Abstract
When face processing studies find sex differences, male infants appear better at face recognition than female infants, whereas female adults appear better at face recognition than male adults. Both female infants and adults, however, discriminate emotional expressions better than males. To investigate if sex and age differences in facial scanning might account for these processing discrepancies, 3–4-month-olds, 9–10-month-olds, and adults viewed faces presented individually while an eye tracker recorded eye movements. Regardless of age, males shifted fixations between internal and external facial features more than females, suggesting more holistic processing. Females shifted fixations between internal facial features more than males, suggesting more second-order relational processing, which may explain females’ emotion discrimination advantage. Older male infants made more fixations than older female infants. Female adults made more fixations for shorter fixation durations than male adults. Male infants and female adults’ greater encoding of facial information may explain their face recognition advantage.
Keywords: face perception, eye fixations, sex differences, age differences, race
There is an intriguing developmental discrepancy in female and male face processing abilities. When sex differences are found in face recognition tasks, female children and adults show an advantage over male children and adults (Herlitz & Rehnman, 2008), but male infants show an advantage over female infants (Cashon & Cohen, 2004; Pascalis, de Haan, Nelson, & de Schonen, 1998). In comparison, regardless of age, females are more sensitive to differences in emotional expressions compared to males (McClure, 2000). Given the importance of face recognition and emotion processing for social interactions, we investigated mechanisms underlying the sex differences.
Processing emotional expressions entails extracting sufficient information about second-order relations (Deruelle & de Schonen, 1998), such as encoding the shape of and spacing between internal (localized) facial features (Maurer, Le Grand, & Mondloch, 2002). Females’ advantage in discriminating emotional expressions (McClure, 2000) may occur because they scan second-order relations more so than males. Sex differences in hemispheric activation during face processing support this hypothesis. Data from infants, children, and adults show females and males rely relatively equally on the right hemisphere (RH) during face processing, but females rely more on the left hemisphere (LH) than males, and LH activity reflects localized processing (de Schonen & Mathivet, 1990; Everhart, Shucard, Quatrin, & Shucard, 2001; Godard & Fiori, 2010).
RH activity reflects gestalt-like processing (Deruelle & de Schonen, 1998; Everhart et al., 2001; Godard & Fiori, 2010). Compared to females, males’ lower reliance on the LH suggests they engage in more holistic processing (i.e., integrating the internal facial features and the internal and external facial features as a gestalt rather than as individual parts; Maurer et al., 2002). This hypothesis helps explain results showing male infants’ advantage in face recognition. For example, Pascalis et al. (1998) familiarized 3-month-olds to a face shown in varying poses and found that only males recognized the familiar face when tested with a new pose. Cashon and Cohen (2004) familiarized 7-month-olds to two different faces and found that females showed less interest in a novel combination face (a face containing the internal features of one of the familiarized faces and the external features of the other familiarized face) than did the males. Successful performance on these tasks required adequately encoding the gestalt of the face.
Male infants may also have an advantage in face recognition because young infants may rely more on external features for face recognition than older children and adults. Four-month-olds scan external features for more than 1/3 of their time viewing a face (Gallay, Baudouin, Durand, Lemoine, & Lécuyer, 2006), and this attention seems essential for their face recognition. Newborns recognize faces when external features remain present during both the habituation and test trials, but the presence or absence of internal features changes between the habituation and test trials. Newborns, however, do not recognize faces when internal features remain present, but the presence or absence of external features changes between the habituation and test trials (Turati, Cassia, Simion, & Leo, 2006). This external feature recognition bias is also evident among 5-month-olds (Rose, Jankowski, & Feldman, 2008). For successful face recognition, both second-order relational and holistic processing are critical for adults (Maurer et al., 2002), but holistic processing may be particularly important for young infants who rely more on encoding external features.
When scanning faces, holistic processing may be evidenced by sequential shifts in fixations between internal features (e.g., eyes, eyebrows, nose, and mouth) and between internal and external features (e.g., hairline, jaw, ears), whereas second-order relational processing may be evidenced by sequential shifts in fixations between internal features only. For both infants and adults, we investigated whether males show more internal-external fixation shifts and less internal-internal fixation shifts than females. If males show more internal-external fixation shifts than females, it may be particularly advantageous for face recognition during infancy. We examined if this was a plausible explanation by investigating whether young infants scanned external features more than older infants and adults.
