Abstract
Rationale
Extinction of drug seeking is facilitated by NMDA receptor (NMDAr) agonists, but it remains unclear whether extinction is dependent on NMDAr activity.
Objectives
We investigated the necessity of NMDArs for extinction of cocaine seeking, and whether extinction altered NMDAr expression within extinction-related neuroanatomical loci.
Methods
Rats were trained to lever press for i.v. infusions of cocaine or sucrose reinforcement prior to extinction training or withdrawal.
Results
Administration of the NMDAr competitive antagonist CPP prior to four brief extinction sessions impaired subsequent extinction retention. In contrast, post-extinction administration of the NMDAr coagonist D-serine attenuated lever pressing across days as compared to saline administration, indicative of facilitated consolidation of extinction. Furthermore, expression of the NMDAr subunits, GluN2A and GluN2B, was not altered in the ventromedial prefrontal cortex. However, both GluN2A and GluN2B subunit expression in the nucleus accumbens was increased following cocaine self-administration, and this increased expression was relatively resistant to modulation by extinction.
Conclusions
Our findings demonstrate that extinction of cocaine seeking is bidirectionally mediated by NMDArs and suggest that selective modulation of NMDAr activity could facilitate extinction-based therapies for treatment of cocaine abuse.
Keywords: D-serine, extinction learning, NR2B, NR2A, medial prefrontal cortex, NMDA receptor, nucleus accumbens, Western blot, substance abuse
Introduction
Cues associated with cocaine use can induce craving and relapse in addicts (Childress et al. 1986; Hyman and Malenka 2001). Therefore, reducing cue reactivity can attenuate craving and prevent relapse. One way to achieve this is through extinction. Extinction results in the formation of a new memory that inhibits the expression of the associations made between the cues and the rewarding effects of the drug (Millan et al. 2011; Quirk and Mueller 2008). Extinction has been studied in multiple paradigms (Millan et al. 2011; Myers et al. 2011; Myers and Davis 2007; Quirk and Mueller 2008), but the mechanisms underlying extinction of cocaine seeking in a cocaine self-administration paradigm are unclear.
Recent evidence indicates that enhancing N-methyl-D-aspartate receptor (NMDAr) function can facilitate extinction. Administration of the NMDAr partial agonist d-cycloserine (DCS) or coagonist D-serine can facilitate extinction of fear-potentiated startle (Fiorenza et al. 2012; Walker et al. 2002), conditioned fear (Langton and Richardson 2008; Ledgerwood et al. 2003; Matsuda et al. 2010) and conditioned place preference (Botreau et al. 2006; Hammond et al. 2012). Furthermore, pre- or post-session administration of DCS facilitates extinction of cocaine seeking in a short-access self-administration paradigm when discrete cues are presented (Nic Dhonnchadha et al. 2010; Thanos et al. 2011a; Thanos et al. 2011b), and D-serine administered before each extinction session without discrete cues reduces lever pressing on days one and three (Kelamangalath et al. 2007). DCS or D-serine administered during extinction can also attenuate reinstatement of a conditioned place preference (Paolone et al. 2009), and following long-access cocaine self-administration and context only extinction, D-serine can reduce low dose cocaine-induced reinstatement (Kelamangalath et al. 2009; Kelamangalath and Wagner 2010). Numerous studies have examined the effect of DCS on multiple aspects of extinction (Nic Dhonnchadha and Kantak 2011); however, it is unclear whether enhancing NMDAr function with D-serine facilitates acquisition or consolidation of extinction.
Despite the evidence that NMDAr agonists can facilitate extinction, there are surprisingly few studies investigating whether NMDArs are necessary for extinction of cocaine seeking. A previous study found that injections of a low dose of the competitive NMDAr antagonist (±)3-(2-carboxypiperazin-4yl)propyl-1-phosphonic acid (CPP) had no effect on extinction of cocaine seeking without discrete cues in a self-administration paradigm (Kelamangalath et al. 2007). However, this dose has been shown to be ineffective at disrupting extinction compared to a higher dose in a fear-learning paradigm (Burgos-Robles et al. 2007). Moreover, previous research has indicated that the NMDAr subunits GluN2A and GluN2B are important in learning (Bannerman et al. 2008; Brigman et al. 2008) and acquisiton or extinction of fear conditioning, respectively (Dalton et al. 2012; Gilmartin et al. 2013; Nakanishi 1992; Sotres-Bayon et al. 2007; Sotres-Bayon et al. 2009; Tang et al. 1999). Furthermore, depending on whether long- or short-access self-administration is conducted, NMDAr expression is differentially altered following withdrawal or extinction in the medial prefrontal cortex (mPFC) or the nucleus accumbens (NAc; Ben-Shahar et al. 2007; Ben-Shahar et al. 2009; Crespo et al. 2002; Lu et al. 2003; Self et al. 2004), but others have reported no change (Ghasemzadeh et al. 2011). These structures, the ventromedial PFC (vmPFC) and NAc, are also involved in extinction of drug seeking (Millan et al. 2011; Peters et al. 2009). Whether extinction changes the expression of NMDAr subunits in these brain regions, however, is unclear. Therefore, we investigated the requirement of NMDArs and their expression following extinction of cocaine seeking.
