Abstract
During central nervous system development, glial cells need to be in the correct number and location, at the correct time, to enable axon guidance and neuropile formation. Repair of the injured or diseased central nervous system will require the manipulation of glial precursors, so that the number of glial cells is adjusted to that of neurons, enabling axonal tracts to be rebuilt, remyelinated and functional. Unfortunately, the molecular mechanisms controlling glial precursor proliferative potential are unknown. We show here that glial proliferation is regulated by interactions with axons and that the Drosophila gene prospero is required to maintain the mitotic potential of glia. During growth cone guidance, Prospero positively regulates cycE promoting cell proliferation. Neuronal Vein activates the MAPKinase signalling pathway in the glia with highest Prospero levels, coupling axon extension with glial proliferation. Later on, Prospero maintains glial precursors in an undifferentiated state by activating Notch and antagonising the p27/p21 homologue Dacapo. This enables prospero-expressing cells alone to divide further upon elimination of neurons and to adjust glial number to axons during development.
Keywords: axon guidance, glia, oligodendrocyte, precursor, Prospero
Introduction
The number of cells in different interacting cell populations needs to be adjusted during development to ensure that correct organ structure, size, shape and function are achieved. In the nervous system, the number of innervating neurons is matched to the size of the target field, as excess neurons are eliminated by apoptosis (Hamburger and Levi-Montalcini, 1949). Similarly, the number of glial cells, such as oligodendrocytes of the vertebrate central nervous system (CNS), is adjusted to the volume of available axons by apoptosis, ensuring correct myelination (Barres and Raff, 1994). Oligodendrocyte precursor proliferation also depends on interactions with axons and on neuronal function, since blocking electrical activity reduces oligodendrocyte proliferation by 80% (Barres and Raff, 1992, 1994). In fact, the attainment of the normal number of cells requires a balance between the control of cell survival and the control of cell proliferation. However, the mechanisms by which this balance is achieved are unknown.
During axon guidance, glial cells have to be in the correct location and number, at the correct time, to organise axonal patterns. Glia occupy choice point positions during axon guidance and trigger fasciculation and defasciculation decisions of axons (Bate, 1976; Silver et al, 1982; Tear, 1999; Hidalgo and Booth, 2000). Once the axonal trajectories are established, glia maintain axonal bundles, neuronal survival and the integrity of neuropiles (Booth et al, 2000). Undoubtedly, glia must be in constrained numbers to allow the emergence of such sophisticated structures. Whereas it is known that neuron–glia interactions maintain the survival of both cell types, it is still much of a mystery what regulates how these cells divide to achieve their normal cell numbers.
Repair of the injured or diseased central nervous system will depend on the restoration of correct glial cell number. For instance, transplantation of glial cells to the site of spinal cord injury is sufficient to promote axonal and functional repair (Ramon-Cueto et al, 2000). Conversely, alterations in glial cell number occur in gliomas, neurodegenerative diseases and upon injury (Fields and Stevens-Graham, 2002; Franklin, 2002; Zhu et al, 2002). Therapeutic restoration of glial cell number will require the directed manipulation of the mitotic potential and differentiation of glial precursors (Ramon-Cueto et al, 2000; Franklin, 2002; Schwab, 2002). Interestingly, the vertebrate adult CNS has a population of immature oligodendrocytes that might be present as a means to adjust cell number when needed (ffrench-Constant and Raff, 1986; Nunes et al, 2003). Unfortunately, nothing is known of what keeps oligodendrocyte precursors in an immature, proliferative state.
The longitudinal glia (LG) of the Drosophila CNS share some features with vertebrate oligodendrocyte precursors. Like oligodendrocytes, LG are also produced in excess and the excess cells are eliminated through apoptosis. The survival of both oligodendrocytes (Raff et al, 1993) and at least some of the LG (Hidalgo et al, 2001) depends on contact with axons and on Neuregulin/Vein. There is also suggestive evidence that LG proliferation may be under non-autonomous control. The LG originate from the segmentally repeated longitudinal glioblasts (LGBs). DiI labelling of the LGB produces a clone of variable number of progeny cells, resulting in between 7 and 10 progeny cells (Schmidt et al, 1997). There is apoptosis in up to three cells in this lineage in normal embryos (Hidalgo et al, 2001), meaning that the resulting progeny of the LG lineage if they were all to survive may be around 12 cells. This suggests that the mitotic profile of the LGB lineage is not simply symmetrical and/or perhaps LG precursor divisions are under non-autonomous control.
Here, we analyse the mechanisms that regulate proliferation of the LG as they interact with pioneer axons. We show that proliferation of the LG is regulated by neurons and that the gene prospero (pros) plays a key role in linking glial proliferation and axon guidance. Early on, Pros enables glial proliferation in response to pioneer neurons. Once the axonal bundles are formed, Pros maintains glial precursors in an arrested, immature state, enabling pros-expressing cells alone to divide further upon elimination of neurons.
Results
LG divide during axon guidance
Here we analyse the mechanisms that regulate LG precursor proliferation from the time in which they interact with neurons during axon guidance.
In the wild-type embryo, the LGB is located at the edge of the neuroectoderm, it divides and the LG progeny invaginate and migrate towards the midline. When the LG reach the pioneer neurons, they stop migrating medially to migrate anteroposteriorly together with the extending axons (Hidalgo and Booth, 2000). The LGBs generate before neuronal contact up to four progeny cells, but most LG divisions occur as the LG contact the axons, during axon guidance (stage 13).
We monitored LG proliferation by colocalisation of anti-pHistone-H3 and anti-Repo antibodies (Figure 1D, E, H and L) or a cytoplasmic LG lineage reporter lacZ (F263) (Jacobs et al, 1989) (Figure 1C, G and I), or with anti-GFP antibodies to Histone YFP expressed under the control of the htlGAL4 promoter (Figure 1J and K). We have previously shown that htlGAL4 expression is restricted to the LG in the CNS (Hidalgo et al, 2001). At a first time point, as the dMP2 pioneer axons begin to extend towards the LG, two of the four LG cells divide first (Figure 1C and G), resulting in a total of six cells. Next, the descending and ascending pioneer axons meet to fasciculate into the first single longitudinal fascicle (Hidalgo and Brand, 1997). Just after these, axons meet, as seen with GAP GFP driven by FTZNGAL4 or with 22c10 antibodies, LG divide at the point of contact between the fascicles (Figure 1H–J). Most often, the two posterior of the six-LG cluster divide next (Figure 1I and J), and the daughter cells migrate with the extending axons. The resulting LG trigger the defasciculation of the first fascicle into two fascicles (Figure 1K) (Hidalgo and Booth, 2000). LG division is then seen again over these fascicles (Figure 1E and L). The resulting LG trigger the final distribution of axonal fascicles into three major bundles, characteristic of the end of embryogenesis (Hidalgo and Booth, 2000) (see also Figure 2J). Since LG are required for all these axon guidance and fasciculation events, the coordination of LG proliferation and axon guidance ensures that LG are in the correct number to shape axonal patterns. This raises the question of whether axons regulate LG proliferation during guidance.
Figure 1.
