (Invitrogen) were as follows: 2100 voltage, 15 width, two pulses, 200 nmol/l siRNA and T buffer. Low GC content siRNA (Invitrogen) was used as a negative control. For siCRBN studies, transfected cells were cultured in an OKT3 (3 μg/ml; eBioscience) coated 10-cm dish with 20 ml media at 37°C for 48 h. Cells were collected for measuring CRBN knockdown efficiency by quantitative reverse transcription polymerase chain reaction (qRT-PCR). For detection of Aiolos expression in the siCRBN-transfected cells, the remaining transfected cells were seeded on OKT3-prebound (3 μg/ml) tissue culture plates, treated with DMSO or drug at 37°C for 6 h, and then harvested for Western analysis. Aiolos protein expression was determined by immunoblot. For siAiolos and siIkaros studies, T cells were transfected and cultured in OKT3 (3 μg/ml; eBioscience) coated 10 cm dishes with 20 ml media at 37°C and treated with drug for 48 h. Cells were collected for measuring CRBN,IKZF1 or IKZF3 knockdown efficiency by Western blot and qRT-PCR and IL2 expression by qRT-PCR after transfection. The Cereblon monoclonal antibody CRBN65 was used as previously described by Lopez-Girona et al (2012), and commercial gene expression assays were from Applied Biosystems (Grand Island, NY, USA). Supernatants were harvested and cytokines were detected by enzyme-linked immunosorbent assy (ELISA; Thermo Scientific, Lafayette, CO, USA) or Milliplex (Millipore).
Healthy volunteer lenalidomide clinical study, sample collection and Aiolos flow cytometry
The study protocol and informed consent form were approved by the institutional review board/independent ethics committees of participating institutions. Written informed consent was obtained from all participants, and the trial was conducted in accordance with the Helsinki declaration. Healthy volunteers were administered lenalidomide at doses of 10 or 50 mg. Blood samples were drawn immediately before dosing (pre-dose) and 6 h after a single dose of lenalidomide. Peripheral blood mononuclear cells were isolated by Ficoll from whole blood samples and viably frozen in DMSO. The cells were washed twice with 2 ml of cold PBS, permeabilized by adding 2 ml of cold Becton Dickinson (BD; Frankin Lakes, NJ, USA) Cytofix/cytoperm buffer (BD Biosciences, San Jose, CA, USA) and incubated on ice for 15 min. The cells were centrifuged, washed twice with BD perm/wash buffer, and then resuspended in 40 μl of BD perm/wash buffer. Cells were stained with anti-CD3 or anti-CD19 antibody, and 20 μl of anti-Aiolos antibody (Santa Cruz Biotechnology) or rabbit polyclonal immunoglobulin G (IgG; Santa Cruz Biotechnology) at 1:200 dilution with staining buffer, or 20 μl of appropriate isotype controls to cells. Cells were mixed thoroughly, incubated at room temperature for 30 min in the dark, washed once with BD perm/wash buffer, and then resuspended in 80 μl of BD perm/wash buffer, and 20 μl of secondary antibody was added before analysis on a flow cytometer.
Statistical analysis
Statistical analyses for the proteomics study were carried out using r software (http://www.r-project.org/). All other statistical analyses were performed using graphpad prism v5.0 (GraphPad Software, La Jolla, CA, USA), with statistical significance defined as *P < 0·05, **P < 0·01 and ***P < 0·001.
Results
Lenalidomide and pomalidomide promote degradation of Ikaros and Aiolos via the proteosome in a concentration- and time-dependent manner
To identify substrates of CRL4CRBN in the presence of a thalidomide analogue, lysates from human peripheral blood mononuclear cells treated with vehicle or a cereblon-binding glutarimide-containing analogue were subjected to immunoprecipitation using the di-glycine-lysine antibody (Kim et al, 2011) and subsequent mass spectrometry to identify peptides uniquely ubiquitinated upon drug treatment. A peptide mapping to the Aiolos protein (EYNEYENIKLER) was enriched 3·35-fold in the presence of the glutarimide analogue compared with vehicle control (Fig 1). To investigate the effect of lenalidomide or pomalidomide on the protein expression of Aiolos and its close family member Ikaros (which has an 88% identical gene sequence), primary human T cells were treated with either of these compounds for 1, 3, 6 and 24 h. Time-dependent degradation of both Aiolos and Ikaros was observed as early as 1 h after drug treatment, with pomalidomide having a stronger effect than lenalidomide (Fig 2A left). The Ikaros antibody detected a dominant band representing the full-length Ikaros isoform 1 (65 kDa) and a lower band (57 kDa). The full-length Ikaros band was efficiently reduced in response to compound treatment while the lower band appeared to be affected to a lesser extent. This lower band probably represents an Ikaros isoform, several of which have been previously reported (Sun et al, 1999; Iacobucci et al, 2008). Densitometric analysis for both Aiolos and Ikaros (full-length size marked with arrow) from multiple experiments is shown in Fig 2A (middle and right). Under identical conditions, there was no change in IKZF1 or IKZF3 gene expression as measured by real-time PCR analysis (data not shown).
Figure 1.