To determine the necessity of NMDAr activity in extinction of cocaine seeking, we examined the effect of NMDAr blockade prior to extinction on subsequent extinction retention. Next, we determined whether enhancing NMDAr function during consolidation of extinction would facilitate extinction. We then examined glutamate receptor expression in the vmPFC and NAc following extinction. Results indicate that NMDArs can bidirectionally mediate extinction, and that cocaine self-administration increases the expression of NMDArs in the NAc.
Materials and Methods
Subjects
Male Long-Evans rats (Harlan) weighing 250-300g were housed and handled as previously described (Otis and Mueller 2011). Rats were food restricted (13-28g/d rat chow) throughout the experiment except during surgery and recovery. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Milwaukee in accordance with National Institutes of Health guidelines.
Lever response training
Sucrose and cocaine self-administration procedures were conducted using 12 sound-attenuated operant conditioning chambers (MED Associates). Chambers were equipped with two retractable levers with a stimulus light above each lever, a food hopper between the levers, a house light, a rod grid floor, and a speaker/tone generator (65 Hz, 65 dB). One week after arrival, rats were trained to press for sucrose on a fixed ratio-1 (FR1) schedule of reinforcement. At the beginning of each session, the house light and right stimulus light turned on, and the right lever extended. Active lever presses resulted in delivery of a 20mg sucrose pellet. When criteria were met (minimum 100 responses in 10 h), sessions were shortened to 90 min on a FR1 schedule with a minimal criterion of 50 responses/session.
Surgery
Following lever response training, rats were implanted with chronic intravenous catheters (assembled by Access Technologies) using a modified procedure described previously (Grigson and Twining 2002). Briefly, rats were anesthetized with a mixture of ketamine/xylazine (87/13 mg/kg, i.p.). A catheter consisting of a small polyurethane tube was inserted into the right jugular vein. The small tube was connected through a larger polyurethane tube to a backmount pedestal (Plastics One) implanted behind the shoulder blades. The catheter had polyester mesh above the side tubing to facilitate tissue regrowth, and a 2.5 by 2.5cm mesh screen (Small Parts) permanently fixed to the base via cyanoacrylic gel to stabilize it subcutaneously. At the end of surgery, a stylet was placed in the exposed catheter port. Following surgery, rats were administered penicillin g procaine (75,000 units in a 0.35ml, s.c.) to control infection and carprofen (5mg in 0.1ml, s.c.) to relieve pain. Rats recovered for at least five days before catheter patency was verified with 0.3ml of 1% Propofol (i.v.), a short acting anesthetic that causes an immediate loss of muscle tone. Rats were given a minimum of seven days to recover prior to behavioral testing. Catheter patency was maintained with heparinized saline (~0.2 cc of 60 i.u./ml) daily during the experiment.
Drugs
Cocaine HCl (National Institute of Drug Abuse) was dissolved in sterile 0.9% saline and administered intravenously (i.v.) at 0.25mg per infusion for 3 s or at 10 mg/kg, i.p. for cocaine-induced reinstatement testing. CPP (Tocris) and D-serine (Sigma-Aldrich) were dissolved in sterile 0.9% saline and administered at a dose of 10 mg/kg, i.p. and 100 mg/kg, i.p., respectively. These doses were determined based on previous research using CPP (Burgos-Robles et al. 2007; Santini et al. 2001) or D-Serine (Kelamangalath et al. 2009).
Cocaine self-administration procedures
Rats were trained to self-administer cocaine daily for 90 min sessions on a FR1 schedule or until the daily cap was reached (initially 20 infusions/session, progressively moved to 35). At the beginning of each session, the house light and right stimulus light turned on, and the right and left lever extended. When a rat pressed the active lever, the stimulus light turned off for 20 s, a tone (65 dB) sounded for 5 s, and a cocaine infusion was initiated for 3 s through a syringe pump located outside of the enclosure that was connected by a single channel swivel (Instech). Another cocaine infusion was not available until the stimulus light turned back on after 20 s. All lever presses, whether reinforced or not, are reported as active lever presses during acquisition, extinction, and reinstatement. Responding on the inactive lever was recorded, but had no programmed consequences. Rats were trained to self-administer cocaine for 18-19 sessions. In order to achieve stable drug seeking across rats, some rats (n=16) received a priming infusion at the start of the session or received additional acquisition sessions. Groups were matched on total number of infusions during self-administration, the average number of infusions over the last three days of self-administration, and if rats received priming infusions or extra sessions. A saline-treated control group was treated identically to the experimental groups (CPP or D-serine treated), and was included with each experiment to control for variability across time and across groups of self-administering rats as previously reported with these procedures (LaLumiere et al. 2010).
Experimental manipulations
Experiment one
Following cocaine self-administration, rats were matched and assigned to two groups, receiving either saline (1 ml/kg, i.p.; n=9) or CPP (10 mg/kg, i.p.; n=9) one h before each of four brief 45 min extinction sessions. Brief extinction sessions were used as previously described (LaLumiere et al. 2010) to better manipulate extinction in this paradigm. Following the brief sessions, rats were given a two-day break before extinction retention was tested during 90 min extinction sessions (days 5+). During extinction, lever presses resulted in the same programmed consequences as during self-administration, but no cocaine was administered.