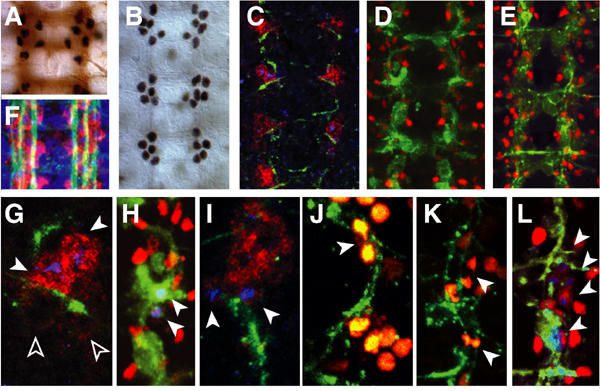
LG divide during during axon guidance. (A, B, F) The stage-16 embryonic neuropile visualised with (A) BP102 (brown) and anti-Pros (black); (B) anti-Pros, labelling subsets of LG; (F) FasII (axons, green) and anti-βgal (red) in the LG-LacZ reporter. (C–L) LG proliferation visualised with: (C, D, E, G, H, I, L) anti-pHistone-H3 (blue) and (J, K) anti-GFP in htlGAL4/UASHistone YFP (yellow); the LG (red) are visualised with: (C, G, I) anti-βgal in the lacZ reporter; (D, E, H, L) anti-Repo and (J, K) with anti-GFP (yellow). The axons (green) are visualised with: (G, I, J, K) 22c10 and (H, L) FTZNGAL4/UAS GAPGFP. (C–E) are lower magnification views of (G, H, L), respectively. (G) The two anterior LG (arrowheads) divide before the posterior (empty arrowheads). In (H–L), arrowheads indicate dividing LG. (A, F) show one segment, (B–E) three segments, (G–L) one hemisegment. Midline to the left, anterior up.
Figure 2.
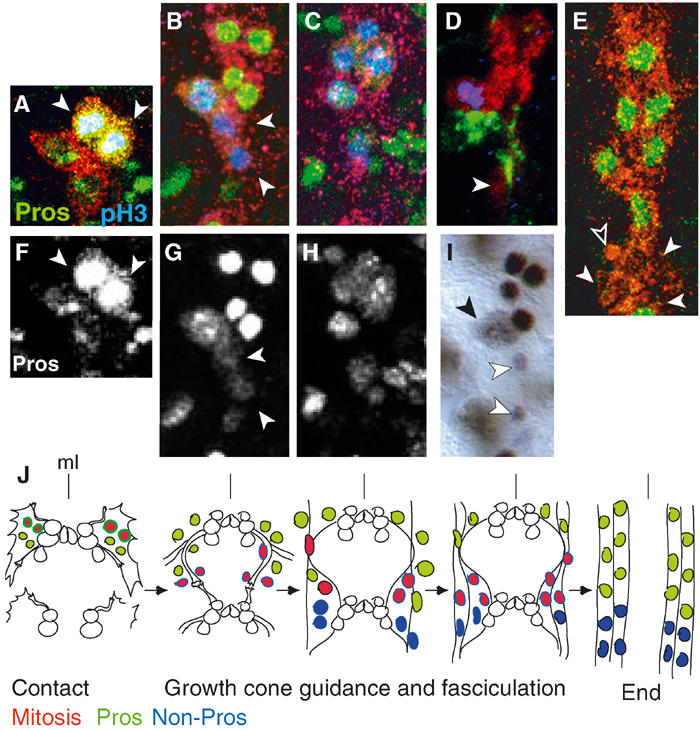
Mitotic profile and Pros distribution in the LG. (A–E) LG are visualised with anti-βgal (red) in LG-lacZ, mitosis with anti-pHistone-H3 (blue) and anti-Pros (green) in wild type, except for (D) where axons are visualised with 22c10 (green). (F–H) Single-channel images of (A–C), respectively, showing only anti-Pros. (I) Anti-Pros visualised with HRP. (A, F) The two anterior LG have higher Pros levels (arrowheads). (B, G) As cells exit mitosis, they downregulate Pros (arrowheads). (D) As cells exit mitosis, they migrate with the axons (arrowhead). In (I), arrowhead indicates dividing LG and white arrowheads indicate cells with lower Pros levels, migrating posteriorly. (E) Stages 15–17: no LG divide, Pros is present in 6/10 LG (arrowheads indicate non-Pros LG and empty arrowhead indicates apoptotic LG). (J) Diagram to illustrate glial proliferation during axon guidance and fasciculation (as in Figure 1G–L) in one segment through time. (A–I) show one hemisegment, midline to the left, anterior up.
We verified the mitotic profile within the LGlioblast lineage only, using LG-lacZ and visualising mitosis with anti-pHistone-H3 antibodies (Figure 2A–E). We confirmed that the two most anterior cells in each four-cell cluster divide most often first (Figure 2A). The resulting six LG divide immediately again, asynchronously, and between one and five LG can divide at once during axon contact (Figure 2B–D). As cells exit mitosis, they migrate with the axons (Figure 2D and I). Later, as the neuropile is formed and the LG overlie the axons, there is no LG cell division in wild type (stage 15 on; Figure 2E). This profile suggests that LG proliferation may be regulated by interactions with axons.
Proliferation of the LG precursors is regulated by neurons
To address this possibility, we tested whether eliminating neurons might affect LG proliferation. We eliminated neurons by targeted genetic ablation with FTZNGAL4, which drives expression in the pioneer neurons and other neurons (Hidalgo and Brand, 1997) (Figure 3C). Although this driver is expressed very transiently in the glioblast lineage, we have previously shown that when used for genetic ablation this driver does not kill the longitudinal glia (Kinrade et al, 2001). We monitored mitosis with pHistone-H3 antibodies in the LG stained with anti-Htl antibodies. Targeted neuronal ablation causes a reduction in mitosis in the LG at the time when LG precursors normally divide upon neuronal contact, and an increase in division later, when LG do not divide in wild-type embryos (Figure 3A).
Figure 3.
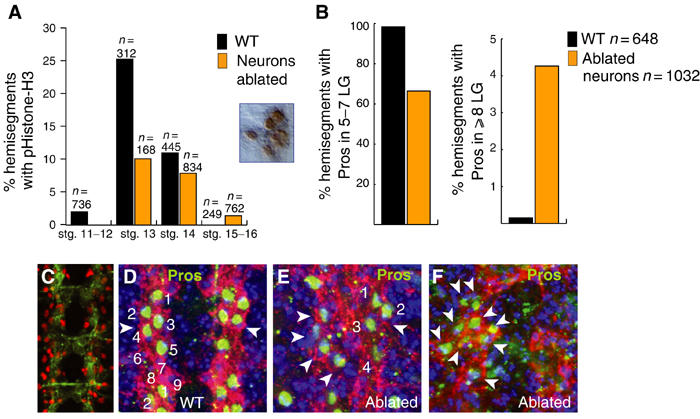
Glial proliferation changes in response to neuronal ablation. (A) Mitosis in the LG monitored with anti-phospho-Histone-H3 antibodies (see inset, example of anti-pHistone H3 with HRP staining) in LG-LacZ flies stained with anti-βgal or with anti-Htl. Quantification of hemisegments with pHistone-H3 in LG in ablated embryos compared to wild type: decrease at stage 13 (P<0.0005) and mild increase at stages 15–16. The inset shows a cluster of mitotic LG (pHistone-H3, brown) at stage 15 in an ablated embryo. (B) Quantification of hemisegments with 5–7 Pros-positive LG upon neuronal ablation, as opposed to four or fewer, compared to wild type (P<0.0005), and of ⩾8 Pros-positive LG upon ablation compared to wild type (P<0.0005). See (D–F) for examples of quantified anti-Pros-stained segments. (C) Expression driven by FTZNGAL4 as seen with UASGAPGFP (anti-GFP). (D–F) LG stained with anti-Heartless (red) and anti-Pros (green) at stage 16: (D) Wild-type segments have 5–7 Pros-positive LG; upon neuronal ablation, these are reduced to four Pros-positive LG (E, arrowheads) or they increase up to 15 (F, arrowheads, here increased to 9). (D–F) show one segment, anterior up. Blue is nuclear staining with TOTO-3. n=number of hemisegments.