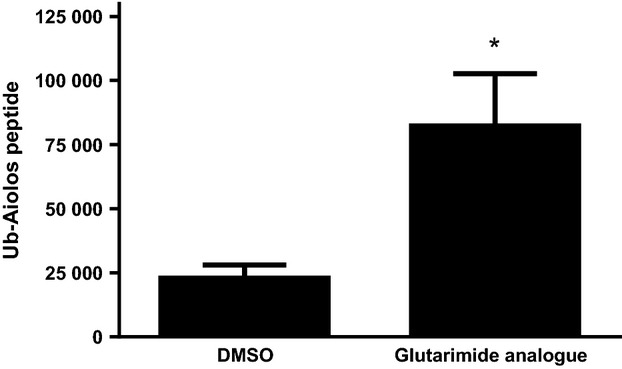
A ubiquitinated Aiolos peptide is enriched in T cells treated with a Cereblon-binding glutarimide analogue. T cells from multiple donors were treated with DMSO or drug for 6 h followed by enrichment for ubiquitinated peptides. Peptides were analysed by LC-MS/MS and data shown are mean Ub-Aiolos peptide peak height ± SD; *P < 0·05 versus DMSO using paired t-test. DMSO, dimethyl sulfoxide; Ub, ubiquitinated; SD, standard deviation.
Figure 2.
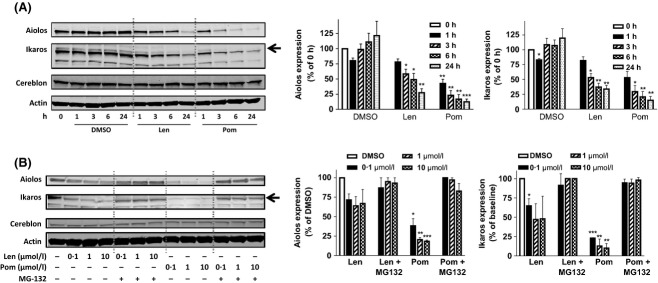
Lenalidomide and pomalidomide induce the degradation of Aiolos and Ikaros in T cells in a time-dependent manner and concentration-dependent manner via proteosomal degradation. (A) T cells were treated with 10 μmol/l lenalidomide or 1 μmol/l pomalidomide for 1, 3, 6 and 24 h. Lysates were separated by SDS-PAGE and immunoblotted for Aiolos, Ikaros, Cereblon and Actin expression; left representative image, middle densitometry analysis of Aiolos, right densitometry analysis of Ikaros expression normalized to Actin; (B) T cells were pre-treated with MG-132 for 30 min then treated with lenalidomide or pomalidomide for 6 h at the indicated concentrations. Lysates were separated by SDS-PAGE and immunoblotted for Aiolos, Ikaros, Cereblon and Actin expression; left representative image, middle densitometry analysis of Aiolos, right densitometry analysis of Ikaros expression normalized to Actin; data shown is mean ± SEM of four experiments; *P < 0·05, **P < 0·01 and ***P < 0·001 using one-way anova. Len, lenalidomide; Pom, pomalidomide; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; SEM, standard error of the mean; anova, analysis of variance.
T cell treatment with lenalidomide or pomalidomide (0·1–10 μmol/l) for 6 h, triggered concentration-dependent degradation of Aiolos and Ikaros. In contrast, 30-min pre-treatment with the proteosome inhibitor MG-132 stabilized Aiolos and Ikaros (Fig 2B left). Densitometric analysis of Aiolos and Ikaros from multiple experiments is shown in Fig 2B (middle and right). These results indicate that lenalidomide or pomalidomide administration promotes the proteasome-dependent degradation of Aiolos and Ikaros in T cells.
Cereblon binds Aiolos and Ikaros in a drug-dependent manner
To determine if Aiolos and Cereblon physically interact, co-immunoprecipitation experiments were performed in HEK-293T cells overexpressing Flag-CRBN and HA-Aiolos (Fig 3A). After immunoprecipitation with anti-Flag antibody, Aiolos protein was co-precipitated, but only in the presence of pomalidomide. Furthermore, overexpression of a mutant CRBN (YW/AA), which can no longer bind to the pomalidomide structural analogue thalidomide (Ito et al, 2010) abrogated the CRL4CRBN co-precipitation with Aiolos. Fig 3B shows that binding of Ikaros to Cereblon similarly occurred in a pomalidomide-dependent manner, with enhanced recovery of Ikaros. Taken together, drug binding promotes Cereblon engagement in interactions with the transcription factors Aiolos and Ikaros, proteins that are not readily detected as substrates in the absence of drug. These interactions lead to their degradation in T cells.
Figure 3.
