Abstract
Summary
Background
It has been suggested that intralesional triamcinolone injections represent a safe and effective therapeutic strategy in controlling the permanent disfiguring swelling of orofacial granulomatosis (OFG). However, robust supporting evidence is lacking, due to the variable and inconsistent design of available studies.
Objectives
To investigate whether a standardized regimen of intralesional triamcinolone has beneficial long-term effects on orofacial swelling of OFG. We also studied potential associations with a number of prognostic factors.
Methods
We designed a retrospective observational study of a homogeneous cohort of 22 well-phenotyped patients with OFG. The primary outcome was defined as a statistically significant decrease in post-treatment disease severity. Statistically significant association with prognostic factors was the secondary outcome. Statistical analysis included Wilcoxon signed-rank tests and logistic regression.
Results
Compared with pretreatment, there were statistically significant decreases in disease severity scores at all time points until 48 months post-treatment (P < 0·01). Logistic regression analysis showed there was no independent prognostic variable of statistical significance (P > 0·05). The majority of patients (14/22, 63·6%) received one course of intralesional triamcinolone and did not experience disease recurrence. The mean disease-free period after the first course of intralesional therapy was 28·9 ± 18 months (95% confidence interval 28·7–29·1). No adverse effects were reported.
Conclusions
This is the first study to have employed robust cohort methodology and sound statistics to demonstrate long-term effectiveness of intralesional triamcinolone in controlling the disfiguring swelling of OFG. Because of limitations inherent in observational studies, further research in the form of randomized case-control trials is needed to confirm the present findings.
What's already known about this topic?
It has been suggested that intralesional corticosteroid therapy is effective in controlling the permanent disfiguring swelling of orofacial granulomatosis (OFG); however, robust supporting evidence is lacking due to the variable and inconsistent design of available studies.
What does this study add?
This is the first cohort study on intralesional therapy to employ robust cohort design, consistent methodology and a standardized regimen of triamcinolone injections. It provides reliable evidence of long-term effectiveness in reducing the orofacial swelling of OFG.
Orofacial granulomatosis (OFG) is a chronic immunologically mediated granulomatous disorder that typically affects the orofacial region. The spectrum of possible clinical manifestations of OFG ranges from subtle oral mucosal swelling and recurrent ulceration to permanent fibrous swelling of the lips and face; neurological manifestations can also occur.1–3 Although variable and multiform in its early stages,3,4 the natural history of OFG is ultimately progressive, and permanent disfiguring labial and/or facial swelling eventually develops in nearly all affected individuals.2–4 Indeed, labial and facial swelling represents the most common reason for which patients with OFG seek medical attention5 and is the major culprit in reduced quality of life and psycho-social distress associated with the disease.6
Long-term remission of the orofacial swelling of OFG by means of intralesional triamcinolone acetonide injections was first reported in 1992 by Sakuntabhai et al.7 A number of subsequent independent reports have further suggested their clinical benefit and overall safety.8–20 However, there is little robust evidence, as available studies are limited by uncontrolled design, small sample size, short-term follow-up and wide variability in administration schedule, drug concentration, concomitant medications, total dose of injected corticosteroids, OFG diagnostic criteria and outcome measures.8–13
In order to clarify the long-term effectiveness of intralesional triamcinolone in the treatment of OFG-related orofacial swelling, we retrospectively studied a homogeneous cohort of well-phenotyped patients with idiopathic OFG who had been homogeneously managed with a predefined therapeutic regimen of triamcinolone injections and reviewed long-term with a predefined set of outcome measures. The present study is reported according to STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) recommendations.21
Patients and methods
Study cohort and data extraction
The study cohort consisted of 22 individuals diagnosed with OFG and treated with intralesional triamcinolone acetonide therapy2,3 at the Oral Medicine Unit of the Eastman Dental Hospital, University College London Hospital Trust between January 2006 and July 2012. Diagnostic and inclusion criteria are reported in Table 1. In order to minimize selection bias, all patients with idiopathic OFG consecutively treated with intralesional therapy from 2006 and 2012 were included in the present cohort study. Systematic data extraction of detailed patient phenotype and management was performed in order to minimize information bias. The following data were extracted from hospital notes: patient demographics, date of disease onset and disease duration before treatment, number and location of orofacial sites involved, date, number of courses and total dose of intralesional corticosteroid therapy, disease-free period and recurrences, length of observation period and adverse events.