By later childhood and adulthood, females’ advantage over males in face recognition (Herlitz & Rehnman, 2008) may occur due to age-related changes. Godard & Fiori (2010) proposed that female adults’ less lateralized face processing results in concurrent processing of localized and configural information, whereas males switch from one type of processing to the other. For females’ concurrent processing to occur, the two hemispheres need to effectively communicate with one another via the corpus callosum, so such processing may not be efficient until childhood when corpus callosum myelination begins to show substantial increases (Durston et al., 2001). Efficient processing is related to shorter fixation durations because more information can be encoded (via more fixations) within a particular timeframe (Colombo, Mitchell, Coldren, & Freeseman, 1991; Rose, Jankowski, & Feldman, 2002). We therefore investigated whether female adults showed more fixations for shorter durations than male adults.
To conduct these investigations, 3–4-month-olds, 9–10-month-olds, and adults viewed individual faces while we recorded their eye movements. We included infants from different age groups because holistic and second-order relational processing emerges between 3 and 5 months of age (Bhatt, Bertin, Hayden, & Reed, 2005; Cashon & Cohen, 2004; Turati, Di Giorgio, Bardi, & Simion, 2010) and becomes refined during the second half of the first year (Schwarzer, Zauner, & Jovanic, 2007). Female and male faces from familiar and unfamiliar races were used as stimuli to determine if sex differences in scanning generalized across different types of faces that infants and adults often process differently (Ferguson, Kulkofsky, Cashon, & Casasola, 2009; Herlitz & Rehnman, 2008; Michel, Rossion, Han, Chung, & Caldara, 2006; Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002; Rhodes, Hayward, & Winkler, 2006).
Method
Participants
Caucasian 3–4-month-olds (N= 21, 13 females), 9–10-month-olds (N= 20, 11 females), and adults aged 18–24 years (N= 29, 16 females) were included in the analyses. All infants had Caucasian female primary caregivers and most adults reported having predominant experience with Caucasian faces. Research assistants used birth information from local newspapers and marketing lists to determine infant eligibility and sent letters followed up with telephone calls to schedule appointments. Adults were recruited through the psychology subject pool. Additional infants’ data were deleted due to: fussiness (n= 16); insufficient data1 (n= 9); developmental delay or born earlier than three weeks prior to their due date (n= 3); and parent interaction (n= 1).
Stimuli
Stimuli included 24 (12 female, 12 male) digitized, color photographs of African, Asian, Caucasian, and Hispanic faces aged 18 to 35 years (M = 20.05, SD = 1.95), providing 3 familiar race females, 3 familiar race males, 9 unfamiliar race females, and 9 unfamiliar race males.2 Using standardized procedures, we photographed individuals who gave permission for their faces to be used for research. Faces were posed with neutral expressions and were without facial hair, glasses, or jewelry. Clothing cues were masked with a white sheet. Research assistants used Adobe Photoshop to standardize image size and background. At a viewing distance of 76 cm, each 23 × 23 cm (450 × 450 pixels) stimulus subtended a 17.2 × 17.2° visual angle.
Apparatus
Infants sat in a car seat (with pillow support if needed) secured to a chair and adults sat in a chair in front of a 94 cm LCD monitor (81.8 cm × 46 cm with 1024 ×768 resolution) that displayed the stimulus faces. A black wooden structure and a curtain or divider occluded other objects in the room. Gazetracker™ Image Analysis software presented the stimuli and recorded data. Infants’ eye movements were measured using Applied Science Laboratories’ (ASL) 504 remote eye tracker system, the Model 5000 or 6000 control unit, a pan/tilt eye camera optics module, and a magnetic head tracker. Adults’ eye movements were measured using ASL’s Eye-Trac6 system, the Model 6000 control unit, and D6 desk mounted optics.
Participants sat approximately 61 cm from the eye tracker, which measured participants’ pupil-corneal reflection at a frequency of 60 Hz. The accuracy of both eye-tracking systems is 0.5°, which approximates to a 0.5 cm area on the screen with a viewing distance of up to 100 cm. It can track head movements within one square foot. The visual range is 50° horizontally and 40° vertically. The experimenter viewed the stimuli and participants’ gaze via monitors.
Procedure
Before the study began, an experimenter explained the procedure to the parent(s) of the infant or to the adult participant and obtained consent and voluntary demographic information. During the study, participants faced toward the stimulus monitor in a dimly lit room. For infant participants, parents sat behind their infant and the experimenter asked parents to not interact with their infant to avoid influencing looking behavior.
