Abstract
We set out to determine the factors responsible for twitch force decline in isolated intact rat cardiac trabeculae. The contractile force of trabeculae declined over extended periods of isometric twitch contractions. The force-frequency relationship within the frequency range of 4–8 Hz, at 37 °C, became more positive and the frequency optimum shifted to higher rates with this decline in baseline twitch tensions. The post-rest potentiation (37 °C), a phenomenon highly dependent on calcium handling mechanisms, became more pronounced with decrease in twitch tensions. We show that the main abnormality during muscle run-down was not due to a deficit in the myofilaments; maximal tension achieved using a K+ contracture protocol was either unaffected or only slightly decreased. Conversely, the sarcoplasmic reticulum (SR) calcium content, as assessed by rapid cooling contractures (from 27 °C to 0 °C), decreased, and had a close association with the declining twitch tensions (R2 ~ 0.76). SR Ca2+-ATPase, relative to Na+/Ca2+ exchanger activity, was not altered as there was no significant change in paired rapid cooling contracture ratios. Furthermore, confocal microscopy detected no abnormalities in the overall structure of the cardiomyocytes and t-tubules in the cardiac trabeculae (~23 °C). Overall, the data indicates that the primary mechanism responsible for force run-down in multi-cellular cardiac preparations is a decline in the SR calcium content and not the maximal tension generation capability of the myofilaments.
Keywords: force-frequency relationship, sarcoplasmic reticulum (SR) calcium content, muscle run-down, myofilaments
Introduction
There are multiple systems which can be used for assessing cardiac contractility in vitro ranging from the whole heart level to permeabilized single cardiomyocytes. The intact cardiac trabeculae model has been used extensively by many investigators in the past couple of decades for assessing cardiac function in animal models and humans(Backx and Ter Keurs 1993; Janssen 2010b; Milani-Nejad and Janssen 2014; Stull et al. 2002). Cardiac trabeculae are small linear bundles of tissue and are located on the endocardial surface of the ventricles of most mammalian species. They contain cardiomyocytes, endothelial cells, and fibroblasts, and can be electrically stimulated to contract in vitro, for up to several days(Janssen et al. 1999). Various aspects of myocardial contraction, including contraction amplitude, contractility, kinetics, ion handling, and even ejection properties(de Tombe and Little 1994) can be assessed in these preparations.
One unexplained phenomenon that has been observed routinely in these isolated cardiac trabeculae is “muscle run-down”, where the peak force of twitch contraction of these trabeculae progressively decreases over prolonged periods of isometric twitch contractions in vitro(Janssen et al. 1998; Taylor et al. 2004; Varian et al. 2009). This phenomenon can potentially involve alterations in calcium handling, cellular integrity, myofilament function, and/or a combination of these. However, which of these factors is mainly, or perhaps solely, responsible for muscle run-down is currently unknown. Closing this gap in knowledge would allow the field to better understand and analyze data obtained from this type of preparation. Here, we utilized various techniques in order to determine the underlying mechanism responsible for muscle run-down in isolated cardiac trabeculae. Our data shows that while myofilament contraction and myocyte integrity remain unaffected, the SR calcium load is decreased during this phenomenon and is most likely the underlying mechanism.
Methods
Isolation of cardiac trabeculae
All experiments were approved by the Institutional Animal Care and Use Committee of The Ohio State University and are in compliance with the laws of The United States of America and conform to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. Male Brown Norway rats (approximately 2–4 months old, ~200–250 grams) were intraperitoneally injected with euthasol (~400 mg/kg pentobarbital sodium and ~50 mg/kg phenytoin sodium). The chest cavity was exposed by bilateral thoracotomy and the heart was quickly removed after it was injected with 1000 units of heparin. The heart was perfused via the aorta in a retrograde fashion with a modified Krebs-Henseleit solution containing (in mM) 137 NaCl, 5 KCl, 20 NaHCO3, 1.2 NaH2PO4, 1.2 MgSO4, 10 glucose, 0.25 CaCl2, and 20 2,3-butanedione monoxime (BDM) at room temperature. This solution was bubbled with 95% O2/5% CO2 where the buffering system results in a pH of 7.4. Trabeculae were isolated from the right ventricle and mounted horizontally in a custom made setup as previously described(Milani-Nejad et al. 2013). Muscle were stimulated at 20% over theshhold voltage, using 2 ms bipolar pulses, typically ~5 V. The perfusion solution during experiments was changed to a modified Krebs-Henseleit solution without (BDM) and containing 2 mM CaCl2. This solution was also continuously bubbled with 95% O2/5% CO2 resulting in a pH of 7.4. Muscles were gradually stretched until reaching optimal length as previously described (Milani-Nejad et al. 2013) and all of the experiments were performed at this length. Muscles for force-frequency relationship and post-rest potentiation experiments were stabilized at a baseline frequency of 4 Hz and 37 °C. Muscles used for rapid cooling contracture experiments were stabilized at 1 Hz and 27 °C, the latter temperature was chosen to allow for a rapid cooling; cooling from 37 °C in this particular set-up led to incomplete cooling of the muscles. Muscle chamber size was ~400 μl, with a flow rate that refreshed the bath once every ~3 seconds. Due to space restrictions of the small bath (needed for rapid cooling), The temperature probe as situated after the muscle, leading to a small lag time in recording of the actual temperature at the muscle level. Confocal imaging experiments were stabilized at 1 Hz and room temperature (~23 °C) to avoid actifacts from solution flow vibrations. All force recordings in all protocols were normalized to cross-sectional area of the muscles.
