Abstract
Rationale
Previous studies indicate that psychosocial stressors could accelerate amyloid-β (Aβ) levels and accelerate plaque deposition in mouse models of Alzheimer disease (AD). Stressors enhanced the release of corticotrophin-releasing factor (CRF) and exogenous CRF administration mimicked the effects of stress on Aβ levels in mouse models of AD. However, whether CRF receptor 1 (CRF1) antagonists could influence the stress-induced acceleration of an AD-like process in mouse models has not been well studied.
Objective
We sought to examine whether CRF1 antagonists inhibit the effects of isolation stress on tissue Aβ levels, Aβ plaque deposition, and behaviors related to anxiety and memory in Tg2576 mice, and to investigate the molecular mechanism underlying such effects.
Methods
Cohorts of Tg2576 mouse pups were isolated or group-housed at 21 days of age, then the subgroups of these cohorts received daily intraperitoneal injections of the CRF1 antagonists, antalarmin or R121919 (5, 10 and 20 mg/kg), or vehicle for 1 week. Other cohorts of Tg2576 mouse pups were isolated or group housed at 21 days of age, and then at 4 months of age, subgroups of these mice were administered antalarmin (20 mg/kg) or vehicle in their drinking water for 6 months. Finally, cultured primary hippocampal neurons from regular Tg2576 pups (P0) were incubated with CRF (0.1, 1 and 10nM), antalarmin (100 nM) or H-89 (1 μM) for 48 hours. Brain tissues or cultured neurons were collected for histological and biochemical analyses, and behavioral measures were collected in the cohorts of mice that were chronically stressed.
Results
Administration of antalarmin at 20 mg/kg dose for 1 week significantly reduced Aβ1-42 levels in isolation stressed mice. Administration of antalarmin for 6 months significantly decreased plasma corticosterone levels, tissue Aβ1-42 levels and Aβ plaque deposition in the brain and blocked the effects of isolation stress on behaviors related to anxiety and memory. Finally, incubation of neurons with 100 nM antalarmin inhibited the ability of 10 nM CRF to increase Aβ1-42 levels and protein kinase A IIβ (PKAIIβ) expression. The effect of CRF1 on Aβ1-42 levels was also diminished by treatment with H-89, a c-AMP/PKA inhibitor.
Conclusions
These results suggest that CRF1 antagonists can slow an AD-like process in Tg2576 mice, and that the c-AMP/PKA signaling pathway may be involved in this effect.
Keywords: Stress, Corticotrophin-releasing hormone, Antalarmin, R121919, Alzheimer disease, Tg2576 mice
Introduction
Alzheimer disease (AD) is the most common type of dementia in the elderly and currently affects approximately 5 million Americans (www.alz.org Facts and Figures). The pathological changes of AD include the formation of extracellular amyloid-β (Aβ) plaques and intracellular phosphorylated tau tangles in the brain (Harrington, 2012). Although the exact causes of these neuropathological changes are unknown, both genetic and environmental factors are thought to be involved (Miller and O’Callaghan, 2008; Migliore and Coppede, 2009). Current pharmacotherapies, such as acetylcholinesterase inhibitors and an N-methyl-D-aspartate (NMDA) antagonist, memantine, can ameliorate some of the behavioral symptoms. However, none of the approved treatments for AD have been shown to slow or halt the progression of this disease (Herrmann et al., 2011; Tayeb et al., 2012). Thus, new strategies to block or delay the pathogenesis and prevent cognitive decline in AD are greatly needed.
Stress is common in daily life and may be especially high in AD patients who are less resilient because of age, other health problems, and limited resources. The results of recent studies suggest that stress can significantly affect the onset and progression of AD. For example, individuals prone to psychological distress are twice as likely to develop AD (Wilson et al., 2005; Wilson et al., 2006). Elevated plasma cortisol levels, often an indicator of stress, have been reported in patients with AD (Swanwick et al., 1998; Umegaki et al., 2000; Rehman, 2002), and higher plasma cortisol levels were correlated with severity of cognitive decline in AD patients (Csernansky et al., 2006). Considering other elements of the HPA axis, investigators have reported a reduction in corticotropin releasing factor (CRF) immunoreactive cells and increases in the density of postsynaptic CRF receptors in the brain of AD patients (Whitehouse et al., 1987; Pomara et al., 1989; Behan et al., 1995; De Souza, 1995). These changes in the hypothalamic-pituitary-adrenal (HPA) axis in AD patients are unlikely to be secondary to depression, as AD patients without depression also have higher cerebrospinal fluid cortisol levels as compared to controls (Hoogendijk et al., 2006). However, hippocampal degeneration could lead to increases in cortisol in AD patients via reductions in negative feedback within the HPA axis (O’Brien et al., 1996).
Genetically manipulated mice that imitate some of the neuropathological changes of AD provide an opportunity to investigate the impact of stress on AD pathogenesis and cognitive function, and reveal the molecular mechanisms underlying these effects. Studies from our group and others indicate that behavioral stressors can accelerate the production of Aβ and its incorporation into Aβ plaques, and increase hippocampal tau phosphorylation (tau-P) in transgenic mouse models (Dong et al., 2004; Dong et al. 2008; Rissman et al., 2007; Carroll et al., 2011; Cuadrado-Tejedor et al., 2012). CRF and CRF receptor 1 (CRF1) play critical roles in regulation of stress-induced neuropathogenesis and behavioral deficits in mouse models of AD (Kang et al., 2007; Dong et al., 2008; Carroll et al., 2011; Rissman et al., 2012). In this study, we examined the effectiveness of CRF1 antagonists on tissue Aβ levels, amyloid plaque deposition, and behaviors related to anxiety and memory in Tg2576 mice under stressed conditions. Also, we investigated possible molecular pathways by which such effects might be mediated using hippocampal neurons from Tg2576 mice in culture.