To calibrate infants’ right eye movements, the experimenter played a video featuring puppets and babies dancing to music. As the video played, the image size shrunk to a 7 cm × 7 cm cube for 5 s, causing infants to attend toward one of four calibration points located in the upper-middle, center-left, center-right, or bottom-middle of the stimulus monitor. To calibrate adults’ right eye movements, the experimenter asked adults to look at each of the four calibration points. For data collection to occur, the experimenter needed to calibrate at least three of the four points with a participant’s looks. The experimenter presented the points a second time to test calibration quality. If gaze was outside the points or no gaze was located, the experimenter repeated the calibration procedure. Experimenters reported calibration quality to be excellent for 77% of participants and fair for 23% of participants included in the analyses.
Following calibration, participants viewed the stimulus faces in randomized orders. Each stimulus face was shown once during the 24 trials and displayed in the center of the screen individually for 5 seconds. A clown’s face (387 × 366 pixels) accompanied by a chime appeared after every fourth face and stayed on screen until participants attended to it. A blue screen displayed for 100 ms between each face.
Data Analyses
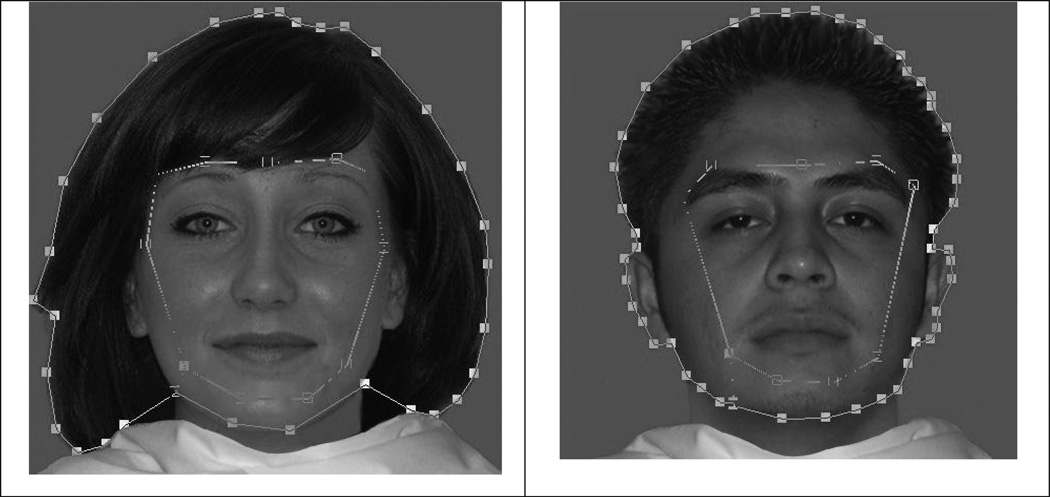
Using Gazetracker, we created areas of interest(AOIs) for internal/external features for each stimulus face following Cashon and Cohen’s (2004) definition. See Figure 1. The area of the external AOIs was naturally larger (M= 144,555 pixels, SE= 4,357) than the internal AOIs (M= 49,350 pixels, SE= 1,139). The area of the internal AOIs did not significantly differ based on face type. The area of the external AOIs significantly differed by sex (p< .0001). Females’ typically greater amount of hair meant their external AOIs (M= 159,387 pixels, SE= 5,520) were larger than male external AOIs (M= 129,724 pixels, SE= 2,985). To maintain ecological validity, we chose not to alter our stimuli, but kept this difference in mind for interpreting results.
Figure 1.
Examples of the internal and external AOIs for two faces used in the study. Facial features located within the inner outline represent the internal AOI. Facial features located outside the inner outline, but within the outer outline, represent the external AOI.
Participants needed to make at least one fixation to the face during the trial for those data to be included in the analyses. Similar to other infant eye tracking research (Liu et al., 2010), we defined a fixation as having a minimum duration of 100 ms within a diameter of 40 pixels. Fixations greater than 1000 ms may be due to tracker loss and were excluded (Pickering & Frisson, 2001). These criteria provided useable data from 1492 out of 1680 trials.
For all analyses, we performed 3 × 2 × 2 × 2 (age group [3–4-month-olds, 9–10-month-olds, adults] × participant sex [female, male] × stimulus race familiarity [familiar, unfamiliar] × stimulus sex [female, male]) mixed linear model analyses with repeated measures. We used differences in least squares means with Tukey-Kramer adjustments to decompose significant interactions. Data were unbalanced, so we report least squares means (SAS Institute Inc., 2008).
