Abstract
Rationale
Smoking lapses (i.e., returns to smoking after quitting) often occur following alcohol consumption with observational data suggesting greater quantities of alcohol lead to greater risk. However, a causal dose-dependent effect of alcohol consumption on smoking lapse behavior has not been established, and the mechanisms that might account for such an effect have not been tested.
Objectives
In a within-subjects design, we examined effects of low (0.4 g/kg) and high (0.8 g/kg) dose alcohol, relative to placebo, on smokers’ ability to resist initiating smoking after acute smoking abstinence.
Methods
Participants were 100 heavy alcohol drinkers, smoking 10–30 cigarettes per day. Across three separate days, participants consumed placebo, low, or high dose alcohol following 3 h of smoking abstinence, and 35 min later were offered the opportunity to smoke while resisting smoking was monetarily reinforced proportional to the amount of time delayed.
Results
Consistent with a dose-response effect, participants smoked 3.35 min (95% CI [−7.09, 0.40], p=.08) earlier following low dose alcohol and 6.36 min (95% CI [−9.99, −2.73], p=.0006) earlier following high dose alcohol compared to drinking a placebo beverage. Effects of dose on smoking behavior were partially mediated by increases in urge to smoke. There was no evidence that alcohol’s effects on urge to smoke or ability to resist smoking were mediated through its stimulating or sedating effects.
Conclusions
Alcohol can reduce the ability to resist smoking in a dose-dependent fashion, in part, due to its effect on increasing the intensity of smoking urges.
Keywords: Alcohol, smoking relapse, craving, urge to smoke, stimulation, alcohol administration
Introduction
Cigarette smokers consume more alcohol than nonsmokers (Anthony and Echeagaray-Wagner, 2000; Chiolero et al., 2006; Falk et al., 2006; Kahler et al., 2008b), and greater alcohol use decreases the odds of smoking cessation both in community samples (Augustson et al., 2008; Dawson, 2000; Dollar et al., 2009; Hymowitz et al., 1997; Kahler et al., 2009; Osler et al., 1999; Sorlie and Kannel, 1990) and in smokers trying to quit (Humfleet et al., 1999; Leeman et al., 2008; Murray et al., 1995; Sherman et al., 1996; Smith et al., 1999). Alcohol appears to precipitate smoking lapses (i.e., a return to smoking after quitting), occurring in about one quarter of initial lapses (Baer and Lichtenstein, 1988; Borland, 1990; Shiffman, 1982) with a temporal association shown in some studies between drinking and a subsequent smoking lapse (Gwaltney et al., 2005; Shiffman and Gwaltney, 2008; Shiffman et al., 1996). The risk of lapsing to smoking on days when light to moderate drinking occurs (1–3 drinks for women, 1–4 drinks for men) is more than four times greater than the risk of lapsing on a nondrinking day, and heavy drinking (4+ drinks for women, 5+ drinks for men) doubles the risk of a lapse compared to moderate drinking, suggesting a dose-response association (Kahler et al., 2010). However, more research is needed to determine (a) whether there is a causal dose-response effect of alcohol consumption on smoking lapse risk and (b) the mechanisms accounting for such an effect.
Controlled alcohol administration studies provide the most direct evidence of alcohol’s effects on smoking lapse risk, but such studies have been rare. McKee et al. (2006) used a laboratory analogue for smoking lapse and found that a priming dose of alcohol (target breath alcohol concentration [BrAC] of 0.03 g/dl) compared to placebo decreased time to initiating smoking when monetary reinforcement was proportionally provided for increasingly greater delay of smoking initiation. In a follow up to that study, we used the same smoking lapse analogue task and a balanced placebo alcohol administration design to separate pharmacologic from stimulus expectancy effects of alcohol on smoking lapse (Kahler et al., 2012). Although expectancy effects of alcohol on smoking were present among women (i.e., women told alcohol smoked sooner than those told placebo), a 0.4 g/kg dose of alcohol did not exert a pharmacologic effect on time to smoke among either men or women and did not increase urge to smoke compared to placebo. These unexpected findings could have resulted from using a lengthy nicotine deprivation period prior to experimental sessions (~15 h); we found that alcohol effects on urge to smoke are stronger when a 3 h deprivation period is used compared to a 15 h period, (Day et al., 2014). Finally, both McKee et al. (2006) and Kahler et al. (2012) used only one dose of alcohol, which was equivalent to about 2 drinks, and neither provided evidence on mechanisms whereby alcohol use might reduce the ability to resist smoking.
Potential Mechanisms of Alcohol Effects on Smoking Lapse Behavior
In this study, we focus on three putative mechanisms that may underlie alcohol-induced smoking lapse risk: urge to smoke, stimulating effects of alcohol, and sedating effects of alcohol. Consistent with studies of alcohol consumption and urge to smoke in the natural environment (e.g., Businelle et al., 2013), administration of alcohol in a lab setting increases urge to smoke (Burton and Tiffany, 1997; Kouri et al., 2004; Sayette et al., 2005) in a dose-dependent fashion where higher doses of alcohol produce greater urge to smoke both during the ascending and descending limbs of the BrAC curve (Epstein et al., 2007; King and Epstein, 2005). Whether increases in urge to smoke account for smoking lapse risk is not known, however.