Materials and methods
Transgenic mouse model
Tg2576 mice of both genders [B6;SJL-Tg(APPSWE)2576Kha; Taconic Farms Inc. Germantown, NY] were used for this study. All animal procedures were performed in accordance with the National Institutes of Health (NIH) Guidelines and Animal Studies Committee at Northwestern University. Tg2576 mice have the K670N-M671L mutation, driven by a hamster prion protein promoter, and over-expressed human APP 695. Heterozygous Tg2576 (+/−) males were bred with C57B6/SJL females (The Jackson Laboratory, Bar Harbor, ME). Screening for transgenic mice in offspring was performed by genotyping DNA obtained from tail biopsies. Generally, the ratio for Tg2576 mice (Tg+) in each litter was 25–40% (i.e., 1–4 Tg2576 positive mice from each litter depending on the litter size). Tg2576 mice showed Aβ plaques at 9 to 10 months of age, and the cognitive deficits occurred earlier (Hsiao et al., 1996)
Animal groups and CRF1 antagonist administration in vivo
A total of 60 Tg2576 mice were used for the one-week stress and CRF1 antagonist treated study (3–5 mice per group with males and females evenly distributed in each group). Tg2576 pups from each litter were randomly divided to isolation stressed and standard group housed (see below) with and without drug administration immediately after weaning to avoid the litter effects. Only 1 Tg2576 positive pup from each litter could be assigned to a treatment group in which every individual animal came from a different litter (3–5 litters for 1 week treatment, 5–7 litters for 6 months treatment). CRF1 antagonists antalarmin and R121919 are nonpeptide compounds that can pass through the blood brain barrier (Sabino et al., 2006; Funk et al., 2007). Drug administered groups were given either antalarmin (suspended in warm water with 20% Tween-80) or R121919 (suspended in warm water) at one of 3 doses (5, 10 and 20 mg/kg) by intraperitoneal (IP) injection once a day for 7 days. Control groups were IP injected the vehicles that were used for dissolving drugs. For the chronic stress and CRF1 antagonist study, a total of 24 Tg2576 pups (5–7 mice per group) were randomly divided to isolation stressed and standard group housing until 4 months old and then were administered antalarmin for 6 months. Antalarmin (20 mg/kg) was suspended in drinking water for drug administration (first suspended in warm water with 20% Tween-80, then diluted in drinking water) and the final concentration of Tween-80 in the drinking water is 0.2%. The same vehicle was used for the control. To ensure the consistency of drug dosing and that the presence of antalarmin did not influence daily water consumption, we measured the amount of water consumed by each mouse weekly and calculated the total and mean volume of drinking water with and without the drug. The mean amount of consumption between groups was not significantly different [F1,21=0.34; P=0.56]. We also measured body weight each week to ensure that antalarmin administration did not affect mouse food chow consumption. The mean body weight between groups was not significantly different [F1,21=0.87; p=0.32]
Isolation stress
Isolated Tg2576 mice were individually housed in cages one-third smaller (10 × 14 × 9.5 cm3) than standard-sized mouse cages (27 × 14 × 11 cm3) and placed in a separate area (our satellite facility) from other animals to prevent visual contact from weaning for 7 days (from weaning (21 days) to 28 days of age) and 9 months (from weaning (21 days) to 4 months of age before drug treatment then continued isolation stress for 6 months during drug treatment until 10 months of age). Control animals (group housed) were housed at 3 animals per standard cage for the same period. Food and water were available ad libitum (Dong et al. 2004; Dong et al., 2008).
Behavioral tests
Animals were habituated in the testing room (next to satellite facility) for at least 30 minutes before each test. All behavioral tests were performed starting at 9:00 am. The testing rooms and housing facility were light controlled to automatically turn on at 6:00 am and off at 6:00 pm.
Elevated plus maze (Handley and Mithani, 1984)
The elevated plus-maze is an apparatus composed of 2 open arms (10 × 50 cm) and 2 perpendicular closed arms [10 × 50 × 40 cm (wall height)] surrounding a 10 × 10-cm center platform 50 cm above the ground. A camera was assembled above the maze for recording. The mouse was placed on the center platform facing one of the closed arms and allowed to freely explore the plus maze for 5 minutes. The following measures were quantified by 2 trained observers blind to the group assignment of the animals: number of entries into the open arms, total number of entries, time spent in the open arms, time spent on the center platform.
Spontaneous alternation (Mitani et al., 2012; Hsiao et al., 1996)
The spontaneous alternation apparatus consists of a three-arm (5 cm wide × 21 cm long × 15.5 cm high) Y-shaped maze. Mice were placed in the central region facing one of the closed arms and were allowed to freely explore the arms for 5 minutes without investigator presence while a camera recorded its movements. A successful alternation was defined as discrete and successive entries into each open arm, including events where the animal directly progresses from one arm to the next in consecutive fashion (that is ABC, ACB, BAC, BCA, CAB, and CBA) without reentering the two previously visited arms. The locomotion index were calculated as the overall number of arm entries, whereas the working memory index (% correct alternation) were calculated by dividing the number of total successful alternations divided by the total number of possible alternations (i.e., the number of total entries minus two) X 100%.
Plasma antalarmin measurement
To confirm chronic administration of antalarmin through drinking water would be sufficient and compatible compared to administration of IP injection, we measured the antalarmin concentration in the plasma when the blood was collected by rapid retro-orbital phlebotomy (less than 1 min) after one-week of antalarmin treatment (two time points: 6 am, 30 mins after antalarmin IP injection, and 6 pm at same day), and 6 pm at the end of the 6 months of antalarmin treatment (antalarmin in drinking water). Antalarmin was extracted from 25.0 μL mouse plasma by liquid-liquid extraction with methyl tert-butyl ether (MTBE). Briefly, internal standard (IS) solution (25.0 μL of 10.0 ng/mL R121919 for antalarmin analysis) was added to thawed plasma samples in a 96 well plate. Following addition of water and mixing, the samples were processed by supported liquid extraction (SLE). The eluates resulting from extraction were evaporated to dryness under a stream of nitrogen (nominal 40°C, 20 psi). The residues were reconstituted in the mobile phase and submitted for analysis by LC-MS/MS. Separation was achieved on a Thermo Electron Betasil Silica-100 column (50 × 3 mm, 5 μm) at a flow rate of 0.4 mL/min with an isocratic mobile phase composed of acetonitrile: water: glacial acetic acid: trifluoroacetic acid (87.5:12.5:0.5:0.05, v/v/v/v). Precursor/fragment ions: 379.3→ 307.2 m/z (Antalarmin) were monitored by tandem mass spectrometry (MS/MS) using electrospray ionization in the positive ion mode on a Sciex API5000. Concentrations of Antalarmin were calculated using peak area ratios (analyte/internal standard). The calibration curves were generated using a linear regression with a weighting of 1/x2. The results showed plasma antalarmin concentrations: 580±117.22ng/ml (30 mins after injection), 40.33±4.50ng/ml (12 h after injection) and 34.267±8.63 (6 month in drinking water), which indicated that the plasma antalarmin concentrations administrated by drinking water are comparable to the concentrations at 12 hours after IP injection.
Plasma corticosterone measurement
The blood was collected by rapid retro-orbital phlebotomy at 6:00 pm before antalarmin treatment, after one-week of antalarmin treatment and at the end of 6 months of antalarmin treatment. Plasma corticosterone levels were measured using EIA kit (Cayman Chemical Company, Ann Arbor, MI) according to the introduction manual and published methods (Zamora et al., 2009). The serum corticosterone content was calculated in picograms per milliliter according to the manufacturer’s recommendations.
Brain tissue Aβ measurement
All animals were deeply anesthetized by 50 mg/kg pentobarbital and were quickly perfused by saline to rinse out the blood. For the 1-week drug treated groups, the cortex and hippocampus in both hemispheres of each animal were dissected on ice and snap-frozen on dry ice then stored in an −80°C freezer. For the 6-month drug treated groups, one hemisphere of each brain was fixed in 4% paraformaldehyde for the histological procedure. The cortex and hippocampus of the other hemisphere were dissected on ice and snap-frozen on dry ice, then stored in a −80°C freezer for biochemical analysis.