Experiment two
Following cocaine self-administration, rats were matched and assigned to two groups receiving either saline (n=6) or D-serine (100 mg/kg, i.p.; n=6) immediately following each of four brief 45 min extinction sessions. Post-extinction injections were used to determine the role of NMDArs in consolidation of extinction and to control for state learning effects. Following the brief sessions, rats were given a two–day break before extinction retention was tested during 90 min extinction sessions (days 5+).
Reinstatement and locomotion procedures
Following extinction, rats received a priming injection of cocaine (10 mg/kg; i.p.) before undergoing normal extinction procedures to test for reinstatement. To determine whether CPP or D-serine altered general behavior, rats were injected with either saline, CPP (10 mg/kg, i.p.), or D-serine (100 mg/kg, i.p.) one h before being placed in locomotor activity chambers and photobeam breaks were recorded for 20 min (saline, n=5; CPP, n=6; D-serine, n=5).
Experiment three
Following cocaine self-administration, rats were matched and assigned to two groups: extinction (coc-ext; n=7) or no extinction (coc-noext; n=7). An additional control group was included, in which rats were treated the same as coc-ext rats but were reinforced with sucrose instead of cocaine (suc-ext; n=10) as previously described (Fischer et al. 2013; Fischer-Smith et al. 2012; Lu et al. 2003; Self et al. 2004; Sutton et al. 2003). Coc-ext and sucext rats underwent extinction procedures as above, whereas coc-noext rats did not undergo extinction (remained in home cages). Following extinction (day 13), all rats (coc-ext, coc-noext, and suc-ext) were tested the next day for extinction retention with the same extinction procedure used throughout the experiments. Extinction retention was tested on this day to conclude that the coc-ext rats had extinguished lever pressing as compared to the coc-noext rats. Immediately following the extinction session, rats were anesthetized with isoflurane gas, sacrificed, and had their brains extracted and frozen at -80°C for Western blot analysis.
Western blotting
The effects of extinction on the expression of glutamate receptors in the vmPFC and the NAc was examined using procedures previously described (Fortress et al. 2013). Samples were re-suspended in a 1:50 weight/volume dilution of lysis buffer containing phosphate and protease inhibitors, and were homogenized using a probe sonicator (Branson Sonifier 250). Protein concentrations of the homogenates were determined using a Bio-Rad protein assay (Bio-Rad). All samples were normalized to 2 μg/μl using 5X SDS/PAGE loading buffer and 1X lysis buffer, and were boiled for five min to denature proteins. Sample (10 μl aliquots) were loaded onto 4-15% Tris-HCl gels (Bio-Rad) and underwent electrophoresis for protein separation. Following separation, samples were transferred from the gel to a polyvinylidene fluoride (PVDF) membrane using a Trans Blot Midi transfer pack and Turbo transfer system (Bio-Rad). Membranes were blocked in 5% milk for one h at room temperature, followed by overnight incubation at 4°C in primary for the following antibodies: GluN2B, GluN2A, GluR1, and β-Actin (1:1000 for GluN2B and GluN2A; 1:800 for GluR1; 1:5000 for β-Actin; Cell Signaling). Subsequently, membranes were incubated in anti-rabbit IgG HRP-linked secondary antibody (1:5000 for GluR1 and GluN2A; 1:20000 for GluN2B and β-Actin; Cell Signaling) for one h, and developed using West Dura chemiluminescence (Pierce Laboratories). Images of protein expression were taken using a Gel Logic 6000 Pro Molecular Imager (Carestream), and were quantified by densitometry using the accompanying Carestream analysis/quantification software. There were no significant differences between groups in β-Actin expression in the vmPFC or the NAc, thus GluR1, GluN2B, and GluN2A expression were normalized to β-Actin levels. Data are expressed as relative average levels of immunoreactivity in arbitrary units (a.u.).
Data analysis
Average active and inactive lever presses, and number of infusions received during the last three days of self-administration were analyzed using independent sample t-tests. Drug seeking during extinction was analyzed by comparing active or inactive lever presses across days between saline and drug groups (CPP or D-serine) using a two-way repeated-measures analysis of variance (ANOVA). The last two extinction sessions for each rat was averaged and shown as the last extinction day to account for variability in the number of extinction sessions to criterion. Criterion was defined as a significant reduction in the average active lever presses during the last two extinction sessions as compared to day 5 with a paired t-test. Reinstatement was measured by comparing the average active lever presses made during the last two extinction sessions to those made after a priming-injection of cocaine and was analyzed separately with two-way repeated measures ANOVAs. For locomotor activity, the total number of photobeam breaks during the 20 min session was analyzed using a one-way ANOVA. From the total number of rats used in these experiments (n=80), 26 rats were removed from the analysis due to blocked or non-patent catheters (n=10), symptoms of illness prior to extinction conditions (n=9), not acquiring self-administration (n=6), or being two or more standard deviations away from the group mean throughout extinction training (n=1). Group sizes throughout the experimental manipulations section are reported as the final size, following the aforementioned removal of animals. For western blot analysis, mean optical densities were determined for each group and normalized to β-Actin, and data were analyzed using a one-way ANOVA. Outliers greater than two standard deviations from the mean were removed from the analysis (vmPFC: n=1 coc-noext was removed from the GluN2B protein expression analysis; NAc: n=1 suc-ext was removed from GluN2A and GluN2B expression analysis). Fisher’s least significant difference (LSD) post hoc tests were used, when appropriate, to identify significant pair-wise differences in lever-pressing behavior or protein expression.
