Abstract
Ovarian cancer is typically accompanied by the occurrence of malignant ascites containing large number of macrophages. It has been suggested that these tumor-associated macrophages (TAMs) are skewed to alternative polarization (M2) and thereby play an essential role in therapy resistance and metastatic spread. In our study, we have investigated the nature, regulation and clinical correlations of TAM polarization in serous ovarian cancer. Macrophage polarization markers on TAMs and ascites cytokine levels were analyzed for 30 patients and associated with relapse-free survival (RFS) in a prospective study with 20 evaluable patients. Surface expression of the M2 marker CD163 on TAMs was inversely associated with RFS (p < 0.01). However, global gene expression profiles determined for 17 of these patients revealed a mixed-polarization phenotype unrelated to the M1/M2 classification. CD163 surface expression also correlated with the ascites levels of IL-6 and IL-10 (p < 0.05), both cytokines induced CD163 expression, and their ascites levels showed a clear inverse association with RFS (p < 0.01). These findings define a subgroup of patients with high CD163 expression, high IL-6 and/or IL-10 levels and poor clinical outcome.
Keywords: ovarian carcinoma, tumor-associated macrophages, CD163, IL-6, IL-10
The cellular microenvironment, consisting predominantly of immune cells, fibroblasts and endothelial cells, is crucial for the growth, progression and metastazation of malignant tumors.1 Under the influence of tumor cell-borne signals, these host-derived cells secrete a plethora of growth factors, cytokines, chemokines and proteases with tumor-promoting properties. Macrophages play a pivotal role in this context, as shown in numerous mouse models and suggested by the correlation of clinical outcome with intratumoral macrophage density in different types of cancer.2 These tumor-associated macrophages (TAMs) are “re-educated” by factors of the tumor microenvironment and can promote basically of all aspects of tumorigenesis and tumor progression, including tumor cell proliferation, invasion, angiogenesis, metastasis formation and immune suppression.2–5
TAMs are recruited form circulating monocytes that are attracted by tumor-derived chemokines and polarized to M2-resembling macrophages by growth factors and cytokines of the tumor microenvironment.5–7 A hallmark of macrophages is their plasticity. Depending on external stimuli, macrophages can acquire different phenotypes with partly opposing properties.8 Classical activation by cytokines such as interferon-γ leads to macrophages with immune stimulatory properties owing to the secretion of proinflammatory cytokines including “tumor necrosis factor-alpha” (TNF-α), interleukin-1β (IL-1β), IL-6, IL-12 und IL-23.6,8,9 In contrast, alternatively activated macrophages are elicited by anti-inflammatory cytokines such as IL-4, IL-10 or IL-13. They comprise a wide spectrum of subtypes with diverse functions in wound healing, tissue repair, angiogenesis and immune suppression. Classically and alternatively activated macrophages have been termed M1 and M2 macrophages, but this classification is a purely operational definition, because macrophages can adopt a large variety of phenotypes deviating from this classification and may even acquire properties of both M1 and M2 cells.4,5,8 However, the role of macrophage subtypes in tumor progression is not clear. Similarly, the contribution of specific signaling molecules and target genes to the protumorigenic polarization of TAMs remains a largely unresolved issue. This applies in particular to human tumors, where because of the lack of systematic studies our knowledge is particularly poor.
What’s new? —
The peritoneal environment plays a critical role in the spread of ovarian cancer, ultimately becoming a malignant ascites, rich in tumor-associated macrophages (TAMs), in advanced stages of disease. TAMs in this context have been implicated in therapy resistance and metastatic spread, though their function remains unclear. Here, TAMs from malignant ascites in patients with serous ovarian carcinoma were found to express a mixed-polarization phenotype and highly variable levels of the surface marker CD163. Elevated CD163 was associated with early disease relapse and increased IL-6 and IL-10 levels. Further investigation of these changes in surface marker expression could provide insight into mechanisms of progression in ovarian cancer.
Serous ovarian carcinoma is the most common ovarian cancer and is the deadliest of all gynecological malignancies.10 Despite good clinical responses to first-line therapy, most ovarian carcinoma patients succumb to their disease because of the outgrowth of chemoresistant tumor cells spreading to serous membranes throughout the peritoneal cavity. This metastatic spread is facilitated by the peritoneal fluid serving as a carrier for the passive dissemination of cells shed from the primary tumor cells.10 The peritoneal environment, which at more advanced stages is formed by a malignant effusion building up in the peritoneal cavity, is therefore an essential determinant of metastatic dissemination. This malignant ascites is rich in tumor-promoting soluble factors, tumor cells and immune cells, including large numbers of protumorigenic TAMs.11 Similar to other tumor types, macrophage density in histological sections correlates with poor prognosis of ovarian cancer,12 confirming the clinical relevance of TAMs.
In our study, we have investigated the polarization of TAMs from first-line patients with advanced serous ovarian carcinoma by determining the expression of surface markers and establishing transcriptional profiles. We show that these TAMs can be categorized with respect to CD163 surface expression, which is associated with early clinical relapse, but is unrelated to the M1/M2 classification. We also show that CD163 expression and clinical outcome are linked to IL-6 and IL-10 present in the malignant ascites of ovarian cancer patients.
Material and Methods
Patient samples
Ascites was collected from 30 patients with high-grade serous ovarian carcinoma undergoing primary surgery at the University Hospital in Marburg. Informed consent was obtained from all patients according to the protocols approved by the local ethical committee. Patient characteristics are presented in Supporting Information Table S1. Clinical courses were evaluated by RECIST criteria13 in patients with measurable disease or profiles of serum CA125 levels14 in patients without measurable lesions, according to the recommendations by the Gynecologic Cancer InterGroup (GCIG, www.gcig.igcs.org/CA-125.html). Peripheral blood mononuclear cells were obtained from healthy adult volunteers for monocyte-derived macrophage (MDM) stimulation.