How alcohol increases urge to smoke is not entirely known. Alcohol can have stimulating properties, possibly reflecting activation of a neurobiological approach motivational system (Gray, 1994; Wise, 1996) that may increase appetitive salience of rewards in general, including smoking (Little, 2000; Ostafin and Palfai, 2006). Consistent with this possibility, the positive association between BrAC and urge to smoke was partially mediated by self-reported stimulation in one study (Epstein et al., 2007). However, no studies have examined whether stimulation resulting from alcohol accounts for its effects on smoking lapse and whether these effects are independent of alcohol’s effects on urge to smoke. It has also been suggested that alcohol may affect smoking behavior because nicotine can partially offset alcohol’s sedating effects (Perkins et al., 1995). However, Epstein et al. (2007) did not find an association between sedation and urge to smoke following administration of low or high dose alcohol.
Aims and Hypotheses
The purpose of the present study was to test the hypothesis that alcohol (0.4 g/kg, 0.8 g/kg) would dose-dependently reduce latency to smoke, relative to placebo, in the smoking lapse analogue task used in the studies cited above. We also tested the hypotheses that alcohol would dose-dependently increase urge to smoke and that this increase would mediate the effects of dose on latency to smoke. Finally, we examined whether self-reported stimulating and sedating effects of alcohol would mediate alcohol’s effects on urge and latency to smoke. Hypotheses were tested using a within-subjects design in which 100 heavy drinking smokers completed 3 beverage administration sessions where they consumed a placebo (a trace amount of alcohol), low dose, or high dose alcohol beverage and then completed the smoking lapse analogue procedure. We experimentally controlled for expectancy effects (i.e., the effect of believing that one is drinking alcohol rather than a non-alcoholic beverage), which may differ by gender (Kahler et al., 2012), by informing participants that their beverage contained alcohol at each session.
Method
Participants
Procedures were approved by the Brown University Institutional Review Board. Participants recruited from the community had to meet the following inclusion criteria: 21 to 65 years of age, smoking 10–30 cigarettes a day, a carbon monoxide (CO) level >10 ppm, current heavy drinking (≥ 5 drinks per occasion for men, ≥ 4 drinks for women) at least twice a month, and report no history or intention to seek alcohol treatment. Exclusion criteria were: using other tobacco products or nicotine replacement therapy, plan to quit smoking in the next month, incapable of abstaining from alcohol for 24 h without significant withdrawal symptoms, current affective disorder or psychotic symptoms, current pregnancy or nursing, illicit drug use on more than four occasions in the past 4 weeks, medical issues or medications contraindicated for alcohol consumption, and weighing greater than 250 lbs.
Potential participants were screened by telephone (N=1427) and if they appeared eligible, were asked to come into the laboratory to complete a baseline interview, at which they signed informed consent. Participants were informed that the study evaluated effects of alcohol on smoking behavior and that they would be consuming beverages containing varying amounts of alcohol at three experimental sessions. Of the 186 potential participants completing a baseline interview, 79 were deemed ineligible at baseline and 7 dropped out prior to completing an alcohol administration session. Results are based on the remaining 100 participants.
Design
Participants took part in a 3-session, within-subjects, repeated measures experimental design in which participants were administered a placebo (trace amount of alcohol), 0.4 g/kg dose or 0.8 g/kg dose of alcohol, with the order of beverage condition randomly assigned. Research assistants were blind to the alcohol content of the beverages.
Procedure
Sessions occurred in an 80 ft2 ventilated smoking room with a one-way mirror window. Participants were instructed to refrain from drinking alcohol for 24 h prior to all study sessions and arrived for each at approximately 11:00 am. On arrival, compliance with alcohol abstinence was confirmed with zero BrAC per an Alco-Sensor IV (Intoximeters, Inc., St Louis, MO, USA). Participants smoked as they normally would prior to each session, and smoked one of their usual-brand cigarettes in the laboratory at 11:30 am. They then consumed a standardized meal consisting of a bagel with cream cheese. At session 1, participants completed a battery of interview and self-report assessments including a diagnostic interview and smoking and alcohol use questions. If eligible, participants continued with the experimental session.
In each experimental session, beverage administration began at 2:30 pm, 3 h after participants had last smoked. Beverages were prepared by the study coordinator who randomized participants to the alcohol dose conditions but had no contact with study participants. The study coordinator prepared drinks according to weight and sex-adjusted measurements (0.4 g/kg or 0.8 g/kg: 90% of this dose was given to women, per Friel et al., 1999), and research assistants brought the pre-made drinks into the testing room for the participants. Participants drank vodka, tonic water, and lime juice in a mixed drink, and the volume of each drink remained the same for each session; only the relative amount of vodka was changed.