Results
NMDArs are necessary for extinction of cocaine seeking
To determine the necessity of NMDArs for extinction of cocaine seeking, rats were injected with either saline or CPP before four 45 min extinction sessions. Extinction retention was tested on days 5-8 with 90-min drug-free extinction sessions. Active and inactive lever presses, and number of infusions were equivalent between groups across the average of the last three days of cocaine self-administration (Table 1). Both groups extinguished across the first four days of extinction (Figure 1A, left), but CPP-treated rats had impaired extinction retention during the 90 min drug-free extinction sessions (Figure 1A, right). For the brief extinction days (1-4; active lever presses), ANOVA revealed a significant effect of day (F3,64=7.554, p<0.0001), but no effect of treatment or day by treatment interaction. For the 90 min extinction days (5-8; active lever presses), ANOVA revealed a significant effect of day (F3,64=3.290, p=0.026) and treatment (F1,64=9.241, p=0.003), but no interaction. Thus, CPP treatment during the brief extinction sessions impaired subsequent extinction retention in the longer extinction sessions. For inactive lever presses during extinction days 1-4, ANOVA revealed a significant effect of day (F3,64=4.137, p=0.010), but no effect of treatment or day by treatment interaction. For inactive lever presses during extinction days 5-8, ANOVA revealed a significant effect of day (F3,64=3.572, p=0.019), treatment (F1,64=4.989, p=0.029), and a day by treatment interaction (F3,64=3.213, p=0.029). A subsequent ANOVA revealed that previously saline- and CPP-treated rats were significantly different on day 5 (F3,64=4.603, p=0.048), but not days 6-8. Furthermore, an additional ANOVA between active and inactive lever presses on day 5 revealed a significant difference between active and inactive levers (F3,64=15.207, p<0.0001), but no treatment by lever interaction. These results indicate that CPP-treated rats lever pressed more on the inactive lever than saline-treated rats on day 5, but both groups pressed the active lever significantly more than the inactive lever demonstrating goal-directed behavior. Taken together, CPP treatment during extinction disrupted subsequent extinction retention.
Table 1.
Average number of active and inactive lever presses made, or infusions received during the last three days of cocaine self-administration.
| Experiment | Treatment | Active lever presses | Inactive lever presses | Infusions |
|---|---|---|---|---|
| 1 | Saline | 42.33 ± 4.10 (9) | 0.89 ± 0.49 | 29.63 ± 0.75 |
| CPP | 39.07 ± 6.06 (9) | 2.19 ± 1.19 | 26.33 ± 2.10 | |
| 2 | Saline | 47.39 ± 12.74 (6) | 0.78 ± 0.46 | 30.00 ± 1.72 |
| D-serine | 35.00 ± 5.18 (6) | 3.06 ± 2.16 | 27.17 ± 2.11 | |
| 3 | Coc-ext | 30.29 ± 4.39 (7) | 3.81 ± 3.48 | 25.38 ± 2.89 |
| Coc-noext | 39.33 ± 5.97 (7) | 1.24 ± 0.57 | 27.05 ± 2.64 | |
| Suc-ext | 80.13 ± 6.53 (10) |
Numbers in the parentheses are group sizes; Coc-ext = extinction of cocaine seeking, Coc-noext = cocaine self-administration without extinction, Suc-ext = extinction of sucrose seeking.
Figure 1.
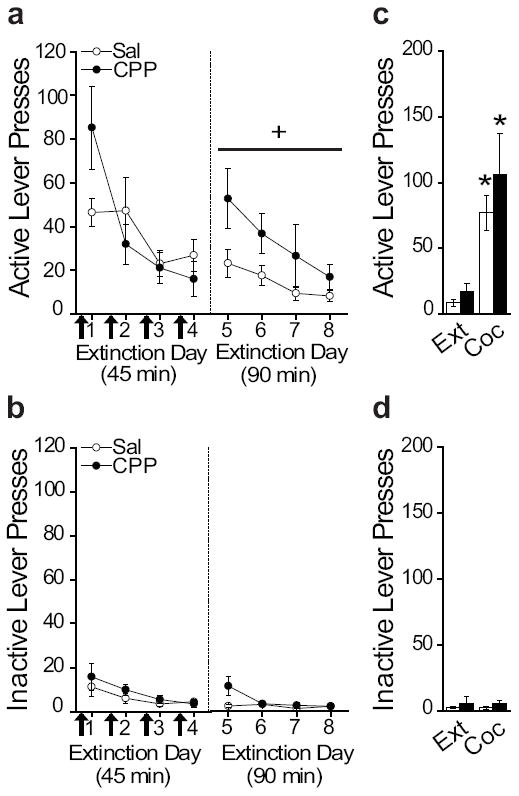
NMDArs are necessary for extinction. (A) Pre-extinction session injections of CPP (arrows) disrupted extinction retention. (B) Previous CPP treatment had no effect on cocaine-induced reinstatement. Inactive lever presses during (C) extinction and (D) reinstatement. *p<0.05, +p<0.05 for overall group difference
Following extinction, all rats were tested for cocaine-induced reinstatement of drug seeking. Rats were given a non-contingent priming injection of cocaine prior to a 90 min extinction session (Figure 1C,D). ANOVA revealed a significant increase in active lever pressing on the cocaine-induced reinstatement day compared to the last extinction day (F1,32=20.714, p<0.0001), but no effect of treatment or day by treatment interaction. Furthermore, ANOVA revealed no significant difference in inactive lever presses. Thus, previous treatment with CPP did not affect reinstatement.