Levels of Aβ1-40 and Aβ1-42 were measured using enzyme-linked immunosorbent assay (ELISA) kits (Biosource Inc., Camarillo, CA) according to the manufacturer’s instructions and protocols from our previous study (Dong et al. 2004; Kang et al., 2007; Dong et al., 2008). Briefly, the cortex or hippocampus from one hemisphere was homogenized in PBS and centrifuged at 10,000 g for 5 minutes. The supernatant was collected and protein quantification was performed using the BCA assay (Bio-Rad Laboratories, Hercules, CA). Samples were analyzed for soluble Aβ using a sandwich ELISA specific for human Aβ1-40 or Aβ1-42 (BioSource International Inc., Camarillo, CA) according to the manufacturer’s instructions. Duplicate standards (Aβ1-40 or Aβ1-42, from 1000, 500, 250, 125, 62.5, 31.25, 15.65 and 0 pg/mL) and tissue samples (120 μg per sample) containing protease inhibitor cocktail tablets (Roche Molecular Biochemicals, Mannheim, Germany) were incubated overnight in antibody-coated plates at 4°C. After washing, the plates were incubated in rabbit anti-human Aβ1-40 or Aβ1-42 for 2 hours at room temperature and then with horseradish peroxidase–conjugated anti-rabbit IgG for another 2 hours. Stabilized chromogen (100 μL) was added to each well and incubated for 30 minutes in the dark. The absorbance in each well was read with a microplate reader (SPECTRAmax Plus, Molecular Devices Corporation, Sunnyvale, CA). The intra-assay coefficient of variation was 2.9%.
Aβ plaque immuno-staining and measurement
The hemispheres fixed in 4% paraformaldehyde for 3 days were transferred into the same fixative with 30% sucrose for 48 hours. The brains were cut into 40-μm-thick serial sections for 4 sets in the coronal plane using a cryostat (Leica CM 1850 UV; Leica, Nussloch, Germany) (average 35 ±3 sections/per set). One set of sections were rinsed with PBS, treated with 0.3% H2O2 in methanol for 30 minutes to eliminate the endogenous peroxidase activity, and the sections were incubated in a blocking solution of 5% normal goat serum 0.3 tritonX-100 in PBS for 1 hour. Sections were then incubated overnight in the primary antibody for Aβ1-40 (rabbit polyclonal pan antibody raised against Aβ synthetic peptide, 1:1,000, Biosource, Camarillo, CA) at 4°C. After washing, the sections were incubated in biotinylated anti-rabbit secondary antibody for 2 hours, then in an avidin-biotin complex for 1 hour at room temperature. Aβ-like immunoreactivity was visualized using a DAB kit (Vector Laboratories, Burlingame, CA). Aβ plaques were counted in every section of one set (35 ±3 sections) using a non-biased stereological system program (Olympus, Albertslund, Denmark). Each Aβ plaque was counted as one plaque regardless of its size but was not connected to other Aβ plaques. The total number summed from one set of sections was multiplied by 4 to obtain an estimate of the total number of Aβ plaques in the brain. Aβ plaque sizes were measured using CAST-GRID by drawing an outline of the Aβ plaque areas for Aβ burden (i.e. total Aβ plaque area/brain area in coronal section).
Primary neuronal culture and related assays
The hippocampi from day 0 (P0) Tg2576 positive newborn pups were dissected and digested with 0.25% trypsin EDTA solution. Neurons were plated into 24-well tissue culture plates coated with 0.1 mg/mL of poly-D-lysine (Sigma-Aldrich, St. Louis, MO) in a high density (4.5 × 105/cm2). Cells were maintained in neurobasal medium supplemented with B-27, 2 mM glutamine, 100 μg/mL of streptomycin, and penicillin (Invitrogen, Carlsbad, CA) in the incubator at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cultured neurons were treated at day 14 with different doses of CRF (0.1, 1 and 10nM), antalarmin (100nM), and PKA inhibitor H-89 (1μM). This experiment was repeated three times with 3 different litters.
MTT cell toxicity assays
To test whether the doses of CRF, antalarmin and H-89 we selected in this study exhibited any neurotoxic effects in the cultured neurons, cells with CRF, antalarmin and H-89 treatment were added with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) 0.5 mg/mL, 4 hours before the end of the treatment. MTT is reduced to purple formazan in living cells. A solubilization solution (dimethyl sulfoxide, DMSO) was added to dissolve the insoluble purple formazan product into a colored solution. The absorbance of this color was measured at a wavelength of 570 nm using a microplate reader. MTT assay showed that exposure to any of the treatments for 48h did not cause dramatic decreases in the viability of neurons compared to vehicle-treated controls.
Aβ1-42 levels
The cultured neurons were collected at different conditions for measurement of Aβ1-42 levels using an ELISA kit (see the detailed method above).
PKAIIβ
The type IIβ isoform of cAMP-dependent protein kinase (PKAIIβ) is a major mediator of the actions of cAMP in the central nervous system; therefore, we selected PKAIIβ as an indicator to investigate the involvement of cAMP/PKA-signaling in the CRF/CRF1 activity. The primary cultured neurons at 16 days after 48 hours of CRF, antalarmin and H-89 administration were collected and homogenized in 5 volumes of ice-cold homogenization buffer (0.2% NP-40 and protease inhibitor in PBS buffer). Homogenates were centrifuged at 15,000g for 20 minutes at 4°C, and the supernatant was used to measure protein levels (bicinchoninic acid method). A total of 25 mg of each sample was further diluted in sample buffer (Bio-Rad, Hercules, CA) and 15% polyacrylamide gels were used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene fluoride membranes probed with rabbit anti-mouse PKAIIβ (1:500) and β-actin (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA) followed by horseradish peroxidase–conjugated secondary antibody binding (1:20,000; BD Diagnostic Systems, Sparks, MD). Immunoreactive proteins were visualized using the enhanced chemiluminescence western blotting detection system (Thermo Fisher Scientific). The light-emitting bands were detected with X-ray film (Thermo Fisher Scientific). Quantification of the blots were performed using Image J software (NIH), by plotting density measurements against the dilution factor in the linear range of signal.