In all conditions, the beverage was divided into three glasses in equal-sized portions. Participants were instructed to consume each glass within 5 min for a total of 15 min. Research assistants were kept unaware of the alcohol content of the beverage by conducting BrAC assessments with the digital readout covered and stored in memory for later retrieval. To minimize interoceptive cues of intoxication, all participants remained seated until the end of the smoking lapse task (per Rohsenow and Marlatt, 1981).
Smoking lapse task
At 35 min after completing drinking (50 min from the start of drinking), participants were presented with a tray containing eight cigarettes of their preferred brand and an ashtray and were informed that they had a $4 ‘smoking tab’ from which they could pay $0.50 for each cigarette they wished to smoke (McKee et al., 2006; McKee et al., 2011). They were further informed that they could commence smoking at any point over the next 50 min, but that for each 5 min they delayed smoking, they would earn $1, for a maximum of $10 during the delay period. Participants were instructed to tell the research assistant when they wanted to smoke; the time at which this occurred during the delay period was recorded as the primary dependent variable for this task (range = 0–50 min). They also were informed that once they initiated smoking (or at the end of the 50 min delay period), cigarettes would be available to smoke for 60 min and then would be unavailable until the session ended at 6:30 pm. Money earned during the delay period and unspent money from their $4.00 tab credit was paid at the end of the session.
Post-experimental procedures
Participants remained in the laboratory until at least 6:30 pm and until their BrAC was below 0.04 g/dl. At 6:15 pm, they completed a post-session questionnaire regarding how much alcohol they believed they consumed that day. They received a light meal and were allowed to watch movies and read until they were transported home by a taxi paid for by the study. At the end of each session, participants were paid $50 per session (total = $150) plus the money earned from the smoking lapse task (up to $14 per session). Participants also received a $150 bonus after completing all sessions. As part of the larger study, participants completed computerized cognitive and impulsivity tasks not described here that could increase their total payment by a maximum of $120 (actual range = $5 to $120). The computerized tasks used varied over the course of the study, but each participant completed the same tasks across each session so that the tasks could not differentially impact the within-subject results reported here. Actual payments for completion of the study ranged from $416 to $462. At the end of their participation, participants received brief advice to reduce drinking and quit smoking and were offered referrals for alcohol treatment and smoking cessation.
Measures
Baseline Measures
DSM-IV Axis I diagnoses, including alcohol use disorders, were determined with the Structured Clinical Interview for DSM-IV Non-Patient Edition (First et al., 1995). Severity of nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND, Heatherton et al., 1991). The Timeline Followback Interview (Sobell and Sobell, 1996) was used to assess past 60-day alcohol use.
Experimental Session Measures
BrAC was assessed at the beginning of the session and then at 15, 30, 45, 75, and 105 min after drinking was completed.
Urge to smoke
Urge to smoke and expected satisfaction from smoking were assessed immediately prior to drinking and again 25 min after completing drinking. Urge to smoke was assessed with a single item 0–100 Visual Analogue Scale (VAS) and with the 10-item Brief Questionnaire of Smoking Urges (BQSU; Cox et al., 2001). BQSU items are rated on a 0=”strongly disagree” to 6=”strongly agree scale,” with higher scores indicating greater urge. As in Kahler et al. (2012), expected satisfaction from smoking was assessed with an adapted version of the 2-item subscale of the Cigarette Evaluation Scale (CES; Westman et al., 1992) using a 0–100 VAS; items were “Would it be satisfying?” and “Would it taste good?”. The three measures (VAS, BQSU, and CES) were highly correlated (rs ranging from .63 to .82 across sessions). Therefore, we converted BQSU total scores to a 0–100 scale by multiplying by 100 and dividing by 6 and averaged the VAS, BSQSU, and CES to form an urge to smoke composite that ranged from 0 to 100. Across sessions, internal consistency for the composite prior to drinking ranged from Cronbach’s α = .87–.89 and after drinking ranged from .90–.93.
Subjective stimulation and sedation
Subjective stimulation and sedation were measured using the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) and a 0–100 VAS. The BAES assesses stimulation and sedative effects of alcohol prior to and after beverage administration. This measure uses 7 adjectives to assess stimulant effects and 7 adjectives to measure sedative effects. It has demonstrated good psychometric properties (Martin et al., 1993) and validity (Davidson et al., 2002; King et al., 2002; King et al., 1997).
Data Analysis Plan
We first examined BrACs following low and high dose alcohol and participants’ perceptions of how much alcohol they consumed at each session. We used generalized estimating equations (GEE) to test the effect of experimental condition on latency to initiate smoking during the delay period. Experimental condition was dummy-coded with placebo as the reference group. Those who delayed for the entire 50-min period received a maximum value of 50. We initially included session number in our analyses in case there were order effects, but session number was not associated with any outcome of interest and was therefore dropped from all analyses.
We next used GEE to test the effects of experimental conditions on changes in the three putative mediators: the urge to smoke composite, stimulation, and sedation. Change scores were calculated by subtracting pre-drinking values from the values obtained 25 min after completing drinking. All models included the pre-drinking value of the respective dependent variable as a time-varying covariate. Dummy-codes for low and high dose alcohol relative to placebo were the primary independent variables of interest. Because each mediator was assessed on a 0–100 scale, the coefficients for the dummy codes can be compared directly to gauge the relative magnitude of alcohol effects on each mediator.