Potentiation of NMDArs with D-serine facilitated subsequent extinction learning
To determine if NMDAr potentiation would facilitate the consolidation of extinction of drug seeking, rats were injected with saline or D-serine immediately following four 45 min extinction sessions. Extinction retention was tested on subsequent 90 min extinction sessions (day 5+). Active and inactive lever presses, and number of infusions were equivalent between groups across the average of the last three days of self-administration (Table 1). Both groups extinguished across the first four days of extinction (Figure 2A, left), but D-serine-treated rats had facilitated extinction across the subsequent 90 min extinction days (Figure 2A, right). For the brief extinction days (1-4; active lever presses), ANOVA revealed a significant effect of day (F3,40=3.254, p=0.031), but not treatment, or day by treatment interaction. During the full extinction days (5-23; active lever presses), ANOVA revealed a significant effect of treatment (F1,190=30.327, p<0.0001), but no effect of day or day by treatment interaction. Thus, D-serine treatment during the brief extinction sessions facilitated subsequent retention of extinction in the longer extinction sessions. For inactive lever presses during extinction days 1-4 (Figure 2B, left), ANOVA revealed no significant differences by day, treatment, or day by treatment interaction. For inactive lever presses during extinction days 5-23 (Figure 2B, right), ANOVA revealed a significant effect of day (F18,190=2.730, p<0.0001), but no effect of treatment or day by treatment interaction. Taken together, rats treated with D-serine after each brief extinction session showed reduced lever pressing across the 90 min extinction days as compared to rats previously treated with saline. Thus, D-serine facilitated subsequent extinction by enhancing consolidation of extinction.
Figure 2.
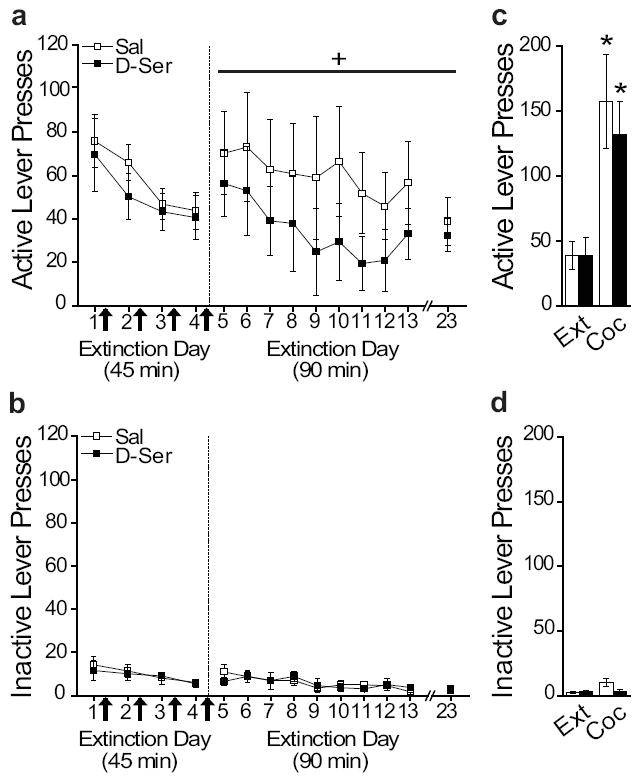
Potentiating NMDArs with D-serine facilitates extinction. (A) Post-extinction session injections of D-serine (arrows) facilitated extinction retention. (B) Previous D-serine treatment had no effect on cocaine-induced reinstatement. Inactive lever presses during (C) extinction and (D) reinstatement. *p<0.05, +p<0.05 for overall group difference
Following extinction, all rats were tested for cocaine-induced reinstatement of lever pressing. Rats received a non-contingent priming injection of cocaine prior to a 90 min extinction session (Figure 2C,D). ANOVA revealed a significant increase in active lever pressing between the average of the last two extinction days and the cocaine-induced reinstatement day (F1,18=17.816, p=0.001), but no effect of treatment or day by treatment interaction. Furthermore, ANOVA for inactive lever presses revealed a significant effect of day (F1,18=6.570, p=0.020), but no effect of treatment or day by treatment interaction. Thus, both groups reinstated similarly with no effect of previous treatment with D-serine. In summary, previous treatment with D-serine did not affect reinstatement.
To determine if CPP or D-serine affected general motor activity, rats were treated with saline, CPP, or D-serine one h prior to being placed in locomotor activity chambers for 20 min. ANOVA revealed no differences in photobeam breaks between treatment groups, demonstrating that locomotor activity was not affected by administration of CPP or D-serine.
