Abstract
Background
Hepatic abscesses are uncommon in nonhuman primates and usually occur as multifocal microabscesses originating from bacteremia.
Methods
Necropsy, histopathology, and bacterial cultures were performed on five sub-adult to adult female rhesus macaques (Macaca mulatta) that died spontaneously.
Results
Necropsy findings included cavitating abscesses in the right central liver lobe of all five animals, with intralesional plant material in four animals.
Conclusions
This is the first report of cavitating hepatic abscesses with intralesional plant material in nonhuman primates.
Keywords: Abscess, Foreign-Body Migration, Inhalation, Liver, Plants, Macaca mulatta
Introduction
Bacterial liver abscesses originate from a variety of pathways, including hematogenous spread via the portal vein, hepatic artery, or umbilical veins in neonates; ascending bacteria via the biliary system; parasitic migration; and direct extension from inflammatory processes in nearby tissues, including migrating foreign bodies [5]. Hepatic abscesses are considered common in domestic species, including neonatal foals, small ruminants, and feedlot cattle [5]. In nonhuman primates, large hepatic abscesses are rarely reported and are typically the result of hematogenous seeding of bacteria that enter the liver via the portal vein [5]. Hepatic microabscesses are reported with Francisella tularensis and Yersinia sp. infections [6, 13]. Type D retroviral infection with consequent immunosuppression has been associated with opportunistic bacterial infections of the liver [8].
The purpose of this report is to describe the gross and histologic findings supporting a proposed pathogenesis of large, cavitating hepatic abscesses in the rhesus macaque.
Case Report
Five outdoor-housed female rhesus macaques ranging in age from 3-8 years died spontaneously. Three of the cases presented are recent necropsies performed from 2007-2013; the remaining cases were identified from a retrospective search of necropsy records over a 20 year period. The animals were of Indian geographic origin, born at the Oregon National Primate Research Center (ONPRC), and had no significant medical history. All were part of the ONPRC breeding colony.
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the ONPRC. The ONPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
At necropsy, the animals had no evidence of physical trauma or penetrating thoracic, abdominal or subcutaneous wounds. The primary gross finding in each case was a large cavitating abscess involving the right central liver lobe and measuring up to 5 cm in diameter (Fig.1). The abscesses contained a copious volume of malodorous purulent exudate. Intralesional plant material was identified grossly in the liver of four cases. The plant material varied in length from 2 to 5.5 cm; the morphology was consistent with grass stem, grass awn, or straw. The diaphragm overlying the abscess was tightly adhered, friable and necrotic in three cases; in the two historical cases, the diaphragm was not described. Three cases exhibited acute fibrinous peritonitis; serous abdominal effusion was present in the other two cases. Pneumonia was present in four cases, and these four animals exhibited pleuritis characterized by pleural effusion and fibrinous to fibrous adhesions. A caudal mediastinal abscess was present in one case. Representative samples of major organs were fixed in 10% neutral buffered formalin. Tissues were routinely processed, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E).
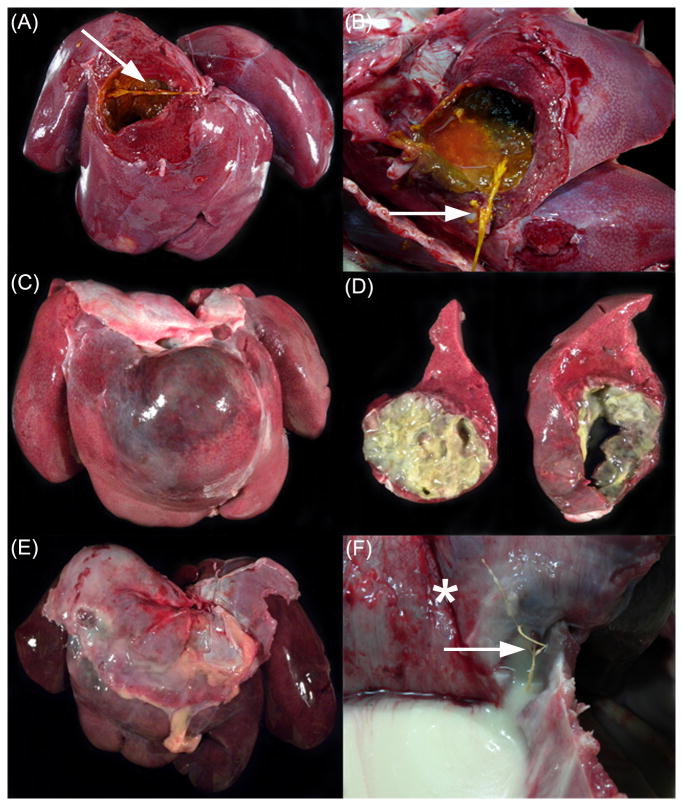
Fig. 1.
Gross hepatic lesions in three rhesus macaques. (A) A large cavitation in the right central lobe, subjacent to the diaphragm contains thin, brown-green exudate. (B) Note the 3 cm long plant foreign body (arrow).