Force-frequency relationship and post-rest potentiation
After muscles were stabilized at 4 Hz, the force-frequency relationship was determined from 4–8 Hz (n = 9, width: 184 ± 12 μm, thickness: 122 ± 8 μm, length: 1.7 ± 0.2 mm). post-rest potentiation was assessed as follows: muscles (n = 7, width: 203 ± 24 μm, thickness: 137 ± 17 μm, length: 1.9 ± 0.3 mm) were stabilized at baseline frequency of 4 Hz and stimulation was stopped for 1, 3, 5, 10, 20, 30, and 60 seconds. After these force-frequency relationship and post-rest potentiation measurements were made, muscles were re-stabilized at 4 Hz. The K+ contracture, sometimes with rate of tension redevelopment (ktr) protocol, was performed as previously described(Milani-Nejad et al. 2013). Following this initial set of experiments (experiment 1), muscles were re-stabilized at 4 Hz for about 15 minutes and this sequence of experiments was performed two more times (experiment 2 and experiment 3).
Rapid-cooling contracture
The SR calcium load of a subset of muscles (n = 10, width: 219 ± 35 μm, thickness: 145 ± 23 μm, length: 2.4 ± 0.3 mm) was determined using rapid-cooling contractures. To measure the rapid-cooling contractures, at 1 Hz and 27 °C, the stimulation pulse was terminated, and simultaneously the muscle bath temperature was reduced from 27 °C to ~ 0 °C (within 1 second) by switching a solenoid valve that controlled the inlet of the bath. Afterwards, the temperature was rapidly switched back to 27 °C, the electrical stimulation was resumed, and the muscles were allowed to stabilize for 5 minutes. The K+ contracture and ktr protocol were performed as previously described (Milani-Nejad et al. 2013) after which the muscles were allowed to re-stabilize at 1 Hz for 15 minutes. Three more rounds (experiments 2,3, and 4) of rapid-cooling contractures and K+ protocols were performed as described above. The reproducibility of the rapid-cooling contractures was investigated in some muscles by performing two rapid-cooling contractures separated by 2 minutes of stabilization at 1 Hz. There was a strong correlation between the developed rapid-cooling contracture tensions in these duplicate experiments (R2 ~ 0.97, n = 19 total experiments on 8 muscles, data not shown).
Paired rapid-cooling contracture experiments were performed in a subset of the rapid-cooling contracture experiments (n = 6 out of 10) by performing an initial rapid-cooling contracture as described above. This was followed by changing the temperature back to 27 °C for a brief period of time (~2–3 s) and then performing a second rapid-cooling contracture while the stimulation is off. The reproducibility of this ratio was determined in some muscles by performing two paired rapid-cooling contracture experiments separated by 2 minutes of stabilization. Both of these ratios (amplitude of the second divided by the amplitude of the first rapid-cooling contracture) were very similar with an R2 of ~ 0.82 (n = 13 total experiments on 5 muscles, data not shown).
Confocal imaging of trabeculae
Muscles were divided into two groups: in one group (n = 3, width: 207 ± 35 μm, thickness: 137 ± 24 μm, length: 2.2 ± 0.6 mm), staining was initiated after obtaining optimal length and a brief stabilization period. In the second group (n = 3, width: 220 ± 44 μm, thickness: 143 ± 29 μm, length: 1.3 ± 0.1 mm), muscles were initially stabilized for 10 minutes followed by 90 minutes of stabilization at optimal length, and then stained. Both groups were stained with RH-237 dye as previously described (Brunello et al. 2013). In some cases muscles were paralyzed with 20 μM of blebbistatin during imaging in order to prevent motion artifact. Multiple z-stacks with an acquisition rate of 200 μs/pixel were taken with and without 3x digital magnification from the surface to the deepest layer that still had adequate staining.
Data and statistical analysis
All force recordings were made using custom-made LabView programs (National Instruments). Confocal images were processed using ImageJ software. ktr tracings were fitted to the equation F = Fmax · (1−e−ktr(t)) + Finitial using Origin 7 software (OriginLab Corporation). Statistical analyses were performed with one-way ANOVA for repeated measures with Bonferroni post-hoc test or two-way ANOVA with statistical significance set at P < 0.05. All data is presented as means ± S.E.M. Muscles were excluded from the final analysis if a complete set of four rapid-cooling contractures (n = 2) and three post-rest potentiation (n = 1) experiments were not obtained.