NMDAr, but not AMPA receptor, expression is altered in the vmPFC and NAc following self-administration
To determine if changes in receptor expression were induced following extinction of cocaine seeking, glutamate receptor expression was measured in the vmPFC and NAc. Following acquisition, coc-ext and suc-ext rats received extinction training while coc-noext rats remained in their home cages. Active and inactive lever presses, and number of infusions were equivalent between cocaine self-administration groups across the average of the last three days of self-administration (Table 1). Rats then underwent cocaine or sucrose extinction (Figure 3A,B). Following extinction, all groups were tested for extinction retention (Figure 3C). ANOVA revealed a significant effect of treatment (F2,21=22.314, p<0.0001), and post hoc analyses confirmed that both coc-ext and suc-ext rats lever pressed significantly less than coc-noext rats (p<0.0001), but coc-ext and suc-ext rats did not differ in active lever presses. Following the extinction retention test, rats were sacrificed and their brains were extracted. The vmPFC and NAc were then collected for analysis (Figure 4).
Figure 3.

Extinction of sucrose and cocaine seeking. Active lever presses during extinction of (A) sucrose or (B) cocaine seeking. (C) Active lever presses during extinction retention test. *p<0.05
Figure 4.

Expression of GluN2A, GluN2B, and GluR1 subunits in vmPFC and NAc following acquisition and extinction of cocaine seeking. (A) Representative regions for tissue collection containing the vmPFC. (B-D top) Representative blots for vmPFC of each group. (B) GluN2B, (C) GluN2A, and (D) GluR1 protein levels in the vmPFC. (E) Representative regions for tissue collection containing the NAc. (F-H top) Representative blots for NAc of each group. (F) GluN2B, (G) GluN2A, and (H) GluR1 protein levels in the NAc. *p<0.05
Glutamate receptor expression was analyzed in the vmPFC of coc-ext, coc-noext, and suc-ext rats. Figure 4A shows a representative area for tissue collection in the vmPFC (bregma 3.24 mm; Paxinos and Watson 2007). ANOVA revealed a trend for altered GluN2B subunit expression between groups (F2,20=2.882, p=0.079; Figure 4B), but this effect was not significant. Additionally, ANOVA revealed no effect of treatment on GluN2A subunit expression (Figure 4C) or GluR1 subunit expression (Figure 4D). Thus, it does not appear, at the selected time point, that cocaine withdrawal or extinction had any effect on GluR1, GluN2A, or GluN2B protein expression in the vmPFC.
Figure 4E shows a representative area for tissue collection in the NAc (bregma 2.76 mm; Paxinos and Watson 2007). In the NAc, ANOVA revealed a significant difference in GluN2B subunit expression between groups (F2,20=5.097, p=0.016). Post hoc analysis confirmed that coc-noext rats had greater GluN2B subunit expression as compared to suc-ext rats (p=0.005), but not compared to coc-ext rats, whereas both coc-ext and suc-ext rats did not differ (Figure 4F). Furthermore, ANOVA revealed a significant effect of GluN2A subunit expression between groups (F2,20=7.534, p=0.004). Post hoc analyses confirmed that coc-ext rats (p=0.04) and coc-noext rats (p=0.001) had increased GluN2A subunit expression compared to suc-ext rats (Figure 4G). However, ANOVA revealed no effect of GluR1 subunit expression between groups (Figure 4H). In summary, GluN2A and GluN2B subunit expression in the NAc was increased following cocaine self-administration, but not altered by extinction. Additionally, GluR1 subunit expression was not altered under any conditions.
Discussion
We demonstrate that NMDAr activation is necessary for extinction of cocaine seeking. NMDAr blockade prior to four brief extinction sessions disrupted extinction retention on subsequent sessions. Conversely, enhancing NMDAr function immediately after four brief extinction sessions resulted in facilitated extinction in later sessions. These findings show that NMDArs can bidirectionally mediate extinction learning, and suggest that NMDArs are necessary for consolidation of extinction of cocaine seeking. Furthermore, we demonstrated that NMDAr subunit expression is modulated by cocaine use. Expression of both GluN2A and GluN2B subunits in the NAc are increased following cocaine self-administration, however GluN2B subunit expression is marginally attenuated, but not significantly, following extinction. There were no significant changes in receptor expression in the vmPFC between groups. Thus, cocaine self-administration increases NMDAr expression in the NAc, but not vmPFC, an effect that is relatively resistant to modulation by extinction.