Statistical Analysis
Data analysis was carried out by two trained and genotype-blind researchers. Given the small number of Tg2576 positive animals (Tg+) from each litter (1–4 Tg+ mice/per litter), only 1 Tg2576 pup from each litter could be assigned to one of four treatment groups. Although no littermates can be included in the same experiment group due to not exact 4 Tg2576 pups in each litter, the assignment of pups to each treatment group was randomized from 3–5 litters for 1-week treatment, 5–7 litters for 6 months treatment. That is, subjects were assigned to treatments in a systematically balanced fashion from each litter. Therefore, in our overall design, except that the corticosterone concentration study is a repeated measure, in all other studies, an individual mouse was approximately represented a variation of treatment. Treatment was not a repeated measurement. However, in order to avoid the potential litter effects confound with treatment effects, we used litter as the unit of analysis and run the model with littler, housing and drug interactions. No litter effects and interaction effects were found. Thus, based on our design, we performed two-way analysis of variance (ANOVA) for tissue Aβ levels, plaques, and behavioral studies and repeated measure ANOVA for corticosterone concentration. (P<. 05; SPSS). When housing (i.e., isolated vs. group housed), drug (i.e., antalarmin or R121919 vs. controls), or housing and drug interactions were found, post hoc (Bonferroni/Dunn tests) analysis was used. Finally, the Prism liner regression test was used for calculation of correlation.
Results
Effect of one-week administration of antalarmin or R121919 on Aβ1-40 and Aβ1-42 levels
Using a sub-acute stress paradigm, we determined whether daily administration of CRF1 antagonists (i.e., antalarmin or R121919, at doses of 5, 10, and 20 mg/kg) versus vehicle could prevent the effects of isolation stress on Aβ levels. Two-way ANOVA indicated a significant effect of housing on both Aβ1-40 (F1,56 = 58.33; P<.0001) and Aβ1-42 (F1,56 = 78.71; P<.0001) levels, but a significant effect of drug treatment on only Aβ1-42 (F2,56 = 5.45; P=.006) levels. The effect of drug treatment on Aβ1-40 only reached a trend level (F2,56 = 3.00; P=.056). Post-hoc tests confirmed that isolation housing significantly increased both tissue Aβ1-40 (Figure 1A, P<.001) and Aβ1-42 levels (Figure 1B, P<.001) as compared to the group housing condition. Administration of antalarmin at a dose of 20 mg/kg, but not at doses of 5 mg/kg or 10 mg/kg, significantly reduced Aβ1-42 levels as compared to the vehicle (P=.0146). The effects of R121919 at 20mg/kg were similar, but only at a trend level (P=.0755) (Figure 1B).
Figure 1. Antalarmin and R121919 reduce tissue Aβ levels in sub-acute stressed Tg2576 mice.
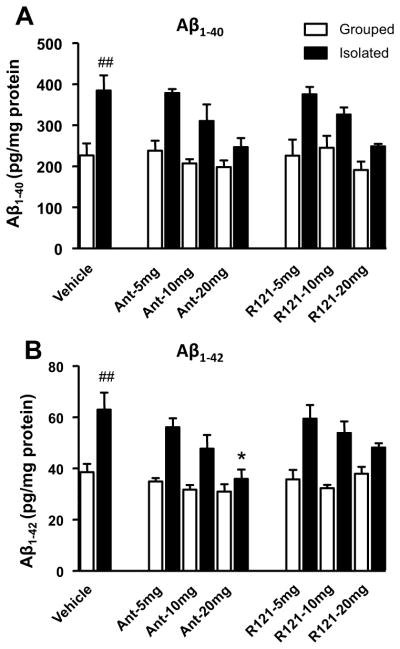
Animals with one week of exposure to isolation stress (isolated) were associated with a significant increase in Aβ1-40 (A, ##P<.001) and Aβ1-42 levels (B: ## P< .001) as compared to group housed (Grouped) mice. Administration of antalarmin showed dose-dependent decreases of Aβ1-42 levels in isolated mice. Post-hoc testing indicated that administration of antalarmin at a dose of 20mg/kg, but not at 10 mg/kg and 5mg/kg, significantly reduced levels of Aβ1-42 (B: *P<.05, drug versus vehicle).
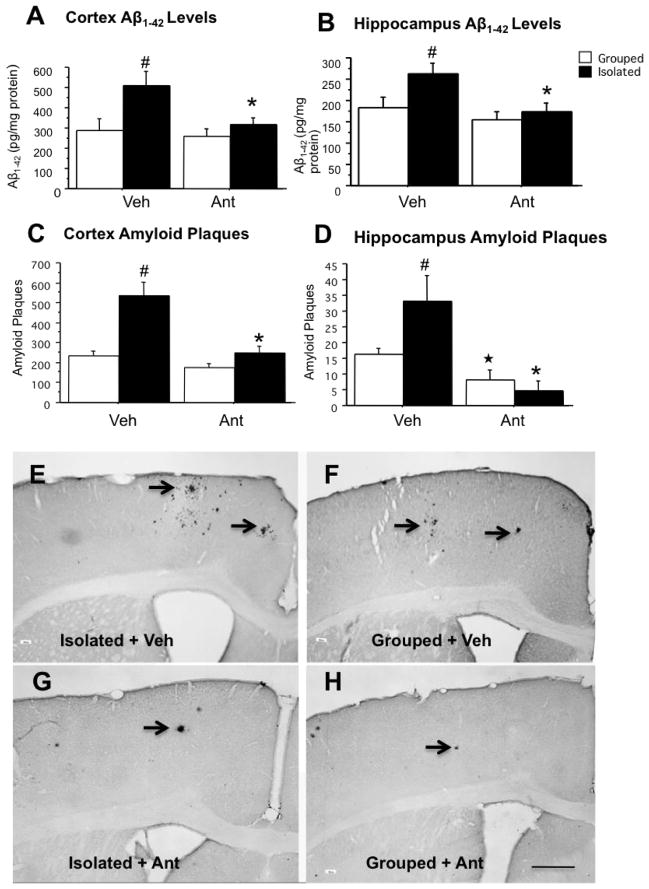
Effect of six-months administration of antalarmin on tissue Aβ1-42 levels and amyloid plaque deposition
Because antalarmin administration at 20 mg/kg was associated with reductions of Aβ1-42 levels in isolated mice under subacute conditions, we used antalarmin at this same dose to test the effects of a CRF1 antagonist on tissue Aβ1-42 levels and amyloid plaque deposition in mice chronically stressed in isolated housing conditions. Two-way ANOVA indicated a significant effect of housing (F1,21=6.17, P=.024), a significant effect of antalarmin administration (F1,21=15.92, P=.001), and a trend towards a significant housing x antalarmin interaction (F1,21=3.45, P=.082) on Aβ1-42 levels. Post-hoc tests indicated that chronic isolation stress significantly increased Aβ1-42 levels in the cortex and hippocampus, and that these increases were blocked by antalarmin administration (Figure 2A and B). Also, there was a significant effect of housing (F1,21=21.001, P<.001), a effect of antalarmin (F1,21=17.585, P<.001), and a housing x antalarmin interaction (F1,21=7.475, P=.015) on Aβ plaque number in the cortex, and an effect of housing (F1,21=14.984, P=.001), and a trend towards a housing x antalarmin interaction (F1,21=4.385, P=.052) on Aβplaque number in the hippocampus. Furthermore, there was a significant effect of housing (F1,21=29.913, P<.0001) and a drug effect (F1,21=12.874, P=.003) on whole brain amyloid plaque burden. Post-hoc tests again indicated that chronic isolation stress significantly increased Aβ plaque deposition in the cortex (P< 0.001) and hippocampus (P< 0.05) (Figure 2C, D, E and F), and that long-term antalarmin administration significantly blocked the effects of isolation on Aβ plaque deposition in both the cortex and hippocampus (Figure 2C, D, G and H).