Because we were interested in participants’ scanning of the whole face in general and the internal and external features in particular, whole face analyses examined the percentage of total looking time (PTLT included fixations and saccades) participants directed to the whole face (internal and external AOIs) relative to the total time tracked, the number of fixations directed toward each face, and the average fixation duration for each face. Internal/external AOI analyses examined the PTLT directed toward the internal AOI relative to total time looking at the whole face, the percentage of internal-external fixation shifts, and the percentage of internal-internal fixation shifts. These percentages were based on the total of all sequential fixation shifts between AOIs and within both the internal and external AOIs. Not all participants who fixated the face showed sequential fixation shifts, so there were 1404 useable trials for the shift analyses.
Results
Whole Face Analyses
Table 1 provides the means, standard error, and summary of findings for the significant age group × sex interactions found for each dependent variable in the whole face analyses.
Table 1.
Whole Face Analyses Results: Means, Standard Error, and Summary of Findings for the Age Group × Participant Sex Interactions
| Dependent variable and participant sex |
3–4-mo-olds M (SE) |
9–10-mo-olds M (SE) |
Adults M (SE) |
Summary of significant findings for age group × participant sex interactions |
|---|---|---|---|---|
| Face PTLT | 9–10-mo-old males & adults > 3–4-mo-old females | |||
| Females | 56.70 (1.46) | 66.28 (1.74) | 82.43 (1.26) | 9–10-mo-old males & adults > 3–4-mo-old males |
| Males | 62.25 (1.89) | 72.32 (1.90) | 78.92 (1.42) | adults > 9–10-mo-old females |
| Females & males | 59.47 (1.19) | 69.30 (1.29) | 80.68 (0.95) | adult females > 9–10-mo-old males |
| Number of fixations | ||||
| Females | 7.53 (0.29) | 6.77 (0.35) | 12.51 (0.26) | adults > 3–4-mo-olds & 9–10-mo-olds |
| Males | 7.17 (0.38) | 8.34 (0.38) | 11.04 (0.29) | 9–10-mo-old males > 9–10-mo-old females |
| Females & males | 7.35 (0.24) | 7.56 (0.26) | 11.77 (0.198) | adult females > adult males |
| Average fixation duration (s) | ||||
| Females | 0.15 (0.006) | 0.14 (0.007) | 0.27 (0.005) | adults > 3–4-mo-olds & 9–10-mo-olds |
| Males | 0.15 (0.008) | 0.15 (0.008) | 0.31 (0.006) | adult males > adult females |
| Females & males | 0.15 (0.005) | 0.15 (0.005) | 0.29 (0.004) |
Percentage of total looking time (PTLT) directed toward the faces
There was a main effect for age group, F(2, 64)= 98.92, p< .0001, ω2= 0.82, and a main effect for participant sex, F(1, 64)= 4.10, p< .05, ω2= 0.13, that were superseded by an age group × participant sex interaction, F(2, 64)= 6.48, p< .005, ω2= 0.20. The 3–4-month-old females showed a lower PTLT toward the faces than 9–10-month-old females, t(64)= −4.22, 9–10-month-old males, t(64)= −6.53, adult females, t(64)= −13.36, and adult males, t(64)= −10.93, ps ≤ .001. The 3–4-month-old males showed a lower PTLT toward the faces than 9–10-month-old males, t(64)= −3.76, adult females, t(64)= −8.87, and adult males, t(64)= −7.05, ps ≤ .005. In addition, 9–10-month-old females showed a lower PTLT than adult females, t(64)= −7.51, and adult males, t(64)= −5.63, ps ≤ .0001. The 9–10-month-old males showed a lower PTLT than female adults only, t(64)= −4.44, p< .001, but trended toward showing a lower PTLT than adult males, p= .07. Within each age group, there were no significant differences in PTLT based on participant sex, ps> .10.
Number of fixations
There was a main effect for age group, F(2, 64)= 137.44, p< .0001, ω2= 0.86, that was superseded by an age group × participant sex interaction, F(2, 64)= 11.01, p< .0001, ω2= 0.32. Adults made more fixations toward the faces than 9–10-month-olds, t(64)= −13.03, and 3–4-month-olds, t(64)= −14.33, ps< .0001, whereas the two infant groups did not differ, p > .10. Within age groups, adult females made more fixations than adult males, t(64)= 3.84, p< .005, and 9–10-month-old males made more fixations than 9–10-month-old females, t(64)= −3.00, p< .05. There was no difference in number of fixations 3–4-month-old females and males made.