We have previously shown that targeted neuronal ablation also induces glial apoptosis, and that one neuronal signal that maintains the survival of LG is neuregulin Vein, produced by pioneer neurons (Hidalgo et al, 2001). To test whether Vein also modulates glial proliferation, we monitored the extent of LG proliferation with pHistone-H3 in vein mutants during axon guidance (stage 13/14) and we found a reduction in LG proliferation (Figure 4K). We confirmed that the decrease in pHistone-H3 spots corresponded to a reduction in cell proliferation and not to cell loss by monitoring cell proliferation with pHistone-H3 in vein mutants in which glial apoptosis is prevented by targeted expression of p35 in the LG (Figure 4K). In these embryos, there is still a reduction in LG proliferation compared to wild type. This means that Vein promotes both the survival and proliferation of the EGFReceptor responsive subset of LG.
Figure 4.
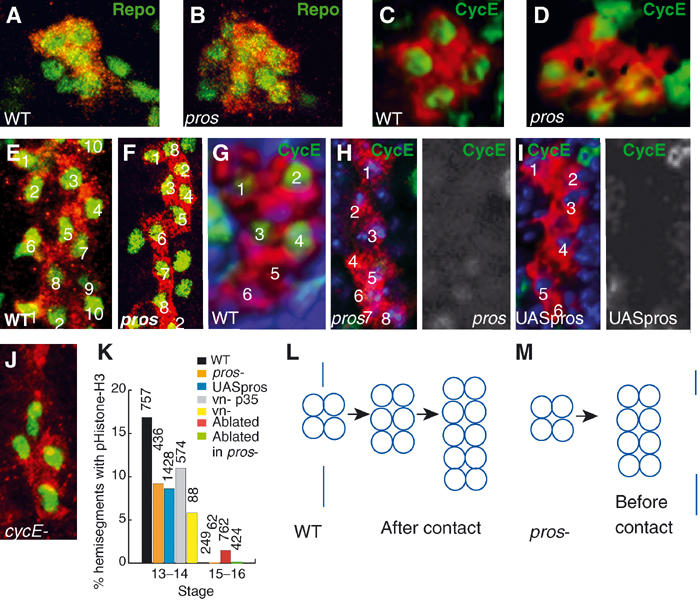
Pros promotes LG cell proliferation after axonal contact. In all images, LG cytoplasms are visualised with anti-βgal (red) in LG-Lacz flies. (A, B, E, F) LG nuclei visualised with anti-Repo (green) in (A) wild type; (B) pros mutants; (E) wild type and (F) pros mutants. (A, B) are state 12 and (E, F) are stage 15. (C, D, G–I) Anti-CycE (green) in stage 12: (C) wild type; (D) pros mutants. (G–I) Stage 13: (G) wild type; (H) pros mutants; (I) embryos expressing pros ectopically in all LG (htlGAL4; F263/UASpros). In gray-scale are single-channel views of (H, I), respectively, showing only anti-CycE. (J) cycE mutant embryo: LG visualised with anti-Heartless (red) and nuclei with anti-Repo (green). (K) Quantification of pHistone-H3 in the LG (anti-βgal): decrease at stage 13 in pros and in vein mutants (planned comparison, pros versus wild type, P<0.0005). Mitosis is also reduced in vn mutants expressing p35 (vnγ4htlGAL4/UAS p35 vnγ3). The increase in glial proliferation at stages 15–16 is abolished in a pros mutant background. (L, M) Diagrams to show the mitotic profile of the LG in (L) wild type and (M) pros mutants after the four-cell stage. Vertical line indicates the time in which LG contact the axons. Blue is nuclear stain TOTO-3. All images are of one hemisegment, midline to the left, anterior up. n=number of hemisegments.
At the end of embryogenesis, when the LG overlie the longitudinal axon fascicles of the CNS, pros is expressed in six out of the approximately 10 LG per hemisegment (Figure 1B and 2E), at the intersection between commissural and longitudinal axons, that is, at the location of highest axonal contact (Figure 1A). When we visualise the LG with anti-Pros antibodies —as well as anti-Htl antibodies—upon neuronal ablation we observe a reduction in Pros-positive LG from six to four (Figure 3B, D and E) and less frequently an excess of Pros-positive LG to up to 15 (Figure 3B and F). Interestingly, we only observe an excess of LG among those LG expressing pros.
The above data suggest that, in the normal embryo, neurons promote glial division during growth cone guidance and they halt glial division when the neuropile is formed.
Pros is expressed in the LG reflecting the mitotic profile
Pros is a DNA-binding protein known as a cell fate determinant in the asymmetric divisions of neuroblasts (Doe et al, 1991; Vaessin et al, 1991; Hirata et al, 1995; Jan and Jan, 2001), but the LGlioblast divides apparently symmetrically and asynchronously, from the time of axon contact (Figure 2A–E). To determine what role might Pros play in the LG, we looked at the expression of pros in the LG lineage. Pros is distributed in the four LG, the two anterior LG have higher Pros levels and most often divide first (Figure 2A and F). Pros is present in all of the resulting six LG, which divide once more asynchronously (Figure 2B, C, G and H). After each division, pros is segregated to both daughter cells but it is downregulated as cells exit mitosis, in the daughter cells that migrate with the axons (Figure 2D and I). Thereafter (stage 15), Pros is maintained in the most anterior six of the 10 LG (Figure 2E), which do not divide further in normal embryos (Figure 2J). This profile raises three questions: (1) why is Pros present in unequal levels at the time when glia contact the axons? (2) Why is Pros present in all the dividing LG? and (3) why is Pros only present in a subset of the LG at a time when no LG divide any more?
Pros promotes cell proliferation in the LG during growth cone guidance
Since Pros is found in all dividing LG, we wondered whether pros mutations might affect LG proliferation. We monitored the effect of pros mutations on the proliferation of the LG with pHistone-H3, and the effect on cell number with anti-Repo antibodies, in flies bearing the LG lacZ reporter. In pros mutants, there is an increase in LG number prior to neuronal contact (3.8%, n=106; Figure 4B) and a reduction in LG number (38%, n=108; Figure 4F) and in mitosis (Figure 4K) after neuronal contact (stage 13). The final number of LG in pros mutants is eight—instead of the normal 10–12—which stretch out over the extent of the axons but do not divide any further. Thus, in pros mutants, the mitotic profile of the LG changes from 4–6–12 during axon guidance to 4–8 happening earlier (Figure 4L and M). This indicates that Pros is required to determine the profile and timing of LG cell divisions.
To test whether Pros is necessary for cell division to proceed, we looked at the expression of cyclinE (cycE)—which induces the transition from G1 to S phase—in pros mutant embryos. In wild-type embryos, at the four-cell stage CycE is present in all four LG (Figure 4C) and subsequently in 4/6 LG (Figure 4G). In pros mutants, there is residual CycE in the abnormal large LG clusters prior to neuronal contact (Figure 4D) and there is no CycE after neuronal contact (Figure 4H), correlating with the drop in cell division at this stage (Figure 4K). In cycE zygotic mutants (Sauer et al, 1995), cell division does not go beyond the four-cell stage (87.5%, n=176; Figure 4J), indicating that from the four-cell stage cycE regulation is under the control of zygotic gene expression. These data suggest that Pros positively regulates cycE from the four-cell stage on. This also indicates that in pros mutants LG divide faster before neuronal contact skipping a G1 phase and that the four-cell stage corresponds to the first G1 phase in the LGlioblast lineage.
At the time the four LG contact the neurons, two of them have higher Pros levels (Figure 2A and F). To test whether Pros levels influence LG cell proliferation, we expressed Pros (Manning and Doe, 1999) in all LG with htlGAL4. We find that ectopic expression of pros in all LG from the four-cell stage on inhibits cycE expression (Figure 4I), causing a reduction in mitosis (Figure 4K) and a final LG number of six. This means that Pros levels influence LG proliferation.