To test mediation, we ran separate GEE models for each mediator predicting latency to smoke with the beverage condition dummy codes, the pre-drinking value of the mediator, and change in the mediator after drinking. Following MacKinnon et al. (2002), mediation was tested by creating a product between the coefficient for the effect of alcohol on the respective mediator (often called the A path, which was calculated in the prior models) and the coefficient for the effect of the mediator on latency to smoke (often called the B path). This product represents the effect of alcohol that goes through a given mediator, referred to as an indirect or mediated effect of alcohol. Significance of this indirect effect was assessed using asymmetric 95% confidence intervals (CI) calculated with the RMediate program (Tofighi and MacKinnon, 2011).
Results
Descriptive Statistics on the Sample
One hundred participants took part in the study, completing at least two experimental sessions. The sample was 43% female with a mean age of 39.4 (SD = 10.1) years, and mean education of 12.4 (SD = 1.9) years. The sample was 75% White, 17% African American, 3% American Indian, 1% Pacific Islander, and 2% multiracial with 2% declining to answer; 9% identified as Hispanic/Latino. Family income was below $20,000 for 62% of participants, 66% were unemployed, and 25% were married or cohabiting. Participants smoked an average of 15.6 cigarettes (SD = 4.9) per day, and the mean on the FTND was 5.0 (SD = 2.0). Participants drank on 54.2% (SD = 26.1) of the 60 days prior to baseline averaging 6.1 (SD = 2.8) drinks per drinking day, and 63% had a history of past DSM-IV alcohol dependence (2 participants met current dependence criteria).
Descriptive Statistics on Experimental Conditions
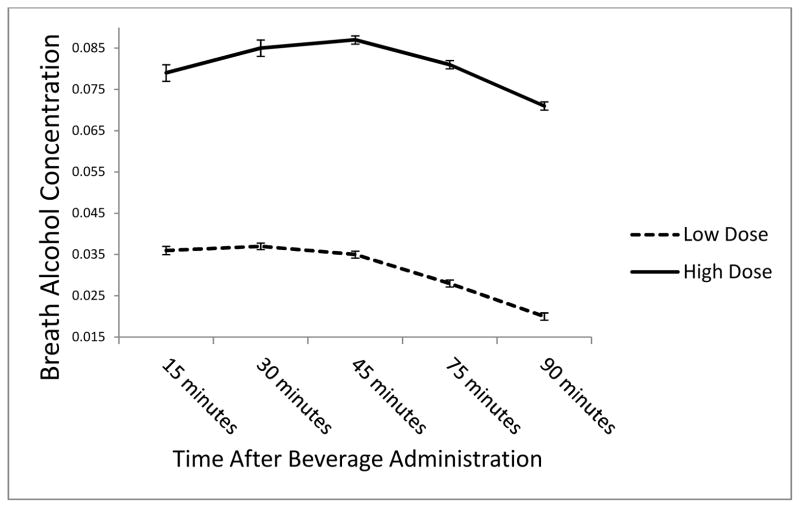
Of the 100 participants, 96 completed all three sessions and 4 completed two sessions; 97 completed the placebo session, 99 completed the low dose session, and 100 completed the high dose session. Mean BrACs in the low and high dose condition over the course of the session are shown in Figure 1. On the post-session questionnaire, the mean numbers of standard alcohol drinks that participants estimated they consumed were 1.86 (1.44), 2.37 (1.20), and 3.32 (1.31) following the placebo, low, and high dose sessions, respectively. Of the 97 participants completing the placebo session, 13 (13.4%) reported that their beverage contained 0 standard alcohol drinks. The minimum number of standard drinks estimated in the low and high dose conditions were 0.5 and 1.0, respectively.
Figure 1.
Mean breath alcohol concentration at 15, 30, 45, 75, 90 and 105 minutes after beverage administration.