Figure 2. Antalarmin reduces tissue Aβ1-42 levels and Aβ plaque deposition in chronically stressed Tg2576 mice.
Tissue Aβ levels. Chronic isolation stress significantly increased Aβ1-42 levels as compared to grouphoused animals in the cortex and hippocampus (A, B: #P< .05) at ten months of age. However, chronic administration of antalarmin (20 mg/kg) significantly decreased Aβ1-42 levels as compared to the vehicle in isolated mice (A, B: *P< .05).
Aβ plaque deposition. The number of Aβ immunohistochemical positive plaques in the cortex and hippocampus (C, D: ## P< .01, #P< .05) were significantly increased in isolated mice as compared to group-housed mice. Antalarmin administration (20 mg/kg) significantly decreased Aβ plaque deposition in the cortex of isolated mice (C: *P<.01: Isolated +Ant versus Isolated +Veh) and decreased Aβ plaque deposition in the hippocampus of both isolated mice; and group-housed mice (D: *P< .05, Isolated +Ant versus Isolated +Veh; ★P<.05: Grouped +Ant versus Grouped + Veh).
Representative Aβ-immunocytochemical stained sections are shown from the cortex of 10-month-old Tg2576 mice from housed in isolation (Isolated, E, G) or under group-housed conditions (Grouped, F, H) and treated with antalarmin. Arrowheads indicate the plaques. Scale Bar =200 μm shown in panel H also applies to panels E, F, and G.
Effect of six-months administration of antalarmin on plasma corticosterone levels
Two-way repeated measure ANOVA indicated a significant effect of housing (F1,24 = 143.79, P< .0001) and a significant housing x antalarmin interaction (F1,24 = 28.109, P= .0002) on plasma corticosterone levels (e.g., baseline, after one week and after 6 months of antalarmin administration). Post-hoc tests indicated that isolation housing significantly increased corticosterone levels after chronic isolation stress and that antalarmin administration for 1 week and 6 months (P< .0001) blocked the effects of isolation increased corticosterone levels (Figure 3).
Figure 3. Antalarmin decreases plasma corticosterone levels in chronically stressed Tg2576 mice.
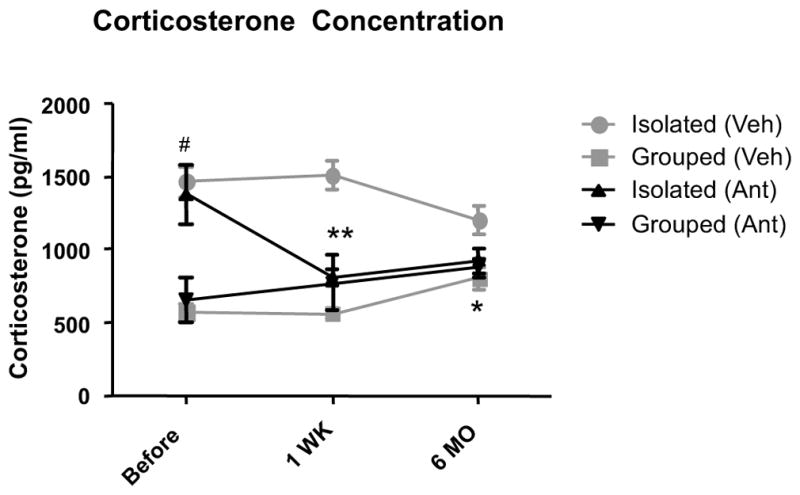
Isolation housing beginning at weaning until 4 months of age significantly increased the plasma corticosterone levels at 4 months of age (#P<.001: Isolated+Veh vs. Grouped+Veh). Antalarmin administration significantly decreased the plasma corticosterone levels in isolated mice as compared to isolated vehicle group after 1 week (**P<.001) and after 6 months(* P< 0.05 Isolated + Veh vs. Isolated +Ant). Antalarmin had no significant effects on plasma corticosterone levels in group-housed mice.
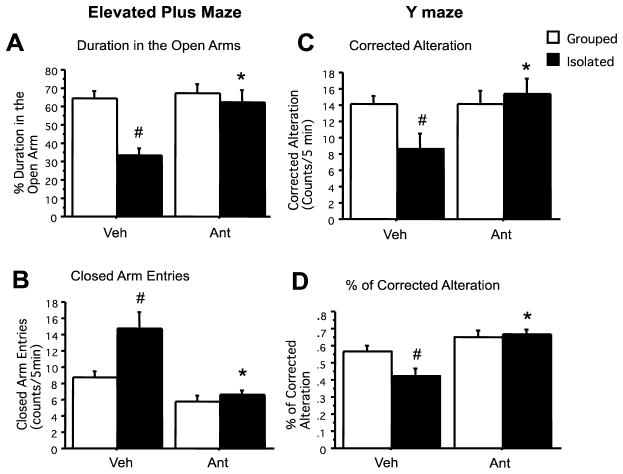
Effect of six-months administration of antalarmin on anxiety- and memory-related behaviors
Using the elevated plus maze test as a measure of anxiety-related behavior, a two-way ANOVA indicated a significant effect of housing (F1,22=12.44, P=.003), a significant effect of antalarmin (F1,22=9.83, P=.006) and a significant housing x antalarmin interaction (F1,22=6.49, P=.022) on the amount of time mice spent in the open arm. Similar effects of housing (F1,22=8.93, P=.009) antalarmin (F1,22=24.21, P<.001), and a housing x antalarmin interaction (F1,22=5.22, P=.036) on closed arm entries were also found. Finally, a significant effect of housing (F1,22 =9.67, P=.007) was found on the amount of time spent in the center. Post-hoc tests indicated that isolation stress reduced the amount of time mice spent in the open arm (P<.01), and that these effects were blocked by antalarmin administration (Figure 4A and B).
Figure 4. Antalarmin blocks stress–induced increases in anxiety and memory-related behavior related behavior in Tg2576 mice.
Chronic isolation stress significantly decreases the amount of time in the open field arm (A: #P<.01) and increases the number of entries in the closed arm compared to group-housed mice (B: #P<.05). Antalarmin administration ameliorated isolation stress–induced anxious behavior by increasing the duration in the open arm and decreasing the number of entries in the closed arm compared to isolated vehicle-treated mice (A, B: *P<.01). After chronic isolation stress, Tg2576 mice demonstrated less alternation and decreased rate of correct alternation as compared to group-housed mice (C, D: #P<.05). Again, antalarmin administration significantly restored number (C) and percentage (D) of correct alternation behaviors as compared to the isolated vehicle group (C, D: *P<.01). There was no difference in the total arm entries between isolated and group-housed animals.