Isolation of CD14+ cells
Mononuclear cells were isolated from ascites and peripheral blood by Ficoll (GE Healthcare/PAA, Cölbe, Germany) density gradient centrifugation and further purified by magnetic cell sorting (MACS) using CD14 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of CD14+ cells was >94%, except for three samples (TAM21, 85%; TAM26, 87% and TAM31, 90%). Purified TAMs and MDMs were directly analyzed by fluorescence activated cell sorting (FACS), lysed in PeqGold (Peqlab, Erlangen, Germany) for RNA preparation or cultured in serum-free macrophage medium (Mph medium; Life Technologies, Darmstadt, Germany). Further details are described in Supporting Information Methods.
FACS phenotyping
After MACS isolation, 1 × 105 CD14+ cells were washed with staining buffer [phosphate buffered saline (PBS) plus 1% fetal calf serum (FCS)], incubated with 100 µg/ml polyclonal mouse IgG (Dianova, Hamburg, Germany) for 15 min at 4°C to block unspecific Fc binding and subsequently stained with the following surface markers for 30 min at 4°C (except for anti-human CCR7, 37°C): FITC-labeled anti-human CD14, (Miltenyi Biotech), PE-labeled anti-CCR7 (BD Biosciences, Heidelberg, Germany), APC-labeled anti-CD206 (BioLegend, San Diego, CA), PE-labeled anti-CD163, PE-labeled anti-CD64, PE-Cy7-labeled anti-CD16, APC-labeled anti-CD32 and APC-labeled anti-HLA-DR (eBioscience, Frankfurt, Germany). Intracellular staining (30 min, 4°C) was performed with FITC-labeled anti-CD68 (eBioscience), FITC-labeled anti-IL-12 and PE-labeled anti-IL-10 (BD Biosciences) after permeabilization for 20 min at 4°C using BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit (BD Biosciences). Isotype control antibodies were purchased from BD Biosciences, Miltenyi Biotech and eBioscience. Cells were analyzed using a FACS Calibur cytometer and CellQuestPro software (BD Biosciences). Results were calculated as percentage of CD14+ cells and mean fluorescence intensities (MFIs).
Cell culture and cytokine treatment of TAMs and MDMs
CD14+ monocytes and TAMs were cultured in serum-free macrophage medium (Mph medium; Life Technologies). MDMs were differentiated from CD14+ monocytes of healthy volunteers for 5–7 days at 1 × 106 cells/ml in Mph medium supplemented with 1,000 U/ml GM-CSF (Immunotools, Friesoythe, Germany). Treatment of MDMs with 50 ng/ml IL-6, 10 ng/ml IL-10, 20 ng/ml IL-4 (all from Immunotools) or 10% ascites was performed either during differentiation for 5–7 days or after differentiation and a 2-day resting period in Mph medium without GM-CSF for 48 hr. TAMs were stimulated with cytokines or ascites for 2 days after plating at 1 × 106 cells/ml in Mph medium, or in some experiments, activation was started after a 2-day resting period in Mph medium.
Quantification of soluble mediators in ascites
IL-1β, Il-4, IL-6, IL-10, IL-12, IL-13, CCL2, LIF, GM-CSF, VEGF-A, VEGF-C, TGF-β and TNF-α were quantified by ELISA kits purchased from eBioscience. Human leptin and ANGPTL4 ELISAs were from RayBiotech (Norcross, GA) and the human CSF-1 ELISA from R&D Systems (Wiesbaden, Germany).
Microarrays
Global gene expression was analyzed on Human Agilent 4-plex Array 44K chips in a reference-design assay. Raw microarray data were normalized using the “loess” method implemented within the marray package of R/Bioconductor as described.15 Probes were assigned to genes using Ensembl release 67. Only probes with sufficient intensity (≥5) and matching a single gene were considered in our analysis. If multiple probes matched a gene, the one showing the largest differences was chosen. For comparison between TAM samples, each probe on the microarray was median centered. To compensate for variable contamination with mesothelial and tumor cells, probes correlating with EPCAM (cosine similarity > 0.15) were removed from all datasets. Raw and normalized microarray data from this publication have been submitted to the EBI ArrayExpress and assigned the identifier (accession number E-MTAB-1661). All data are MIAME compliant.
Bioinformatics
Meier–Kaplan plots and Pearson’s r correlations were calculated with GraphPad Prism (version 6). Heat maps were drawn using R/ggplot/ggdendro. Missing values were replaced by the median. Hierarchical clustering was performed using the complete method of R’s hclust, based on the cosine distance between genes/markers and the hamming distance on a binarized (≥median) vector between arrays.