Latency to Initiate Smoking
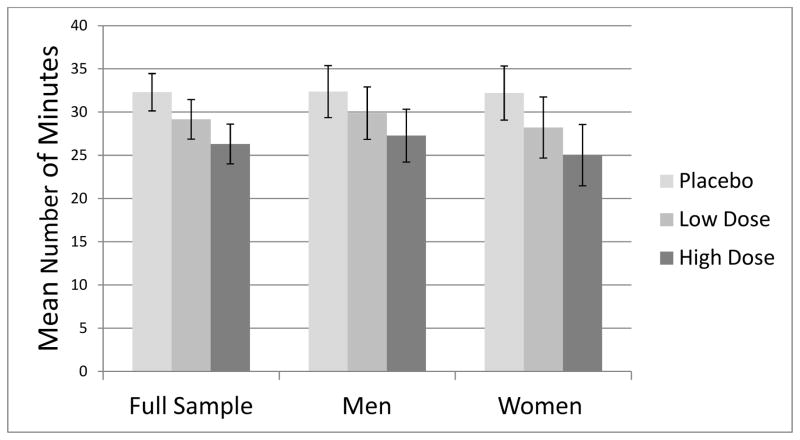
Mean number of minutes before initiating smoking during the 50-minute delay period is presented in Figure 2 by experimental condition for the sample as a whole and by gender. At the placebo session, 47.4% of participants chose to smoke during the delay period compared to 50.5% at the low dose session (odds ratio [OR] vs. placebo = 1.15, 95% CI [0.84, 1.58] and 56.0% (OR vs. placebo = 1.46, 95% CI [1.07, 2.01] at the high dose session. The patterns of results were very similar for men and women, so gender was not considered in further analyses. During the 60-min smoking period (which began as soon as participants chose to smoke or at the end of the delay period), participants smoked an average of 1.91 (SD = 0.85) cigarettes after receiving placebo (including 3 participants who never smoked), smoked 2.18 (SD = 1.05) cigarettes after low dose alcohol (1 did not smoke), and 2.21 (SD = 0.97) after high dose alcohol (1 did not smoke). Differences in number of cigarettes smoked between placebo and both low and high dose alcohol were significant at p < .01.
Figure 2.
Mean number of minutes to choosing to smoke a cigarette during the 50-minute delay period by experimental condition within the full sample and within men and women, respectively.
Results of the GEE model predicting latency to choosing to smoke indicated that low dose alcohol compared to placebo resulted in a latency that was 9.7% shorter, but the effect did not reach significance, B = −3.35 minutes, 95% CI (−7.09, 0.40), p=.08. High dose alcohol compared to placebo significantly reduced latency to smoke by 18.5%, B = −6.36 minutes, 95% CI (−9.99, −2.73), p=.0006. The difference between low and high dose alcohol was nonsignificant, B = −3.02 minutes, 95% CI (−6.66, 0.63), p=.10). We repeated analyses using only those latencies for participants who chose to smoke at a given session; the effects of dose were similar in this model: low dose vs. placebo (B = −3.80 minutes, 95% CI [−7.90, 0.29], p=.07); high dose vs. placebo (B = −5.44 minutes, 95% CI [−9.66, −1.22], p=.01). We also examined self-estimated number of alcoholic drinks consumed at each session as a predictor of latency. Controlling for beverage condition, estimated number of drinks did not significantly predict latency, B = −0.56 minutes, 95% CI [−1.41, 0.30], p=.21; however, estimated drinks did predict latency when beverage condition was not in the model, B = −0.94 minutes, 95% CI [−1.62, −0.27], p=.006.
Analysis of Mediators
Changes in the three putative mediators (urge to smoke, subjective stimulation, and subjective sedation) from immediately prior to drinking to 25 min after completing drinking are shown in Figure 3. Results of GEE analyses predicting change in these three mediators controlling for pre-drinking levels of the respective mediator are shown in column 2 of Table 1. For stimulation, there was a clear dose-response effect where the high dose of alcohol led to an increase that was about twice the magnitude of the increase associated with the low dose. This was not the case for urge to smoke, where both doses resulted in a similar increase of just over 4 points relative to placebo. For sedation, the low but not the high alcohol dose was associated with a significant increase relative to placebo. Controlling for beverage condition, higher self-estimated number of drinks consumed was associated with higher urge to smoke, B = 0.86, 95% CI [0.15, 1.57], p=.017. It was not associated significantly with stimulation or sedation.
Figure 3.
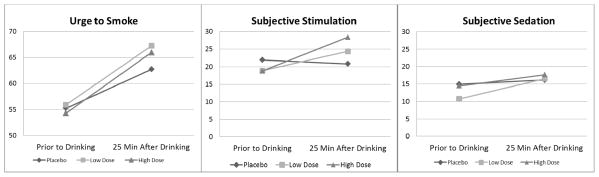
Mean levels of urge to smoke, subjective stimulation, and subjective sedation prior to drinking and at 25 min after drinking by experimental condition.
Table 1.
Effects of alcohol dose on putative mediators and associated direct and indirect effects on latency to smoke with 95% confidence intervals
| Mediator | Effect of Dose on the Mediator | Effect of Mediator on Latency to Smoke1 | Indirect Effect of Dose Through the Mediator2 | Direct Effect of Dose on Latency to Smoke3 |
|---|---|---|---|---|
| Change in Urge to Smoke | −0.24 (−0.37, −0.10)*** | |||
| Low Dose vs. Placebo | 4.37 (1.22, 7.51)** | −1.04 (−2.14, −0.23)* | −2.34 (−5.96, 1.28) | |
| High Dose vs. Placebo | 4.04 (0.33, 7.76)* | −0.96 (−2.17, −0.07)* | −5.40 (−8.95, −1.85)** | |
| Change in Stimulation | −0.008 (−0.16, 0.14) | |||
| Low Dose vs. Placebo | 5.16 (2.06, 8.27)** | −0.04 (−0.90, .0.80) | −2.86 (−6.54, 0.82) | |
| High Dose vs. Placebo | 8.95 (4.89, 13.02)*** | −0.07 (−1.50, 1.34) | −5.76 (−9.49, −2.03)** | |
| Change in Sedation | −0.008 (−0.13, 0.12) | |||
| Low Dose vs. Placebo | 3.28 (0.49, 6.07)* | −0.03(−0.54, 0.47) | −3.06 (−6.74, 0.63) | |
| High Dose vs. Placebo | 1.79 (−1.71, 5.29) | −0.01 (−0.40, 0.34) | −5.96 (−9.52, −2.34)** |
Note. Latency to smoke ranges from 0 to 50 min.