Using a spontaneous alternation test to assess memory-related behavior, a two-way ANOVA indicated a significant effect of antalarmin (F1,22= 18.76 P<.001) and a significant housing x antalarmine interaction (F1,22= 5.12. P=0.043) on the number and percentage (%) of correct alternations. Post-hoc tests indicated that isolation stress decreased the number and percentage of correct alternations as compared to group-housing (P<.05), and that these effects were blocked by antalarmin administration. (Figure 4C and D).
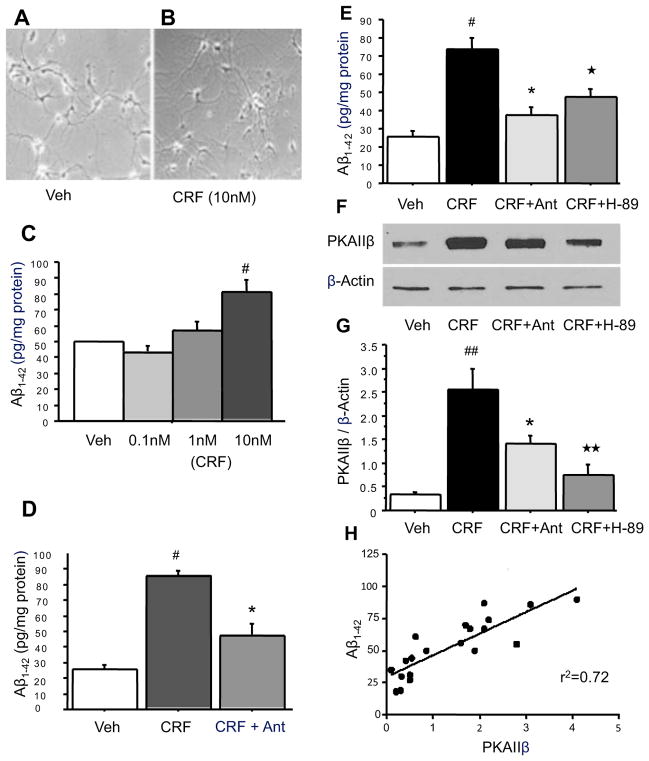
Effects of CRF and antalarmin on Aβ levels and the cAMP/PKA signaling pathway in cultured hippocampal neurons
Because CRF1 is a G-protein–coupled receptor that alters cellular function through the cAMP/PKA signaling pathway in neuronal systems (Jedema and Grace, 2004; Hauger et al., 2006), we measured Aβ1-42 and PKAIIβ levels after 48 hours of exposure to CRF, antalarmin and H-89, using ELISA and western blot analyses in hippocampal neurons cultured from Tg2576 mice. MTT cell toxicity assays indicated there was no effect of CRF administration on neuronal growth at 16 days with CRF for 48 hours in cultured hippocampal neurons [Figure 5A (vehicle without CRF) vs. B (10nM CRF for 48 hours)].
Figure 5. Antalarmin inhibits the effect of CRF on Aβ1-42 levels through the cAMP/PKA signaling pathway.
Primary hippocampal neurons derived from Tg2576 mice cultured at 16 days without CRF (A) and with CRF for 48 hours (B). ELISA analysis indicates that 10nM but not 0.01nM or 1nM CRF administered to medium of primary neurons at 14 days significantly increased Aβ1-42 levels after 48 hours of CRF exposure as compared to vehicle control (C: #P<.05). Treatment with 10nM CRF plus 100nM antalarmin blocked the increases of Aβ1-42 levels compared to CRF treated groups (D: #P<.001: CRF vs. Veh; *P<.01: CRF vs CRF+Ant). H-89, a PKA inhibitor, co-treated with CRF blocked CRF effect on Aβ1-42 levels (E: ★P<.05: CRF vs CRF+H-89). Western blotting analysis indicates that CRF significantly increased PKAIIβ expression, and antalarmin can block this effect. Treatment of PKA inhibitor H-89 also blocked CRF induced increase of PKAIIβ expression (F, G: #P<.001: CRF vs. Veh; *P<.05: CRF vs CRF+Ant; ★★P<.01: CRF vs CRF+H-89). Linear regression analysis indicates that Aβ1-42 levels are highly correlated with PKAIIβ expression (J: r2 = 0.72, P<.0001).
Two-way ANOVA indicated a significant effect of CRF administration on Aβ1-42 (F3,16 = 15.791, P< .0001) and PKAIIβ levels (F3,16 = 27.470, P< .0001) in cultured hippocampal neurons. Administration of CRF (10nM) into the cultured medium at 14 days for 48 hours resulted in a significant increase in Aβ1-42 levels (P=.007) (Figure 5C), and administration of 100nM antalarmin in addition to 10nM CRF blocked this increase in Aβ1-42 levels (P=.001) (Figure 5D). Moreover, administration of H-89 in addition to CRF also blocked the effect of CRF on Aβ1-42 levels (P<.0001, CRF vs. CRF+H-89, Figure 5E). Similarly, antalarmin and H-89 also blocked CRF-induced increases in PKAIIβ levels in cultured hippocampal neurons (P<.005, CRF vs. CRF+H-89, Figure 5F and G). There was a significant correlation between Aβ1-42 and PKAIIβ levels in cultures during CRF administration (r2 = 0.72, P<.0001) (Figure 5H).
Discussion
In this study, we evaluated the effect of CRF1 antagonists on tissue Aβ levels and Aβ plaque deposition, as well as behaviors related to anxiety and memory in Tg2576 mice, a commonly used animal model of AD. The effects of CRF1 antagonists were evaluated as a means of reducing the effects of isolation stress under both subacute and chronic conditions. In the subacute study, main effects of both housing and drug administration were observed, but not a significant housing x drug administration interaction, which suggests that CRF1 antagonists may be able to reduce Aβ levels through both stress-related and stress-unrelated mechanisms under subacute conditions. Because antalarmin at a dose of 20 mg/kg displayed the strongest effect in this paradigm, we then used this drug dose to investigate the long-term effects of CRF1 antagonists Aβ plaque deposition and behavior. We then found that 6-months of administration of antalarmin to isolated and group-housed mice significantly decreased not only tissue Aβ1-42 levels, but also amyloid plaque deposition and stress-related changes in anxiety- and memory-related behaviors. Under these conditions significant housing x drug administration effects were often observed, suggesting that antalarmin was exerting its effects mainly by blocking the stress-related effects. The observed changes in plasma corticosterone with and without CRF1 antagonist treatment also support the hypothesis that CRF-mediated effects on the HPA axis function may play a critical role involved in the process of amyloid deposition and the pathogenesis of AD. Finally, in our study of hippocampal neurons in culture, we found that CRF could directly stimulate Aβ production without the involvement of the HPA axis, and that CRF1 could reverse the effects of CRF on its signal transduction elements and on Aβ production. These results suggest that CRF and CRF1 may regulate Aβ production both through the HPA axis and as a direct neuromodulator of neuronal activity throughout the cortex and hippocampus (de Souza, 1988; Orozco-Cabal et al., 2006; Gallagher et al., 2008).