Average fixation duration
There was a main effect for age group, F(2, 64)= 395.42, p< .0001, ω2= 0.95, and a main effect for participant sex, F(1, 64)= 10.30, p< .005, ω2= 0.30, that were superseded by an age group × participant sex interaction, F(2, 64)= 4.57, p< .05, ω2= 0.14. Adults showed longer fixation durations than 9–10-month-olds, t(64)= −22.92, p< .0001, and 3–4-month-olds, t(64)= −23.60, p< .0001, whereas the two infant groups did not differ, p > .10. Within age groups, adult females showed shorter fixation durations than adult males, t(64)= −4.97, p< .0001, but females and males within the two infant groups did not differ, ps> .10.
Internal/External AOI Analyses
Table 2 provides the means, standard error, and summary of findings for significant main effects and interactions found for each dependent variable in the internal/external AOI analyses.
Table 2.
Internal/External AOI Analyses Results: Means, Standard Error, and Summary of Findings for the Age Group Main Effects, the Age Group × Race Familiarity Interaction, and the Race Familiarity × Stimulus Sex Interactions
| Dependent variable | 3–4-mo-olds M (SE) |
9–10-mo-olds M (SE) |
Adults M (SE) |
Summary of significant findings for main effects of age group and the age group × race familiarity interaction |
|---|---|---|---|---|
| Internal AOI PTLT | 63.04 (1.51) | 82.90 (1.63) | 72.09 (1.20) | 9–10-mo-olds & adults > 3–4-mo-olds |
| 9–10-mo-olds > adults | ||||
| % Internal-internal fixation shifts | 55.94 (2.09) | 73.64 (2.18) | 61.26 (1.55) | 9–10-mo-olds > 3–4-mo-olds & adults |
| % Internal-external fixation shifts | 9–10-mo-olds: familiar race faces > | |||
| Familiar race faces | 21.96 (2.27) | 21.20 (2.38) | 22.88 (1.69) | unfamiliar race faces |
| Unfamiliar race faces | 17.04 (1.29) | 11.47 (1.36) | 22.10 (0.96) | unfamiliar race faces: adults > 3–4- |
| mo-olds > 9–10-mo-olds |
| Dependent variable | Familiar race females |
Familiar race males |
Unfamiliar race females |
Unfamiliar race males |
Summary of significant findings for race familiarity × stimulus sex interactions |
|---|---|---|---|---|---|
| Internal AOI PTLT | 71.70 (2.08) | 69.07 (2.05) | 71.90 (1.19) | 78.03 (1.18) | unfamiliar race males > all other face types |
| % Internal-internal fixation shifts | 65.07 (2.82) | 56.03 (2.75) | 63.14 (1.60) | 70.23 (1.57) | unfamiliar race males > familiar race males & unfamiliar race females |
| % Internal-external fixation shifts | 19.64 (1.77) | 24.38 (1.72) | 17.77 (1.00) | 15.96 (0.98) | familiar race males > unfamiliar race females & unfamiliar race males |
PTLT scanning the internal AOI
There was a main effect for age group, F(2, 64)= 39.99, p< .0001, ω2= 0.64. The 3–4-month-olds spent a lower PTLT scanning internal features (and a higher PTLT scanning external features) relative to 9–10-month-olds, t(64)= −8.94, p< .0001, and adults, t(64)= −4.69, p< .0001. Adults, however, showed a lower PTLT scanning internal features (and a higher PTLT scanning external features) relative to 9–10-month-olds, t(64)= 5.35, p< .0001.
There was also a main effect for race familiarity, F(1, 64)= 7.40, p< .01, ω2= 0.23, that was superseded by a race familiarity × stimulus sex interaction, F(1, 63)= 6.78, p< .05, ω2= 0.21. Participants spent a higher PTLT scanning internal features (and a lower PTLT scanning external features) of unfamiliar race males relative to all other face types: unfamiliar race females, t(63)= −3.65, p< .005, familiar race males, t(63)= 3.79, p< .005, and familiar race females, t(63)= −2.65, p< .05.
Internal-internal fixation shifts
The predicted main effect for participant sex almost reached significance, F(1, 64)= 3.52, p= .065, ω2= 0.11. Females (M= 65.74, SE= 1.47) made a greater percentage of internal-internal fixation shifts than males (M= 61.49, SE= 1.72).