Results
Muscle run-down is associated with pronounced relative force-frequency relationship
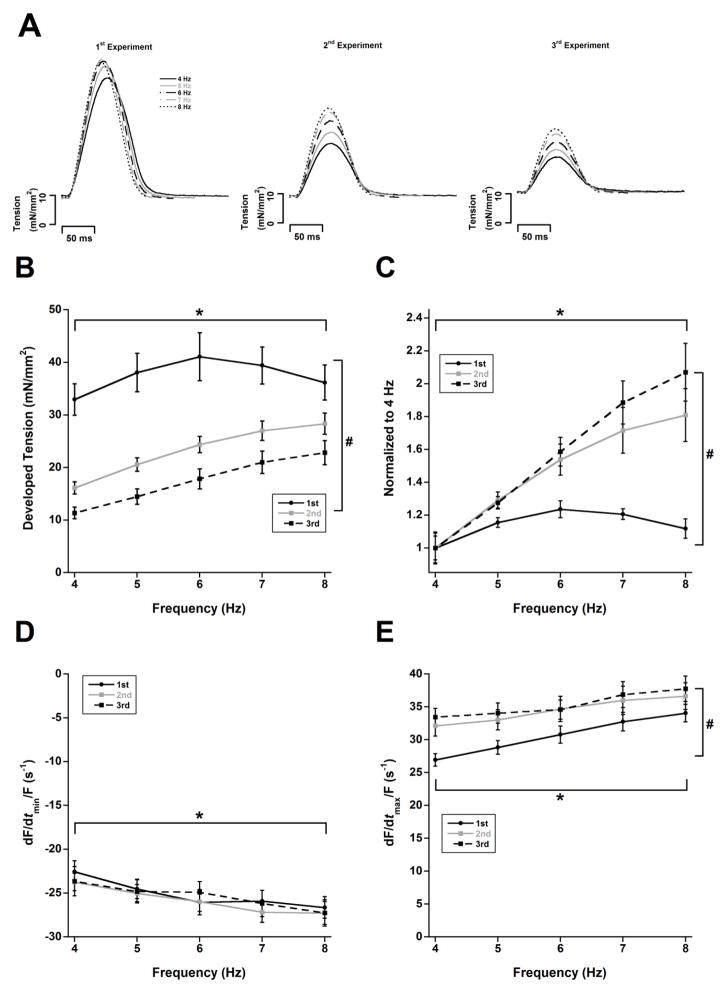
Rat cardiac muscles had an overall positive force-frequency relationship near their in vivo heart rate range during the initial control experiment (1st experiment in Figure 1A). The peak occurred at 6 Hz followed by a slight decrease at 7 Hz and a more prominent decrease at 8 Hz. We next investigated the effects of muscle run-down on the force-frequency relationship by repeating the experiments after the muscles were stabilized for additional time periods. The muscles did exhibit “muscle run-down” with each additional experiment as the developed forces decreased in the 2nd and 3rd experimental series (ANOVA, P < 0.05); however, the decline was more dramatic at lower stimulation frequencies than at higher ones (Figure 1A). Furthermore, the twitch tension in the 2nd and 3rd experiments continued to increase up to 8 Hz in contrast to the 1st experiment. Therefore, the force-frequency relationship of the 2nd and 3rd experiments were more positive when normalized to their corresponding baseline frequency of 4 Hz (ANOVA, P < 0.05). Increasing the stimulation frequency from 4 Hz to 8 Hz resulted in relative increases of 12%, 81%, and 107% for the 1st, 2nd and 3rd force-frequency relationship experiments, respectively (Figure 1B, ANOVA P < 0.05).
Figure 1.
The force-frequency relationship becomes more positive with decreasing twitch tension. A) Original recordings of the same muscle in a typical experiment at 5 frequencies during the 1st, 2nd, and 3rd consecutive repeat of the force-frequency relationship assessment, showing a decline of twitch force over time. B) Non-normalized twitch data at various frequencies during the 1st, 2nd, and 3rd consecutive repeat of the force-frequency relationship assessment. B) Normalization of data to 4 Hz reveals an increase in the relative force-frequency relationship. D) No change in relaxation kinetic parameter −dF/dt/Force across all frequencies. E) Contractile kinetic parameter +dF/dt/Force becomes accelerated with declining twitch tension. * and # indicate P < 0.05 with respect to frequency and experiment succession number respectively, as determined with two-way ANOVA. n = 9. All experiments performed at 37 °C.
We analyzed parameters of maximal rate of tension rise normalized to developed force (+dF/dt/Force) and maximal rate of tension decline normalized to developed force (−dF/dt/Force) in these experiments. It was necessary to divide the kinetic measurements +dF/dt and −dF/dt by the developed forces in order to 1) obtain a purely kinetic measurement (in units of s−1)(Janssen 2010a; Janssen 2010b), and 2) normalization purposes accounting for the decline in twitch tension over time. Increasing stimulation frequency resulted in increases in both +dF/dt/Force and −dF/dt/Force (ANOVA, P < 0.05). The −dF/dt/Force did not change from the 1st to the 3rd experiments (Figure 1C, ANOVA P = 0.68). Interestingly, the +dF/dt/Force of the 2nd and 3rd experiments became accelerated as compared to the 1st experiment (Figure 1D, ANOVA, P < 0.05). Similar to developed tensions, alterations in +dF/dt/Force were more pronounced at lower frequencies.