Results
Characteristics of TAMs and ascites from ovarian carcinoma patients
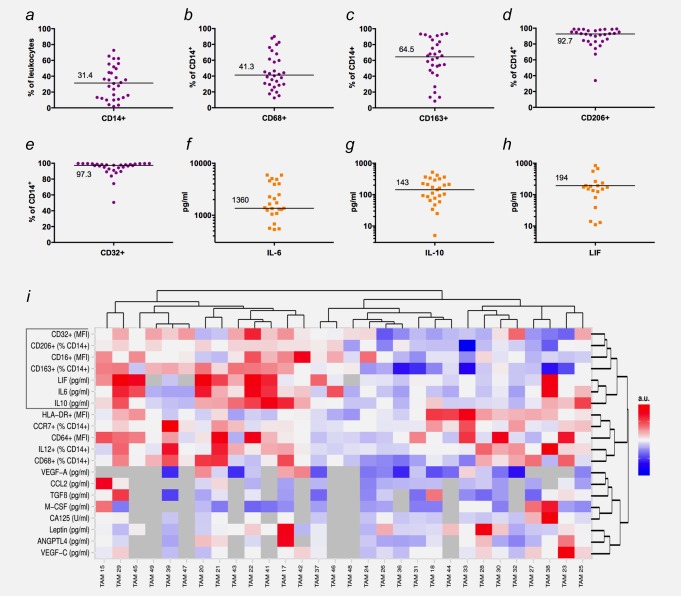
Our prospective study was performed with 30 patients (Supporting Information Table S1) suffering from serous ovarian carcinoma and the formation of malignant ascites. All patients underwent surgery followed by standard chemotherapy in 2011–2013 as first-line therapy. TAMs were purified by MACS as CD14+ mononuclear cells from ascites collected at the time of surgery and analyzed by FACS for expression of a range of surface markers linked to macrophage activation or polarization (Figs. 1a–1e; complete list in Supporting Information Dataset S1). We also determined the concentrations of several key mediators in the ascites fluid from the same patients previously proposed to play a role in tumor progression and/or the protumorigenic skewing of macrophages (Figs. 1f–1h; Supporting Information Dataset S1). This dataset was used to construct the heat map in Figure 1i placing TAM samples with the most similar expression patterns at adjacent positions. Although most surface markers and cytokines were expressed in wide concentration ranges, there was also clear evidence for clustered expression in subgroups of patients. Furthermore, several markers and cytokines showed very similar expression patterns relative to each other across all patients, which are particularly evident for the first seven top rows of the heat map, including CD32, CD163, CD206, IL-6, IL-10 and LIF. These observations suggest that patients can be distinguished and possibly grouped according to the expression patterns of these surface markers and cytokines. Therefore, we studied potential associations with the relapse of ovarian carcinoma.
Figure 1.
Expression patterns of surface markers on TAMs and cytokine ascites levels in ovarian cancer patients. (a–e) Expression of surface markers associated with macrophage polarization and tumor progression (n = 30; FACS analyses). (f–h) Distribution of IL-6, IL-10 and LIF levels (n = 30; ELISA). (i) Heat map generated by hierarchal clustering of expression data. Data were median centered and scaled from −1 to +1 for each marker or cytokine, with bright red indicating the highest expression levels and bright blue the lowest expression level. Missing values are indicated in gray. Samples with more than 25% of markers/cytokines missing were not considered for clustering of markers or cytokines and replaced by 0 when clustering samples.
Association of relapse-free survival with CD163 surface expression
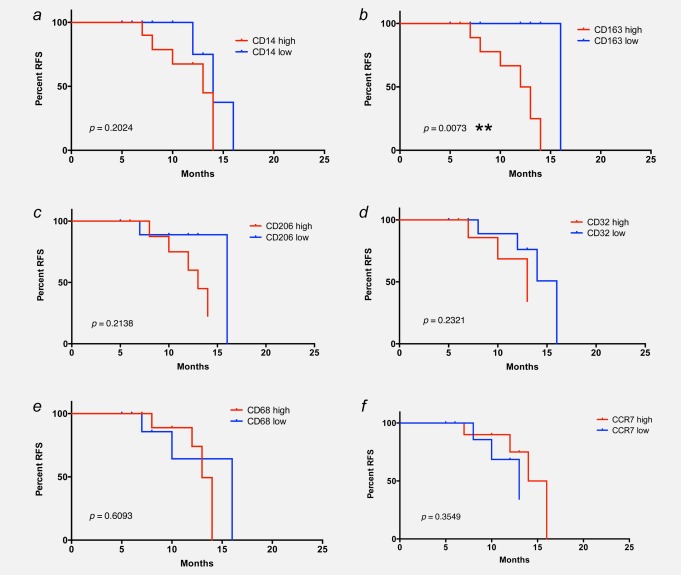
We first analyzed potential correlations between the expression of macrophage polarization surface markers and clinical progression. All patients with a postsurgery period of at least 6 months (range 6–24 months) were included in our study (n = 20). For each marker, patients were grouped as “high” or “low” using the respective median as a cutoff point, as shown in Figures 1a–1f. These datasets were analyzed for association with relapse-free survival (RFS). Although macrophage density (% CD14+ cells of total leukocytes) was not associated with RFS (log-rank test, p = 0.7241; Fig. 2a), we found an inverse association between RFS and expression of the M2 marker CD163 (hemoglobin/haptoglobin scavenger receptor; p = 0.0496; Fig. 2b). In contrast, no association was observed for another M2 marker, the mannose receptor CD206 (p > 0.7; Figs. 1d and 2c). Similarly, other markers connected to macrophage polarization did not show any association with clinical progression (CD32, CD68 and CCR7 in Figs. 2d and 2e; all p-values > 0.8). A similar negative result was obtained with CD16, CD64, HLA-DR and intracellular IL-10/IL-12 ratio (all p-values > 0.6). Furthermore, no correlation was seen between histological grading and RFS (p > 0.1).
Figure 2.
Association of RFS with CD163 surface expression on ovarian carcinoma TAMs. Kaplan–Meier plots showing the RFS of patients with high or low levels (median centered) of CD14 (% of leukocytes; panel a), CD163 (% of CD14+; panel b), CD206 (% of CD14+; panel c), CD32 (MFI; panel d), CD68 (% of CD14+; panel e) and CCR7 (% of CD14+; panel f) expression on their TAMs. p-Values were determined by Mantel–Cox log-rank test.