To date, no studies have demonstrated the necessity of NMDArs for extinction of cocaine seeking in a self-administration paradigm. Previously, a low dose of CPP was found to be ineffective at impairing extinction of cocaine seeking (Kelamangalath et al. 2007). In contrast, we found that a higher dose of CPP, that has been shown to be effective in other extinction paradigms (Burgos-Robles et al. 2007; Santini et al. 2001), disrupted extinction of cocaine seeking. Our findings agree with recent data showing that infusions of the NMDAr antagonist APV into the NAc inhibited Pavlovian cue-extinction learning following cocaine selfadministration (Torregrossa et al. 2013), and are consistent with the finding that NMDArs are necessary for extinction in other paradigms (Burgos-Robles et al. 2007; Hsu and Packard 2008; Liu et al. 2009; Santini et al. 2001). Our results also agree with previous research indicating that potentiating NMDAr function facilitates extinction. Post-session injections of D-serine facilitate extinction of inhibitory avoidance (Fiorenza et al. 2012), and pre-session injections of D-serine facilitate extinction of conditioned fear (Matsuda et al. 2010). Furthermore, D-serine given prior to each extinction session has been shown to reduce lever pressing during extinction (Kelamangalath et al. 2007) and to facilitate extinction of a conditioned place preference (Hammond et al. 2012). Taken together, D-serine facilitates extinction when given before or after an extinction session, suggesting it could enhance learning during either acquisition or consolidation. Our results show that D-serine administered immediately after each brief extinction session facilitated subsequent extinction, indicating that D-serine acted to enhance consolidation of extinction.
We found that neither CPP nor D-serine treatment during extinction had an effect on cocaine-induced reinstatement. These results conflict with previous research in which pre-extinction session administration of a low dose of CPP (5 mg/kg) for five days had no effect on context-only extinction following short-access cocaine self-administration, but potentiated low dose I.V. cocaine-induced reinstatement (Kelamangalath et al. 2007). Moreover, low-dose cocaine-induced, but not cue- or context-induced, reinstatement is reduced when D-serine is administered during one or five days of context only extinction training following both short- and long-access cocaine self-administration (Kelamangalath et al. 2009; Kelamangalath and Wagner 2010). Following short-access cocaine self-administration, DCS administered systemically or into the NAc core after cue extinction can reduce cue-induced reinstatement in a context-independent manner (Torregrossa et al. 2010). However, using a different route of administration and a higher dose of cocaine to induce reinstatement, we observed no effect of previous CPP or D-serine treatment. Procedural differences may account for these discrepancies. CPP or D-serine administered during extinction may only be effective at potentiating or reducing, respectively, cocaine-induced reinstatement when the cocaine dose is low, or when the route of administration matches the original self-administration parameters. Overall, these results indicate that NMDArs are necessary for extinction, but the effectiveness of enhancing these receptors on reducing subsequent reinstatement needs further exploration.
Additionally, our results indicate that NMDAr expression in the NAc, but not the vmPFC, is altered following cocaine self-administration, and extinction appears to have little effect on glutamatergic receptor expression. GluR1 expression is not altered in either structure or any groups. These results are unexpected as previous research, using a long-access model of self-administration, found an increase in GluR1 expression in the NAc shell after one week of extinction with (Sutton et al. 2003) or without discrete cues (Self et al. 2004), and an increase in GluN2B expression in the PFC following 13 days of context extinction (Tang et al. 2004). Furthermore, using a short-access model, GluR1 expression is increased in the vmPFC, but not NAc, following a single 2 h extinction session with discrete cues (Nic Dhonnchadha et al. 2013). However, the NMDAr subunit GluN1 was initially increased following cocaine self-administration, but was progressively decreased following 1, 5, or 10 days of extinction with discrete cues in the mPFC and NAc (Crespo et al. 2002). In contrast, others found no change in GluR1 or GluN1 in the vmPFC after 10 days of extinction following long-access cocaine self-administration with discrete cues (Ghasemzadeh et al. 2011). Thus, the amount of exposure an animal receives to cocaine and cocaine-related cues could affect glutamate receptor expression.
Another potential factor that could account for our results is the amount of time animals undergo withdrawal. Previous research has indicated that following 20 min of withdrawal from short-access, but not long-access, cocaine self-administration there was an increase in NMDAr expression in the vmPFC (Ben-Shahar et al. 2007). Furthermore, withdrawal from long-access self-administration for 14 days increased GluN2B expression in the mPFC and NAc shell, and after 60 days, increased GluN2A expression in the mPFC (Ben-Shahar et al. 2009). Interestingly, when a cue test (extinction session) was given following 30 days of withdrawal, there was a decrease in mGluR1 expression in the vmPFC, but no change following 3 days of withdrawal or when a cue test was not given (Ben-Shahar et al. 2013). These results suggest that reactivating the memory could affect glutamate receptor expression in the vmPFC following prolonged withdrawal. Taken together, it seems likely that the amount of exposure to cocaine and cocaine-related cues, as well as time undergoing extinction or withdrawal may influence glutamate receptor expression.
Accumulating evidence suggests that GluN2A- and GluN2B-containing NMDArs serve different functions in learning. For instance, selective blockade of the GluN2B-containing NMDAr impairs extinction of conditioned fear (Dalton et al. 2012; Sotres-Bayon et al. 2007; Sotres-Bayon et al. 2009; Tang et al. 1999), but over-expression in the forebrain can facilitate extinction (Tang et al. 1999). Conversely, selective blockade of the GluN2A-containing NMDAr impairs retention of conditioned fear (Dalton et al. 2012; Gilmartin et al. 2013). Furthermore, GluN2B-containing NMDArs have a greater potential for inducing plasticity than GluN2A-containing NMDArs as they have greater channel conductance, resulting in a greater influx of Ca2+ (Erreger et al. 2005; Flint et al. 1997; Vicini et al. 1998). Thus, stimulating GluN2B-containing NMDArs alone during extinction may enhance consolidation of extinction, resulting in greater inhibition of drug seeking and reducing relapse susceptibility.