Muscle run-down is associated with enhanced post-rest potentiation
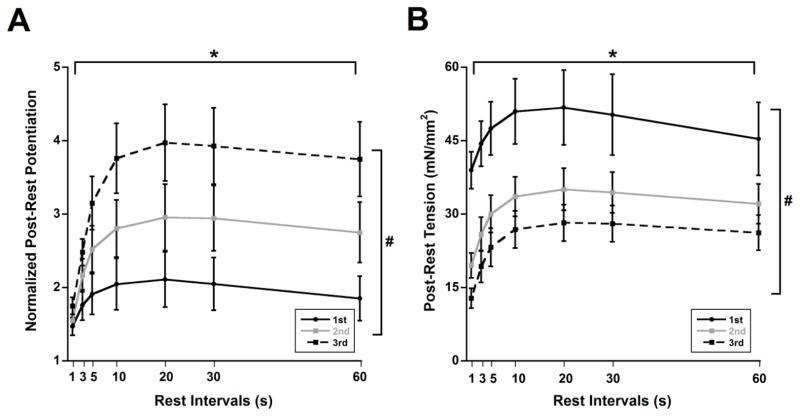
Rat cardiac trabeculae (n = 7) exhibited post-rest potentiation during the initial control experiment (1st experiment in Figure 2A) where rest periods resulted in an increase in post-rest tension relative to pre-rest values. The muscle run-down phenomenon was also present in these muscles after additional periods of stabilization similar to the previous force-frequency relationship experiments. The non-normalized post-rest tensions declined over time in the 2nd and 3rd post-rest potentiation experiments as compared to the 1st (Figure 2B, ANOVA P < 0.05). This post-rest potentiation was not only maintained but became increasingly pronounced with muscle run-down in the 2nd and 3rd post-rest potentiation experiments (Figure 2A, ANOVA P < 0.05). We also kept track of non-stimulated contractions during rest periods which can potentially signify abnormal calcium release. Non-stimulated contraction was recorded in one muscle during the 2nd post-rest potentiation which was not observed during the 1st or 3rd post-rest potentiation in the same muscle (data not shown).
Figure 2.
Post-rest potentiation becomes more pronounced with declining twitch tension. A) Post-rest twitches normalized to pre-rest twitches indicate an enhanced post-rest potentiation with muscle “run-down”. B) Non-normalized post-rest twitches show the decline in tensions. * and # indicate P < 0.05 with respect to rest interval and experiment succession number respectively, as determined with two-way ANOVA. n = 7. All experiments performed at 4 Hz and 37 °C.
Myofilament capability of generating tension is not/slightly decreased
As discussed above, there was a profound decrease in the amount of developed tension of electrically stimulated twitches over time during the course of both force-frequency relationship and post-rest potentiation protocols. We performed K+ contractures in between each successive force-frequency relationship and post-rest potentiation experiments in order to assess the maximum force generation capability of the myofilaments. K+ contracture data from all of the force-frequency relationship and post-rest potentiation experiments from Figures 1 and 2 were combined and analyzed together. In some muscles (4 out 15), we did not achieve a proper apparent level of myofilament activation during contracture (i.e. contracture tension was much lower than twitch tension), these were excluded from this analysis. The tension developed during the K+ contracture did not significantly change (ANOVA, P = 0.21) during the course of these experiments in stark contrast to the developed twitch tensions discussed previously. The contracture tensions (in mN/mm2) were 45.9 ± 4.3 (1st experiment), 41.7 ± 3.7 (2nd experiment), and 41.2 ± 3.8 (3rd experiment).
Decline in SR calcium load contributes to the decrease in twitch tension over time during muscle run-down
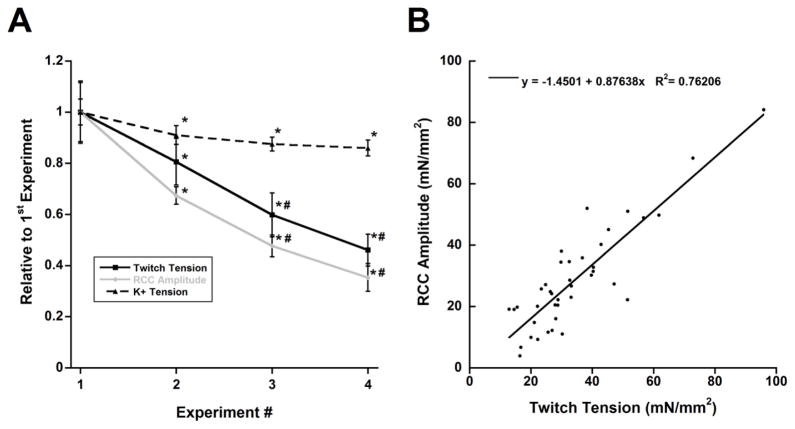
We used rapid-cooling contractures in order to determine SR calcium content as a factor in the decrease of the twitch tension over time in a separate set of muscles. These muscles also presented muscle run-down over time similar to the previous experiments. The developed twitch tensions of the 2nd, 3rd, and 4th experiments were significantly decreased, on average, (n = 10, ANOVA P < 0.05) to 81%, 60%, and 46% of the 1st experiment, respectively (Figure 3A). Interestingly, this decrease in twitch tension was accompanied by a similar decrease in SR calcium content (n = 10, ANOVA P < 0.05) which were made immediately after measuring the amplitude of the twitch tensions. rapid-cooling contracture amplitudes of the 2nd, 3rd, and 4th experiments were, on average, 67%, 48%, and 35% of the 1st experiment, respectively (Figure 3A). The resting tension of these measurements decreased slightly but did not reach statistical significance (ANOVA P = 0.20, data not shown). The K+ contracture tension in these muscles, measured in between each successive rapid-cooling contracture experiment, was also decreased slightly over the course of the experiments (Figure 3A) as the 2nd, 3rd, and 4th experiments were 91%, 88%, and 86% of the 1st experiments, respectively (n = 6, ANOVA P < 0.05). However, this decrease was much less than changes in the twitch tensions and rapid-cooling contracture amplitudes. Four muscles were excluded from this contracture analysis since a proper level of activation was not achieved. We further analyzed the relationship between rapid-cooling contracture amplitude and twitch tension immediately before the rapid-cooling contracture protocol (40 data points from 10 muscles) and found a linear correlation with an R2 ~ 0.76 (Figure 3B). Additionally, there was no difference in the ktr values across all four experiments in a subset of these muscles (n = 5, ANOVA P = 0.23).