Table 1.
Diagnostic and inclusion criteria
| Presence of permanent swelling of orofacial tissues due to orofacial granulomatosis |
| Orofacial granulomatosis diagnosed on the bases of currently accepted criteria2,3 |
| No history and no clinical evidence of Crohn disease or sarcoidosis |
| No reported clear history of hypersensitivity to cinnamon and benzoate |
| Lack of response to topical corticosteroids or immunosuppressants |
| No concomitant systemic/topical corticosteroid or other immunosuppressive therapy |
Intralesional triamcinolone acetonide therapy
All patients were treated with at least one full course of intralesional corticosteroid injections using highly concentrated triamcinolone acetonide 40 mg mL−1.11 The therapeutic regimen is described in Figures1 and 2. We used a 1 mL subcutaneous syringe and no trigeminal nerve anaesthetic block. A thin film of anaesthetic cream was applied onto the affected lip immediately before the injections in most patients. After completion of one full course, post-treatment hospital appointments were arranged at 2 weeks and 1 month from completion of therapy, followed by reviews at 6-monthly intervals. Further courses of therapy were performed in case of recurrent disease occurring more than 6 months after treatment completion. Patients experiencing recurrences or disease progression within the first 6 months after therapy were offered alternative treatment options (not reported in this study).
Figure 1.
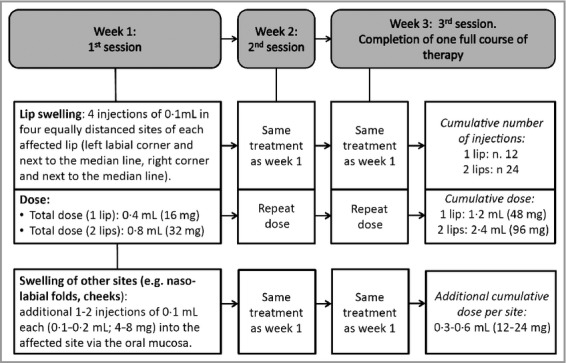
Regimen of intralesional injections of triamcinolone acetonide
Figure 2.
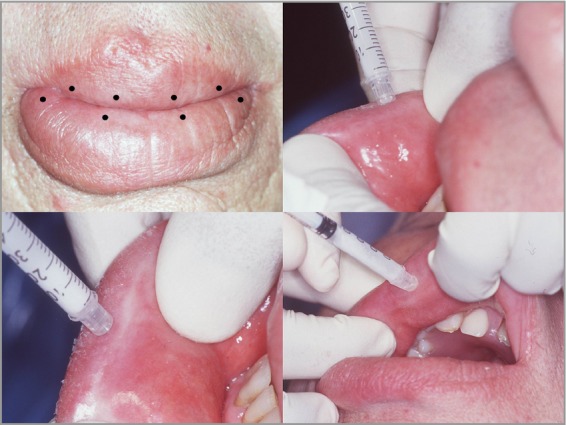
Sites of intralesional injections in a patient with swelling of upper and lower lip.
Primary outcome and outcome measure
The primary aim of this study was to investigate whether intralesional triamcinolone therapy has beneficial long-term effects upon the orofacial swelling of OFG. The primary outcome was defined as a statistically significant decrease in disease severity post-treatment, compared with pretreatment and between review appointments at 2 weeks, 1 month and every 6 months after therapy. A dedicated four-point disease severity score was used as the outcome measure (Table 2). The disease severity score was attributed on the basis of (i) the clinician's judgement during a hospital appointment, (ii) the patient's own opinion, (iii) four standardized clinical photographs (frontal face, frontal lips, left face and right face), taken before therapy and during review appointments and used to support the clinician's and patient's judgement in allocating outcome scores to each time point. Clinical photographs of each patient's face prior to OFG onset, where available, were used for comparison.
Table 2.
