Abstract
Objectives
Kaposi's sarcoma (KS), invasive cervical carcinoma (ICC) and non-Hodgkin lymphoma (NHL) have been listed as AIDS-defining cancers (ADCs) by the Centers for Disease Control and Prevention since 1993. Despite this, HIV screening is not universally mentioned in ADC treatment guidelines. We examined screening practices at a tertiary centre serving a population where HIV seroprevalence is 0.4%.
Methods
Patients with KS, ICC, NHL and Hodgkin lymphoma (HL), treated at Lausanne University Hospital between January 2002 and July 2012, were studied retrospectively. HIV testing was considered part of the oncology work-up if performed between 90 days before and 90 days after the cancer diagnosis date.
Results
A total of 880 patients were examined: 10 with KS, 58 with ICC, 672 with NHL and 140 with HL. HIV testing rates were 100, 11, 60 and 59%, and HIV seroprevalence was 60, 1.7, 3.4 and 5%, respectively. Thirty-seven patients (4.2%) were HIV-positive, of whom eight (22%) were diagnosed at oncology work-up. All newly diagnosed patients had CD4 counts < 200 cells/μL and six (75%) had presented to a physician 12–236 weeks previously with conditions warranting HIV testing.
Conclusions
In our institution, only patients with KS were universally screened. Screening rates for other cancers ranged from 11 to 60%. HIV seroprevalence was at least fourfold higher than the population average. As HIV-positive status impacts on cancer patient medical management, HIV screening should be included in oncology guidelines. Further, we recommend that opt-out screening should be adopted in all patients with ADCs and HL.
Keywords: AIDS-defining cancers, HIV screening, HIV testing recommendations
Introduction
Although Kaposi's sarcoma (KS), invasive cervical cancer (ICC) and non-Hodgkin lymphoma (NHL) have been listed as AIDS-defining cancers (ADCs) since 1993 1, HIV testing is not universally proposed in ADC treatment guidelines. Testing is implicit for KS, as part of staging, recommended for NHL 2, but not mentioned for ICC 3,4. Testing is also recommended in Hodgkin lymphoma (HL) 5, given its high incidence in HIV-positive patients 6. Testing in ADC patients is beneficial and logical: in lymphoma, outcome is optimized when co-existing HIV infection is also treated 7,8; in ICC, the leading cause, human papillomavirus, and HIV share transmission routes.
In 2006, the Centers for Disease Control and Prevention recommended nontargeted ‘opt-out’ HIV testing in all settings where local undiagnosed HIV seroprevalence is ≥ 0.1% 9. In Europe, most national testing recommendations are ‘opt-in’: the 2008 British HIV Association guidelines recommend diagnostic testing and targeted screening 10; in Switzerland, where estimated HIV seroprevalence is 0.4% 11, physician-initiated counselling and testing (PICT) has been recommended since 2007 12. The Swiss PICT recommendations list ADCs among testing indications. However, the degree of translation of these recommendations into clinical practice is unclear: there was no change in testing rates in our 1300-bed hospital when the recommendations were updated, and rates among oncology patients remained very low (< 5%) 13. The primary objective of this study was therefore to determine HIV testing rates among patients treated for ADCs and HL at our centre.
Methods
This study was approved by the ethics committee on human scientific research of the canton of Vaud, Switzerland. Patients aged ≥ 18 years treated for KS, ICC, NHL and HL between 1 January 2002 and 31 July 2012 were studied retrospectively.
HIV testing was considered as related to oncology work-up if performed within 90 days before and 90 days after the cancer diagnosis date, based on local clinical experience (SP and CA). Patients were classified as HIV ‘tested’ upon identification of 1 documented testing in the hospital database or 2 a reference to testing performed elsewhere. Testing date, result and, where positive, baseline CD4 count were recorded. Patients were further cross-referenced against the Lausanne arm of the Swiss HIV Cohort Study (SHCS), with an ongoing, continuous enrolment 14, to identify HIV-positive diagnoses made after the oncology event. Patients not ‘tested’ were classified as ‘not tested’, or ‘missing data’ when records were insufficiently comprehensive to determine testing status. Patient demographic data were recorded.
HIV testing rate was calculated using a modified equation described previously 13:
HIV seroprevalence was also calculated:
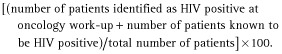 |
Data analysis was performed after patient anonymization. Given the different demographic profiles of the ADCs examined, each ADC was analysed separately. Means were compared using Student's t-test and proportions compared using the χ2 test. All analyses were performed using Microsoft Excel 2008 (Microsoft Corporation, Redmond, WA).
Results
Of 880 patients attending our centre during the study period, 10 had KS, 58 ICC, 672 NHL and 140 HL. A total of 610 patients (of 880; 69%) were ‘tested’, most (79%) within 90 days before and 90 days after their cancer diagnosis date. Patients tested > 90 days after their cancer diagnosis were mostly NHL patients (78%).