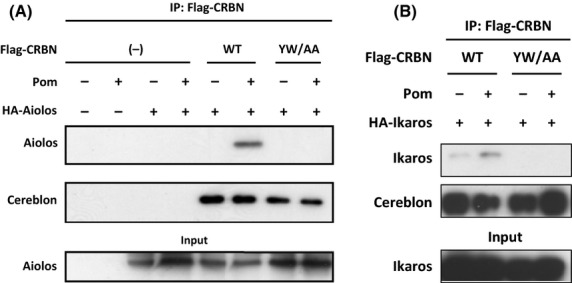
Cereblon binds Aiolos and Ikaros and requires the thalidomide binding domain on Cereblon. (A) HEK-293T cells were transfected with Flag-WT Cereblon or Flag-YW/AA Cereblon with or without HA-Aiolos for 48 h with pomalidomide added to cells for the last hour. Lysates were made and immunoprecipitation with Flag antibody followed by SDS-PAGE separation and immunoblotting with anti-Aiolos or anti–Cereblon was performed. (B) HEK-293T cells were transfected with Flag-WT Cereblon or Flag-YW/AA Cereblon with or without HA-Ikaros for 48 h with pomalidomide added to cells for the last hour. Lysates were made and immunoprecipitation with Flag antibody followed by SDS-PAGE separation and immunoblotting with anti–Cereblon or anti-Ikaros was performed. IP, immunoprecipitation; CRBN, Cereblon; WT, wildtype; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis.
Lenalidomide and pomalidomide accelerate Aiolos and Ikaros protein turnover
CRL4CRBN is an E3 ubiquitin ligase complex known to modify target substrates with ubiquitin. Therefore, because lenalidomide- or pomalidomide-mediated degradation of Aiolos and Ikaros requires the proteosome (Fig 2B), we investigated whether lysine ubiquitination was required for drug-mediated Aiolos degradation. To prevent ubiquitination of Aiolos, a Flag-tagged mutant IKZF3 (Flag-MT-IKZF3), in which all 30 lysine residues were conservatively substituted with arginines, was constructed. Jurkat cells were transfected with either a Flag-tagged WT IKZF3, Flag-MT-IKZF3, or an empty vector control. Cells were treated with lenalidomide or pomalidomide for 24 h and extracts were analysed by Western blot for Aiolos. Drug treatment caused degradation of endogenous Aiolos and recombinant Flag-WT-Aiolos, but not of Flag-MT-Aiolos, consistent with a mechanism of degradation that involves drug-induced lysine ubiquitination (Fig 4A).
Figure 4.
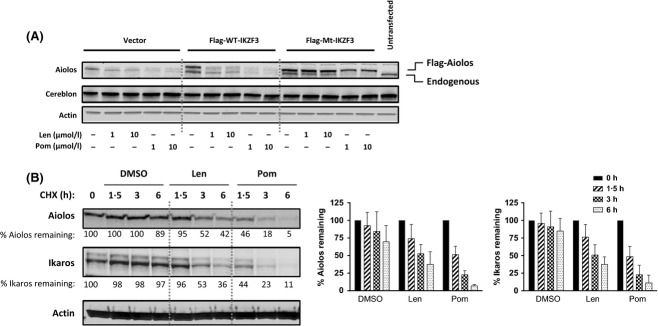
Lenalidomide and pomalidomide stimulate Aiolos degradation via ubiquitination and protein turnover. (A) Jurkat cells were transfected with control empty vector, wildtype IKZF3 or mutant IKZF3 for 6 h then treated with either DMSO, 1 or 10 μmol/l lenalidomide or pomalidomide for 24 h. Cell lysates were separated by SDS-PAGE and immunoblotted for Aiolos and Actin. (B) U266 cells were pulsed with 10 μg/ml cycloheximide and treated with either DMSO, 10 μmol/l lenalidomide or 1 μmol/l pomalidomide for 1·5, 3 or 6 h. Cell lysates were separated by SDS-PAGE and immunoblotted for Aiolos, Ikaros and Actin. Densitometry analysis of Aiolos and Ikaros expression normalized to Actin; data shown is mean ± SEM of three experiments. Len, lenalidomide; Pom, pomalidomide; CHX, cycloheximide; Mt, mutant; WT, wildtype; DMSO, dimethyl sulfoxide; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis.
We next evaluated the effect of lenalidomide and pomalidomide on the half-life of Aiolos and Ikaros. Primary human T cells were pulsed with cycloheximide to inhibit new synthesis of Aiolos and Ikaros proteins, and treated with either DMSO, lenalidomide, or pomalidomide in a time course. Aiolos and Ikaros appeared relatively stable in vehicle-treated cells, indicating a half-life of more than 6 h (Fig 4B, middle and right panels). By contrast, the addition of lenalidomide or pomalidomide accelerated the turnover of the proteins, resulting in a half-life for Aiolos and Ikaros of approximately 3 and 1·5 h, respectively (Fig 4B).
Lenalidomide and pomalidomide induced degradation of Aiolos and Ikaros is Cereblon-dependent in T Cells
The results described above suggest that lenalidomide or pomalidomide binding enables CRL4CRBN to associate with Ikaros and Aiolos, thus enabling their ubiquitination. To investigate whether drug-induced degradation of Aiolos and Ikaros in T cells occurs in a Cereblon-dependent manner, primary T cells were transfected with an siRNA against CRBN or control siRNA. CRBN siRNA resulted in a 75% decrease in CRBN mRNA levels compared with a non-targeting siRNA control (Fig 5A). Previous studies have established that a similar degree of knockdown leads to abrogation of lenalidomide- or pomalidomide-induced IL2 upregulation in T cells (Lopez-Girona et al, 2012). Figure5B demonstrates that the ability of lenalidomide or pomalidomide to induce Aiolos and Ikaros degradation in T cells was abrogated in the presence of CRBN siRNA, but not a non-targeting control siRNA. These data show that lenalidomide and pomalidomide induce degradation of Aiolos and Ikaros in a Cereblon-dependent manner.