Outcome measure and prognostic factors
| Disease severity score | |
| 0 | No swelling |
| 1 | Mild swelling – aesthetically acceptable and requiring no treatment. |
| 2 | Moderate swelling |
| 3 | Severe swelling |
| Prognostic factors | |
| Patient-related factors | Age |
| Gender | |
| Disease-related factors | Duration of disease before treatment |
| Severity of disease before treatment | |
| Number of affected orofacial sites | |
| Presence or absence of facial (nonlabial) swelling | |
| Treatment-related factors | Total dosage of triamcinolone |
| Total number of courses of therapy | |
| Observation period after first course of treatment | |
Secondary outcome
The secondary aim was to investigate the potential association between primary outcome and multiple confounding prognostic factors. Confounding prognostic factors were defined as disease, patient or treatment-related covariates (Table 2) that could potentially influence OFG response to intralesional therapy. The secondary outcome was defined as a statistically significant association between post-therapy disease severity status and any of the possible confounding factors.
Ancillary outcomes
Ancillary outcomes included length of disease-free period after the first and additional courses of intralesional therapy, time to outcome, number and timing of recurrences and adverse effects.
Statistical analysis
Primary outcome
Pairwise comparisons of score between pre- and corresponding post-treatment at 2 weeks, 1, 6, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66 and 72 months were performed using a series of Wilcoxon signed-rank tests, with an adjusted significance level of 0·01 for multiple testing. All analyses were conducted using SPSS version 20.0 (IBM, Armonk, NY, U.S.A.).
Secondary outcomes
Longitudinal disease severity scores from the initial to the most recent time points were clustered at the individual patient level. Repeated measures univariable longitudinal ordinal logistic regression analysis was performed with the disease severity score as the dependent variable and each of the prognostic confounding factors as independent variables (Table 2). The intention was to enter independent variables with statistically significant odds ratios at the 10% level in the univariable regression analyses into a multivariable regression analysis using a significance level of 5%. Robust standard errors were employed to take into account the longitudinal nature of the data and all analyses were conducted using Stata version 11.0 (StataCorp, College Station, TX, U.S.A.).
Ancillary analyses
Descriptive analyses were performed to report ancillary outcomes. We considered disease-free those patients who had post-treatment disease severity score 0 (no swelling) or 1 (mild, aesthetically acceptable swelling requiring no therapy) and calculated the length of disease-free period after the first course of intralesional therapy and subsequent to additional courses in individuals who had disease recurrences. We also calculated the time needed for patients to become disease-free (time to outcome).
Disease recurrence was defined as development of clinically significant swelling (e.g. disease severity score 2 or 3) subsequent to the completion of a full course of intralesional therapy and after at least 6 months of disease severity 0 or 1. Adverse effects during and after intralesional triamcinolone injections were recorded.
Results
Details of study cohort
There was no information missing in the data collected, and full details of the study cohort are provided in Table 3. There were five (23%) female and 17 (77%) male patients, with mean age 39·1 ± 22·1 years [95% confidence interval (CI) 38·8–39·4]. The mean disease duration before intralesional therapy was 60·3 ± 86·6 months (95% CI 59·1–61·4). In particular, before receiving intralesional corticosteroid, the vast majority of patients (19, 86%) had had OFG for at least 12 months, and more than half of them (12, 54%) for 36 months or longer. Three patients (4%) had had OFG for less than a year and about one-third (6, 27%) for more than 6 years, before commencing intralesional therapy.
Table 3.