The two anterior cells with higher Pros levels express the EGFReceptor that signals through the Ras/MAPKinase pathway (Hidalgo et al, 2001). The activation of this pathway can be visualised with anti-dpERK antibodies in these two cells (Figure 5A–C). In pros mutants, dpERK is not activated (85%, n=20; Figure 5D). Thus, in pros mutants, the EGFR/dpERK-dependent cell divisions are missed. In normal embryo, the MAPKinase pathway is activated in the LG upon binding of the neuronal signalling molecule Vein to the EGFR (Hidalgo et al, 2001). As shown above, LG cell division is reduced in mutants lacking Vein (Figure 4K). This suggests that high Pros levels positively regulate the EGFR/MAPKinase pathway in two of the LG, and this enables these LG only to respond to Vein.
Figure 5.
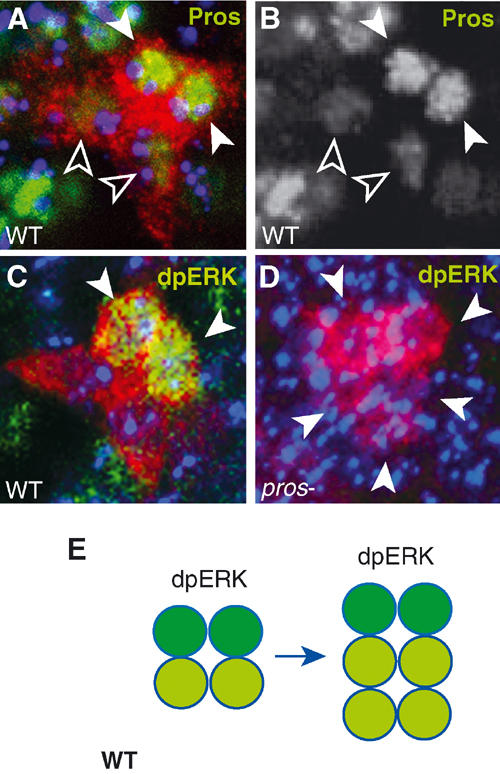
No activation of ERK in LG in pros mutants. LG-lacZ visualised with anti-βgal (red). (A, B) Wild type: (A) high levels of anti-Pros (green, white arrowheads) in two cells and lower levels in the posterior two (empty arrowheads); (B) single-channel view showing only anti-Pros. (C, D) anti-dpERK (green, arrowheads) in the two LG with higher Pros levels: (C) in wild type; (D) no dpERK (arrowheads indicate larger cluster of LG at stage 12) is detected in pros mutants. (E) Diagram illustrating the segregation of dpERK (dark green) among the Pros-positive LG (green). All images represent one hemisegment, blue is TOTO-3, midline to the left, anterior up.
Pros maintains LG precursors in a proliferative state in the mature neuropile
Pros mutants show a decrease in mitosis from stage 13 on due to the loss of cycE expression and dpERK signalling, implying that Pros promotes cell proliferation. However, two observations question whether this is the only way in which Pros promotes cell proliferation: first, why is Pros still present at a time when no LG divide in wild type? Second, why is overproliferation of LG upon neuronal ablation induced only among the Pros-positive LG?
To investigate whether the six Pros-positive LG are different from the rest in any other way, we searched for other genes expressed in these cells. We find that Notch is present in high levels in the same LG that express Pros and in reduced levels in the posterior, Pros-negative LG (Figure 6A). Furthermore, Notch is absent in pros mutant embryos, implying that Pros positively regulates Notch expression in these cells (Figure 6B). Since Notch is known in other contexts as a promoter of the proliferative and stem cell state (Hitoshi et al, 2002), this suggests that the Pros-positive LG may be in a different proliferative state from the Pros-negative cells.
Figure 6.
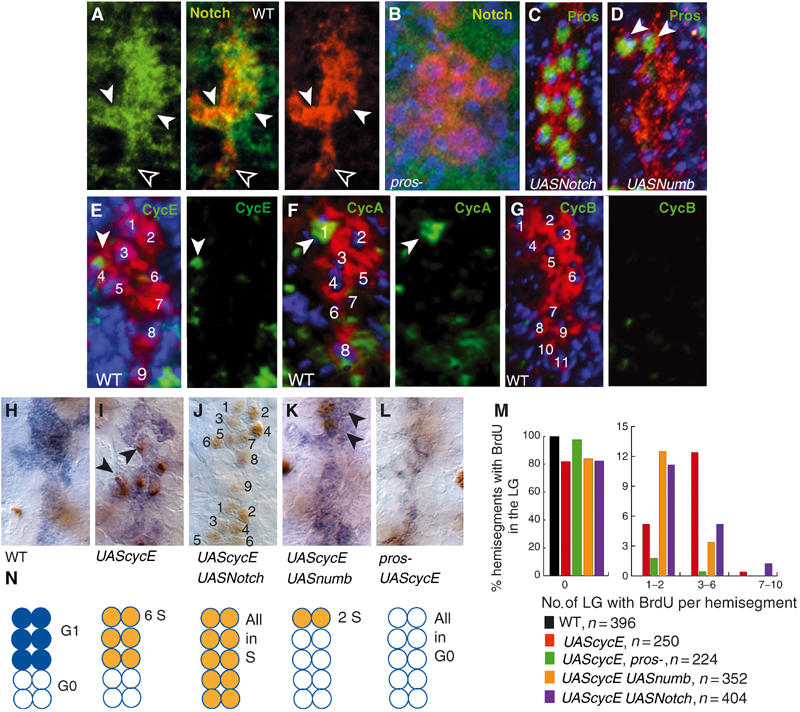
Pros maintains LG precursors proliferative by controlling G1 arrest. LG-lacZ visualised with anti-βgal (A–G) in red; (H–L) in blue. (A) Anti-Notch (green) in wild type (stage 14), single-channel and merged images. (B) Anti-Notch (green) undetectable in pros mutant embryos. (C) Expression of activated Notch in the LG causes upregulation of Pros in all LG (anti-Pros, green) (htlGAL4; F263/UASNotchintra). (D) Expression of numb in the LG causes downregulation of Pros in all LG except for the most anterior two (arrowheads, anti-Pros, green) (htlGAL4;F263/UASnumb). (E) Anti-CycE (green) in one LG (stage 14). (F) Anti-CycA (green) in one LG (stage 14). (G) No anti-CycB (green) in the LG at stage 15. Single-channel views of (E, F, G) show only the cyclins. (H–M) Induction of BrdU incorporation (anti-BrdU, brown, arrowheads) at stage 14 in the LG (blue) upon the expression of cycE in LG in: (H) control (F263): no BrdU incorporation; (I) control expressing cycE in LG (htlG4; F263/UAScycE); (J) embryo expressing in LG cycE and Notch-intra (htlG4;F263/UAScycE UASNotch-intra), 1.5 hemisegments shown; (K) embryo expressing in LG numb and cycE (htlG4;F263/UAScycE UASnumb). (L) pros mutant expressing cycE in LG (htlG4; F263 pros-/UAScycE pros-). (M) Quantification of BrdU incorporation. Compared to the UAScycE control, there is an increase in the frequency of hemisegments with (1) no BrdU in pros mutants (planned comparison, P<0.0005), (2) BrdU in only 1–2 LG when expressing UASNumb (planned comparison, P<0.005), (3) BrdU in >7 LG when expressing UASNotch-intra. There is a decrease in the frequency of hemisegments with BrdU in 3–6 LG when expressing UASNumb (planned comparison, P<0.0005). (N) Summarising diagram, showing in wild type the six LG in G1 arrest (blue) and the remaining LG in G0, and the incorporation of BrdU (orange, i.e. cells in S phase, S) in the experiments shown in the photos immediately above. In (A–G), blue is TOTO-3.All images show one hemisegment, midline to the left, anterior up; n=number of hemisegments.