(C) The right central lobe is distorted by a large, bulging abscess. (D) The abscess is filled with copious yellow exudate and the surrounding parenchyma is multifocally necrotic, firm and white.
(E) The liver is markedly enlarged, diffusely congested and friable. The right central lobe is expanded by a large cavitating abscess filled with copious yellow exudate. (F) View from the thoracic cavity. The pleural surface of the diaphragm (*) overlying the right central lobe abscess is firmly adhered to the liver capsule. Note the 5.5 cm long plant foreign body (arrow) protruding from the abscess, through the diaphragm. Purulent exudate from the ruptured hepatic abscess fills the thoracic cavity.
Microbial cultures of liver revealed mixed bacterial populations including Escherichia coli, Staphylococcus aureus, nonhemolytic Streptococcus, gram negative bacillus, nonspecified gram negative bacterium and nonspecified anaerobic bacterium. Lung cultures revealed growth of Neisseria sp., alpha hemolytic Streptococcus, E. coli, S. aureus, and nonspecified anaerobic bacterium. Streptococcus anginosus was isolated on culture of abdominal effusion in one case.
Histologic findings included changes in the liver, the overlying diaphragm, peritoneum, lungs, mediastinum, and pleura. Four cases exhibited chronic-active hepatitis characterized by severe, diffuse necrosuppurative hepatitis with abscess formation, fibrin deposition, and variable numbers of bacterial colonies. The remaining case exhibited severe, diffuse, chronic, necrotizing hepatitis with abundant granulation tissue and fibrosis.
Three cases with sampled diaphragmatic tissue adjacent to the abscess exhibited severe, diffuse, chronic-active transmural pyogranulomatous and lymphoplasmacytic myositis with foreign body-type multinucleated giant cells and fibrosis. In one case, the diaphragm was discontinuous and segmentally replaced by well-organized granulation tissue.
Lung lesions were classified as either necrotizing, fibrinosuppurative bronchointerstitial pneumonia (three cases), or chronic-active lymphoplasmacytic, histiocytic, neutrophilic interstitial pneumonia (one case). All four of these cases exhibited pulmonary edema and hemorrhage. The fifth case had no significant pulmonary pathology, but did have intralesional hepatic plant material. Pulmonary vessel walls in the four cases with lung lesions frequently exhibited fibrinoid necrosis with luminal fibrin thrombi, and in two cases, septic emboli. Multifocal intralesional refractile plant material was detected microscopically under polarized light. One animal displayed a chronic-active lymphoplasmacytic and neutrophilic mediastinitis with few intralesional bacterial colonies, and abundant fibrosis. In three cases, pleural inflammation was characterized by severe, chronic-active, proliferative, fibrinosuppurative lymphoplasmacytic pleuritis, with fibrosis, congestion and acute hemorrhage.
Fibrin thrombi and acute hemorrhage in numerous organs, consistent with disseminated intravascular coagulation, were present in three animals.
Discussion
Hepatic abscesses of varying pathogenesis are well described in the veterinary and human literature. The five cases presented here were relatively young, healthy, and presumptively active female rhesus macaques. These animals were all housed outdoors in corrals where plant material was readily accessible. In four of the five cases presented, there was grossly visible plant material within the hepatic abscess, pinpointing the source of infection, but not the pathway of foreign body migration. Our hypothesis is that the plant material was introduced via inhalation and migrated caudally through the lung and diaphragm, and lodged in the liver.
Inhaled plant material typically either remains at the site of entry, or migrates through tissue [1, 4]. The migratory path tends to be unidirectional and is assisted by the individual's movements, including respiration [1, 3, 7, 9, 10, 14]. There are reports in humans of aspirated grass material migrating to a subcutaneous chest wall location and the pleural cavity overlying the diaphragm [4, 10, 14]. Similarly, there are reports of canine lumbar abscessation with plant foreign bodies and previous histories of coughing, pneumonia, and radiographic pulmonary changes, supporting inhalation as the route of plant material entry, with subsequent migration through the lung and diaphragm to reach the lumbar soft tissue [7]. In this report, one animal had microscopically visible intralesional refractile plant material within the pulmonary parenchyma, supporting a possible pulmonary route of migration.
The proposed pathway for intrahepatic plant material begins with plant aspiration down the trachea and preferentially following the right mainstem bronchus. Inspired material would favor the course of the right mainstem bronchus because this bronchus branches off the trachea in a linear fashion in rhesus macaques; whereas, the left mainstem bronchus branches at an abrupt angle [11]. From there, the plant material migrated caudally through the pulmonary parenchyma, diaphragm, and into the right central liver lobe, where it became locally sequestered. Over time, the lung lesions gradually transitioned to a state of chronic-active interstitial pneumonia and fibrosis, but the body's inability to degrade the plant material and its chronic irritation in the liver, exacerbated by diaphragmatic motion during respiration, created a regionally extensive abscess that resulted in sepsis, as evidenced grossly and microscopically by multi-organ microvascular thrombosis. Intravascular fibrin thrombi and hemorrhage were observed in multiple organs of each animal, suggesting disseminated intravascular coagulopathy leading to cardiovascular collapse as the cause of death.