Details of study cohort
| Patient number | Age (years) | Gender | Duration of the disease before intralesional therapy (months) | Site affected | Severity of disease before therapy | Duration of follow-up after first course of intralesional therapy (months) | Total number of courses of intralesional therapy | Total dose of triamcinolone (including recurrences), mL (mg) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper lip | Lower lip | Right face | Left face | ||||||||
| 1 | 17 | Male | 12 | + | 2 | 18 | 1 | 1·2 (48) | |||
| 2 | 63 | Female | 24 | + | 2 | 36 | 2 | 2·4 (96) | |||
| 3 | 19 | Male | 84 | + | 2 | 60 | 1 | 1·2 (48) | |||
| 4 | 50 | Male | 216 | + | + | 3 | 54 | 1 | 2·4 (96) | ||
| 5 | 25 | Male | 120 | + | + | + | 3 | 30 | 3 | 4 (160) | |
| 6 | 55 | Female | 24 | + | 3 | 60 | 1 | 1·2 (48) | |||
| 7 | 20 | Male | 36 | + | 2 | 18 | 1 | 1·2 (48) | |||
| 8 | 67 | Male | 5 | + | 3 | 42 | 1 | 1·2 (48) | |||
| 9 | 63 | Male | 30 | + | 3 | 18 | 1 | 1·2 (48) | |||
| 10 | 13 | Male | 12 | + | + | 3 | 48 | 1 | 2·4 (96) | ||
| 11 | 18 | Male | 4 | + | + | + | + | 3 | 60 | 1 | 2·4 (96) |
| 12 | 75 | Male | 384 | + | 3 | 30 | 1 | 1·2 (48) | |||
| 13 | 49 | Female | 36 | + | + | + | 3 | 66 | 2 | 4·2 (168) | |
| 14 | 17 | Female | 48 | + | 3 | 54 | 3 | 3·6 (144) | |||
| 15 | 40 | Male | 24 | + | + | 3 | 48 | 2 | 4·8 (192) | ||
| 16 | 11 | Male | 36 | + | 3 | 36 | 1 | 1·2 (48) | |||
| 17 | 73 | Male | 36 | + | + | 3 | 72 | 2 | 4·8 (192) | ||
| 18 | 15 | Male | 36 | + | + | 3 | 30 | 1 | 2·4 (96) | ||
| 19 | 46 | Male | 72 | + | + | + | + | 3 | 30 | 2 | 5·4 (216) |
| 20 | 18 | Male | 4 | + | 3 | 18 | 1 | 1·2 (48) | |||
| 21 | 61 | Female | 12 | + | 3 | 13 | 1 | 1·2 (48) | |||
| 22 | 46 | Male | 72 | + | + | 3 | 24 | 2 | 1·8 (72) | ||
The observation period after completion of the first course of intralesional treatment ranged from 13 to 72 months with a mean of 39·3 ± 17·9 months (95% CI 39·1–39·5). Data from review appointments were available for analysis at the following time points: 2 weeks, 1, 6 and 12 months (22/22 patients, 100%), 18 months (21/22, 95%), 24 months (17/22, 77%), 30 months (16/22, 73%), 36 months (12/22, 55%), 42 months (10/22, 45%), 48 months (9/22, 41%), 54 months (7/22, 32%), 60 months (5/22, 23%), 66 months (2/22, 9%) and 72 months (1/22, 5%). Therefore, review data for up to 24 months post-treatment were available for the vast majority (> 70%) of patients and for up to 36 months for more than half of them (> 50%). Approximately a quarter of patients were observed for more than 5 years.
Regarding topography and extension of orofacial swelling, approximately half of the patients had OFG limited to a single labial or facial site, whilst the remaining patients had multiple-site involvement. Twelve (55%) patients had one site affected by OFG: upper lip (four patients, 18%), lower lip (seven patients, 32%) and right face (one patient, 4%). The remaining 10 patients (45%) had more than one site affected: both lips (five patients, 23%) and combined labial and facial swelling (five patients, 23%).
The total number of courses of intralesional injections performed was 30, with a mean of 1·45 ± 0·7 (95% CI 1·44–1·46). The majority of patients (14/22, 64%) had a single course, six (27%) had two courses, and only two (9%) had three courses. All courses but one consisted of three consecutive weekly sessions of injections. The mean dose of triamcinolone per session was 0·55 ± 0·2 (95% CI 0·46–0·65), equivalent to 22 ± 8 mg (range 18·4–26). The mean cumulative dose per 3-week full course was 1·68 ± 0·6 mL (95% CI 1·38–1·97), equivalent to 67·2 ± 2·4 mg (95% CI 55·2–78·8). The mean total cumulative dose per patient, including treatment of recurrences, was 2·39 ± 1·4 mL (95% CI 2·37–2·40), equivalent to 95·6 ± 56 mg (95% CI 94·8–96).
Primary outcome: changes in disease severity score
Compared with pretreatment, there was a statistically significant decrease in disease severity scores at all post-treatment time points, i.e. 2 weeks, 1, 6, 12, 18, 24, 30, 36, 42 and 48 months (P < 0·01) (Table 4; Fig.3). Results after 48 months were censored due to the small number of patients.
Table 4.