To determine if Pros LG have a different mitotic potential from Pros-negative LG, we first asked whether LG have exited the cell cycle at the end of embryogenesis or not. At the end of embryogenesis in wild type, none of the LG divide, implying that these cells have either exited the cell cycle or they are in cell cycle arrest. We last observe CycE and CycA in one cell at mid-embryogenesis (stage 14; Figure 6E and F) and there is no CycB (Figure 6G) nor pHistone-H3 (Figure 2E) from stage 15 on in the LG. Therefore at this stage, the LG are not in G1/S transition, S phase nor M phase. They do not incorporate BrdU (Figure 6H); thus, the LG have not gone through S phase and they are not arrested in G2. Therefore, at the end of embryogenesis, after axon guidance, LG are either arrested in G1 or they have exited the cell cycle and are in G0. When neurons are ablated, LG can overproliferate at this stage (Figure 3A, B and F); thus, at least some LG are arrested in G1. However, when neurons are ablated in pros mutant embryos, no overproliferation of LG is induced (Figure 4K). Thus, Pros is needed for LG to divide in response to neuronal ablation, suggesting that Pros maintains LG arrested in G1.
So does Pros control the distinction between G1 arrest and G0 cell cycle exit? The following evidence demonstrates that Pros-positive LG precursors are in G1 arrest and they retain mitotic potential. There is no incorporation of BrdU in wild-type embryos at stage 14, and LG do not divide from this point in normal embryos (Figure 6H, M and N). However, if we express cycE in all LG with htlGAL4, BrdU is incorporated in up to six cells per hemisegment (Figure 6I, M and N). Thus, six cells were arrested in G1 and were able to enter S phase immediately following targeted expression of cycE. If we trigger expression of pros in all LG by expressing Notch-intra together with cycE (Notch positively regulates pros expression; Figure 6C), up to nine cells per hemisegment incorporate BrdU (Figure 6J, M and N). Conversely, when we express numb ectopically in the LG, which causes downregulation of pros in all except two LG (Figure 6D), together with cycE, BrdU incorporation declines and it can be limited to two LG that retain Pros in these embryos (Figure 6K, M and N). In pros mutant embryos, LG do not incorporate BrdU despite the ectopic expression of cycE (Figure 6L, M and N). Thus, in the absence of Pros, CycE cannot push the cell through a G1/S transition. This demonstrates that cells that do not have Pros exit the cell cycle and are in a G0 differentiated state, whereas cells that have Pros are G1-arrested precursors with mitotic potential.
Pros prevents cell cycle exit in the LG by antagonising Dacapo
Dacapo (Dap) is the Drosophila cyclin-dependent kinase inhibitor p21/p27 homologue and it promotes cell cycle exit (de Nooij et al, 1996; Lane et al, 1996). In vertebrates, p27 promotes cell cycle exit of oligodendrocyte precursors (Casaccia-Bonnefil et al, 1997; Durand et al, 1997, 1998). Thus, we asked whether Pros may prevent cell cycle exit by regulating dap.
During normal cell proliferation upon neuronal contact, the LG acquire two distinct morphologies: the anterior cells are rounder than the posterior LG (Figure 7A). The anterior cells are the dividing, Pros-positive cells, whereas the posterior cells are the LG that downregulate Pros. Later on, all LG spread out (Figure 7B). In pros mutants, all LG have large cytoplasmic projections (Figure 7C), indicative of differentiation. When pros is expressed in all LG, they round up (Figure 7D), indicative of incomplete differentiation. In dap mutants, the LG are round (Figure 7E), and when all LG express dap they form extensive cytoplasmic projections (Figure 7F). Furthermore, in dap mutants, the glial differentiation marker Repo is reduced or lost (19%, n=226; Figure 7G and M), and there is an excess of mitosis at a time when no LG divide in wild type (15.4%, n=208 at stage 15). This suggests that Pros prevents complete LG differentiation, whereas Dap promotes it.
Figure 7.
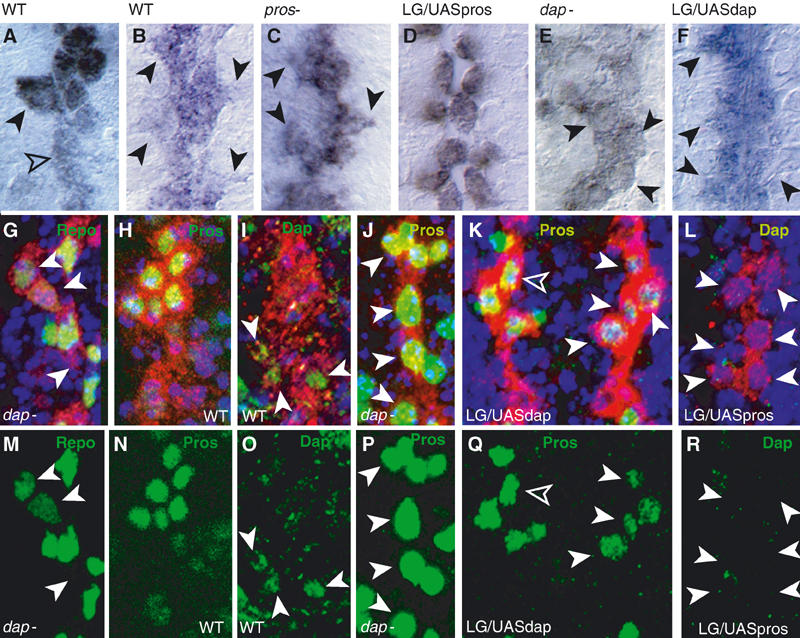
Pros and Dap antagonise each other in the LG. (A–F) LG (anti-βgal, blue) in: (A) wild type at stage 13 (black arrowhead, Pros-LG; empty arrowhead, non-Pros LG) and (B) stage 16 (arrowheads indicate projections); (C) pros mutant (arrowheads as in (B)); (D) htlGAL4; F263/UASpros; (E) dap mutant (arrowheads point to round cells); (F) htlGAL4;F263/UASdap (arrowheads indicate projections); (B–F), all stage 16. (G–L) LG in red (anti-βgal): (G) Repo (green, arrowheads denote loss) in dap mutant; (H) wild-type anti-Pros (green); (I) wild-type anti-Dap (green, arrowheads); (J) anti-Pros (green, arrowheads) in dap mutant; (K) anti-Pros (green) is downregulated (arrowheads) in htlGAL4;F263/UASdap embryos (note weaker effect on the left side, empty arrowhead); (L) anti-Dap (green) is absent (arrowheads) in htlGAL4;F263/UASpros embryos. (M–R) Single-channel images of (G–L), respectively, to show only anti-Repo, anti-Pros and anti-Dap. All images show one hemisegment, except for (K), which shows one segment, midline to the left, anterior up.
Dap is found transiently in the posterior LG that do not express pros (Figure 7H, I, N and O). In dap mutants, Pros can be found in all LG (32.4%, n=34; Figure 7J and P), whereas expression of dap in all LG downregulates Pros (14.5%, n=234; Figure 7K and Q). Conversely, expression of pros in all LG prevents dap expression (Figure 7L and R). Thus, in the LG, Pros and Dap expression and function are complementary and mutually exclusive. Our results demonstrate that Dap promotes cell cycle exit and the terminal differentiation of the subset of LG that do not express pros, and that Pros maintains LG precursors in an immature state, with mitotic potential, by antagonising Dap.
Discussion
We have shown that neuron–glia interactions regulate LG proliferation during axon guidance (Figure 8A). As axons extend, neurons promote LG division; however, once the axonal tracts are mature, neurons prevent LG proliferation. We have shown that Pros is the key regulator in the LG that enables this dual response of LG to neurons.
Figure 8.