Genome-wide expression profiling reveals a mixed-polarization phenotype of ovarian carcinoma TAMs
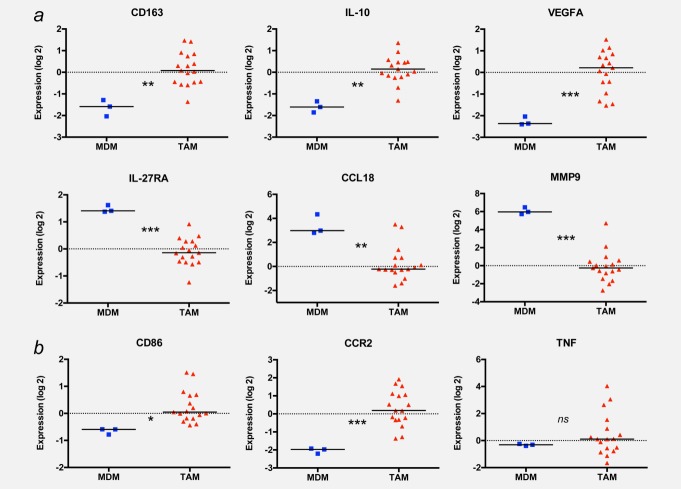
The above data clearly suggest that patient subgroups can be distinguished by the surface expression of CD163 on their TAMs, and that CD163 levels are associated with a less favorable clinical course. As CD163 surface expression is an established marker for alternative macrophage polarization (M2), we sought to investigate whether CD163 expression is an indicator of M2-skewed polarization in ovarian carcinoma TAMs. Toward this end, we determined the transcriptomes of 17 TAM samples and of human MDMs considered largely nonpolarized (M0) cells. TAMs and MDMs differed in the expression of 1,275 genes (threshold 1.5-fold; Supporting Information Dataset S2; corrected p < 0.05), suggesting that the global gene expression profile of ovarian carcinoma TAMs substantially differs from that of normal macrophages. Many of these differentially expressed genes have previously been found in other cancer-associated transcriptomes (see functional annotation in Supporting Information Dataset S3). Furthermore, a large number of these genes have been linked to different modes of macrophage activation, so that we could use this information to assess the polarization state of ovarian carcinoma TAMs. As expected,16 CD163 mRNA levels were higher in TAMs compared to MDM (Fig. 3a). The expression of other genes characteristic of M2-polarized cells17–19 followed a similar pattern (IL10 and VEGFA). In contrast, multiple other M2 markers were downregulated in TAMs (IL27RA, CCL18, CCL22 and MMP9; Fig. 3a; Supporting Information Dataset S2), and several M1 markers were expressed at similar or higher levels in TAMs relative to MDMs (CD86, CCR2 and TNF; Fig. 3b).
Figure 3.
Expression of polarization-associated genes in TAMs and MDMs. RNAs from 17 TAM and three MDM samples were analyzed by transcriptional profiling on Agilent 44K microarrays, and relative expression levels were plotted for a range of established M1 (a) and M2 (b) markers. Horizontal bars indicate the respective median; p-values were determined by t-test (*p < 0.05; **p < 0.01; ***p < 0.001).
The genes differentially expressed between MDMs and TAMs did not include any key genes associated with the regulation of glycolysis and energy metabolism (e.g., HK, PFK, PKM2 and PDK1; Supporting Information Dataset S2) or cell cycle progression (e.g., CCNA, CCNB, PCNA and Ki67), suggesting that cell culture conditions (including oxygen supply) only had a limited impact on the gene expression profile of MDMs. Taken together, our observations are therefore consistent with the view that human ovarian carcinoma TAMs represent a mixed-polarization phenotype.
Surface expression of CD163 is not an indicator of the extent of M2 polarization
We then analyzed potential correlations of CD163 surface expression with two previously published M2 gene signatures by hierarchal clustering: (i) the “Ghassabeh signature,” a gene set common to type II cytokine-associated myeloid cells from mice under different pathologic conditions, including tumor,16 and (ii) the “Beyer signature,” discriminating M1- and M2-polarized human MDMs exposed to interferon-γ and IL-4, respectively.19 First, we constructed heat maps that place TAM samples with the highest degree of similarity at adjacent positions (Supporting Information Fig. S1). Then, patients were categorized according to the surface expression of CD163 (according to Supporting Information Dataset S1), and one of these categories was assigned to each sample in the heat maps. It is easily recognizable that the rank order did not show any similarity with the category of CD163 surface expression (shown at the bottom) for both the Ghassabeh signature (Supporting Information Fig. S1A) and the Beyer signature (Supporting Information Fig. S1B). These results led us to conclude that CD163 surface expression on ovarian carcinoma TAMs is not an indicator of the extent of M2-skewed polarization.
Correlation of CD163 surface expression with ascites cytokine levels
The data described above raised the possibility that the expression of CD163 on TAMs and the ascites levels of cytokines might be linked. Therefore, we tested this hypothesis for pairs of the respective datasets (i.e., CD163 and cytokine values for the same patient) using Pearson’s r (Supporting Information Table S2). This analysis indeed confirmed a significant correlation of CD163 expression with the concentration of both IL-6 (p = 0.0483; correlation coefficient r = 0.1471; Fig. 4a) and IL-10 (p = 0.0459; r = 0.1501; Fig. 4b). None of the other cytokines tested was significantly associated with CD163 expression (Supporting Information Table S2).
Figure 4.