Figure 5.
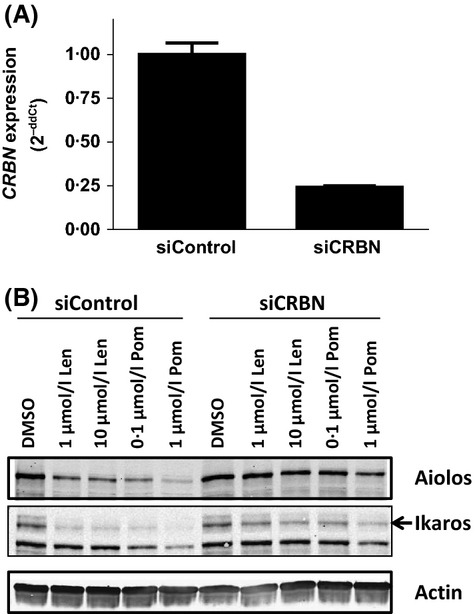
Aiolos and Ikaros degradation by lenalidomide and pomalidomide is Cereblon-dependent. (A) Primary human CD3+ T cells were transfected with control siRNA or CRBN siRNA for 48 h and CRBN knockdown efficiency was measured 24 h post-transfection by qRT-PCR. (B) CRBN siRNA knockdown T cells were treated with lenalidomide or pomalidomide at the indicated concentrations for 24 h. Cell lysates were separated by SDS-PAGE and immunoblotted for Aiolos, Ikaros and Actin expression. Len, lenalidomide; Pom, pomalidomide; CRBN, Cereblon gene; siCRBN, CRBN siRNA; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis.
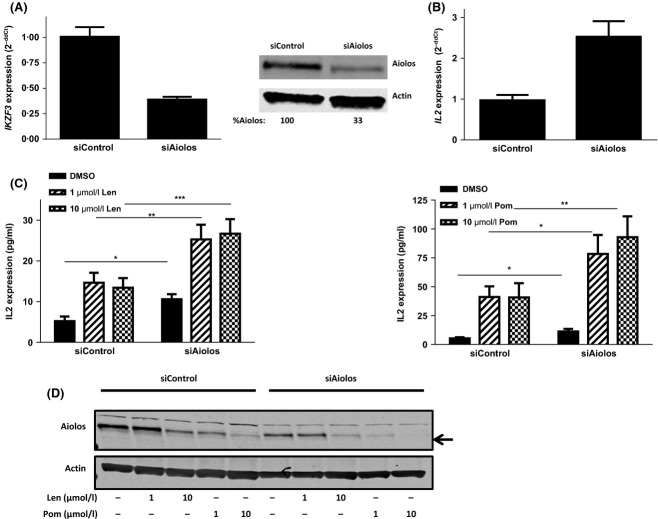
Aiolos knockdown mimics lenalidomide- or pomalidomide-induced IL2 elevation in T cells
Aiolos and Ikaros are known negative regulators of the IL2 promoter in T cells (Bandyopadhyay et al, 2007; Gandhi et al, 2010; Quintana et al, 2012). To investigate whether they are direct mediators of the known lenalidomide- and pomalidomide-induced upregulation of IL2, we knocked down Aiolos in T cells alone or in combination with drug treatment. T cells transfected with Aiolos siRNA for 24 h displayed a 61% decrease in IKZF3 RNA and a 67% decrease in Aiolos protein (Fig 6A). Reduction of Aiolos resulted in a corresponding 2·5 fold increase in IL2 mRNA (P < 0·0001; Fig 6B), confirming that Aiolos is a negative transcriptional regulator of the IL2 gene. There was a concomitant twofold increase in IL2 protein expression (P < 0·01; Fig 6C, compare black bars within each graph). Control siRNA-treated T cells resulted in the expected enhancement of IL2 protein production as previously reported (Fig 6C, left half of graphs; Lopez-Girona et al, 2012). The Aiolos siRNA-mediated reduction in Aiolos protein expression was further augmented by the addition of drug (Fig 6D). This correlated with the finding that the combination of lenalidomide or pomalidomide treatment and Aiolos siRNA expression further enhanced IL2 protein expression beyond that of Aiolos siRNA or drug treatment alone (Fig 6C, right half of graphs). Even a partial loss of Aiolos resulted in the induction of IL2 and stimulation of T cells. Additional cytokines upregulated by Aiolos siRNA and further enhanced by drug treatment include IL4, IL6, IL10, IL13, granulocyte macrophage colony-stimulating factor (GM-CSF) and interferon γ (IFN-γ; Fig S1). However, cytokine levels of IL8 and IL12 remained unchanged by Aiolos siRNA and/or drug treatment (Fig S2). The Aiolos expression levels after drug treatment in the presence or absence of Aiolos siRNA indicate a reverse correlation between Aiolos expression and IL2 production (Fig 6D). Therefore, Aiolos knockdown mimics the pharmacological effects of lenalidomide or pomalidomide on T cells.
Figure 6.