Comparison between pre- and corresponding post-treatment disease status scores
| Time points after first treatment | Patients (n) | Median | Minimum | Maximum | Adjusted P-value* |
|---|---|---|---|---|---|
| Pretreatment | 22 | 3 | 2 | 3 | |
| 2 weeks | 22 | 0 | 0 | 2 | < 0·001 |
| 1 month | 22 | 0 | 0 | 1 | < 0·001 |
| 6 months | 22 | 0 | 0 | 3 | < 0·001 |
| 12 months | 22 | 0 | 0 | 2 | < 0·001 |
| 18 months | 21 | 0 | 0 | 3 | < 0·001 |
| 24 months | 17 | 0 | 0 | 1 | < 0·001 |
| 30 months | 16 | 0 | 0 | 1 | < 0·001 |
| 36 months | 12 | 0 | 0 | 2 | < 0·001 |
| 42 months | 10 | 0 | 0 | 1 | 0·002 |
| 48 months | 9 | 0 | 0 | 1 | 0·004 |
| 54 months | 7 | 0 | 0 | 1 | 0·016 |
| 60 months | 5 | 0 | 0 | 1 | 0·063 |
| 66 months | 2 | 0 | 0 | 0 | 0·500 |
| 72 months | 1 | 0 | 0 | 0 | Not available |
Significant results, P < 0·01.
Figure 3.
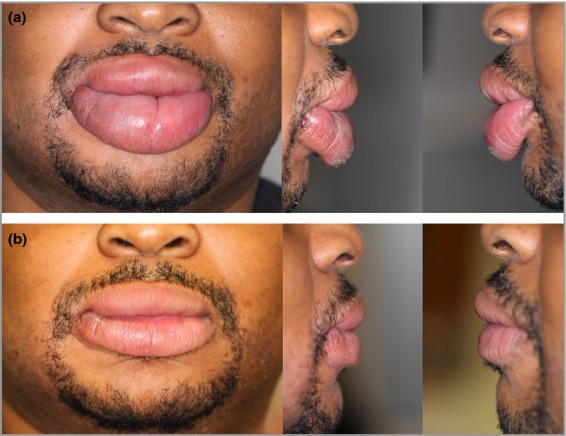
Frontal and lateral views of lip swelling before (a) and after (b) intralesional therapy.
Secondary outcome: association with prognostic confounding factors
The results of the univariable analyses showed no statistically significant association between any independent variable and the severity score (P > 0·1) with the exception of ‘Total number of courses of therapy’ (P < 0·1). Post hoc multivariable regression analysis was not indicated as there was only one relevant factor emerging from univariable analysis; no independent variable was therefore statistically significant at the 5% level (Table 5).
Table 5.
Relationship between longitudinal disease severity scores and patient-, disease- and treatment-related prognostic confounding factors (n = 22)
| Independent variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Patient-related | |||
| Age | 1·000 | 0·981–1·021 | 0·961 |
| Gender | 1·677 | 0·892–3·150 | 0·108 |
| Disease-related | |||
| Duration of disease before treatment | 1·000 | 0·995–1·011 | 0·440 |
| Severity of disease before treatment | 0·883 | 0·364–2·144 | 0·783 |
| Number of affected orofacial sites | 1·060 | 0·634–1·772 | 0·823 |
| Presence or absence of facial (nonlabial) swelling | 1·098 | 0·386–3·121 | 0·861 |
| Treatment-related | |||
| Total dose of triamcinolone | 1·002 | 0·996–1·008 | 0·441 |
| Total number of courses of therapy | 1·573 | 0·946–2·614 | 0·081 |
| Observation period after first course of treatment | 1·010 | 0·985–1·036 | 0·450 |
Ancillary outcomes
Disease recurrence
Fourteen individuals (64%) of the study cohort had not experienced any recurrence of orofacial swelling at the time of data collection. Among the eight patients (36%) who did experience disease recurrence, six (6/22, 27%) had only one recurrence occurring at month 6 (n = 1), 12 (n = 2), 18 (n = 1), 30 (n = 1) and 36 (n = 1). Two individuals (2/22, 9%) had two disease recurrences each occurring at 6 and 18 months and 18 and 36 months respectively.