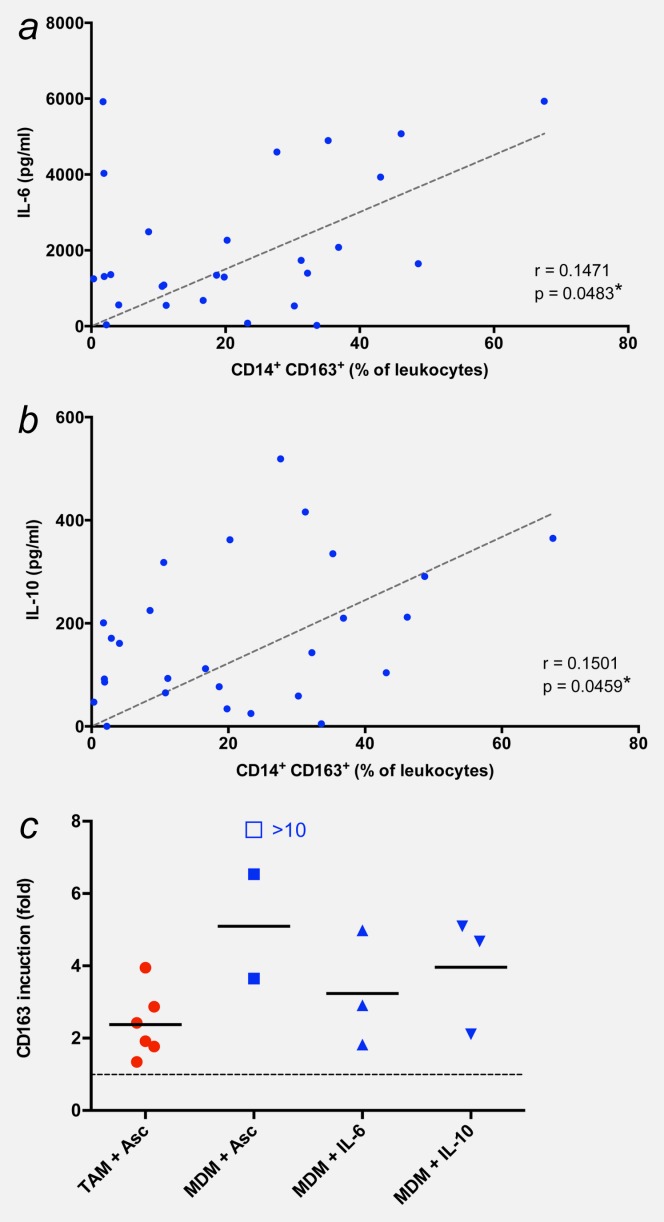
CD163 surface expression is associated with IL-6 and IL-10. (a and b) Correlation of CD163 surface expression with ascites levels of IL-6 and IL-10. *p < 0.05; **p < 0.01 by Pearson’s r correlation. (c) Induction of CD163 in TAMs and MDMs by ascites and/or IL-10 and IL-10. TAMs were cultured for 2 days with or without cell-free ascites (10% of total culture medium). MDMs from healthy donors were differentiated in the presence of GM-CSF and either cell-free ascites (10% of total culture medium) or the indicated recombinant cytokines for 5 days. Expression of surface marker CD163 was determined by FACS and is expressed as fold induction, i.e., normalized to 1 for the respective untreated control (untreated TAMs and MMDs, respectively, are represented by the dotted line). Each data point within the same treatment group represents a different donor. Horizontal bars indicate averages. The induction value represented by the open box could not be precisely determined because of an extremely low expression value in untreated MDMs. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We also observed significant correlations between IL-6 and LIF (p = 0.0002; r = 0.6958) and between IL-10 and LIF (p = 0.0079; r = 0.5094), but not between IL-6 and IL-10 (Supporting Information Table S3), suggesting that the correlation of CD163 expression with the ascites levels of IL-6 and IL-10 is not simply due to their coexpression. Surprisingly, there were also clear correlations of ANGPLT4 with IL-10, leptin and VEGF-A (all p-values < 0.01; Supporting Information Table S3). Weak correlations were also seen between the levels of IL10 and TGF-β (p = 0.0224) and between CSF-1 and CCL2 (p = 0.0485). Other correlations were not detectable (Supporting Information Table S3).
CD163 expression is upregulated by cytokines present in malignant ascites
Consistent with the correlative observations described above, CD163 surface expression was upregulated in ascites-deprived TAMs in short-term primary culture after exposure to cell-free malignant ascites (average 2.4-fold, respectively; Fig. 4c). A considerably stronger induction was observed in “naïve” MDMs from healthy donors stimulated with ascites (5.1-fold; Fig. 4c), IL-6 (>3.2-fold; Fig. 4c) or IL-10 (4.0-fold; Fig. 4c). Taken together, these findings suggest that the upregulation of CD163 in TAMs is, at least in part, caused by IL-6 and IL-10 present in the ascites of ovarian cancer patients. The functional relevance of these findings is suggested by the observed correlation of IL-6 and IL-10 levels with the potency of cell-free ascites to stimulate the proliferation of the human ovarian carcinoma cell line SKOV-3 (Supporting Information Fig. S2).