Aiolos is a negative regulator of IL2 in T cells and silencing Aiolos mimics lenalidomide or pomalidomide treatment. (A) Primary T cells were transfected with control siRNA or Aiolos siRNA for 24 h then assayed for IKZF3 gene expression or lysates were separated by SDS-PAGE and immunoblotted for Aiolos and Actin expression. Values represent Aiolos densitometry values normalized to Actin. (B) T cells transfected with control siRNA or Aiolos siRNA for 24 h were assayed for IL2 gene expression. (C) 24 h post-siRNA transfection, T cells were treated with drug for 48 h (left lenalidomide, right pomalidomide), harvested and IL2 protein expression was measured by ELISA. Data shown are mean of three donors tested in triplicate. (D) Aiolos and Actin expression was measured after drug treatment by immunoblot. *P < 0·05. **P < 0·01 and ***P < 0·001 using paired t-test. Len, lenalidomide; Pom, pomalidomide; siAiolos, Aiolos siRNA; DMSO, dimethyl sulfoxide; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; ELISA, enzyme-linked immunosorbent assay.
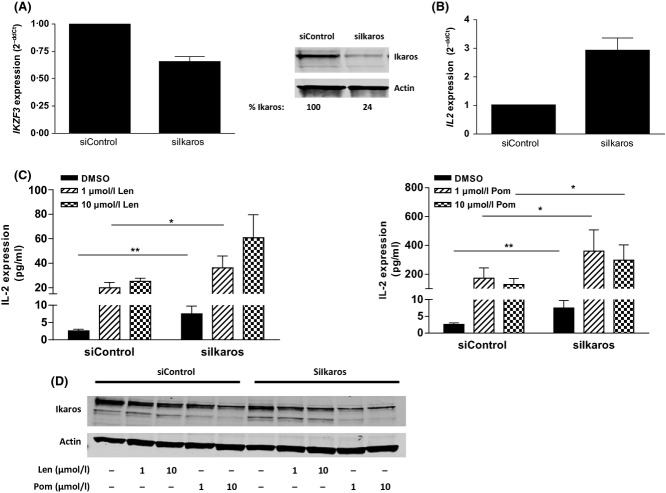
Ikaros knockdown mimics lenalidomide or pomalidomide-induced IL2 elevation in T cells
The impact of reducing Ikaros levels in primary T cells alone or in combination with lenalidomide or pomalidomide treatment was investigated. T cells transfected with Ikaros siRNA for 24 h displayed a 35% decrease in IKZF1 mRNA and 76% decrease in Ikaros protein (Fig 7A), resulting in an approximately threefold increase in IL2 mRNA (P < 0·001; Fig 7B). A concomitant increase in IL2 protein expression by approximately twofold (P < 0·05) was observed (Figs 7C, D, compare black bars). In control siRNA transfected cells, lenalidomide and pomalidomide induced IL2 protein 10- and approximately 150-fold respectively (Figs 7C, D, left half of graphs), whereas lenalidomide and pomalidomide treatment of T cells transfected with Ikaros siRNA further enhanced IL2 protein expression beyond that of DMSO by approximately 12- and 90-fold, respectively (Figs 7C, D, right half of graphs). Similar to the results obtained for Aiolos levels above and in line with previously reported data showing that Ikaros levels also negatively regulates IL2 in T cells, even a partial loss of Ikaros levels resulted in the induction of IL2 and co-stimulation of T cells as evidenced by the increased levels of T cell cytokines (Fig S2). The Ikaros expression levels after drug treatment in the presence or absence of Ikaros siRNA were measured (Fig 7D) and, similar to Aiolos levels, a reverse correlation between Ikaros expression and IL2 production was observed. The IL2 effect in the presence of drug was likely also to be increased due to the simultaneous reduction of Aiolos and Ikaros levels. Given that reduction of either Aiolos or Ikaros levels is sufficient to elicit co-stimulation of IL2 and other cytokines in T cells, we conclude that T cell co-stimulation by lenalidomide or pomalidomide is mediated by de-repression, via induced degradation of these IKZF transcription factors.
Figure 7.
Ikaros is a negative regulator of IL2 in T cells and silencing Ikaros mimics lenalidomide or pomalidomide treatment. (A) Primary T cells were transfected with Control siRNA or Ikaros siRNA for 48 h then assayed for IKZF1 gene expression or lysates were separated by SDS-PAGE and immuno blotted for Ikaros expression. Values represent Ikaros densitometry values normalized to Actin. (B) T cells transfected with control or Ikaros siRNA for 24 h were assayed for IL2 gene expression (n = 4). (C) 24 h post-siRNA transfection, T cells were drug treated for 48 h (left, lenalidomide; right, pomalidomide), harvested and IL2 expression was measured by ELISA. Data shown are mean of three donors tested in triplicate. (D) Ikaros and Actin expression was measured by immunoblot after drug treatment. *P < 0·05. and **P < 0·01 using paired t-test. Len, lenalidomide; Pom, pomalidomide; siIkaros, Ikaros siRNA; DMSO, dimethyl sulfoxide; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; ELISA, enzyme-linked immunosorbent assay.