Disease-free period
The mean disease-free period after the first course of intralesional therapy was 28·9 ± 18 months (95% CI 28·7–29·1). The eight individuals who experienced disease recurrence and received a second course of intralesional therapy had a subsequent mean disease-free period of 28·3 ± 17·2 months (95% CI 27·9–28·7). The two individuals who experienced two disease recurrences and received a third full course of intralesional therapy had an additional mean disease-free period of 15 ± 4·2 months (95% CI 14·8–15·2).
Time to outcome
The vast majority of patients (20/22; 91%) reached disease-free status at 2 weeks after the first course of therapy. All patients did so at 1 month after treatment. Patients who experienced recurrent disease reached disease-free status at 6 months after additional courses of therapy.
Adverse effects
There were no significant adverse effects, with patients reporting only minimal transient discomfort associated with the injections. No patient reported short-term transient worsening of orofacial swelling immediately after the injections, and there were no cases of perioral steroid-induced cutaneous hyperpigmentation.
Discussion
Treatment of the disfiguring orofacial swelling of OFG has proven exceedingly difficult and remains unsatisfactory.22 A recent review of the literature concluded that no uniform and predictable therapeutic model has been demonstrated to be effective in reducing the permanent labial or facial enlargement of OFG.22 Immunosuppressants, tumour necrosis factor alpha inhibitors and other agents, as well as surgical cheiloplasty (reviewed in Banks et al.),22 have been used as single or combined therapy with some positive, although overall inconsistent, results in a variety of cases reports and small case series.22 Similarly, the encouraging results of a benzoate- and cinnamon-free diet reported by White et al.23 have never been replicated by other groups and need further research.
Currently, corticosteroids remain the mainstay of OFG management in many treatment regimens and can be provided either topically, systemically or as intralesional therapy.22 The latter was initially introduced in 1971,24 and almost 20 years later was modified by Sakuntabhai et al.,7 who reported remission of lip swelling for up to 10–12 months. Intralesional therapy was further modified in subsequent years with the introduction of highly concentrated triamcinolone acetonide (40 mg mL−1). This formulation allows injection of a high dose of triamcinolone within a reduced drug volume, thereby increasing efficacy, reducing associated pain and avoiding the need for anaesthetic block.8–11 However, there remains wide variety and little consistency among studies regarding the best intralesional triamcinolone regimen to adopt. Significant differences exist in administration schedules, drug concentration, concomitant medications and total dose of injected corticosteroids.8–13 Evidence is also limited by small sample size, OFG diagnostic criteria, outcome measures and short-term follow-up.8–13 A recent review concludes that although success of intralesional triamcinolone has been reported, it is often temporary and the long-term efficacy remains unclear.22
The present study, although being limited by its retrospective and noncomparative design, is characterized by a number of methodological strengths leading to more robust conclusions than in previous publications.
This is the largest cohort of patients with OFG treated with intralesional triamcinolone injections and the only study that adopted a standardized therapeutic regimen of single agent intralesional, highly concentrated triamcinolone acetonide with minimal variability of dosage among patients. Indeed, the mean cumulative dose per patient per 3-week full course was 1·68 ± 0·6 mL and the mean total cumulative dose, including treatment of recurrences, was 2·39 ± 1·4 mL. In contrast, previous studies were either single case reports, small case series or failed to use a well-defined, consistent single-agent treatment regimen with homogeneous triamcinolone dosage throughout the study cohort.8–10,13–20 Mean post-treatment observation ranged from 13 to 72 months with a mean of 39·3 ± 17·9, therefore assuring assessment of long-term outcomes.
Another robust feature of the present study is its homogeneous cohort. All patients were carefully phenotyped so as to include only individuals with idiopathic OFG and exclude those with evidence of systemic granulomatous disease (e.g. Crohn disease or sarcoidosis). Also, we included all consecutive patients with idiopathic OFG referred to our unit, including those with a long history of persistent orofacial swelling (mean disease duration being 60·3 ± 86·6 months) and failure of other topical and systemic therapies (including corticosteroids), who are traditionally considered difficult to manage due to permanent fibrotic changes of affected tissues. Exclusion of these patients, as done by other authors,8 carries the risk of affecting study outcomes due to biased selection of individuals with short-term, treatment-free disease who are more likely to respond to any given therapy. In addition, some of the perceived benefits of therapy could, in fact, represent spontaneous fluctuations of the disease, which are well known to occur, especially in the early stages of OFG as part of its natural history.3
We adopted a predefined four-point disease severity system as an outcome measure that was based on clinicians' and patients' judgement, as well as on clinical photographs.