Figure 3.
Decrease in SR calcium load is a contributing factor for the decline of developed twitch tension. A) Each measurement was normalized to its corresponding value in the 1st experiment. There was a dramatic decline in developed twitch tension accompanied by rapid-cooling contracture amplitude (n = 10). K+ contracture tension decreased to a lesser degree (n = 6). * and # indicate P < 0.05 vs. 1st and 2nd repeat of measurements respectively, as performed by one-way ANOVA for repeated measures with Bonferroni post-hoc test. B) Correlation between twitch tensions and rapid-cooling contracture amplitudes. All experiments performed at a baseline of 1 Hz and 27 °C.
Relative SR Ca2+-ATPase/Na+/Ca2+ exchanger activity is not likely responsible for the decline in SR calcium load
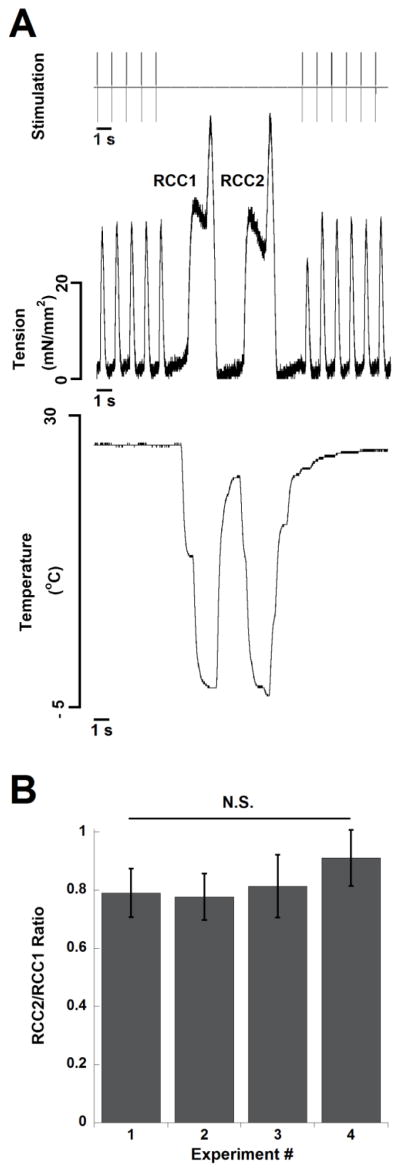
In a subset of the muscles used for the rapid-cooling contracture experiments (n = 6), we also performed paired rapid-cooling contracture protocols (for example, see Figure 4A). The ratios were not statistically different over the course of all four experiments (ANOVA, P = 0.25, Figure 4B).
Figure 4.
Decline in relative SR Ca2+ATPase/Na+/Ca2+ exchanger activity is not responsible for a decrease in twitch tension A) Simultaneous recording of stimulation pulse, muscle tension, and temperature in a single cardiac trabecula. The small time lag between temperature and force results from the placement of the temperature probe behind the muscle. B) Ratio of the amplitude of the second to the first rapid-cooling contracture was not different in successive experiments. n = 6, N.S. signifies not significant. At baseline, muscles were stimulated at 1 Hz and 27 °C.
No change in overall shape of cardiomyocytes and t-tubules
We were able to successfully visualize cardiomyocyte membranes and their t-tubules in intact cardiac trabeculae (Figure 5A). We did not detect any significant alterations in the overall structure of cardiomyocytes or t-tubules after 90 minutes of continuous contraction (n = 3) as compared to control (n = 3) (Figure 5B). It should be noted that with this type of dye and protocol, we were able to properly stain the trabeculae within a maximum distance of ~20–30 μm (variable depending on the trabeculae) from the surface.
Figure 5.
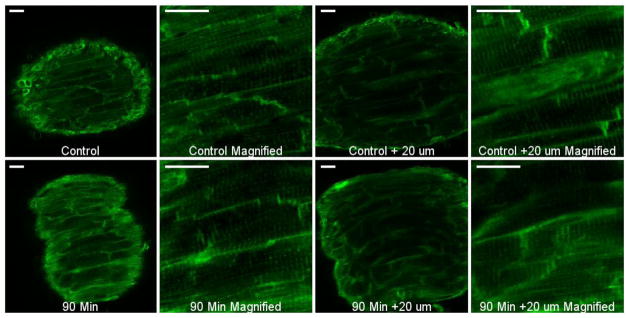
No change in cardiomyocyte shape or t-tubules after long period (90 minutes) of isometric contractions. Multiple z-stacks were taken from each muscle. Images shown are from the most superficial layer (left) and 20 μm deep (right) of each muscle. All scale bars shown are 20 μm. Both muscles were stimulated at 1 Hz and at room temperature (~23 °C).