Association of RFS with cytokine levels in ovarian carcinoma ascites
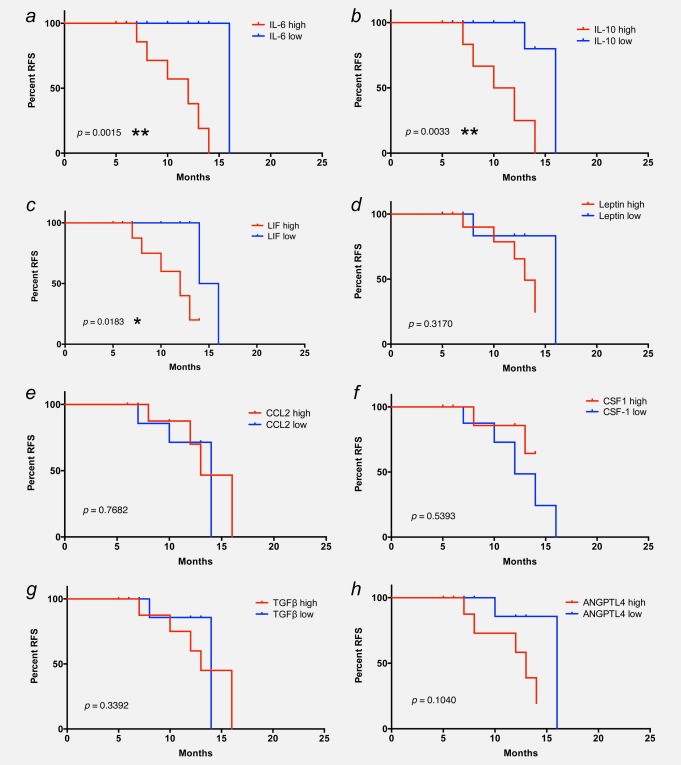
The association of CD163 surface expression with cytokine levels (see above) prompted us to investigate potential associations between RFS and the concentrations of cytokines in the ascites fluid from the same patients. Patients were grouped as “high” or “low” for each cytokine (cutoff 1,500 ng/ml for IL-6; 200 ng/ml for IL-10 and LIF; Supporting Information Dataset S1). A clear inverse association was observed for IL-6 (log-rank test, p = 0.0015; Fig. 5a), IL-10 (p = 0.0033; Fig. 5b) and LIF (p = 0.0183; Fig. 5c), all three of which are STAT3-inducing cytokines. In contrast, no association was detectable between RFS and the ascites level of leptin, CSF-1 and CCL2, TGF-β or ANGPTL4 (Figs. 5d and 5f) or VEGF-A, VEGF-C and CA125 (graphs not shown; all p-values > 0.3).
Figure 5.
Association of RFS with cytokine levels in ovarian carcinoma ascites. Kaplan–Meier plots showing the RFS of patients with high or low ascites levels of IL-6 (a), IL-10 (b), LIF (c), leptin (d), CCL2 (e), CSF1 (f), TGF-β (g) and ANGPTL4 (h). Cutoff values were 1,500 ng/Ml for IL-6 and CSF-1, 200 ng/Ml for IL-10 and LIF, 100 ng/Ml for leptin and 150 ng/Ml for CCL2. p-Values were determined by Mantel–Cox log-rank test.
In view of the observed correlations of RFS, CD163 surface expression and STAT3-activating cytokines (Fig. 6), we searched for associations of the TAM transcriptomes with STAT signaling. Kang et al. recently published a meta-analysis of STAT-binding sites identified by ChIP-Seq analyses.20 We used a 65-gene “STAT signature” derived from our study for hierarchal clustering of the 17 TAM samples analyzed by expression profiling. As shown by the heat map in Supporting Information Figure S3, the resulting rank order did not show any significant association with CD163 surface expression or IL-6 or IL-10 ascites levels, suggesting that elevated CD163 expression or high cytokine levels are not indicative of an activated STAT3 pathway.
Figure 6.
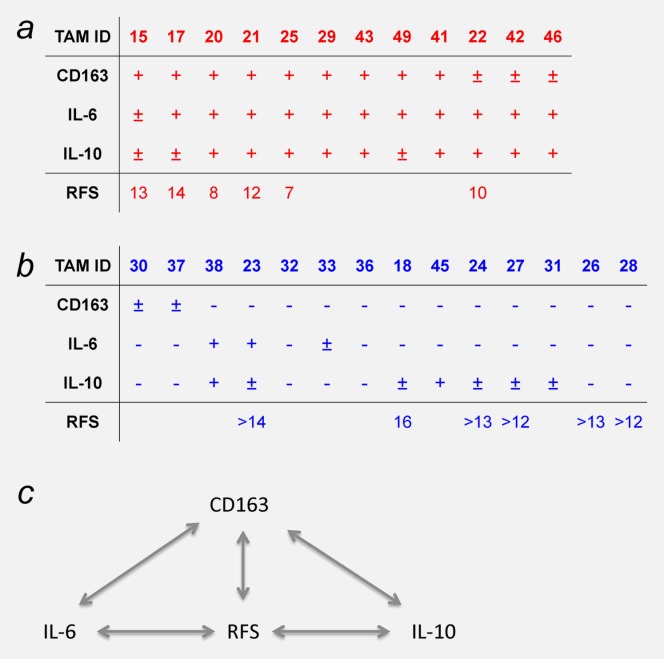
Summary of the correlations identified in our study. Patients were categorized according to the levels of CD163, IL-6 and IL-10 (high in panel a and low in panel b). The bottom row shows the RFS times after first-line surgery. Empty field represent patients that are not yet evaluable (relapse-free <12 months postsurgery). Panel c shows a schematic representations of the correlations between CD163, IL-6, IL-10 and RFS. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
Even though the peritoneal fluid plays a crucial role in the spread of ovarian cancer, the contribution of its host-derived cellular constituents is poorly understood. In our study, we have analyzed the nature, regulation and clinical significance of TAMs isolated from the ascites of patients with serous ovarian cancer. In brief, surface expression of the M2 marker CD163 on TAMs was inversely associated with RFS, yet transcriptional profiling of TAMs revealed a mixed-polarization phenotype unrelated to the M1/M2 classification. CD163 expression also correlated with the ascites levels of IL-6 and IL-10, both cytokines induced CD163 expression, and their ascites levels showed an inverse association with RFS. These findings led to the definition of a subgroup of patients with high CD163 expression, high IL-6 and IL-10 levels and poor clinical outcome (Fig. 6).