The present study is the first to adopt sound and robust statistics to assess primary and secondary treatment outcomes at predefined time-points; the Wilcoxon signed-rank test is appropriate for comparison of two sets of scores from the same participants. We found that all patients of the cohort had a statistically significant reduction in their disease severity at all studied time points up to 4 years post-treatment, therefore demonstrating the long-term clinical benefit of intralesional triamcinolone therapy in reducing orofacial swelling and restoring cosmetic appearance. Of note, logistic regression analysis showed no association at the 5% level between disease severity and prognostic confounding factors, therefore demonstrating that likelihood of response to intralesional triamcinolone is not dependent on factors such as duration and severity of disease before therapy, number of affected orofacial sites, presence or absence of facial (nonlabial) swelling, total dose of triamcinolone or total number of courses of therapy. There was also no significant association with the length of observation period after the first course of treatment, suggesting that the beneficial effects of intralesional therapy can persist in the long term.
Descriptive analyses showed that the mean disease-free period after the first course of intralesional therapy was 28·9 ± 18 months, therefore confirming further its long-term effectiveness. The majority of patients (14/22, 63·6%) remained disease-free until data collection whereas approximately one-third of patients (8/22, 36·4%) experienced disease recurrence but responded to a second course of intralesional therapy with a subsequent mean disease-free period of 28·3 ± 17·2 months. These data demonstrate that disease recurrence can be effectively managed with a second course of intralesional triamcinolone and the second disease-free period can last as long as the first one. Out of these seven patients, two had a further disease recurrence at 12 and 18 months after the second course of therapy and responded to a third course of intralesional triamcinolone.
Analysis of time to outcome showed that the vast majority of patients (20/22, 91%) reached disease-free status within 2 weeks from completion of intralesional therapy and all did so within 4 weeks, thus demonstrating that intralesional triamcinolone injections are fast acting in reducing orofacial swelling in OFG.
In conclusion, the present study, although limited by its observational, retrospective and single-arm design, employed a robust cohort design and consistent methodology for the first time in this field and provided sound evidence of the long-term effectiveness of intralesional injections in reducing the disfiguring orofacial swelling of OFG. Further research in the form of well-designed case-control randomized trials is needed to confirm the present data. Given the rarity of the disease, multicentre collaboration is recommended.
References
- 1.Leão JC, Hodgson T, Scully C, Porter S. Review article: orofacial granulomatosis. Aliment Pharmacol Ther. 2004;20:1019–27. doi: 10.1111/j.1365-2036.2004.02205.x. [DOI] [PubMed] [Google Scholar]
- 2.Al Johani KA, Moles DR, Hodgson TA, et al. Orofacial granulomatosis: clinical features and long-term outcome of therapy. J Am Acad Dermatol. 2010;62:611–20. doi: 10.1016/j.jaad.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 3.Al Johani K, Moles DR, Hodgson T, et al. Onset and progression of clinical manifestations of orofacial granulomatosis. Oral Dis. 2009;15:214–19. doi: 10.1111/j.1601-0825.2009.01512.x. [DOI] [PubMed] [Google Scholar]
- 4.Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L. The multiform and variable patterns of onset of orofacial granulomatosis. J Oral Pathol Med. 2003;32:200–5. doi: 10.1034/j.1600-0714.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 5.McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696–704. doi: 10.1111/j.1601-0825.2011.01826.x. [DOI] [PubMed] [Google Scholar]