Discussion
In this study we show that 1) the relative magnitude of the force-frequency relationship and post-rest potentiation becomes enhanced with concurrent decrease in twitch tensions, 2) there is a dissociation between twitch and maximum contracture tensions, and 3) decline in SR calcium load is at least one contributing factor for the decrease in twitch tension. Comparison of all protocols allowed us to determine for the first time that the “run-down” of twitch contractile force typically observed in the first few hours in isolated muscle experiments occurs as a result of changes in excitation- contraction coupling, and is not due to a primary dysfunction in the myofilaments’ capability of generating force.
The positive force-frequency relationship in our study here is in agreement with previous studies which have shown that rats have a positive force-frequency relationship from 4 to 8 Hz at 37 °C(Hiranandani et al. 2006; Layland and Kentish 1999; Monasky and Janssen 2009; Monasky et al. 2008). However, the maximal tension in this study occurred at 6 Hz while tension in previous studies continued to increase from 6 Hz to 8 Hz. We have previously shown that core hypoxia can develop when muscle thickness exceeds 150 μm(Raman et al. 2006). Since the average thickness of muscles used for force-frequency relationship experiments in this study was 122 μm (range of 90–150 μm), the decline in tension from 6–8 Hz is most likely not due to core hypoxia. This difference in force-frequency relationship between studies can be explained by the higher extracellular calcium concentration (2 mM) in this study than these reference studies (range of 1–1.5 mM). It has been shown that increasing extracellular calcium can dampen the positive force-frequency relationship (Layland and Kentish 1999; Schouten and ter Keurs 1986) which is the likely explanation for the slight differences between our results here and previous reports (Hiranandani et al. 2006; Layland and Kentish 1999; Monasky et al. 2008). Our rat samples also exhibited a positive post-rest potentiation, in agreement with previously published reports for this species (Bers 1989; Maier et al. 2000; Maier et al. 1998). Therefore, the force-frequency relationship and post-rest potentiation experiments under the initial control conditions are in close agreement with previously published studies.
Run-down in stimulated twitch contractile force occurs during long periods of isometric contraction in intact cardiac trabeculae(Bupha-Intr et al. 2009; Janssen et al. 1999; Janssen et al. 1998; Taylor et al. 2004). The progressive enhancement of the force-frequency relationship and post-rest potentiation with concurrent muscle run-down in our study here signifies an alteration in calcium handling mechanism(s) during this phenomenon. The positive force-frequency relationship, which is found in most, if not all, mammalian species under physiologically relevant conditions(Janssen and Periasamy 2007), is primarily achieved via increases in calcium transients and SR calcium content(Endoh 2004). This is due to multiple mechanisms such as increased L-type calcium channel flux, pronounced (SR Ca2+-ATPase activity, and relief of the inhibitory activity of phospholamban on SR Ca2+-ATPase (Endoh 2004; Hasenfuss and Pieske 2002; Janssen and Periasamy 2007). post-rest potentiation is dependent upon the SR calcium load and the amount of calcium released upon resumption of stimulation(Bers 2001). Since both force-frequency relationship and post-rest potentiation are highly dependent on calcium handling processes, changes in both of these parameters suggest alterations in the cardiomyocyte’s calcium handling mechanisms(Biesiadecki et al. 2014). We should point out that the enhanced force-frequency relationship and post-rest potentiation after prolonged incubation periods have been previously reported by another group of investigators(Taylor et al. 2004), although the interpretation of the data between this study and ours is different. In this report, the authors showed that rat left papillary muscles initially exhibit a negative force-frequency relationship from 0.2–6 Hz at 30 °C. However, after incubation either in presence or absence of fura-2 dye for 3–4 hours, twitch tensions dropped at all frequencies and the negative force-frequency relationship was converted into a positive one and the post-rest potentiation became enhanced.
In order to investigate the role of myofilaments in muscle run-down further, we performed contracture experiments in order to assess the tension generation capability of the myofilaments without the confounding effects of the calcium handling mechanisms during muscle run-down. In our study, twitch tensions decreased dramatically while contracture tensions were not (muscles from Figures 1 and 2) or only slightly affected (muscles from Figure 3). This is in agreement with our previous report where we showed that the contracture tensions in rat cardiac trabeculae are stable over time at 4 Hz and 37 °C (Milani-Nejad et al. 2013). It is thought that a high extracellular K+ concentration during this type of contracture induces slow membrane depolarization, activation of calcium channels, and influx of calcium which in turn activates the myofilaments. It effectively bypasses the normal excitation-contraction coupling mechanisms of the myocytes. Therefore, if adequate amounts of calcium are provided, the myofilaments are capable of generating the appropriate amount of force. The dissociation between twitch and contracture tensions indicates preserved myofilament function while implicating abnormalities in calcium handling mechanisms in the event of muscle rundown during prolonged in vitro experiments. The contractile parameter +dF/dt/Force accelerated with declining twitch tensions; however this is most likely not due to cross-bridge kinetics as ktr in both this study and a previous one(Milani-Nejad et al. 2013) remain unchanged overtime. Furthermore, this acceleration was more pronounced at lower stimulation frequencies suggesting that force-frequency relationship dependent processes are responsible.