Stress has been increasingly recognized as an external factor that can impact the onset and progression of AD (Dong et al., 2004; Wilson et al. 2006; Csernansky et al., 2006; Huang et al., 2010; Alkadhi, 2012). Although the mechanisms involved in the effects of stress on the pathogenesis of AD remain unclear, there is growing evidence that CRF and CRF1 receptors play a critical role in the production and aggregation of both Aβ and tau (Kang et al., 2007; Rissman et al., 2007; Carroll et al., 2011; Rissman et al., 2012). Thus, improving our understanding of the precise cellular pathways involved in mediating the effects of CRF and CRF1 receptor on Aβ metabolism will help to identify new drug therapy targets.
CRF exerts its cellular effects by activating one of two known G-protein–coupled receptors: CRF1 and CRF2. Since CRF binds to CRF1 with ~40-fold higher affinity, most of its activity in the CNS has been attributed to the activation of CRF1(Wood and Woods, 2007). CRF1 receptor stimulation primarily activates the cAMP/PKA-signaling cascade in neuronal systems (Jedema and Grace, 2004; Hauger et al., 2006). However, CRF can also couple with the phospholipase C (PLC)/PKC pathway (Ungless et al., 2003; Hauger et al., 2006) through CRF receptors. Activation of CRF1 generally stimulates adenylate cyclase activity, thereby increasing levels of cAMP.
Stress can impact prefrontal cortical function by increasing catecholamine release in the prefrontal cortex, which in turn increases cAMP and PKC intracellular signaling. Under certain conditions, this increase can lead to reductions in prefrontal neuronal firing and impairments in working memory (Birnbaum et al., 2004, Hains and Arnsten, 2008). The highest level of CRF-induced adenylate cyclase activation is in the cerebral cortex, an area that is profoundly involved in AD (de Souza, 1988; Martin et al., 2005). Recent studies indicate that PKC plays an important role in the pathophysiology of AD (Choi et al., 2006; Takashima, 2006; Takashima, 2006; Nelson et al., 2009), and increases in PKC activity can stimulate Aβ peptide production and tau protein hyperphosphorylation. In addition, other studies have demonstrated that GPCRs and PKA/PKC signaling pathways play an important role in the pathophysiology of AD (Lee et al., 2004; Choi et al., 2006; Takashima, 2006; da Cruz e Silva et al., 2009; Nelson et al., 2009; de Barry et al., 2010; Kim et al., 2011; Thathiah and De Strooper, 2011;; Thathiah et al., 2013). For example, some GPCRs directly influence the amyloid cascade through modulation of α-,β- and secretases, thereby affecting the proteolysis of the APP (Xu et al., 1996; Robert et al., 2001; Thathiah and De Strooper, 2011; Thathiah et al., 2013).
In our study, we found that CRF-induced increases of amyloid Aβ1-42 levels in cultured hippocampal neurons were highly correlated with increase in PKAIIβ expression. PKAIIβ is a major mediator of the actions of cAMP in the central nervous system of mammals (Li et al., 1996) and a key factor of the downstream in the CRF/CRF1 pathway. Further introduction of antalarmin into the medium of cultured neurons for 48 hours significantly blocked CRF-induced increases of Aβ1-42, as well as increases in PKAIIβ expression. Similarly, treatment with H-89, a PKA inhibitor, blocked CRF-induced increases of Aβ1-42 and PKAIIβ expression. These results suggest that the cAMP/PKA signaling pathway mediates at least some of the effects of CRF and CRF1 on amyloid production in a mouse model of AD. A limitation of the present study is that only PKAIIβ expression and one PKA inhibitor H-89 was tested. These results need be confirmed by the use of other PKA inhibitors, and the study of other elements of the cAMP/PKA signaling pathway, such as the phosphorylation of cAMP response element binding (CREB) transcription factors.
Preclinical studies and clinical trials on CRF1 antagonists have been tested in the treatment of depression, anxiety and irritable bowel disorder. Unfortunately, CRF1 antagonists have had a poor record of success as anti-depressants, perhaps because of problems with bioavailability (Zorrilla and Koob, 2004, 2010; Nielsen, 2006, Binneman et al., 2008). Nevertheless, new CRF1 antagonists are being developed, perhaps with better pharmacodynamics and pharmacokinetic properties and may be available for clinical testing soon (Zorrilla and Koob, 2010). The therapeutic potential of CRF1 antagonists as treatments for neurodegenerative disorders has not yet been evaluated although previous studies on Tg2576 mice shown some evidence CRF1 antagonists block stress-induced Aβ accumulation (Kang et al., 2007; Lee et al., 2009), and there have been recent reports of the beneficial effects of CRF1 antagonists on tau phosphorylation (Carroll et al., 2011; Rissman et al., 2012). The present study is, to our knowledge, the first to evaluate the long-term effects of CRF1 antagonist administration on Aβ production, plaque deposition and behavior in an animal model of AD. Our results add to the rationale for the further study of CRF1 antagonists in animal models of AD, and in time, clinical trials in AD patients. Since it is not yet possible to interfere with genetic predisposition to AD, the possibility of delaying the onset or progression of neuropathology and concomitant cognitive decline by overcoming a common and substantial environmental factor (ie, psychosocial stress) may be an attractive strategy.
In summary, our results demonstrate that CRF1 antagonists can decrease Aβ levels and slow Aβ plaque deposition in Tg2576 mice under both stressed and unstressed conditions. CRF1 antagonists also preserve memory-related behaviors in isolation-stressed mice. On the basis of an in vitro experiment, it appears likely that CRF1 and CRF1 antagonists regulate Aβ and behavior through cAMP/PKA signaling pathways. These studies suggest the possibility that these agents, by virtue of their ability to mitigate the effects of stress on the pathogenesis of AD, should be considered as treatments for delaying the onset or progression of AD.
Acknowledgments
This work was supported by the Alzheimer’s Drug Discovery Foundation (grant 20111208, JGC). A portion of this research was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism, NIH, US Department of Health and Human Services (KCR).
Drs. Csernansky and Dong have received research grants from the NIMH, NIA, and Dr. John G. Csernansky has served as a Data Safety and Monitoring Board (DSMB) member for Eli Lilly and Sanofi-Aventis, and has received funding for his research from Genentech.
Footnotes
The rest of the authors declare that they have no competing financial interests.