Aiolos is a pharmacodynamic marker in human volunteers receiving lenalidomide
Healthy volunteers were administered either placebo or lenalidomide at doses of 10 or 50 mg. Blood samples were drawn immediately prior to dosing (pre-dose) and 6 h post-single dose of placebo or lenalidomide. Peripheral blood mononuclear cells were isolated by Ficoll from whole blood samples and viably frozen in DMSO prior to flow cytometric analysis of Aiolos expression. Our results indicate that Aiolos expression in peripheral T cells was reduced by 18% and 32% upon administration of 10 or 50 mg lenalidomide, respectively (Fig 8). Therefore, the effects of lenalidomide on Aiolos protein levels observed in vitro were also detected in human volunteers, suggesting that the measurement of Aiolos protein levels in peripheral blood cells can be used as a clinical biomarker of lenalidomide activity and may be used to define the dose and schedule for novel indications and next-generation compounds.
Figure 8.

Dose-dependent Aiolos inhibition in peripheral T cells from healthy volunteers administered with lenalidomide. Healthy volunteers were administered either placebo, 10 or 50 mg of lenalidomide and blood was drawn 6 h post-dose. Aiolos levels were measured in CD3+ T cells by flow cytometry (n = 5–8 for each treatment arm). MEFL, mean equivalent fluorochrome labelling; SEM, standard error of the mean.
Discussion
Following the identification of Cereblon as a target of thalidomide, it was suggested that drug binding to Cereblon inhibits the E3 ligase activity of CRL4CRBN (Ito et al, 2010). In contrast, data here demonstrate that, depending upon cellular context, binding of lenalidomide or pomalidomide can promote the E3 ligase activity towards specific substrates. Here we identify the important regulators of haematological cell fate and differentiation, Ikaros and Aiolos, as substrates that interact with CRL4CRBN consequent to lenalidomide or pomalidomide treatment. We report that lenalidomide and pomalidomide promote the concentration- and time-dependent degradation of Aiolos and Ikaros in activated T cells within hours of drug treatment (Fig 2). This rapid degradation is independent of new protein synthesis and occurs via protein ubiquitination and proteosomal degradation (Figs 1, 2 and 4). Drug binding to Cereblon promotes its coprecipitation with Aiolos and Ikaros (Fig 3). Furthermore, our studies confirm that Cereblon is required for Aiolos and Ikaros degradation by lenalidomide or pomalidomide, as Cereblon knockdown in T cells protects the substrate proteins from drug-induced degradation (Fig 5). Finally, we find that lenalidomide- and pomalidomide-induced T cell co-stimulation can be the consequence of Aiolos and Ikaros degradation, consistent with effects mediated through increased association of these substrates to the E3 ligase complex upon drug binding to Cereblon. Aiolos and Ikaros knockdown studies show that both are negative regulators of IL2 production in pan CD3+ T cells (Figs 6 and 7).
Here we propose a model whereby lenalidomide or pomalidomide binding to CRBN results in the increased ability of CRL4CRBN to adopt Aiolos and Ikaros as substrates, thereby shifting and/or expanding the spectrum of substrates that are bound to and targeted for degradation (Fig 9). This may be due either to a drug-induced neomorphic activity or bias towards a subset of normal substrates. X-ray crystallographic structural studies are underway to address this in more detail.
Figure 9.
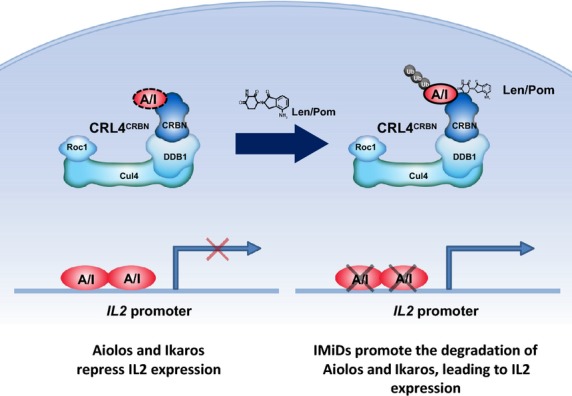
Model of lenalidomide and pomalidomide Co-stimulation of T cells via Degradation of Aiolos and Ikaros. In the absence of lenalidomide and pomalidomide, Aiolos and Ikaros are expressed and function as repressors of the IL2 promoter. Aiolos and Ikaros may or may not be normal substrates of Cereblon as represented by the dashed line, however the presence of lenalidomide and pomalidomide increases the ability of CRL4CRBN to interact with Aiolos and Ikaros, leading to their ubiquitination and degradation resulting in de-repression of the IL2 promoter and increased expression of IL2. CRL4, cullin ring 4 ligase; CRBN, Cereblon; A/I, Aiolos/Ikaros; Cul4, cullin 4; DDB1, DNA damage binding protein-1; IMiD, immunomodulatory drug; Len, lenalidomide; Pom, pomalidomide; Roc1, regulator of cullins 1; Ub, ubiquitin.