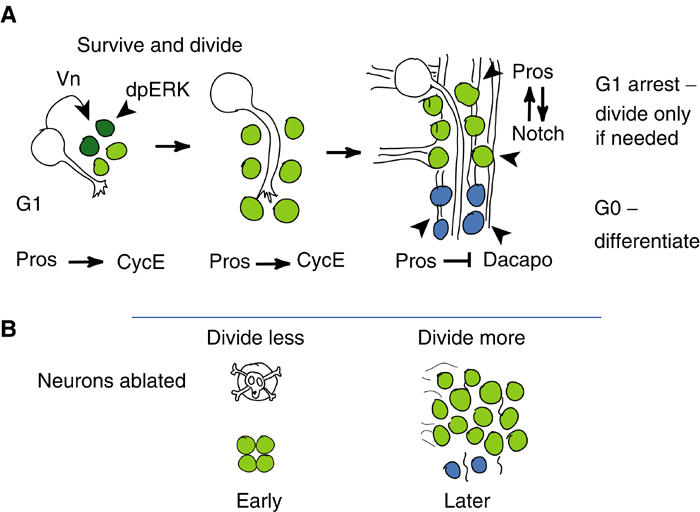
Summarising diagram to illustrate the function of Pros in the control of LG proliferation. Pros-LG are in green, high levels of Pros in dark green and Pros-negative LG in blue. (A) Wild type; (B) upon neuronal ablation.
We have found novel roles of Pros in promoting cell proliferation and preventing cell cycle exit. We have shown that the glia reach the extending growth cones in clusters of four cells, when cell division halts for some time. Normally, two of these LG then divide, resulting in a total of six, which then divide again, but since some LG die the real final number ranges between 8 and 11 cells. In pros mutants, the glia contact the pioneer neurons in clusters of eight cells rather than the normal four, suggesting that LG divided faster than normally in the presence of maternal CycE, skipping a G1 phase. The division of four LG into six is also missed, thus changing the mitotic pattern from its normal 4–6–12 to 4–8.
Loss of pros function causes a reduction in LG proliferation, which is manifested in three ways: (1) In pros mutants, the first division of the two anterior LG with highest Pros levels is missed, because there is no dpERK. (2) LG do not divide at the normal times during axon guidance and fasiculation in pros mutants, because in the absence of pros CycE is not produced. Thus, although LG divided earlier in pros mutants, these divisions are uncoupled from axon guidance. Thus, Pros changes the mitotic profile in the LG from a simple symmetric pattern to a pattern in which the LG respond to incoming axons. (3) In the absence of Pros, LG do not have the potential to overproliferate when neurons are ablated.
Pros protein is present in all dividing LG and in LG that retain mitotic potential. During growth cone guidance and axonal fasciculation, Pros promotes LG proliferation of the two LG that are able to respond to Vein and activate the MAPKinase pathway. Vein induces LG cell division as well as cell survival of the two DER-positive LG. Knock-down of Vein function with targeted RNAi in the MP2 neurons only is sufficient to cause LG apoptosis (Hidalgo et al, 2001). We have shown here that loss of Vein function in genetic null embryos reduces mitosis, also when apoptosis is blocked. Thus, the EGFR/MAPKinase signalling pathway controls both cell survival and cell proliferation in these two LG. The EGFR also controls both cell survival and cell proliferation in the retina, in response to the ligand Spitz (Baker and Yu, 2001). Later on, when the axonal fascicles are formed, Pros maintains the mitotic potential in the LG by preventing them from exiting the cell cycle. In fact, only Pros-positive LG can enter S phase upon ectopic expression of cycE. In this way, at the end of embryogenesis, the LG are divided into Pros-positive G1-arrested LG and Pros-negative LG, which have exited the cell cycle and are in G0. Pros maintains the LG in the G1-arrested undifferentiated, immature precursor state by positively regulating Notch and by antagonising Dacapo.
Novel roles for Pros in the divisions of the LG
Our findings on the roles of Pros in the LG during axon guidance differ from its neuroblast functions. In neuroblast lineages, Pros protein is located in a crescent and it is distributed asymmetrically to the daughter cell upon the division of the neuroblast. In the ganglion mother cell, Pros is internalised into the nucleus, where it determines cell fate and it restricts cell division (Jan and Jan, 2001). However, the progeny of the LGlioblast from the time in which they contact the pioneer axons (four-cell stage) divide apparently symmetrically, although asynchronously. During these divisions, Pros is present in the nuclei of all dividing LG, and not in crescents. Upon cell division, Pros is segregated symmetrically to the two daughter cells and it is downregulated after cell division as the posterior LG migrate with the axons. Finally, during axon guidance, pros mutations cause a reduction in LG proliferation rather than an excess, meaning that pros is necessary for cell division to proceed.
Pros and its vertebrate homologue Prox1 can inhibit cell proliferation and promote cell cycle exit (Wigle et al, 1999; Li and Vaessin, 2000; Dyer et al, 2003). In fact, both in pros and Prox1 mutants, cell proliferation and the expression of cyclin increase, and both Prox1 and Pros can promote p27/dap expression. We have shown that in the LG Pros promotes cell proliferation and it prevents cell cycle exit by antagonising Dap. Therefore, Pros controls cell cycle genes in different ways in different cellular contexts. Moreover, temporal regulation is crucial and Liu et al (2002) have shown that Pros can both promote and antagonise dap expression at different time points. We also observe, like others (Li and Vaessin, 2000; Dyer et al, 2003), that upon ectopic pros expression the LG divide less and do not express cycE. However, in the LG this may not be due to the promotion of cell cycle exit but to the earlier halt of precursors in cell cycle arrest.
Our findings also contrast with the roles of Pros in mixed neuro-glioblast lineages, where Pros is segregated asymmetrically to the daughter cell that will become a glial cell (Gho et al, 1999; Akiyama-Oda et al, 2000; Freeman and Doe, 2000). The LG is a glial-only lineage. In the LG, Pros may control the fate of the two LG with higher Pros levels, which signal through MAPKinase/dpERK. Our results show that during axon guidance Pros plays a primary role in the maintenance of the proliferative and undetermined state.
Timing and plasticity of cell number regulation
Our findings on the non-autonomous regulation of glial proliferation contrast with previous work that envisioned a cell-autonomous proliferation profile determined by lineage identity. Accordingly, the LGB would divide in a straightforward symmetrical fashion, into 2–4–8 cells (Badenhorst, 2001). This conclusion was based on the finding that BrdU is incorporated in four Repo-positive cells. Our data show that the incorporation of BrdU into four LG represents a narrow time window in the LG lineage, and not the final division. In fact, we detect mitosis in up to five LG at the same stage.
The finding of a different LG profile has important implications. It means that the final number of LG is not fixed at eight cells, but variable between 8 and 11, depending on how many LG die. A final fixed number of eight LG could be achieved faster through simple symmetrical divisions without considerable influence on final glial cell mass. In fact, in Pros mutants a final number of eight cells is achieved at an earlier time point, and these eight cells stretch out to occupy the whole length of the segmental neuropile. However, the sequential increase and adjustment in LG number deploys a restricted number of LG at sequential steps in axonal patterning. This enables glia to be in the correct number at discrete time points to enable axon guidance and fasciculation.
The first event in growth cone guidance occurs at the four-cell stage, when LG stop dividing for some time and wait for the pioneer growth cones to extend. At this time, the LG are in the first G1 phase in the lineage. The G1 phase is a characteristic time in which cells respond to growth factors to signal through ERK (Roovers and Assocan, 2000, #86), and in the retina axons approach selectively precursors that are in G1 (Selleck et al, 1992). As the growth cones approach, the two anterior LG (with higher Pros levels) of the four-cell clusters divide in response to Vein. Vein is produced by the MP2 pioneer neurons, which require LG for axon guidance (Hidalgo and Booth, 2000; Hidalgo et al, 2001). By regulating both cell survival and cell proliferation, Vein ensures that LG are present in the correct number to enable growth cone guidance. Pros regulates the zygotic expression of CycE in LG, thus introducing the first G1–S transition, and the fate of the EGFR signalling cells. In this way, Pros modulates the timing of the response of glia to a neuronal signal to divide. Subsequently, the LG continue to divide at times in which axons undergo fasciculation and defasciculation. In this way, LG are deployed in restricted numbers to enable sorting out of axons through time.
