Abstract
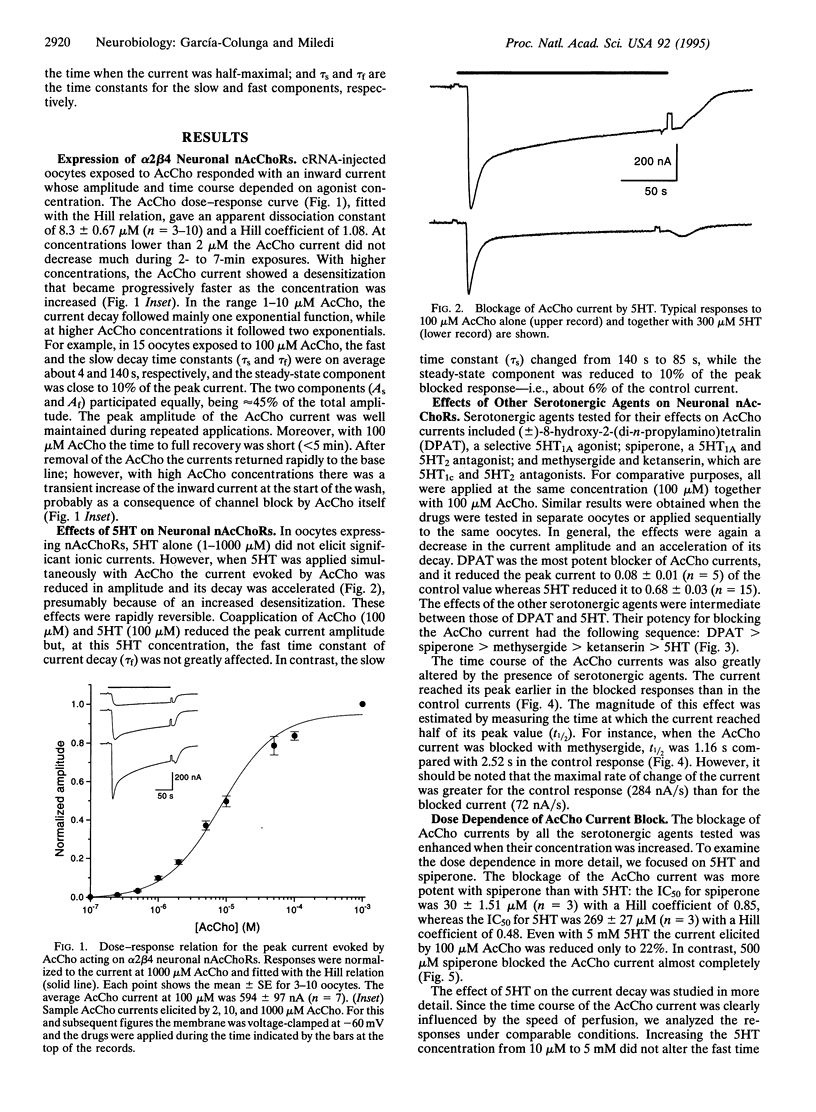
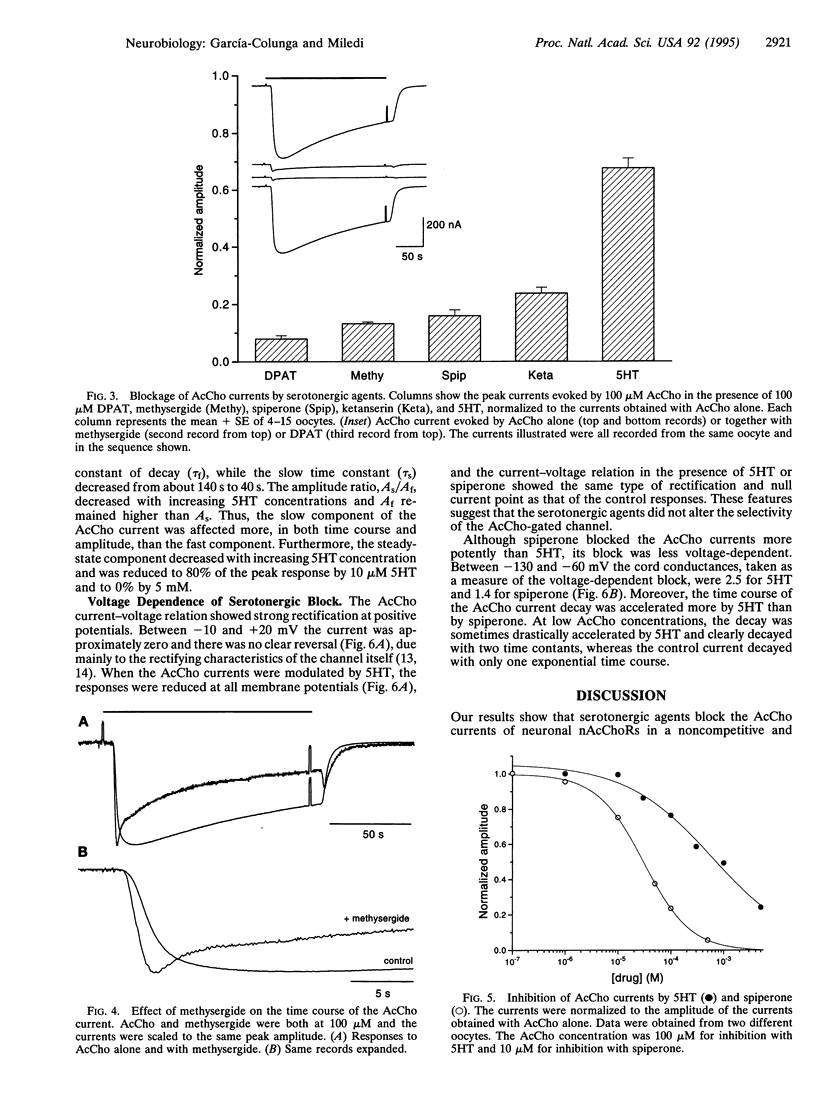
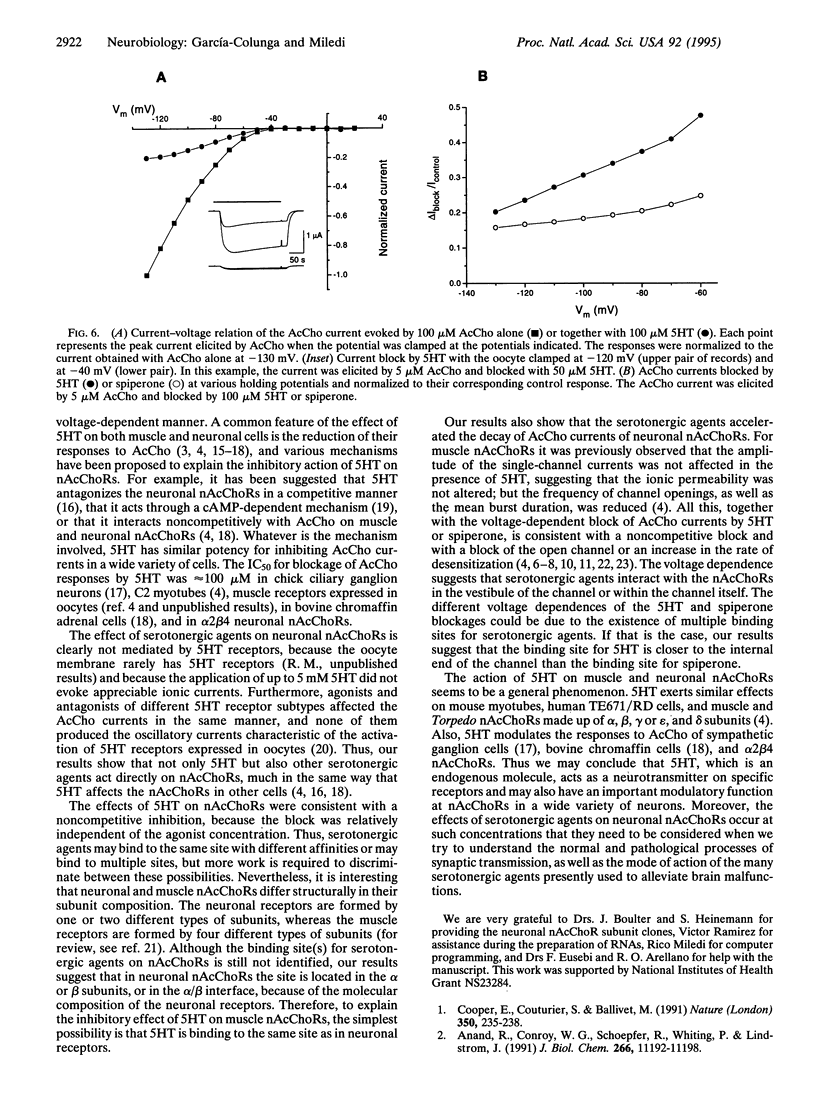
In Xenopus oocytes expressing neuronal nicotinic acetylcholine receptors (nAcChoRs), made up of alpha 2 and beta 4 subunits, acetylcholine (AcCho) elicited ionic membrane currents (AcCho currents) that were modulated by serotonergic agents. Both agonists and antagonists specific for various serotonin (5-hydroxytryptamine, 5HT) receptor subtypes interacted directly with alpha 2 beta 4 nAcChoRs: 5HT, (+/-)-8-hydroxy-2-(di-n-propylamino)tetralin, methysergide, spiperone, and ketanserin reversibly reduced the amplitude of AcCho currents and accelerated their decay. The AcCho-current time course decayed with two exponential functions. In the presence of 5HT, the fast time constant of current decay (tau f) was not greatly modified, but the slow time constant (tau s) was reduced. With AcCho and 5HT both at 100 microM, tau s was reduced from 140 s to 85 s. The order of potency for inhibition of AcCho current amplitudes was (+/-)-8-hydroxy-2-(di-n-propylamino)tetralin > methysergide > spiperone > ketanserin > 5HT. The inhibition was voltage-dependent but the magnitude of the voltage dependence for the different blockers did not correspond to their blocking potency: e.g., the block with spiperone was stronger than with 5HT, but it was less voltage-dependent. Our results suggest that serotonergic agents block neuronal nAcChoRs in a noncompetitive manner, similar to the block of muscle nAcChoR by curare and other substances. These results show that neuronal nAcChoR channels that have been activated by