The state of both T cell anergy and activation as well as T cell lineage differentiation are tightly controlled, and the Ikaros family of zinc-finger transcription factors are essential contributors to T cell fate decisions (Thomas et al, 2007). Ikaros has been identified as a key regulator of haematopoiesis, specifically lymphoid lineage development, and is expressed from early lymphoid progenitor stages to mature T and B cells. Ikaros-deficient mice have retarded T cell development, indicating a crucial role in early lymphoid development, while Aiolos-deficient mice exhibit normal T cell development (Wang et al, 1996, 1998; Cai et al, 2009). Both Ikaros and Aiolos have been shown to interact with repressive nucleosome remodelling and histone deacetylation complexes (NuRD; Kim et al, 1999; Koipally et al, 1999; Gandhi et al, 2010). In CD4+ Th1 T cells, Ikaros is a transcriptional repressor of both IL2 and IFNγ, while Aiolos is specific for IL2. It is also reported that Ikaros binds and represses the IL2 promoter in anergic CD8+ T lymphocytes (Thomas et al, 2007). In our studies in pan-CD3+ T cells, similar to Aiolos, Ikaros functions as a negative regulator of multiple cytokines including IL4, IL6, IL10, IL13, GM-CSF, TNFα and IFNγ (Fig S3) but not all cytokines were affected by Ikaros siRNA (Fig S4). The effect of lenalidomide and pomalidomide on Aiolos and Ikaros in various T cell subsets is under investigation.
The presence of these substrates in relevant cell types is as important as the presence of Cereblon for lenalidomide or pomalidomide efficacy. Therefore Aiolos and Ikaros themselves may serve as biomarkers of response which is supported by the human volunteer data (Fig 8). In this context, it is important to note that the effects on Ikaros and Aiolos in vitro are observed at concentrations well below the clinically achievable plasma levels of both drugs doses (lenalidomide Cmax at the 25 mg dose is 2·2 μmol/l and pomalidomide Cmax at the 4 mg dose is 270 nmol/l; Chen et al, 2007; http://www.pomalyst.com/docs/prescribing_information.pdf). Finally, these biomarkers will be used to define dosing and scheduling for the next generation of drugs.
In conclusion, we have furthered our knowledge of the molecular mechanism of T cell immunomodulation by lenalidomide and pomalidomide. Our findings show that these drugs promote degradation of two members of the Ikaros family of transcription factors, which probably has major implications not only for normal T cells, but also for normal B cell development, as Ikaros and Aiolos play an important role in B cell lineage commitment and differentiation (Thompson et al, 2007; Billot et al, 2010; Ma et al, 2010; Ferreirós-Vidal et al, 2013). In addition, Aiolos is expressed in haematological tumours including, non-Hodgkin lymphoma, CLL and acute myeloid leukaemia, although their relevance in disease requires further clarification (Nückel et al, 2008). Aiolos expression has been correlated with disease progression in CLL, and splice variants of Ikaros have been identified in acute lymphocytic leukaemia (Sun et al, 1999; Iacobucci et al, 2008; Nückel et al, 2008). These results may have further implications in epithelial tumours where Ikaros is expressed (Yang et al, 2010). Whether Ikaros is recruited as a substrate by the ubiquitously expressed CRL4CRBN in solid tumours in response to lenalidomide or pomalidomide treatment remains to be shown. Understanding the full spectrum of effects of lenalidomide and pomalidomide on this important family of transcription factors and additional potentially relevant novel CRL4CRBN substrates is critical for optimal use of these drugs in haematological malignancies, as well as for immunotherapy in oncology and autoimmune/inflammatory diseases. Defining additional substrates of consequence will be important for customizing spectra of activity with E3 ligase modulation as a novel approach.
Acknowledgments
The authors thank Patrick Hagner (Celgene Corporation) for helpful advice and Garth McGrath (Celgene Corporation) for clinical sample collection and management. Flow cytometry was performed at LabCorp (Cranford, NJ, USA). Editorial assistance was provided by MediTech Media funded by Celgene Corporation.
Author contribution
JK, TC, CH, MW and TI designed and performed experiments and/or analysed data. YN analysed proteomics data. LW developed the Aiolos flow cytometry assay. AKG, AK and RC wrote the manuscript. AKG, AT, AK, CH, HH, TOD, PHS, and RC provided intellectual input and advice on experimental design and analysis.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig S1. Aiolos is a negative regulator of multiple cytokines in T cells, and silencing Aiolos mimics lenalidomide or pomalidomide treatment.
Fig S2. Aiolos is not a regulator of IL8 or IL12 p70 in T cells nor do lenalidomide or pomalidomide significantly affect expression of these cytokines.
Fig S3. Ikaros is a negative regulator of multiple cytokines in T cells and silencing Ikaros mimics lenalidomide or pomalidomide treatment.
Fig S4. Ikaros is not a regulator of IL8 or IL12 p70 in T cells nor do lenalidomide or pomalidomide affect expression of these cytokines.