This effect controls for alcohol dose.
This represents the product of the A path (dose effect on the respective mediator) and the B path (mediator effect on latency).
This represents the effect of dose on latency to smoke when the mediator is included in the model, which represent the effect of alcohol dose that does not go through the given mediator.
p < .05;
p < .01;
p < .001
As shown in column 3, greater increases in urge to smoke after drinking predicted a shorter latency to smoke. Changes in stimulation and sedation did not significantly predict latency to smoke. Analysis of indirect effects of alcohol dose on latency to smoke are presented in columns 4 and 5. There was a significant indirect effect of both low and high dose alcohol through urge to smoke, indicating that urge was a partial mediator of these effects; urge to smoke accounted for 30.1% and 15.2% of the effects of low and high dose alcohol, respectively. The indirect effects of dose through stimulation and sedation were nonsignificant. Additional analyses not shown in Table 1 indicated that change in stimulation and in sedation were not significantly associated with changes in urge to smoke. Therefore, changes in stimulation or sedation did not account for the effects of alcohol on urge to smoke.
Discussion
This study presents the most comprehensive experimental examination to date of alcohol effects on the ability to resist smoking. Results indicated that alcohol had a pharmacologic effect on reducing the ability to resist smoking and that alcohol relative to placebo increased the number of cigarettes smoked, consistent with results of McKee et al. (2006). The present study extended the results of the McKee et al. study by showing an alcohol effect when controlling for stimulus expectancy effects. That is, all participants were told that they were receiving alcohol even when they were given a placebo beverage, and the vast majority of participants believed that the placebo beverage did contain alcohol.
Results demonstrated a dose-response association between alcohol and ability to resist smoking, which has not been examined before. The high dose of alcohol significantly increased the odds of initiating smoking and reduced latency to smoke compared to placebo, with the low dose showing effects that were intermediate between placebo and high dose, although not significantly different from either. Kahler et al. (2012) did not find a significant effect of alcohol on ability to resist smoking when only low dose alcohol was compared to placebo; that null result is consistent with the current study’s finding that low dose alcohol has a less robust effect than high dose alcohol. The effect of high dose alcohol vs. placebo was modest, of similar size to the effect of stress on this task (McKee et al., 2011). The dose-response association, however, suggests that higher doses of alcohol, which would not be uncommon for this population of smokers averaging over 6 drinks per drinking day, may produce more robust effects on the ability to resist smoking. Furthermore, a difference of a few minutes in delaying smoking when in the natural environment may be sufficient for smokers to wait out or “surf” urges (Marlatt and Gordon, 1985) or to engage other coping responses, which are essential to resisting temptations to smoke (Shiffman et al., 1996).
Mediation analyses indicated that urge to smoke was a mediator of the effects of both low and high dose alcohol on latency to smoke. This mediated effect accounted for a relatively greater proportion of the low dose effect of alcohol than the high dose effect, suggesting that mechanisms other than craving may play an increasing role in smoking lapse as dose of alcohol increases. Consistent with this idea, and contrary to some prior studies (Epstein et al., 2007; King and Epstein, 2005), we did not see evidence of a linear dose-response association between alcohol consumption and urge to smoke. Both low and high dose alcohol increased urge to smoke, as hypothesized, but high dose alcohol did not appear to have an incremental effect on urge to smoke relative to low dose. In addition, after 3 h of smoking abstinence, the effect of consuming alcohol on urge to smoke was relatively small leading to an increase in urge to smoke of only about 5% compared to placebo beverage. It may be that alcohol more robustly increases urge to smoke in intermittent and light smokers, whose smoking may be more cue-dependent (Epstein et al., 2007; Shiffman et al., 2012). Self-estimated number of alcoholic drinks consumed at a given session was associated with greater urge to smoke at that session, controlling for beverage condition effects; that is, the more participants believed that had drunk (presumably due to stronger subjective effects), the more they wanted to smoke, consistent with a learned association between perceived alcohol effects and smoking.
Alcohol had a clear dose-response association with subjective stimulation as was expected. However, contrary to a prior study (Epstein et al., 2007), change in stimulation was not associated with change in urge to smoke after drinking; again, this divergence in findings may reflect the fact that the Epstein et al. study focused on intermittent smokers rather than daily smokers. Furthermore, change in stimulation did not predict latency to initiate smoking. Therefore, change in stimulation due to drinking did not appear an important mechanism in explaining the effects of alcohol on smoking lapse behavior in daily smokers, suggesting that other mechanisms may play a more important role. It also may be that subjective stimulation did not provide a reliable proxy of activation of a general approach motivational system.