Later on, neurons prevent further glial proliferation. Thus, glial number is achieved by the dual response of glia to neuronal signals: earlier on neurons promote glial proliferation and later on they prevent it. At the later stages, Pros confers developmental plasticity by maintaining a subset of the LG in an undifferentiated state, since G1-arrested LG enable cell number adjustment. This confers robustness to the establishment of the axonal bundles through development.
By regulating G1 arrest Pros could enable a repair-like response in glia
We have found that Pros promotes G1 arrest, it prevents both cell cycle exit and terminal differentiation of LG precursors and it maintains them in an immature state, with mitotic potential. Pros-positive LG might be the only ones to divide further during metamorphosis, in the restructuring of the neuropile to form the adult CNS. Or perhaps, Pros-positive LG constitute a population of glial precursors with the capacity to divide further if required, for instance upon variations occurring during development, in response to different environmental conditions or to limited damage.
The maintenance of a subset of the LG in an immature state by Pros allows them to divide further when neurons are ablated (Figure 8B). The increase in LG proliferation upon neuronal ablation resembles an in vivo repair-like response in glia. In vertebrates, neuronal injury causes limited glial overproliferation and spontaneous remyelination (Redwine and Armstrong, 1998; Franklin, 2002; Miller, 2002). The therapeutic implementation of CNS repair will require the manipulation of oligodendrocyte precursors, and the controlled adjustment of their number relative to the regenerating axons. This requires knowledge of what controls oligodendrocyte precursor differentiation and proliferation relative to neuronal contact. Our results have demonstrated that Pros plays this role in Drosophila, and it invites research into the role of Prox1 in oligodendrocyte precursor proliferation and differentiation.
The adult mammalian brain has oligodendrocyte precursors (ffrench-Constant and Raff, 1986; Nunes et al, 2003), and just like dap induces cell cycle exit and terminal differentiation in the LG, p27 and p21 induce cell cycle exit and terminal differentiation of oligodendrocyte precursors (Casaccia-Bonnefil et al, 1997; Durand et al, 1998). In fact, p27 is part of the cell cycle timer that restricts the number of times that oligodendrocyte precursors can divide (Durand and Raff, 2000). Interestingly, Notch maintains the stem cell state in vertebrates (Hitoshi et al, 2002), and we also find that Notch is restricted to the immature LG precursors. Remarkably, activation of Notch in oligodendrocyte precursors by Jagged1 from optic nerve axons prevents oligodendrocyte differentiation (Wang et al, 1998). Perhaps Prox1 could maintain oligodendrocyte precursors in an immature state by regulating Notch and by antagonising p21 and p27. Upon injury or disease in the CNS, Prox1 could be a key molecule coordinating glial cell number and the re-establishment of axonal trajectories to enable repair.
We have shown that Pros plays a fundamental role in adjusting glial cell number to the extending axons during guidance and fasciculation, and in maintaining glial precursors in an undifferentiated state that enables them to respond to neurons. This interactive mechanism provides robustness to the formation of the axonal trajectories, essential for the structural stability of the CNS during development. Our finding that Pros enables the glia to respond to neuronal ablation by overproliferating provokes further research into the potential use of the Drosophila CNS for the study of repair.
Materials and methods
Flies
LG were visualised using the reporter lacZ line F263 (Jacobs et al, 1989). Stocks were generated by conventional genetics to bear F263 in the background. The following mutants were used in the study: (1) prosJ013 F263/TM6BlacZ; (2) veinγ3 F263/TM3lacZ; (3) veinγ4 F263/TM3lacZ (vein mutant embryos were trans-heterozygotes veinγ3 F263/veinγ4 F263); (4) cycEAR95/CyolacZ; (5) dacapo04454/CyOlacZ; F263; (6) htlG4; F263 prosJ013/TM6BlacZ. Targeted ectopic expression was driven in all the LG with GAL4 from stage 12.2 using htlGAL4; F263 flies, which were crossed to flies driving downstream of UAS the following genes: (1) w;UAS prospero; (2) w;UAS cycE; (3) w;UAS dacapo; (4) w;UAScycE UAS Notch-intra (i.e. activated Notch); (5) w;UAS cycE UASnumb; (6) w;UAScycE prosJ013/TM6BlacZ. To drive ectopic expression of cycE in pros mutants, the following progeny embryos were used: htlG4; F263 prosJ013/UAS cycE -pros J013. Targeted neuronal ablation (Hidalgo et al, 1995; Hidalgo and Brand, 1997) was carried out by crossing FTZNGAL4 flies, which drive GAL4 expression in the pioneer neurons and other neurons, to the catalytic subunit of UASRicin A (UFR1.1) flies. To ablate neurons in pros mutant embryos, the following stocks were crossed to each other: (1) FTZNGAL4 prosJ013/TM6BlacZ; (2) UFR1.1; F263 prosJ013/SM6aTM6B. Balancer chromosomes were marked with a reporter lacZ.
Immunohistochemistry
Antibody stainings were performed following a standard protocol (Patel, 1994), except for embryos stained with dpERK or anti-Dap, which were fixed in 8% formaldehyde for 30 min. Antibodies were used in the following dilutions for fluorescence detection (for HRP detection, primary antibody concentrations were halved): rabbit anti-βgal (Cappel) 1:2500; rabbit anti-Repo 1:100; mouse anti-Repo 1:10; mouse anti-Pros 1:1; mouse FasII 1:2; mouse anti-Notch-intra 1: 10 (C17.9C6); rabbit anti-pHistone-H3 1:300; rabbit anti-Heartless 1:500; mouse anti-dpERK 1:50 (Sigma); mouse anti-CyclinA 1:3; mouse anti-CyclinB 1:3; mouse anti-CyclinE 1:1; mouse anti-Dacapo 1:2. As secondary antibodies we used biotinylated antibodies at 1:300 (Jackson and Vector labs) followed by streptavidin Alexa 488, Alexa 594, Alexa 546 and Alexa 660 at 1:250 or the Elite kit (Vector Labs), or directly conjugated to Alexa 488, Alexa 546 and Alexa 594 at 1:250 (Molecular Probes). Embryos were soaked in TOTO-3 (Molecular Probes) at 1:1000 for 10 min in PBS.
BrdU incorporation
Following dechorionation, embryos were soaked in octane for 8 min and then incubated in BrdU (1 mg/ml)/FdU (0.1 mg/ml) (Amersham Pharmacia) in PBS for 1 h at 25°C. Immediately thereafter, the embryos were rinsed and fixed for 20 min in 4% formaldehyde:heptane 1:1. Embryos were then stained for other antibodies following standard procedures. Subsequently, embryos were treated with protease K at 50 μg/ml in PBTritonX for 1 min and 30 s, and the reaction was stopped with 2 mg/ml glycine in PBTritonX. Embryos were fixed again in 4% formaldehyde, and they were then blocked with 10% normal goat serum and 2% BSA prior to incubation with anti-BrdU antibodies (anti-BrdU, clone BU-1 from Amersham Pharmacia with nuclease) for 2 h. Washing and incubation with biotinylated mouse secondary antibody was carried out in blocking solution as above.
Microscopy and imaging
Confocal microscopy was performed using BioRad 1024 and Radiance 2000 laser scanning confocal microscopes. 3-D imaging was carried out using Volocity (Improvision).