CD163 is a surface receptor on cells of the monocytic lineage.21 It was initially identified as a scavenger receptor that internalizes hemoglobin/haptaglobin complexes, but also interacts with erythroblasts, distinct pathogens and molecular ligands. CD163+ cells are a hallmark of the tumor microenvironment6,16 and are associated with poor prognosis in different types of cancer. In ovarian tumor sections, the number of CD163+ cells is higher in malignant compared to benign lesions and correlates with the histological grading of malignancy.12 Moreover, the frequency of CD163+ TAMs in paraffin sections of advanced ovarian cancer correlates with poor survival.22 Our data extend these findings by showing that the number of CD163+ cells in the malignant ascites of serous ovarian carcinoma patients is clearly associated with early relapse after first-line therapy (Figs. 2b and 6). This is the first demonstration of an association of clinical outcome with CD163+ cells in malignant effusion.
The expression of CD163 is tightly regulated.21 Although proinflammatory signals tend to inhibit its expression, the exposure to anti-inflammatory mediators leads to an increase in both CD163 transcription and CD163 protein levels on the cell surface, consistent with the classification of CD163 as a M2 marker.6,16 Several reports, including our own study (Supporting Information Dataset S1), have shown that a number of cytokines, chemokines and growth factors are present in the tumor microenvironment and malignant ascites of ovarian carcinoma patients.23–27 The concentrations of these cytokines fluctuate over a wide range between individual patients (Fig. 1), concomitantly with large variations in both the numbers of CD163+ TAMs, the level of CD163 surface expression (MFI) and CD163 mRNA levels (Supporting Information Dataset S1; Figs. 1 and 3b). Statistical analyses identified significant correlations of CD163 expression specifically with the concentration of both IL-6 and IL-10 in contrast to all other cytokines tested (Figs. 4a, 4b and 6; Supporting Information Table S2). In keeping with these correlations, we observed an induction of CD163 surface expression by both cytokines in cultured TAMs and MDMs (Fig. 4). This is in line with literature data showing an induction of CD163 by IL-6 and IL-10 in cultured human macrophages and/or monocytes.28,29
Consistent with these connections between CD163 expression, IL-6 and Il-10, RFS of ovarian carcinoma patients was clearly associated with the ascites levels of these cytokines (Figs. 5a, 5b and 6). Circulating IL-6 is a well-known prognostic factor for poor survival of carcinomas of the breast,30,31 prostate,32 stomach,33,34 colon35 and ovaries.36 An inverse association between IL-6 ascites levels and RFS has been described for ovarian cancer,26 consistent with the data in Figure 5b.
Similarly, IL-10 plasma concentrations are associated with the clinical course of different malignant tumors, including colon carcinoma,35 lung cancer,37 hepatocellular carcinoma,38 melanoma39 and lymphoma.40 However, an association between plasma or ascites levels and RFS of ovarian cancer has not been described to date. As IL-6 and IL-10 levels did not significantly correlate in our study (Supporting Information Table S3), the associations of RFS with these two cytokines are likely to be independent. This is different for LIF, which was not only associated with RFS (Fig. 5c) but also showed a strong correlation with IL-6 and IL-10 levels (Supporting Information Table S3). Therefore, it is possible that the observed association of LIF with RFS mirrors the effect of IL-6 and/or IL-10.
Both IL-6 and IL-10 exert a plethora of effects on tumor and immune cells that might explain their apparent protumorigenic effect in ovarian carcinoma.41 These include an induction of tumor cell proliferation, as suggested by our own observation that the proliferation-promoting potential of malignant ascites from ovarian cancer patients correlated with its content in IL-6 and IL-10 levels (Supporting Information Fig. S2). This is consistent with previous studies showing that the antibody-mediated depletion of IL-6 and IL-10 in ascites abrogates its stimulatory effect on tumor cell proliferation.11
CD163 is a widely used marker for alternatively activated (M2) macrophages,6,16,42 suggesting that TAMs from ovarian cancer might be skewed toward M2 polarization. The data in Figure 3 argue against this hypothesis, because other M2 markers are downregulated in TAMs, whereas M1 markers are unchanged or elevated. Furthermore, transcriptional profiling did not show any correlation of CD163 surface expression with two different published M2 signatures16,19 (Supporting Information Fig. S1). On the basis of these findings, we conclude that ovarian carcinoma TAMs have a mixed-polarization monocytic phenotype. Similar conclusions have been derived from mouse models of fibrosarcoma43 and mammary carcinoma.44
Consistent with this mixed phenotype is the cytokine composition of ovarian cancer ascites, which provides the stimuli determining TAM polarization. As shown in Supporting Information Dataset S1, ascites contains both inflammatory (like IL-6) and anti-inflammatory mediators (e.g., IL-10). IL-10 triggers a specific form of alternatively polarized macrophages, M2c, characterized by immune suppressive and tissue remodeling properties, as would be expected for TAMs.6 However, the gene expression profile does not match this classification, because genes typically upregulated in M2c cells, such as CCL18, are expressed at low levels in ovarian carcinoma TAMs (Fig. 3a). On the other hand, IL-10 has also been shown to induce a mixed-polarization phenotype in macrophages after infection with Haemophilus ducreyi45 and might therefore similarly contribute to the phenotype of TAMs.