Arguably, the most important calcium mediated determinant of twitch tension in all mammalian species is SR calcium content, especially in rats where it is estimated that SR calcium contributes to 92% of activating calcium(Bers 2002). Therefore, we measured the SR calcium content during muscle run-down using rapid-cooling contractures which is an established technique for measuring SR calcium content in multi-cellular cardiac preparations where a rapid temperature decline results in an increase in tension due to release of the SR calcium content (Bers 1989; Maier et al. 2000; Raman et al. 2006). The decrease in twitch tension in our samples was accompanied with a similar decline in rapid-cooling contracture amplitude over the course of the experiments. The contracture tension in this series of experiments did decline over time. However, this decline of 14% was much less when compared to the 54% decrease in twitch tension and 65% decrease in SR calcium load. Our data suggests that one mechanism for the observed decrease in twitch tension is alteration in SR calcium content and not the tension generation capability of the myofilaments. It should be pointed out that other factors can contribute, although they are currently not investigated in this study. Note that the rapid-cooling contracture amplitude is often used as an index of SR calcium content, but it may also depend on the ability of the myofilaments to bind calcium. If over time a significant decrease in myofilament calcium sensitivity would occur, it could present an alternative explanation for a decline in rapid-cooling contracture amplitude. However, the decline of twitch tension and rapid-cooling contracture amplitude is so large that if a decline in myofilament calcium sensitivity was the only factor, we would have expected to see a significant change in kinetics. We did not observe any change in kinetics of relaxation (−dF/dt/Force) whereas the kinetics of contraction (+dF/dt/Force) are only slightly increased. Thus, although we did not directly measure myofilament calcium sensitivity as a function of time, lack of accelerated twitch kinetics would not support a major impact of this parameter.
Rat cardiac myocytes are heavily dependent on SR Ca2+-ATPase activity for re-sequestration of calcium back into the SR where the SR Ca2+-ATPase is responsible for removal of 92% of calcium during relaxation(Bassani et al. 1994). Therefore, a prime candidate for a decrease in SR calcium content is an alteration in the relative SR Ca2+-ATPase and Na+/Ca2+ exchanger activities. These two systems are in competition with each other and an alteration in this relationship can affect SR calcium content. The ratio of the paired rapid-cooling contracture (second divided by first) is an established indicator of the relative SR Ca2+-ATPase and Na+/Ca2+ exchanger activities (Bers et al. 1993; Maier et al. 2000) in multi-cellular cardiac preparations. Our paired rapid-cooling contracture data indicates that there is no change in the relative SR Ca2+-ATPase and Na+/Ca2+ exchanger activities and the observed decrease in SR calcium load is not likely primarily mediated by changes in SR Ca2+-ATPase function. The positive force-frequency relationship is highly dependent on SR Ca2+-ATPase function as it is necessary to increase SR calcium content at higher stimulation frequencies(Hasenfuss et al. 1994; Janssen and Periasamy 2007; Pieske et al. 1999). Therefore, the positive force-frequency relationship that is maintained and even enhanced, during muscle run-down is dependent in part by having a normal SR Ca2+-ATPase function, but with an enhanced initial SR load. This is somewhat in contrast with the conclusion that was reached in the study by Taylor and colleagues which showed muscle run-down occurring in rat papillary muscles(Taylor et al. 2004). They concluded that SR Ca2+-ATPase activity is initially very high at low frequencies and after incubation of muscles with fura-2 for 3–4 hours, this SR Ca2+-ATPase activity decreases. Although SR Ca2+-ATPase activity was not assessed directly, this conclusion was reached by the observation that relaxation velocity of twitches decreased at low frequencies (0.5–2 Hz) but not at higher frequencies (3–6 Hz) after such incubation. Some differences between these two studies can be partially attributed to various factors including a lower temperature, larger muscle dimensions, and slightly lower stimulation frequencies in the study by Taylor and colleagues (Taylor et al. 2004). Several findings are however similar: the lack of significant change in relaxation kinetics at higher frequencies of 3–6 Hz found by Taylor and colleagues (Taylor et al. 2004) agrees with our data at 4–8 Hz.
The cardiac trabeculae model is a multi-cellular system consisting of several hundreds of individual cardiomyocytes. Therefore, it is possible that some of these cells become damaged or de-tubulated while others are unaffected during muscle run-down which can contribute to the decline in twitch tensions. Qualitatively, we did not detect any significant changes in the shape of cardiomyocytes or t-tubules after long periods of contractions as compared to control. This indicates that the alteration is mostly sub-cellular which fits well in with the data showing that decline in SR calcium load is the underlying mechanism.
In summary, our data for the first time provides an explanation for the force run-down phenomenon that has been routinely observed in intact multi-cellular cardiac preparations. We show that a decline in SR calcium load is at least one primary mechanism contributing to this phenomenon. The findings here will provide better understanding and interpretation of data gathered from this type of preparation.
Acknowledgments
This study was supported by NIH RO1 HL113084 to P.M.L.J. and American Heart Association Greater Rivers Affiliate Predoctoral Fellowship Award 1148008 to N.M.