There was a main effect for age group, F(2, 64)= 18.21, p< .0001, ω2= 0.46. The 9–10-month-olds made a greater percentage of internal-internal fixation shifts than 3–4-month-olds, t(64)= −5.86, p< .0001, and adults, t(64)= 4.62, p< .0001. The 3–4-month-olds and adults, however, did not significantly differ in percentage of internal-internal fixation shifts, p > .10.
There was a main effect for race familiarity, F(1, 64)= 7.34, p< .01, ω2= 0.24, that was superseded by a race familiarity × stimulus sex interaction, F(1, 59)= 12.70, p< .001, ω2= 0.37. This interaction occurred because participants made a greater percentage of internal-internal fixation shifts when viewing unfamiliar race males relative to familiar race males, t(59)= 4.40, p< .0005, and unfamiliar race females, t(59)= −3.17, p< .05, but there were no significant differences when viewing familiar race females, p > .10.
Internal-external fixation shifts
There was a main effect for participant sex, F(1, 64)= 7.46, p< .01, ω2= 0.24. Males (M= 21.38, SE= 1.08) made a greater percentage of internal-external fixation shifts than females (M= 17.50, SE= 0.92).
There was a main effect for age group, F(2, 64)= 6.91, p< .005, ω2= 0.23, that was superseded by an age group × race familiarity interaction, F(2, 64)= 3.63, p< .05, ω2= 0.11. The 9–10-month-olds made a greater percentage of internal-external fixation shifts when viewing familiar race faces relative to unfamiliar race faces, t(64)= −3.55, p< .01, whereas 3–4-month-olds and adults’ percentage of internal-external fixation shifts did not significantly differ when viewing familiar and unfamiliar race faces, ps> .10. When viewing unfamiliar race faces, adults made a greater percentage of internal-external fixation shifts than 3–4-month-olds, t(64)= −3.15, p< .05, who made a greater percentage of internal-external fixation shifts than 9–10-month-olds, t(64)= 2.97, p< .05. The age groups did not differ in their percentage of internal-external fixations shifts when viewing familiar race faces ps> .10.
There was a main effect for race familiarity, F(1, 64)= 13.15, p< .001, ω2= 0.37, that was superseded by a race familiarity × stimulus sex interaction, F(1, 59)= 5.34, p< .05, ω2= 0.18. Participants showed a greater percentage of internal-external fixation shifts for familiar race males than unfamiliar race males, t(59)= −4.25, p< .0005, and unfamiliar race females, t(59)= −3.32, p< .01. Participants’ percentage of internal-external fixation shifts for familiar race females did not significantly differ from other stimuli, ps> .10.
Discussion
This study examined if sex differences in facial scanning during infancy and adulthood served as a mechanism to explain sex differences in face processing abilities. As predicted, differences in how the two sexes shifted their fixations were consistent during infancy and adulthood. Males made more internal-external fixation shifts than females, suggesting more holistic processing of faces, whereas females showed a trend toward more internal-internal fixation shifts than males, suggesting more second-order relational processing of faces. These differences did not interact with face type, suggesting that sex differences in facial scanning occur regardless of the face’s sex or race familiarity. Females’ scanning patterns appear advantageous for discriminating emotional expressions during infancy and adulthood. Males’ scanning patterns appear advantageous for face recognition during infancy, but not necessarily during adulthood.
Our findings provide potential explanations for why males shift from being more skilled than females at face recognition during infancy to being less skilled than females at face recognition during adulthood. Compared to females, males’ greater percentage of internal-external fixation shifts appears advantageous for face recognition at 3–4 months of age when they attend more to external facial features than 9–10-month-olds and adults. Males’ early advantage in face recognition was also evidenced in their showing more fixations toward faces than females at 9–10 months. By adulthood, however, females made more fixations and showed shorter fixation durations than males, but there were no sex differences in the percentage of time adults attended to faces. Females therefore encoded more information during this timeframe, which may explain female adults’ greater advantage in recognizing faces compared to male adults (Herlitz & Rehnman, 2008). Female adults were more efficient at scanning faces than male adults, perhaps due to their ability to simultaneously process localized and configural information (Godard & Fiori, 2010).
Other age group differences showed that attention toward faces increased with age, as would be expected with development. Unexpectedly, 9–10-month-olds spent more time scanning internal features and showed more internal-internal fixation shifts than adults. One possible reason for 9–10-month-olds’ particularly heightened attention toward internal features is that infants typically begin to locomote independently around this age, their fear system develops, and they increase their attention to distal persons and their social referencing (Braungart-Rieker, Hill-Soderlund, & Karrass, 2010; Campos et al., 2000; Kutsuki et al., 2007).