References
- Bandyopadhyay S, Duré M, Paroder M, Soto-Nieves N, Puga I. Macián F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billot K, Parizot C, Arrouss I, Mazier D, Debre P, Rogner UC. Rebollo A. Differential Aiolos expression in human hematopoietic subpopulations. Leukemia Research. 2010;34:289–293. doi: 10.1016/j.leukres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Cai Q, Dierich A, Oulad-Abdelghani M, Chan S. Kastner P. Helios deficiency has minimal impact on T cell development and function. Journal of Immunology. 2009;183:2303–2311. doi: 10.4049/jimmunol.0901407. [DOI] [PubMed] [Google Scholar]
- Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R. Laskin OL. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. Journal of Clinical Pharmacology. 2007;47:1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI. Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. Journal of Immunology. 1999;163:380–386. [PubMed] [Google Scholar]
- Dredge K, Marriott JB, Todryk SM, Muller GW, Chen R, Stirling DI. Dalgleish AG. Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Thl-type immunity. Journal of Immunology. 2002;168:4914–4919. doi: 10.4049/jimmunol.168.10.4914. [DOI] [PubMed] [Google Scholar]
- Ferreirós-Vidal I, Carroll T, Taylor B, Terry A, Liang Z, Bruno L, Dharmalingam G, Khadayate S, Cobb BS, Smale ST, Spivakov M, Srivastava P, Petretto E, Fisher AG. Merkenschlager M. Genome-wide identification of Ikaros targets elucidates its contribution to mouse B-cell lineage specification and pre-B-cell differentiation. Blood. 2013;121:1769–1782. doi: 10.1182/blood-2012-08-450114. [DOI] [PubMed] [Google Scholar]
- Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. Dalgleish AG. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunology Immunotherapy. 2008;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL. Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nature Immunology. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi AK, Shi T, Li M, Jungnelius U, Romano A, Tabernero J, Siena S, Schafer PH. Chopra RImmunomodulatory. Effects in a phase II study of lenalidomide combined with cetuximab in refractory KRAS-mutant metastatic colorectal cancer patients. PLoS ONE. 2013;8:e80437. doi: 10.1371/journal.pone.0080437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci I, Lonetti A, Messa F, Cilloni D, Arruga F, Ottaviani E, Paolini S, Papayannidis C, Piccaluga PP, Giannoulia P, Soverini S, Amabile M, Poerio A, Saglio G, Pane F, Berton G, Baruzzi A, Vitale A, Chiaretti S, Perini G, Foà R, Baccarani M. Martinelli G. Expression of spliced oncogenic Ikaros isoforms in Philadelphia-positive acute lymphoblastic leukemia patients treated with tyrosine kinase inhibitors: implications for a new mechanism of resistance. Blood. 2008;112:3847–3855. doi: 10.1182/blood-2007-09-112631. [DOI] [PubMed] [Google Scholar]
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y. Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T. Kingst R. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW. Gygi SP. Systematic and quantitative assessment of the ubiquitin modified proteome. Molecular Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koipally J, Renold A, Kim J. Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO Journal. 1999;18:3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, Cathers B, Rychak E, Gaidarova S, Chen R, Schafer PH, Handa H, Daniel TO, Evans JF. Chopra R. Cereblon is a direct target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Pathak S, Mandal M, Trinh L, Clark MR. Lu R. Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Molecular and Cellular Biology. 2010;30:4149–4158. doi: 10.1128/MCB.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nückel H, Frey UH, Sellmann L, Collins CH, Dührsen U. Siffert W. The IKZF3 (Aiolos) transcription factor is highly upregulated and inversely correlated with clinical progression in chronic lymphocytic leukaemia. British Journal of Haematology. 2008;144:268–270. doi: 10.1111/j.1365-2141.2008.07442.x. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K. Kuchroo VK. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nature Immunology. 2012;13:770–778. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, Byrd JC. Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. Journal of Clinical Investigation. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, Wolbring G, Govinda S, Corral LG, Payvandi F, Muller GW. Stirling DI. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. Journal of Pharmacology and Experimental Therapeutics. 2003;305:1222–1232. doi: 10.1124/jpet.102.048496. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology. 2004;3 doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Sun L, Goodman PA, Wood CM, Crotty ML, Sensel M, Sather H, Navara C, Nachman J, Steinherz PG, Gaynon PS, Seibel N, Vassilev A, Juran BD, Reaman GH. Uckun FM. Expression of aberrantly spliced oncogenic Ikaros isoforms in childhood acute lymphoblastic leukemia. Journal of Clinical Oncology. 1999;17:3753–3766. doi: 10.1200/JCO.1999.17.12.3753. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S. Wells AD. Ikaros enforces the costimulatory requirement for IL-2 gene expression and is required for anergy induction in CD4+ T lymphocytes. Journal of Immunology. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- Thompson EC, Cobb BS, Sabbattini P, Meixlsperger S, Parelho V, Liberg D, Taylor B, Dillon N, Georgopoulos K, Jumaa H, Smale ST, Fisher AG. Merkenschlager M. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26:335–344. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M. Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA. Georgopoulos K. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–553. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P. Bartlett JB. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clinical Cancer Research. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- Yang L, Luo Y. Wei J. Integrative genomic analyses on Ikaros and its expression related to solid cancer prognosis. Oncology Reports. 2010;24:571–577. doi: 10.3892/or_00000894. [DOI] [PubMed] [Google Scholar]
- Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ. Stewart AK. Cereblon expression is required for the anti-myeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Aiolos is a negative regulator of multiple cytokines in T cells, and silencing Aiolos mimics lenalidomide or pomalidomide treatment.
Fig S2. Aiolos is not a regulator of IL8 or IL12 p70 in T cells nor do lenalidomide or pomalidomide significantly affect expression of these cytokines.
Fig S3. Ikaros is a negative regulator of multiple cytokines in T cells and silencing Ikaros mimics lenalidomide or pomalidomide treatment.
Fig S4. Ikaros is not a regulator of IL8 or IL12 p70 in T cells nor do lenalidomide or pomalidomide affect expression of these cytokines.