For HIV testing rate and seroprevalence calculations, ‘missing data’ patients [22 of 672 patients (3.3%) with NHL and six of 140 patients (4.3%) with HL] were grouped with ‘not tested’ patients. Calculations pertain to the whole study period, as rates remained static between calendar years (data not shown). Testing rates and seroprevalence figures are shown in Table 1. Mean age was lower in ‘tested’ compared with ‘not tested’ NHL patients (55 vs 62 years, respectively; P < 0.0001), and testing rate was higher among younger (≤ 55 years old) than older (> 55 years old) NHL patients (68% vs 54%, respectively; P = 0.01). No age difference was observed in other cancer categories. Testing rates did not differ with sex, marital status or origin (Table 1).
Table 1.
Demographic characteristics of study patients, HIV testing rates and HIV seroprevalence, grouped by cancer diagnosis
| ICC |
NHL |
HL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tested | Not tested | Pa | Tested | Not tested | Pa | Tested | Not tested | Pa | |
| Number of patients [n (%)] | 6 (11) | 51 (89) | 392 (60) | 261 (40) | 79 (59) | 54 (41) | |||
| Number of men [n (%)] | NA | NA | – | 230 (59) | 161 (62) | > 0.5 | 44 (56) | 32 (59) | > 0.5 |
| Age (years) [median (IQR)] | 49 (36–58) | 54 (44–65) | > 0.5 | 57 (45–66) | 62 (52–74) | 0.01 | 32 (27–51) | 36 (27–59) | 0.6 |
| Married/widowed [n (%)] | 2 (33) | 26 (51) | > 0.5 | 276 (70) | 187 (72) | > 0.5 | 40 (51) | 29 (54) | > 0.5 |
| Origin [n (%)] | |||||||||
| Switzerland and North/West Europe | 4 (67) | 37 (73) | > 0.5 | 300 (77) | 219 (84) | 0.27 | 53 (67) | 38 (70) | > 0.5 |
| Other* | 2 (33) | 14 (27) | 92 (23) | 42 (16) | 26 (33) | 16 (30) | |||
| HIV-positive patients [total (n diagnosed at oncology work-up)] | 1 (0) | 23 (4) | 7 (0) | ||||||
| HIV seroprevalence, based on all patients as denominator [% (95% CI)]† | 1.7 (NA; < 5 HIV-positive patients†) | 3.4 (2.0–4.8) | 5 (1.4–8.6) | ||||||
| HIV seroprevalence, based on ‘tested’ patients only as denominator [% (95% CI)]† | 16 (NA; < 5 HIV-positive patients†) | 5.9 (3.6–8.2) | 8.9 (5.7–12.1) | ||||||
ICC, invasive cervical carcinoma; IQR, interquartile range; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; IQR, interquartile range; NA, not applicable.
P-values are specified where P < 0.5 (χ2 test); those for patient origin are calculated for patients from Switzerland and North/West Europe against those of all other origin.
Asia, Africa, South/East Europe, Middle East and America.
For cancer groups with testing rates < 100%, seroprevalence was calculated taking tested as well as all patients as the denominator, as assuming that all ‘not tested’ patients were HIV negative could underestimate true HIV seroprevalence. The 95% confidence interval (CI) is calculated for cancer groups with > 5 HIV-positive patients. The 10 Kaposi's sarcoma patients are not included in this table as HIV testing rate was 100%. Among these patients, nine (90%) were men, median age was 59 years (IQR 49–62 years), and seven (70%) were from Switzerland or North/West Europe; seroprevalence was six of 10 (60%), with four of the six patients being newly diagnosed at oncology work-up.
Of 37 HIV-positive patients, eight (22%) were diagnosed at oncology work-up, all with CD4 counts < 200 cells/μL. Seven (88%) had consulted a doctor a median of 76 weeks (range 8-236 weeks) prior to their ADC presentation, and six (75%) had presented with criteria for HIV testing according to national recommendations (Table S1). No patient ‘not tested’ at cancer diagnosis was subsequently identified as HIV positive.
Discussion
In this 10-year retrospective analysis of oncology patients, we observe HIV testing rates of 59-60% in lymphoma patients, 11% in ICC patients, and 100% in KS patients. Among NHL patients, we observe a significant inverse relationship between testing rate and patient age. Eight new HIV-positive diagnoses were made at oncology work-up. Assuming all untested patients were HIV negative, HIV seroprevalence among ICC, NHL and HL patients was 1.7, 3.4 and 5%, respectively; seroprevalence among KS patients was 60%.
The low testing rate in ICC patients is surprising, given that ICC is an ADC. The authors of the HIV Indicator Diseases across Europe Study (HIDES 1) recently concluded that HIV screening in patients with cervical cancer of any stage is cost effective and should be recommended 15. However, neither European nor American ICC guidelines currently mention testing 3,4.
The testing rate in lymphoma patients is suboptimal, when treatment guidelines recommend testing 2,5. The inverse relationship between testing rates and patient age in NHL patients goes against the emerging trend of increasing numbers of adults aged > 50 years accessing HIV care 16. Older individuals often present at late stages of infection, with increased associated morbidity, mortality and health care costs 16,17. Undiagnosed, and therefore untreated, infection also favours onward transmission.