- 6.Riordain RN, Meaney S, McCreary C. Impact of chronic oral mucosal disease on daily life: preliminary observations from a qualitative study. Oral Dis. 2011;17:265–9. doi: 10.1111/j.1601-0825.2010.01734.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakuntabhai A, MacLeod RI, Lawrence CM. Intralesional steroid injection after nerve-block in orofacial granulomatosis. Lancet. 1992;340:969. doi: 10.1016/0140-6736(92)92851-6. [DOI] [PubMed] [Google Scholar]
- 8.Mignogna MD, Pollio A, Leuci S, et al. Clinical behaviour and long-term therapeutic response in orofacial granulomatosis patients treated with intralesional triamcinolone acetonide injections alone or in combination with topical pimecrolimus 1% J Oral Pathol Med. 2013;42:73–81. doi: 10.1111/j.1600-0714.2012.01186.x. [DOI] [PubMed] [Google Scholar]
- 9.Alajbeg I, Rogulj AA, Hutinec Z. Orofacial granulomatosis treated with intralesional triamcinolone. Acta Dermatovenerol Croat. 2011;19:165–9. [PubMed] [Google Scholar]
- 10.Martini MZ, Galletta VC, Pereira EM, et al. Orofacial granulomatosis of the lip: a report of 2 cases with histological and immunohistochemical analyses and intralesional corticotherapy. Minerva Stomatol. 2010;59:579–81. [PubMed] [Google Scholar]
- 11.Mignogna MD, Fedele S, Lo Russo L, et al. Effectiveness of small-volume, intralesional, delayed-release triamcinolone injections in orofacial granulomatosis: a pilot study. J Am Acad Dermatol. 2004;51:265–8. doi: 10.1016/s0190-9622(03)00769-2. [DOI] [PubMed] [Google Scholar]
- 12.Sakuntabhai A, MacLeod RI, Lawrence CM. Intralesional steroid injection after nerve block anesthesia in the treatment of orofacial granulomatosis. Arch Dermatol. 1993;129:477–80. [PubMed] [Google Scholar]
- 13.van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up–results of management. Int J Dermatol. 2002;41:225–9. doi: 10.1046/j.1365-4362.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- 14.Bacci C, Valente ML. Successful treatment of cheilitis granulomatosa with intralesional injection of triamcinolone. J Eur Acad Dermatol Venereol. 2010;24:363–4. doi: 10.1111/j.1468-3083.2009.03466.x. [DOI] [PubMed] [Google Scholar]
- 15.Coskun B, Saral Y, Cicek D, Akpolat N. Treatment and follow-up of persistent granulomatous cheilitis with intralesional steroid and metronidazole. J Dermatolog Treat. 2004;15:333–5. doi: 10.1080/09546630410015538. [DOI] [PubMed] [Google Scholar]
- 16.Sobjanek M, Michajłowski I, Zelazny I, et al. What is the most effective treatment of cheilitis granulomatosa in Melkersson-Rosenthal syndrome? J Eur Acad Dermatol Venereol. 2010;24:364–5. doi: 10.1111/j.1468-3083.2009.03540.x. [DOI] [PubMed] [Google Scholar]
- 17.Sobjanek M, Włodarkiewicz A, Zelazny I, et al. Successful treatment of Melkersson-Rosenthal syndrome with dapsone and triamcinolone injections. J Eur Acad Dermatol Venereol. 2008;22:1028–9. doi: 10.1111/j.1468-3083.2008.02834.x. [DOI] [PubMed] [Google Scholar]
- 18.Lynde CB, Bruce AJ, Orvidas LJ, et al. Cheilitis granulomatosa treated with intralesional corticosteroids and anti-inflammatory agents. J Am Acad Dermatol. 2011;65:e101–2. doi: 10.1016/j.jaad.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Calderón R, Gonzalo-Garijo MA, Chaves A, de Argila D. Cheilitis granulomatosa of Melkersson-Rosenthal syndrome: treatment with intralesional corticosteroid injections. Allergol Immunopathol (Madr) 2004;32:36–8. doi: 10.1016/s0301-0546(04)79221-6. [DOI] [PubMed] [Google Scholar]
- 20.El-Hakim M, Chauvin P. Orofacial granulomatosis presenting as persistent lip swelling: review of 6 new cases. J Oral Maxillofac Surg. 2004;62:1114–17. doi: 10.1016/j.joms.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE: explanation and elaboration. Ann Intern Med. 2007;147:163–94. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 22.Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934–7. doi: 10.1111/j.1365-2133.2011.10794.x. [DOI] [PubMed] [Google Scholar]
- 23.White A, Nunes C, Escudier M, et al. Improvement in orofacial granulomatosis on a cinnamon- and benzoate-free diet. Inflamm Bowel Dis. 2006;12:508–14. doi: 10.1097/00054725-200606000-00011. [DOI] [PubMed] [Google Scholar]