Nine-10-month-olds, but not 3–4-month-olds, also showed significantly fewer internal-external fixation shifts when viewing unfamiliar race faces compared to when viewing familiar race faces. These data complement Ferguson et al.’s (2009) finding that 8-month-olds holistically process familiar race faces, but not unfamiliar race faces, whereas 4-month-olds holistically process both familiar and unfamiliar race faces. Such scanning differences likely contribute to 9–10-month-olds’ better recognition of familiar than unfamiliar race faces (Kelly et al., 2007).
Interestingly, when viewing unfamiliar race males, all age groups attended more toward internal features compared to other face types and made more internal-internal fixation shifts compared to unfamiliar race females and familiar race males.3 Participants also made less internal-external fixation shifts when viewing unfamiliar race faces compared to familiar race males, which fits with other research showing less holistic processing of unfamiliar race faces (Ferguson et al., 2009; Michel et al., 2006). Spending additional time attending to features that provide information about eye gaze and emotional expression may be adaptive when the person’s face type is less familiar and expectations about behavior are uncertain. Race familiarity may make perceivers more comfortable shifting their attention away from internal features toward external features. Ease in shifting attention could also result from having better-developed top-down representations of familiar race faces than unfamiliar race faces.
Importantly, participants’ percentage of internal-external and internal-internal fixation shifts when viewing familiar race females were not at either of the extreme ends of processing (i.e., not significantly higher or lower than when viewing other face types), but rather somewhere in between. This happy medium may be what is most ideal for face processing given the advantages infants and adults show in recognizing exemplars of familiar race female faces (Lovén et al., 2012; Quinn et al., 2002).
This study demonstrated differences in females and males’ fixation shifts when scanning faces, regardless of participant age or face type. The number of fixations participants directed toward faces also varied as a function of their sex and age. Work investigating facial scanning during emotional expression discrimination and face recognition tasks is needed to verify that these sex and age differences are related to performance. It is also important to investigate when and why females become more efficient at encoding facial information than males.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development [HD48467]; the National Science Foundation [0645761]; and the College of Liberal Arts Center for Advanced Research at the University of Nevada, Las Vegas.
We thank Noelle Lefforge for comments on a previous version of this manuscript.
Footnotes
Portions of these data were previously presented at the International Conference on Infant Studies in Vancouver, B.C. in March 2008.
Participants needed to provide eye-tracking data for at least one face from the familiar race females, familiar race males, unfamiliar race females, and unfamiliar race males, and from each of the four races for their data to be included in analyses.
Although the design led to an imbalance in the number of familiar and unfamiliar race faces displayed, it was intended to provide participants from the four most locally represented racial groups an opportunity to view both familiar and unfamiliar race faces during the study. Unfortunately, our samples of African, Asian, and Hispanic participants are not yet large enough for comparison to the Caucasian participants.
Although Liu et al. (2010) found that attention toward internal features of unfamiliar races decreased from 4 to 9 months of age, the faces in their study were shown for 30 s via video, whereas the faces in our study were shown for 5 s via static photographs. Moreover, the internal AOIs were defined differently in the two studies and their findings applied to Asian infants viewing unfamiliar race females, whereas ours applied to Caucasian infants viewing unfamiliar race males. Given these differences, and evidence that Asian and Caucasian adults scan faces differently (Blais, Jack, Scheepers, Fiset, & Caldara, 2008), one or more of these factors could contribute to the differences in results.