Sedative effects of alcohol did not appear relevant to the ability to resist smoking given the methods used in this study. Subjective sedation was assessed about 25 minutes after finishing beverage consumption. When consuming a low dose of alcohol, this assessment was just after the peak of the BrAC curve, and sedation was significantly, though only marginally, higher than when consuming a placebo beverage. However, in the high alcohol dose condition, BrAC had not yet peaked when sedation was assessed, and sedation was not significantly higher than in the placebo condition. Sedation may be more important as a predictor of the ability to resist smoking if the choice to smoke is given well into the descending limb of the BrAC curve when sedation may be more pronounced. However, that sedation did not predict either urge to smoke or latency to smoke suggests its importance to the ability to resist smoking may be limited.
Limitations
The primary limitation of this study is that it examined an analogue for smoking lapse behavior in smokers who were not making an attempt to quit. However, it is likely that this is the only feasible approach to studying causal dose-response associations between alcohol consumption and the ability to resist smoking, while avoiding the confound of alcohol expectancies and environmental context. Furthermore, irrespective of the clinical-translational value of this task, it provides information about a putatively important behavioral process proximal to alcohol’s effect on smoking—the reward value of initiating smoking relative to abstinence for alternative reinforcement (i.e., money). We examined only a limited set of mediators in this paper, and future studies are needed to determine mechanisms other than urge to smoke that might account for the effects of high doses of alcohol on smoking lapse behavior. For example, measures of response inhibition, delay discounting, and working memory all may be relevant in this regard. Also, the smoking choice task was administered at one fixed time after alcohol administration near the peak of BrAC, and different effects may be more pronounced in either the ascending or descending limbs of the curve.
Potential Clinical Implications
Study results, combined with those of McKee et al. (2006) and of observational studies of smokers trying to quit, have implications for counseling individuals trying to quit smoking. With these results, smokers can now be informed that alcohol has a causal pharmacologic effect on increasing craving for cigarettes and that that increase results in a reduced ability to resist smoking. Consumption of greater quantities of alcohol further reduces the ability to resist smoking, independent of environmental and stimulus properties of alcohol that also may contribute to lapse risk, and independent of increased craving. These messages have been incorporated in prior counseling approaches that present data to heavy drinking smokers on the risks associated with drinking alcohol when trying to quit smoking (e.g., Kahler et al., 2008a), but can now be presented in more causal terms than prior data allowed and with more clarity around the role of craving.
Given the importance of smokers’ limiting alcohol consumption when trying to quit smoking, pharmacotherapy to reduce drinking, such as naltrexone or varenicline (among others), may be beneficial. In addition, naltrexone may reduce urge to smoke resulting from drinking (Ray et al., 2007). Although naltrexone has not been found effective for smoking cessation overall (David et al., 2013), one study did suggest a potential smoking cessation benefit for naltrexone in heavy drinkers (King et al., 2009). Varenicline has shown to reduce both urge to smoke (Brandon et al., 2011) and drinking in non-alcoholic smokers (McKee et al., 2009). Any medication that reduces smoking urges may be useful for the smokers who will not reduce their drinking. Future studies to understand better who is most susceptible to the effects of alcohol on smoking lapse behavior and the mediators of alcohol’s effects on smoking lapse may inform development of more effective smoking cessation treatments for the substantial proportion of smokers who drink heavily.
Acknowledgments
Source of funding: This study was funded by the National Institute on Alcohol Abuse and Alcoholism, grant R01AA016978 to Dr. Kahler, by a Senior Research Career Scientist award from the Department of Veterans Affairs to Dr. Rohsenow, by National Institute on Drug Abuse grant K08-DA025041 to Dr. Leventhal, and by National Institute on Drug Abuse grant K08-DA029094 to Dr. Spillane.