References
- Alkadhi KA. Chronic psychosocial stress exposes Alzheimer’s disease phenotype in a novel at-risk model. Front Biosci (Elite Ed) 2012;4:214–229. doi: 10.2741/371. [DOI] [PubMed] [Google Scholar]
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ. Chronic Stress Exacerbates Tau Pathology, Neurodegeneration, and Cognitive Performance through a Corticotropin-Releasing Factor Receptor-Dependent Mechanism in a Transgenic Mouse Model of Tauopathy. J Neurosci. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wang D, Yu GQ, Zhu G, Kharazia VN, Paredes JP, Chang WS, Deitchman JK, Mucke L, Messing RO. PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice. Proc Natl Acad Sci U S A. 2006;103:8215–8220. doi: 10.1073/pnas.0509725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, Ricobaraza A, Frechilla D, Franco R, Perez-Media villa A, Garcia-Osta A. Chronic mild stress accelerates the onset and progression of the Alzheimer’s disease phenotype in Tg2576 mice. J Alzheimers Dis. 2012;28:567–578. doi: 10.3233/JAD-2011-110572. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva OA, Rebelo S, Vieira SI, Gandy S, da Cruz e Silva EF, Greengard P. Enhanced generation of Alzheimer’s amyloid-beta following chronic exposure to phorbol ester correlates with differential effects on alpha and epsilon isozymes of protein kinase C. J Neurochem. 2009;108:319–330. doi: 10.1111/j.1471-4159.2008.05770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barry J, Liegeois CM, Janoshazi A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp Gerontol. 2010;45:64–69. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- de Souza EB. CRH defects in Alzheimer’s and other neurologic diseases. Hosp Pract (Off Ed) 1988;23:59–71. doi: 10.1080/21548331.1988.11703535. [DOI] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Harrington CR. The molecular pathology of Alzheimer’s disease. Neuroimaging Clin N Am. 2012;22:11–22. vii. doi: 10.1016/j.nic.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Chau SA, Kircanski I, Lanctot KL. Current and emerging drug treatment options for Alzheimer’s disease: a systematic review. Drugs. 2011;71:2031–2065. doi: 10.2165/11595870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, Meynen G, Endert E, Hofman MA, Swaab DF. Increased cerebrospinal fluid cortisol level in Alzheimer’s disease is not related to depression. Neurobiol Aging. 2006;27:780e781–780 e782. doi: 10.1016/j.neurobiolaging.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Liang KC, Chang YY, Ke HC, Lin JY, Hsieh-Li HM. The interaction between acute oligomer Abeta(1-40) and stress severely impaired spatial learning and memory. Neurobiol Learn Mem. 2010;93:8–18. doi: 10.1016/j.nlm.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Hinton DJ, Choi DS. Protein kinase C-regulated abeta production and clearance. Int J Alzheimers Dis. 2011;2011:857368. doi: 10.4061/2011/857368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- Lee W, Boo JH, Jung MW, Park SD, Kim YH, Kim SU, Mook-Jung I. Amyloid beta peptide directly inhibits PKC activation. Mol Cell Neurosci. 2004;26:222–231. doi: 10.1016/j.mcn.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Li Y, Ndubuka C, Rubin CS. A kinase anchor protein 75 targets regulatory (RII) subunits of cAMP-dependent protein kinase II to the cortical actin cytoskeleton in non-neuronal cells. J Biol Chem. 1996;271:16862–16869. doi: 10.1074/jbc.271.28.16862. [DOI] [PubMed] [Google Scholar]
- Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med. 2005;7:3–36. doi: 10.1385/nmm:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2009;667:82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism. 2008;57(Suppl 2):S44–49. doi: 10.1016/j.metabol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Yarimizu J, Saita K, Uchino H, Akashiba H, Shitaka Y, Ni K, Matsuoka N. Differential effects between gamma-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012;32:2037–2050. doi: 10.1523/JNEUROSCI.4264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TJ, Cui C, Luo Y, Alkon DL. Reduction of beta-amyloid levels by novel protein kinase C(epsilon) activators. J Biol Chem. 2009;284:34514–34521. doi: 10.1074/jbc.M109.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DM. Corticotropin-releasing factor type-1 receptor antagonists: the next class of antidepressants? Life Sci. 2006;78:909–919. doi: 10.1016/j.lfs.2005.06.003. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer’s disease. Br J Psychiatry. 1996;168:679–687. doi: 10.1192/bjp.168.6.679. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Shinnick-Gallagher P, Gallagher JP. Regulation of synaptic transmission by CRF receptors. Rev Neurosci. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Pomara N, Singh RR, Deptula D, LeWitt PA, Bissette G, Stanley M, Nemeroff CB. CSF corticotropin-releasing factor (CRF) in Alzheimer’s disease: its relationship to severity of dementia and monoamine metabolites. Biol Psychiatry. 1989;26:500–504. doi: 10.1016/0006-3223(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Rehman HU. Role of CRH in the pathogenesis of dementia of Alzheimer’s type and other dementias. Curr Opin Investig Drugs. 2002;3:1637–1642. [PubMed] [Google Scholar]
- Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci. 2007;27:6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Staup MA, Lee AR, Justice NJ, Rice KC, Vale W, Sawchenko PE. Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. Proc Natl Acad Sci U S A. 2012;109:6277–6282. doi: 10.1073/pnas.1203140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert SJ, Zugaza JL, Fischmeister R, Gardier AM, Lezoualc’h F. The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J Biol Chem. 2001;276:44881–44888. doi: 10.1074/jbc.M109008200. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Swanwick GR, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s disease: lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmacotherapies for Alzheimer’s disease: beyond cholinesterase inhibitors. Pharmacol Ther. 2012;134:8–25. doi: 10.1016/j.pharmthera.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Horre K, Snellinx A, Vandewyer E, Huang Y, Ciesielska M, De Kloe G, Munck S, De Strooper B. beta-arrestin 2 regulates Abeta generation and gamma-secretase activity in Alzheimer’s disease. Nat Med. 2013;19:43–49. doi: 10.1038/nm.3023. [DOI] [PubMed] [Google Scholar]
- Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: a cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Vale WW, Zweig RM, Singer HS, Mayeux R, Kuhar MJ, Price DL, De Souza EB. Reductions in corticotropin releasing factor-like immunoreactivity in cerebral cortex in Alzheimer’s disease, Parkinson’s disease, and progressive supranuclear palsy. Neurology. 1987;37:905–909. doi: 10.1212/wnl.37.6.905. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- Wood SK, Woods JH. Corticotropin-releasing factor receptor-1: a therapeutic target for cardiac autonomic disturbances. Expert Opin Ther Targets. 2007;11:1401–1413. doi: 10.1517/14728222.11.11.1401. [DOI] [PubMed] [Google Scholar]
- Xu H, Sweeney D, Greengard P, Gandy S. Metabolism of Alzheimer beta-amyloid precursor protein: regulation by protein kinase A in intact cells and in a cell-free system. Proc Natl Acad Sci U S A. 1996;93:4081–4084. doi: 10.1073/pnas.93.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora BM, Jiang M, Wang Y, Chai M, Lawson PT, Lawson GW. Decreased blastocyst production in mice exposed to increased rack noise. J Am Assoc Lab Anim Sci. 2009;48:486–491. [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]