Finally, it is concerning that the eight newly diagnosed patients had to develop cancer to be diagnosed with HIV infection, despite the fact that six had previously consulted for reasons that should have prompted earlier HIV testing.
This study has limitations. We examined testing performed rather than testing offered, so may have misclassified patients declining testing as ‘not tested’. Against this, we have observed that most patients are agreeable to routine testing pre-surgery 18. Among lymphoma patients, we did not examine whether testing was prompted by diagnosis or anticipated treatment as we examined whether HIV testing was performed, not why. If testing is performed in anticipation of risk-related treatment options, this could explain the low testing rate in ICC, where chemotherapy schedules have less impact on host immunity.
In conclusion, we observed suboptimal HIV testing rates among patients with ICC and lymphoma, and lower testing rates with increasing age among NHL patients. HIV seroprevalence was at least fourfold higher than the population average. To our knowledge, this is the first study examining a decade of HIV testing rates for all three ADCs. Given the impact of HIV-positive status on the medical management of cancer patients, we propose that treatment guidelines should mention HIV testing for ICC patients, and that opt-out testing should be adopted in all patients with ADCs and HL. Exploring barriers to testing oncology patients, beyond the lack of guidelines, is a crucial parallel measure.
Acknowledgments
The authors thank the Swiss HIV Cohort Study for access to the database of patients followed up in Lausanne.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by the Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1 Previous consultation histories of the eight patients newly diagnosed as HIV-positive as a result of their oncology work-ups. Abbreviations: KS, Kaposi's sarcoma; NHL, non-Hodgkin lymphoma.
References
- 1.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 2.Tilly H, Vitolo U, Walewski J, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii78–vii82. doi: 10.1093/annonc/mds273. [DOI] [PubMed] [Google Scholar]
- 3.Colombo N, Carinelli S, Colombo A, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–vii32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 4.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 5.Eichenauer DA, Engert A, Dreyling M, Group EGW. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi55–vi58. doi: 10.1093/annonc/mdr378. [DOI] [PubMed] [Google Scholar]
- 6.Goedert JJ, Bower M. Impact of highly effective antiretroviral therapy on the risk for Hodgkin lymphoma among people with human immunodeficiency virus infection. Curr Opin Oncol. 2012;24:531–536. doi: 10.1097/CCO.0b013e3283560697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro JT, Lloveras N, Ribera JM, Oriol A, Mate JL, Feliu E. The prognosis of HIV-infected patients with diffuse large B-cell lymphoma treated with chemotherapy and highly active antiretroviral therapy is similar to that of HIV-negative patients receiving chemotherapy. Haematologica. 2005;90:704–706. [PubMed] [Google Scholar]
- 8.Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30:4111–4116. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE1-4. [PubMed] [Google Scholar]
- 10.Palfreeman A, Fisher M, Ong E, et al. Testing for HIV: concise guidance. Clin Med. 2009;9:471–476. doi: 10.7861/clinmedicine.9-5-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2009. UNAIDS epidemiology figures 2009. Switzerland. Available at http://www.unaids.org/en/regionscountries/countries/switzerland/. (accessed 5 February 2014)
- 12.Office fédéral de la santé publique. 2007. Dépistage du VIH et conseil initiés par les médecins. Bulletin. 371–3. Available at http://www.bag.admin.ch/hiv_aids/12472/12474/?lang=fr. (accessed 5 February 2014)
- 13.Darling KE, Hugli O, Mamin R, et al. HIV testing practices by clinical service before and after revised testing guidelines in a Swiss University Hospital. PLoS ONE. 2012;7:e39299. doi: 10.1371/journal.pone.0039299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swiss HIV Cohort Study Schoeni-Affolter F. Ledergerber B, Rickenbach M, Rudin C, et al. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39:1179–1189. doi: 10.1093/ije/dyp321. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan AK, Raben D, Reekie J, et al. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study) PLoS ONE. 2013;8:e52845. doi: 10.1371/journal.pone.0052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith RD, Delpech VC, Brown AE, Rice BD. HIV transmission and high rates of late diagnoses among adults aged 50 years and over. AIDS. 2010;24:2109–2115. doi: 10.1097/QAD.0b013e32833c7b9c. [DOI] [PubMed] [Google Scholar]
- 17.Wolbers M, Bucher HC, Furrer H, et al. Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med. 2008;9:397–405. doi: 10.1111/j.1468-1293.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht E, Frascarolo P, Meystre-Agustoni G, et al. An analysis of patients' understanding of ‘routine’ preoperative blood tests and HIV screening. Is no news really good news? HIV Med. 2012;13:439–443. doi: 10.1111/j.1468-1293.2012.00993.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Previous consultation histories of the eight patients newly diagnosed as HIV-positive as a result of their oncology work-ups. Abbreviations: KS, Kaposi's sarcoma; NHL, non-Hodgkin lymphoma.