Acknowledgments
We thank J Drummond, I Robinson, J Heath and members of our group for advice, discussions and comments on the manuscript; J Fenton for technical assistance; and C Doe, C Goodman, I Hariharan, Iowa Hybridoma Bank, R Jacobs, J Knoblich, C Lehner, F Matsusaki, N Patel, H Richardson, K Saigo, A Travers and H Vaessin for reagents. RG held a BBSRC Studentship and AH a Wellcome Trust CDF, MRC CEG and EMBO YIP.
References
- Akiyama-Oda Y, Hotta Y, Tsukita S, Oda H (2000) Mechanism of glia neuron cell fate switch in the Drosophila thoracic neuroblast 6-4 lineage. Development 127: 3513–3522 [DOI] [PubMed] [Google Scholar]
- Badenhorst P (2001) Tramtrack controls glial number and identity in the Drosophila embryonic CNS. Development 128: 4093–4101 [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu S-Y (2001) The EGFReceptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell 104: 699–708 [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff M (1992) Proliferation of oligodendrocyte precursors depends on electrical activity in axons. Nature 361: 258–260 [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron 12: 935–942 [DOI] [PubMed] [Google Scholar]
- Bate CM (1976) Pioneer neurones in an insect embryo. Nature 260: 54–55 [DOI] [PubMed] [Google Scholar]
- Booth G, Kinrade E, Hidalgo A (2000) Glia maintain follower neuron survival in the Drosophila CNS. Development 127: 237–244 [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V Jr, Chao MV, Koff A (1997) Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27kip1. Genes Dev 11: 2335–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij JC, Letendre MA, Hariharan IK (1996) A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell 87: 1237–1247 [DOI] [PubMed] [Google Scholar]
- Doe CQ, Chu-La-Graff Q, Wright DM, Scott MP (1991) The prospero gene specifies cell fate in the Drosophila central nervous system. Cell 65: 451–464 [DOI] [PubMed] [Google Scholar]
- Durand B, Fero ML, Roberts JM, Raff MC (1998) p27 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr Biol 8: 431–440 [DOI] [PubMed] [Google Scholar]
- Durand B, Gao F-B, Raff M (1997) Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J 16: 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B, Raff M (2000) A cell-intrinsic timer that operates during oligodendrocyte development. BioEssays 22: 64–71 [DOI] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G (2003) Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 34: 53–58 [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Raff MC (1986) Proliferating bipotential glial progenitor cells in the adult rat optic nerve. Nature 319: 499–502 [DOI] [PubMed] [Google Scholar]
- Fields R, Stevens-Graham B (2002) New insights into neuro-glia communication. Science 298: 556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3: 705–714 [DOI] [PubMed] [Google Scholar]
- Freeman M, Doe CQ (2000) Asymmetric Prospero localisation is required to generate mixed neuronal/glial lineages in the Drosophila CNS. Development 128: 4103–4112 [DOI] [PubMed] [Google Scholar]
- Gho M, Bellaiche Y, Schweisguth F (1999) Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development 126: 3573–3584 [DOI] [PubMed] [Google Scholar]
- Hamburger V, Levi-Montalcini RJ (1949) Proliferation, differentiation and degeneration in the spinal ganglion of the chick embryo under normal and experimental conditions. J Exp Zool 111: 457–502 [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Booth GE (2000) Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS. Development 127: 393–402 [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Brand AH (1997) Targeted neuronal ablation: the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development 124: 3253–3262 [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Kinrade EFV, Georgiou M (2001) The Drosophila Neuregulin Vein maintains glial survival during axon guidance in the CNS. Dev Cell 1: 679–690 [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Urban J, Brand AH (1995) Targeted ablation of glia disrupts axon tract formation in the Drosophila CNS. Development 121: 3703–3712 [DOI] [PubMed] [Google Scholar]
- Hirata J, Nakagoshi H, Nabeshima Y, Matsuzaki F (1995) Asymmetric segregation of the homeodomain protein Prospero during Drosophila development. Nature 377: 627–630 [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D (2002) Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16: 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R, Hiromi Y, Patel NH, Goodman CS (1989) Lineage, migration and morphogenesis of longitudinal glia in the Drosophila CNS as revealed by a molecular lineage marker. Neuron 2: 1621–1635 [DOI] [PubMed] [Google Scholar]
- Jan Y-N, Jan LY (2001) Asymmetric cell division in the Drosophila nervous system. Nat Rev Neurosci 2: 772–779 [DOI] [PubMed] [Google Scholar]
- Kinrade EFV, Brates T, Tear G, Hidalgo A (2001) Roundabout signalling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development 128: 207–216 [DOI] [PubMed] [Google Scholar]
- Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H (1996) Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87: 1225–1235 [DOI] [PubMed] [Google Scholar]
- Li L, Vaessin H (2000) Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev 14: 147–151 [PMC free article] [PubMed] [Google Scholar]
- Liu T-H, Li L, Vaessin H (2002) Transcription of the Drosophila CK1 gene dacapo is regulated by a modular array of cis-regulatory sequences. Mech Dev 112: 25–36 [DOI] [PubMed] [Google Scholar]
- Manning L, Doe CQ (1999) Prospero distinguishes sibling cell fate without asymmetric localization in the Drosophila adult external sense organ lineage. Development 126: 2063–2071 [DOI] [PubMed] [Google Scholar]
- Miller RH (2002) Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol 67: 451–467 [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung M, Goodman RR, McKhann G II, Jiang L, Kang J, Nedergaard M, Goldman SA (2003) Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9: 439–447 [DOI] [PubMed] [Google Scholar]
- Patel NH (1994) Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. In Drosophila melanogaster: Practical Uses in Cell and Molecular Biology, Goldstein LSB, Fyrberg EA (eds) Vol. 44, pp 446–485. San Diego: Academic Press [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD (1993) Programmed cell death and the control of cell survival: lessons from the nervous system. Science 262: 695–700 [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero M, Santos-Benito F, Avila J (2000) Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25: 425–435 [DOI] [PubMed] [Google Scholar]
- Redwine J, Armstrong R (1998) In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol 37: 413–428 [DOI] [PubMed] [Google Scholar]
- Roovers K, Assoian RK (2000) Integrating the MAP kinase signal into the G1 phase cell cycle machinery. BioEssays 22: 818–826 [DOI] [PubMed] [Google Scholar]
- Sauer K, Knoblich JA, Richardson H, Lehner CF (1995) Distinct modes of cyclin E/cdc2 kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev 9: 1327–1339 [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM (1997) The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol 189: 186–204 [DOI] [PubMed] [Google Scholar]
- Schwab ME (2002) Repairing the injured spinal cord. Science 295: 1029–1031 [DOI] [PubMed] [Google Scholar]
- Selleck SB, Gonzalez C, Glover DM, White K (1992) Regulation of the G1–S transition in postembryonic neuronal precursors by axon ingrowth. Nature 355: 253–255 [DOI] [PubMed] [Google Scholar]
- Silver J, Lorenz SE, Wahlstein D, Coughlin J (1982) Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies in vivo on the role of the preformed glial pathways. J Comp Neurol 210: 10–29 [DOI] [PubMed] [Google Scholar]
- Tear G (1999) Neuronal guidance: a genetic perspective. Trends Genet 15: 113–118 [DOI] [PubMed] [Google Scholar]
- Vaessin H, Grell E, Wolff E, Bier E, Jan LY, Jan YN (1991) prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell 67: 941–953 [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofsiger D (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21: 63–75 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G (1999) Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet 21: 318–322 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF (2002) Neurofibromas in NF1: Schwann cell origin and the role of tumor environment. Science 296: 920–922 [DOI] [PMC free article] [PubMed] [Google Scholar]