their specific neurotransmitter may be modulated by nonspecific neurotransmitters and their antagonists. These effects may help to better understand brain functions as well as the mode of action of the many serotonergic agents that are used in medical practice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Feltz A. Quinacrine (mepacrine) action at frog end-plate. J Physiol. 1980 Sep;306:261–281. doi: 10.1113/jphysiol.1980.sp013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. 5-Hydroxytryptamine decreases the sensitivity of nicotinic acetylcholine receptor in bull-frog sympathetic ganglion cells. J Physiol. 1986 Nov;380:93–109. doi: 10.1113/jphysiol.1986.sp016274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Conroy W. G., Schoepfer R., Whiting P., Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991 Jun 15;266(17):11192–11198. [PubMed] [Google Scholar]
- Carter A. A., Oswald R. E. Channel blocking properties of a series of nicotinic cholinergic agonists. Biophys J. 1993 Aug;65(2):840–851. doi: 10.1016/S0006-3495(93)81103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomo F., Rahamimoff R., Stefani E. An action of 5-hydroxytryptamine on the frog motor end-plate. Eur J Pharmacol. 1968 Jun;3(3):272–274. doi: 10.1016/0014-2999(68)90143-x. [DOI] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Costa A. C., Patrick J. W., Dani J. A. Improved technique for studying ion channels expressed in Xenopus oocytes, including fast superfusion. Biophys J. 1994 Jul;67(1):395–401. doi: 10.1016/S0006-3495(94)80494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Polenzani L., Mileo A. M., Caratsch C. G., Eusebi F., Miledi R. Blockage of nicotinic acetylcholine receptors by 5-hydroxytryptamine. J Neurosci Res. 1993 Apr 1;34(5):562–570. doi: 10.1002/jnr.490340508. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Kozousek V., Mizobe F., Dean D. M. Substance P inhibits nicotinic activation of chromaffin cells. Nature. 1979 Mar 15;278(5701):256–257. doi: 10.1038/278256a0. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schmid H. A., Vijayaraghavan S. Inhibition of the nicotinic acetylcholine response by serotonergic and muscarinic agents in chick ciliary ganglion neurones. Neuropharmacology. 1992 Oct;31(10):1001–1008. doi: 10.1016/0028-3908(92)90101-t. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Patrick J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A. Curare can open and block ionic channels associated with cholinergic receptors. Nature. 1982 Jul 15;298(5871):272–275. doi: 10.1038/298272a0. [DOI] [PubMed] [Google Scholar]
- Turpaev T. M., Yurchenko O. P., Grigoriev N. G. Alteration of the acetylcholine response by intra- and extracellular serotonin application in intracellularly perfused neurons of Lymnaea stagnalis. Cell Mol Neurobiol. 1987 Dec;7(4):381–390. doi: 10.1007/BF00733790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino S., Amador M., Luetje C. W., Patrick J., Dani J. A. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992 Jan;8(1):127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S., Schmid H. A., Mapp K. S. Serotonin modulates nicotinic responses of adrenal chromaffin cells. J Neurochem. 1993 Jul;61(1):324–331. doi: 10.1111/j.1471-4159.1993.tb03571.x. [DOI] [PubMed] [Google Scholar]