Pulmonary migration is further supported by the bacterial cultures obtained at necropsy. Several bacterial species isolated from the liver and or lung are considered normal microbial inhabitants of nasopharyngeal region of nonhuman primates, suggesting that the inoculating material passed through the pharyngeal region en route to the liver [2, 12].
To our knowledge, this is the first report describing focal hepatic abscessation following migration of aspirated plant material through the lung and diaphragm to the liver in any species. We propose that evaluation of future cases of right-central liver lobe abscesses in non-human primates include extensive examination of the pulmonary parenchyma, diaphragm and liver for plant foreign bodies.
Acknowledgments
Dr. Michael Axthelm
Funding: This work was supported through funding by the National Institutes of Health Grant P51OD011092.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Baethge BA, Eggerstedt JM, Olash FA., Jr Group F streptococcal empyema from aspiration of a grass inflorescence. Ann Thorac Surg. 1990;49:319–20. doi: 10.1016/0003-4975(90)90162-y. [DOI] [PubMed] [Google Scholar]
- 2.Bowers LC, Purcell JE, Plauché GB, Denoel PA, Lobet Y, Philipp MT. Assessment of the nasopharyngeal bacterial flora of rhesus macaques: moraxella, Neisseria, haemophilus, and other genera. J Clin Microbiol. 2002;40:4340–2. doi: 10.1128/JCM.40.11.4340-4342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan KE, Ihrke PJ. Grass awn migration in dogs and cats: a retrospective study of 182 cases. J Am Vet Med Assoc. 1983;182:1201–4. [PubMed] [Google Scholar]
- 4.Caglar KK, Yeginsu A, Ozer I. Chest wall abscess due to aspiration of grass inflorescence. West Indian Med J. 2007;56:312–4. doi: 10.1590/s0043-31442007000300031. [DOI] [PubMed] [Google Scholar]
- 5.Cullen JM, Brown DL. In: Hepatobiliary and exocrine pancreas In: Pathologic Basis of Veterinary Disease. 5th. McGavin, Zachary, editors. Elsevier Mosby; 2012. pp. 431–434. [Google Scholar]
- 6.Ferrecchia CE, Colgin LM, Andrews KR, Lewis AD. An outbreak of tularemia in a colony of outdoor-housed rhesus macaques (Macaca mulatta) Comp Med. 2012;62:316–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Frendin J, Funkquist B, Hansson K, Lönnemark M, Carlsten J. Diagnostic imaging of foreign body reactions in dogs with diffuse back pain. J Small Anim Pract. 1999;40:278–85. doi: 10.1111/j.1748-5827.1999.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 8.Henrickson RV, Maul DH, Lerche NW, Osborn KG, Lowenstine LJ, Prahalada S, Sever JL, Madden DL, Gardner MB. Clinical features of simian acquired immunodeficiency syndrome (SAIDS) in rhesus monkeys. Lab Anim Sci. 1984;34:140–5. [PubMed] [Google Scholar]
- 9.Hopper BJ, Lester NV, Irwin PJ, Eger CE, Richardson JL. Imaging diagnosis: pneumothorax and focal peritonitis in a dog due to migration of an inhaled grass awn. Vet Radiol Ultrasound. 2004;45:136–8. doi: 10.1111/j.1740-8261.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 10.Karagöz B, Köksal Y, Varan A, Haliloglu M, Ekinci S, Büyükpamukçu M. An unusual case of grass inflorescence aspiration presenting as a chest wall tumour. Pediatr Radiol. 2006;36:434–6. doi: 10.1007/s00247-005-0088-8. [DOI] [PubMed] [Google Scholar]
- 11.Lineback P. The respiratory, digestive, and urinary systems. In: Hartman, Straus, editors. The Anatomy of the Rhesus Monkey (Macaca Mulatta) Hafner Pub. Co.; 1961. pp. 210–229. [Google Scholar]
- 12.Mugisha L, Köndgen S, Kaddu-Mulindwa D, Gaffikin L, Leendertz FH. Nasopharyngeal colonization by potentially pathogenic bacteria found in healthy semi-captive wild-born chimpanzees in Uganda. Am J Primatol. 2014;76:103–10. doi: 10.1002/ajp.22212. [DOI] [PubMed] [Google Scholar]
- 13.Soto E, Griffin M, Verma A, Castillo-Alcala F, Beierschmitt A, Beeler-Marfisi J, Arauz M, Illanes O. An outbreak of Yersinia enterocolitica in a captive colony of African green monkeys (Chlorocebus aethiops sabaeus) in the Caribbean. Comp Med. 2013;63:439–44. [PMC free article] [PubMed] [Google Scholar]
- 14.Steele NB, Hague JR. Migration of inflorescence: complications of grass head aspiration. Am J Dis Child. 1980;134:704–6. doi: 10.1001/archpedi.1980.02130190070019. [DOI] [PubMed] [Google Scholar]