References
- Backx PH, Ter Keurs HE. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol Heart Circ Physiol. 1993;264:H1098–1110. doi: 10.1152/ajpheart.1993.264.4.H1098. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. SR Ca loading in cardiac muscle preparations based on rapid-cooling contractures. Am J Physiol. 1989;256:C109–120. doi: 10.1152/ajpcell.1989.256.1.C109. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM, Bassani RA, Bassani JW, Baudet S, Hryshko LV. Paradoxical twitch potentiation after rest in cardiac muscle: increased fractional release of SR calcium. J Mol Cell Cardiol. 1993;25:1047–1057. doi: 10.1006/jmcc.1993.1117. [DOI] [PubMed] [Google Scholar]
- Biesiadecki BJ, Davis JP, Ziolo MT, Janssen PML. Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophysical Reviews. 2014 doi: 10.1007/s12551-014-0143-5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello L, et al. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proc Natl Acad Sci U S A. 2013;110:10312–10317. doi: 10.1073/pnas.1300052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupha-Intr T, Haizlip KM, Janssen PM. Temporal changes in expression of connexin 43 after load-induced hypertrophy in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H806–814. doi: 10.1152/ajpheart.01058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe PP, Little WC. Inotropic effects of ejection are myocardial properties. Am J Physiol Heart Circ Physiol. 1994;266:H1202–1213. doi: 10.1152/ajpheart.1994.266.3.H1202. [DOI] [PubMed] [Google Scholar]
- Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- Hiranandani N, Varian KD, Monasky MM, Janssen PM. Frequency-dependent contractile response of isolated cardiac trabeculae under hypo-, normo-, and hyperthermic conditions. J Appl Physiol. 2006;100:1727–1732. doi: 10.1152/japplphysiol.01244.2005. (1985) 01244.2005 [pii] [DOI] [PubMed] [Google Scholar]
- Janssen PML. 54th Bowditch Lecture: Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol. 2010a;299:H1741–1749. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML. Kinetics of Cardiac Muscle Contraction and Relaxation are Linked and Determined by Properties of the Cardiac Sarcomere. Am J Physiol Heart Circ Physiol. 2010b;299:H1092–1099. doi: 10.1152/ajpheart.00417.2010. ajpheart.00417.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML, Lehnart SE, Prestle J, Hasenfuss G. Preservation of contractile characteristics of human myocardium in multi-day cell culture. J Mol Cell Cardiol. 1999;31:1419–1427. doi: 10.1006/jmcc.1999.0978. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Lehnart SE, Prestle J, Lynker JC, Salfeld P, Just H, Hasenfuss G. The trabecula culture system: a novel technique to study contractile parameters over a multiday time period. Am J Physiol Heart Circ Physiol. 1998;274:H1481–1488. doi: 10.1152/ajpheart.1998.274.5.H1481. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43:523–531. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol Heart Circ Physiol. 1999;276:H9–H18. doi: 10.1152/ajpheart.1999.276.1.H9. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM, Pieske B. Differences in Ca(2+)-Handling and Sarcoplasmic Reticulum Ca(2+)-Content in Isolated Rat and Rabbit Myocardium. J Mol Cell Cardiol. 2000;32:2249–2258. doi: 10.1006/jmcc.2000.1252. [DOI] [PubMed] [Google Scholar]
- Maier LS, Brandes R, Pieske B, Bers DM. Effects of left ventricular hypertrophy on force and Ca2+ handling in isolated rat myocardium. Am J Physiol. 1998;274:H1361–1370. doi: 10.1152/ajpheart.1998.274.4.H1361. [DOI] [PubMed] [Google Scholar]
- Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther. 2014;141:235–249. doi: 10.1016/j.pharmthera.2013.10.007. S0163–7258(13)00212-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PM. Effect of muscle length on cross-bridge kinetics in intact cardiac trabeculae at body temperature. J Gen Physiol. 2013;141:133–139. doi: 10.1085/jgp.201210894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasky MM, Janssen PML. The positive force-frequency relationship is maintained in absence of sarcoplasmic reticulum function in rabbit, but not in rat myocardium. J Comp Physiol B. 2009;179:469–479. doi: 10.1007/s00360-008-0331-3. [DOI] [PubMed] [Google Scholar]
- Monasky MM, Varian KD, Janssen PML. Gender comparison of contractile performance and beta-adrenergic response in isolated rat cardiac trabeculae. J Comp Physiol [B] 2008;178:307–313. doi: 10.1007/s00360-007-0223-y. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Raman S, Kelley MA, Janssen PML. Effect of muscle dimensions on trabecular contractile performance under physiological conditions. Pflugers Arch. 2006;451:625–630. doi: 10.1007/s00424-005-1500-9. [DOI] [PubMed] [Google Scholar]
- Schouten VJ, ter Keurs HE. The force-frequency relationship in rat myocardium. The influence of muscle dimensions. Pflugers Arch. 1986;407:14–17. doi: 10.1007/BF00580714. [DOI] [PubMed] [Google Scholar]
- Stull LB, Leppo M, Marban E, Janssen PML. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J Mol Cell Cardiol. 2002;34:1367–1376. doi: 10.1006/jmcc.2002.2065. [DOI] [PubMed] [Google Scholar]
- Taylor DG, Parilak LD, LeWinter MM, Knot HJ. Quantification of the rat left ventricle force and Ca2+-frequency relationships: similarities to dog and human. Cardiovasc Res. 2004;61:77–86. doi: 10.1016/j.cardiores.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Varian KD, Xu Y, Torres CA, Monasky MM, Janssen PML. A random cycle length approach for assessment of myocardial contraction in isolated rabbit myocardium. Am J Physiol Heart Circ Physiol. 2009;297:H1940–1948. doi: 10.1152/ajpheart.01289.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]