Although unbiased hierarchical clustering of global gene expression profiles of 17 patients did not yield informative clusters (not shown), we were able to identify 110 probes whose expression pattern followed the surface expression of CD163 (Supporting Information Dataset S3). These do not include any genes previously been linked to macrophage polarization. Consistent with our data described above (Fig. 3), established human M2 marker genes were not present in this dataset. CD163 itself is not the part of this gene set, which is due to a weak correlation of protein (FACS) and RNA (microarray) expression. We attribute this to the existence of 11 potential CD163 transcript variants (ENSEMBL), which only partly match the single probe on the microarray chip, and/or the involvement of other regulatory mechanisms acting on translation, protein translocation and/or receptor recycling.
Even though CD163, IL-6 and IL-10 have previously been associated with the clinical outcome of different types of cancer, our study provides the first integrated picture of macrophage polarization, cytokines and clinical outcome. Our data also pave the way to further analyses investigating the precise nature and function of CD163high TAMs in the malignant ascites of patients suffering from serous ovarian carcinoma. Key to solving this question may be the application of high-resolution proteomics and genomics technologies.
Acknowledgments
The authors are grateful to T. Plaum, A. Allmeroth and M. Alt for expert technical assistance. This work was supported by grants from the Wilhelm-Sander-Stiftung to S.M.-B., S.R. and U.W and from UKGM to S.R. and U.W.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbe H, Chakravarty P, Leinster DA, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010;101:2128–36. doi: 10.1111/j.1349-7006.2010.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Komohara Y, Takaishi K, et al. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int. 2009;59:300–5. doi: 10.1111/j.1440-1827.2009.02369.x. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Rustin GJ, Timmers P, Nelstrop A, et al. Comparison of CA-125 and standard definitions of progression of ovarian cancer in the intergroup trial of cisplatin and paclitaxel versus cisplatin and cyclophosphamide. J Clin Oncol. 2006;24:45–51. doi: 10.1200/JCO.2005.01.2757. [DOI] [PubMed] [Google Scholar]
- Adhikary T, Kaddatz K, Finkernagel F, et al. Genomewide analyses define different modes of transcriptional regulation by peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta. PLoS One. 2011;6:e16344. doi: 10.1371/journal.pone.0016344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabeh GH, De Baetselier P, Brys L, et al. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–83. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80:705–13. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- Lolmede K, Campana L, Vezzoli M, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–87. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- Beyer M, Mallmann MR, Xue J, et al. High-resolution transcriptome of human macrophages. PLoS One. 2012;7:e45466. doi: 10.1371/journal.pone.0045466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Robinson GW, Hennighausen L. Comprehensive meta-analysis of Signal Transducers and Activators of Transcription (STAT) genomic binding patterns discerns cell-specific cis-regulatory modules. BMC Genomics. 2013;14:4. doi: 10.1186/1471-2164-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–60. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Lan C, Huang X, Lin S, et al. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259–67. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Cui ZM, Zhang J, et al. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chin J Cancer. 2011;30:336–43. doi: 10.5732/cjc.010.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D, Matte I, Rancourt C, et al. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matte I, Lane D, Laplante C, et al. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res. 2012;2:566–80. [PMC free article] [PubMed] [Google Scholar]
- Buechler C, Ritter M, Orso E, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- Sulahian TH, Hogger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–21. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- Bachelot T, Ray-Coquard I, Menetrier-Caux C, et al. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–6. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–32. [PubMed] [Google Scholar]
- Nakashima J, Tachibana M, Horiguchi Y, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–6. [PubMed] [Google Scholar]
- Ashizawa T, Okada R, Suzuki Y, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124–31. doi: 10.1007/s10120-005-0315-x. [DOI] [PubMed] [Google Scholar]
- Necula LG, Chivu-Economescu M, Stanciulescu EL, et al. IL-6 and IL-11 as markers for tumor aggressiveness and prognosis in gastric adenocarcinoma patients without mutations in Gp130 subunits. J Gastrointest Liver Dis. 2012;21:23–9. [PubMed] [Google Scholar]
- Galizia G, Orditura M, Romano C, et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169–78. doi: 10.1006/clim.2001.5163. [DOI] [PubMed] [Google Scholar]
- Scambia G, Testa U, Benedetti Panici P, et al. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer. 1995;71:354–6. doi: 10.1038/bjc.1995.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita F, Orditura M, Galizia G, et al. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. 2000;117:365–73. doi: 10.1378/chest.117.2.365. [DOI] [PubMed] [Google Scholar]
- Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552–8. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisu-Itakura H, Lee JH, Huynh Y, et al. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J Immunother. 2007;30:831–8. doi: 10.1097/CJI.0b013e318158795b. [DOI] [PubMed] [Google Scholar]
- Rautert R, Schinkothe T, Franklin J, et al. Elevated pretreatment interleukin-10 serum level is an International Prognostic Score (IPS)-independent risk factor for early treatment failure in advanced stage Hodgkin lymphoma. Leuk Lymphoma. 2008;49:2091–8. doi: 10.1080/10428190802441339. [DOI] [PubMed] [Google Scholar]
- Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138:657–64. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- Ambarus CA, Krausz S, van Eijk M, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Gangi L, Paul S, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation. Blood. 2006;107:2112–22. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Torroella-Kouri M, Silvera R, Rodriguez D, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69:4800–9. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- Li W, Katz BP, Spinola SM. Haemophilus ducreyi-induced interleukin-10 promotes a mixed M1 and M2 activation program in human macrophages. Infect Immun. 2012;80:4426–34. doi: 10.1128/IAI.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.