Footnotes
Conflicts of interest
The authors have no financial relationship with the study sponsor, and no conflicts of interest to disclose.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201–208. [PMC free article] [PubMed] [Google Scholar]
- Augustson EM, Wanke KL, Rogers S, Bergen AW, Chatterjee N, Synder K, Albanes D, Taylor PR, Caporaso NE. Predictors of Sustained Smoking Cessation: A Prospective Analysis of Chronic Smokers From the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Public Health. 2008;98:549–555. doi: 10.2105/AJPH.2005.084137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addictive behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Businelle MS, Lam CY, Kendzor DE, Cofta-Woerpel L, McClure JB, Cinciripini PM, Wetter DW. Alcohol consumption and urges to smoke among women during a smoking cessation attempt. Experimental and clinical psychopharmacology. 2013;21:29–37. doi: 10.1037/a0031009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: A population-based survey. Prev Med. 2006;42:348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- David SP, Lancaster T, Stead LF, Evins AE, Prochaska JJ. Opioid antagonists for smoking cessation. The Cochrane database of systematic reviews. 2013;6:CD003086. doi: 10.1002/14651858.CD003086.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Hutchison K, Dagon C, Swift R. Assessing the stimulant effects of alcohol in humans. Pharmacol Biochem Behav. 2002;72:151–156. doi: 10.1016/s0091-3057(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Day AM, Kahler CW, Spillane NS, Metrik J, Rohsenow DJ. Length of smoking deprivation moderates the effects of alcohol administration on urge to smoke. Addictive Behaviors. 2014;39:976–979. doi: 10.1016/j.addbeh.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and alcohol dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Dollar KM, Homish GG, Kozlowski LT, Leonard KE. Spousal and alcohol-related predictors of smoking cessation: a longitudinal study in a community sample of married couples. Am J Public Health. 2009;99:231–233. doi: 10.2105/AJPH.2008.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl) 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Friel PN, Logan BK, O’Malley D, Baer JS. Development of dosing guidelines for reaching selected target breath alcohol concentrations. J Stud Alcohol. 1999;60:555–565. doi: 10.15288/jsa.1999.60.555. [DOI] [PubMed] [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Davidson RJ, Ekman P, editors. The nature of emotion: Fundamental questions. Oxford University Press; New York: 1994. pp. 243–247. [Google Scholar]
- Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. J Abnorm Psychol. 2005;114:649–660. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Humfleet G, Munoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addictive behaviors. 1999;24:149–154. doi: 10.1016/s0306-4603(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6:S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug and alcohol dependence. 2009;100:214–220. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, LaChance HR, Ramsey SE, Abrams DB, Monti PM, Brown RA. Addressing heavy drinking in smoking cessation treatment: a randomized clinical trial. J Consult Clin Psychol. 2008a;76:852–862. doi: 10.1037/a0012717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Leventhal AM, McKee SA, Tidey JW, McGeary JE, Knopik VS, Rohsenow DJ. Sex differences in stimulus expectancy and pharmacologic effects of a moderate dose of alcohol on smoking lapse risk in a laboratory analogue study. Psychopharmacology. 2012;222:71–80. doi: 10.1007/s00213-011-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2010;12:781–785. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Papandonatos GD, Colby SM, Clark MA, Boergers J, Niaura R, Abrams DB, Buka SL. Cigarette smoking and the lifetime alcohol involvement continuum. Drug and alcohol dependence. 2008b;93:111–120. doi: 10.1016/j.drugalcdep.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcoholism, clinical and experimental research. 2009;33:1044–1050. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcoholism, clinical and experimental research. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcoholism, clinical and experimental research. 2002;26:827–835. [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug and alcohol dependence. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Leeman RF, McKee SA, Toll BA, Krishnan-Sarin S, Cooney JL, Makuch RW, O’Malley SS. Risk factors for treatment failure in smokers: relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:1793–1809. doi: 10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ. Behavioral mechanisms underlying the link between smoking and drinking. Alcohol Res Health. 2000;24:215–224. [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism, clinical and experimental research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SM. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RP, Istvan JA, Voelker HT, Rigdon MA, Wallace MD. Level of involvement with alcohol and success at smoking cessation in the lung health study. Journal of studies on alcohol. 1995;56:74–82. doi: 10.15288/jsa.1995.56.74. [DOI] [PubMed] [Google Scholar]
- Osler M, Prescott E, Godtfredsen N, Hein HO, Schnohr P. Gender and determinants of smoking cessation: A longitudinal study. Prev Med. 1999;29:57–62. doi: 10.1006/pmed.1999.0510. [DOI] [PubMed] [Google Scholar]
- Ostafin BD, Palfai TP. Implicit cognition and cross-addictive behaviors. In: Weirs RW, Stacy AW, editors. Hanbook of Implicit Cognition and Addiction. Sage Publications; Thousand Oaks, CA: 2006. [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, Stiller RL. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berl) 1995;119:205–12. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Kahler CW, Leventhal AM, Monti PM, Swift R, Hutchison KE. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology. 2007;193:449–456. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Marlatt GA. The balanced placebo design: methodological considerations. Addictive behaviors. 1981;6:107–122. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SE, Wang MM, Nguyen B. Predictors of success in a smoking cessation clinic. Journal of general internal medicine. 1996;11:702–704. doi: 10.1007/BF02600163. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ. Does heightened affect make smoking cues more salient? J Abnormal Psychol. 2008;117:618–624. doi: 10.1037/0021-843X.117.3.618. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcos M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Experimental and Clinical Psychopharmacology. 2012;20:264–77. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Kraemer HC, Miller NH, Debusk RF, Taylor CB. In-hospital smoking cessation programs: Who responds, who doesn’t? Journal of Consulting and Clinical Psychology. 1999;67:19–27. doi: 10.1037//0022-006x.67.1.19. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Canada: 1996. [Google Scholar]
- Sorlie PD, Kannel WB. A description of cigarette smoking cessation and resumption in the Framingham Study. Prev Med. 1990;19:335–345. doi: 10.1016/0091-7435(90)90033-g. [DOI] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman E, Levin E, Rose J. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful? Clinical Resarch. 1992;40:871A. [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]