In conclusion, few studies have aimed to determine the role of NMDArs in extinction of cocaine seeking or their specific role in consolidation of extinction using a self-administration paradigm. The current findings suggest that consolidation of extinction of cocaine seeking is mediated by NMDArs as pre-extinction session injections of CPP disrupts extinction retention and post-extinction injections of D-serine facilitate extinction. Moreover, these results further support a use for D-serine in a clinical setting. Extinction-based exposure therapy alone has had limited success as a treatment for addiction (Conklin and Tiffany 2002; Kantak and Nic Dhonnchadha 2011), however, DCS has been shown to enhance therapeutic outcome for treatment of anxiety disorders and phobias (Hofmann and Smits 2008). Unfortunately, DCS has a number of limitations (e.g., instability at room temperature; Davis 2011) and has been shown to be ineffective in the few studies that applied DCS to the treatment of addiction (Kantak and Nic Dhonnchadha 2011). Our results suggest that D-serine would be a useful adjuvant for extinction-based therapies to improve success rates without some of the limitations of DCS.
Acknowledgments
This research was supported by DA027870 and a grant from the University of Wisconsin—Milwaukee Research Growth Initiative to DM. We thank John Schneider, Ashley Fortress, Tim Jarome, and Jake Burkard for technical assistance, and Dr. Karyn Frick for generous use of equipment.
Footnotes
The authors declare no conflict of interest.
References
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, Kohr G, Rawlins JN. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–30. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–8. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495–506a. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–8. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–4. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–80. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr Clin North Am. 1986;9:413–25. [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Oliva JM, Ghasemzadeh MB, Kalivas PW, Ambrosio E. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Ann N Y Acad Sci. 2002;965:78–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wu DC, Wang YT, Floresco SB, Phillips AG. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology. 2012;62:797–806. doi: 10.1016/j.neuropharm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Davis M. NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci. 2011;13:463–74. doi: 10.31887/DCNS.2011.13.4/mdavis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–58. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232:210–6. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci. 2013;33:9319–27. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–9. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–76. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Schram SL, Tuscher JJ, Frick KM. Canonical Wnt signaling is necessary for object recognition memory consolidation. J Neurosci. 2013;33:12619–26. doi: 10.1523/JNEUROSCI.0659-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res. 2011;1413:60–71. doi: 10.1016/j.brainres.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20:290–4. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–33. [PubMed] [Google Scholar]
- Hammond S, Seymour CM, Burger A, Wagner JJ. D-serine facilitates the effectiveness of extinction to reduce drug-primed reinstatement of cocaine-induced conditioned place preference. Neuropharmacology. 2012;64:464–71. doi: 10.1016/j.neuropharm.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–32. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem. 2008;89:504–12. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Nic Dhonnchadha BA. Pharmacological enhancement of drug cue extinction learning: translational challenges. Ann N Y Acad Sci. 2011;1216:122–37. doi: 10.1111/j.1749-6632.2010.05899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelamangalath L, Seymour CM, Wagner JJ. D-serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol Learn Mem. 2009;92:544–51. doi: 10.1016/j.nlm.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelamangalath L, Swant J, Stramiello M, Wagner JJ. The effects of extinction training in reducing the reinstatement of drug-seeking behavior: involvement of NMDA receptors. Behav Brain Res. 2007;185:119–28. doi: 10.1016/j.bbr.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelamangalath L, Wagner JJ. D-serine treatment reduces cocaine-primed reinstatement in rats following extended access to cocaine self-administration. Neuroscience. 2010;169:1127–35. doi: 10.1016/j.neuroscience.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–75. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton JM, Richardson R. D-cycloserine facilitates extinction the first time but not the second time: an examination of the role of NMDA across the course of repeated extinction sessions. Neuropsychopharmacology. 2008;33:3096–102. doi: 10.1038/npp.2008.32. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–9. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, Li YQ, Wang W. A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS One. 2009;4:e7548. doi: 10.1371/journal.pone.0007548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–13. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Nakazawa K, Sutoh C, Ohtsuka H, Ishii D, Tomizawa H, Iyo M, Shimizu E. D-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:895–902. doi: 10.1016/j.pnpbp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–62. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–93. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav. 2011;99:229–44. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Lin A, Leite-Morris KA, Kaplan GB, Man HY, Kantak KM. Alterations in expression and phosphorylation of GluA1 receptors following cocaine-cue extinction learning. Behav Brain Res. 2013;238:119–23. doi: 10.1016/j.bbr.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–67. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Mueller D. Inhibition of beta-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology. 2011;36:1912–20. doi: 10.1038/npp.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–9. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–17. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–40. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19:474–82. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–5. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–33. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–9. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011a;65:938–44. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Subrize M, Lui W, Puca Z, Ananth M, Michaelides M, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in C57 mice. Synapse. 2011b;65:1099–105. doi: 10.1002/syn.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Gordon J, Taylor JR. Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of pavlovian cocaine cue extinction. J Neurosci. 2013;33:8370–7. doi: 10.1523/JNEUROSCI.0489-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30:10526–33. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–66. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–51. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