References
- Bhatt RS, Bertin E, Hayden A, Reed A. Face processing in infancy: Developmental changes in different kinds of relational information. Child Development. 2005;76(1):169–181. doi: 10.1111/j.1467-8624.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- Blais C, Jack RE, Scheepers C, Fiset D, Caldara R. Culture shapes how we look at faces. PLoS One. 2008;3(8):e3022, 1–8. doi: 10.1371/journal.pone.0003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, Karrass J. Fear and anger reactivity trajectories from 4 to 16 months: The roles of temperament, regulation, and maternal sensitivity. Developmental Psychology. 2010;46(4):791–804. doi: 10.1037/a0019673. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel broadens the mind. Infancy. 2000;1(2):149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Cashon CH, Cohen LB. Beyond U-shaped development in infants’ processing of faces: An information-processing account. Journal of Cognition and Development. 2004;5(1):59–80. [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Differences in infant visual attention: Are short lookers faster processors or feature processors. Child Development. 1991;62(6):1247–1257. [PubMed] [Google Scholar]
- de Schonen S, Mathivet E. Hemispheric asymmetry in a face discrimination task in infants. Child Development. 1990;61(4):1192–1205. [PubMed] [Google Scholar]
- Deruelle C, de Schonen S. Do the right and left hemispheres attend to the same visuospatial information within a face in infancy? Developmental Neuropsychology. 1998;14(4):535–554. [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: What have we learned? Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Everhart DE, Shucard JL, Quatrin T, Shucard DW. Sex-related differences in event-related potentials, face recognition, and facial affect processing in prepubertial children. Neuropsychology. 2001;15(3):329–341. doi: 10.1037//0894-4105.15.3.329. [DOI] [PubMed] [Google Scholar]
- Ferguson KT, Kulkofsky S, Cashon CH, Casasola M. The development of specialized processing of own-race faces in infancy. Infancy. 2009;14(3):263–284. doi: 10.1080/15250000902839369. [DOI] [PubMed] [Google Scholar]
- Gallay M, Baudouin J, Durand K, Lemoine C, Lécuyer R. Qualitative differences in the exploration of upright and upside-down faces in four-month-old infants: An eye-movement study. Child Development. 2006;77:984–996. doi: 10.1111/j.1467-8624.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- Godard O, Fiori N. Sex differences in face processing: Are women less lateralized and faster than men? Brain and Cognition. 2010;73(3):167–175. doi: 10.1016/j.bandc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Rehnman J. Sex differences in episodic memory. Current Directions in Psychological Science. 2008;17(1):52–56. [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuki A, Egami S, Ogura T, Kuroki M, Itakura S, Nakagawa K. Developmental changes of referential looks in 7- and 9-month-olds: A transition from dyadic to proto-referential looks. Psychologia: An International Journal of Psychology in the Orient. 2007;50(4):319–329. [Google Scholar]
- Liu S, Quinn PC, Wheeler A, Xiao N, Ge L, Lee K. Similarity and difference in processing of same- and other-race faces as revealed by eye tracking in 4- to 9-month-olds. Journal of Experimental Child Psychology. 2010;108(1):180–189. doi: 10.1016/j.jecp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovén J, Rehnman J, Wiens S, Lindholm T, Peira N, Herlitz A. Who are you looking at? The influence of face gender on visual attention and memory for own- and other-race faces. Memory. 2012;20(4):321–331. doi: 10.1080/09658211.2012.658064. [DOI] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Sciences. 2002;6(6):255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression and processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126(3):424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- Michel C, Rossion B, Han J, Chung C, Caldara R. Holistic processing is finely tuned for faces of one’s own race. Psychological Science. 2006;17(7):608–615. doi: 10.1111/j.1467-9280.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24(1):249–260. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Pickering MJ, Frisson S. Processing ambiguous verbs: Evidence from eye movements. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27(2):556–573. doi: 10.1037/0278-7393.27.2.556. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Hayward WG, Winkler C. Expert face coding: Configural and component coding of own-race and other-race faces. Psychonomic Bulletin & Review. 2006;13(3):499–505. doi: 10.3758/bf03193876. [DOI] [PubMed] [Google Scholar]
- Rose SA, Jankowski JJ, Feldman JF. Speed of processing and face recognition at 7 and 12 months. Infancy. 2002;3:435–455. [Google Scholar]
- Rose SA, Jankowski JJ, Feldman JF. The inversion effect in infancy: The role of internal and external features. Infant Behavior & Development. 2008;31(3):470–480. doi: 10.1016/j.infbeh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.; 2008. [Google Scholar]
- Schwarzer G, Zauner N, Jovanovic B. Evidence of a shift from featural to configural processing in infancy. Developmental Science. 2007;10(4):452–463. doi: 10.1111/j.1467-7687.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Turati C, Cassia VM, Simion F, Leo I. Newborns’ face recognition: Role of inner and outer facial features. Child Development. 2006;77:297–311. doi: 10.1111/j.1467-8624.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Turati C, Di Giorgio E, Bardi L, Simion F. Holistic face processing in newborns. 3-month-old infants, and adults: Evidence from the composite face effect. Child Development. 2010;81(6):1894–1905. doi: 10.1111/j.1467-8624.2010.01520.x. [DOI] [PubMed] [Google Scholar]