Abstract
Objectives
To determine patient independency, health-related and disease-specific quality of life (QOL), gait pattern, and muscle strength in patients after salvage arthroplasty for failed internal fixation of a femoral neck fracture.
Design
Secondary cohort study to a randomized controlled trial.
Setting
Multicenter trial in the Netherlands, including 14 academic and non-academic hospitals
Patients
Patients after salvage arthroplasty for failed internal fixation of a femoral neck fracture were studied. A comparison was made with patients who healed uneventfully after internal fixation.
Intervention
None (observatory study)
Main outcome measurements
Patient characteristics, SF-12, and WOMAC scores were collected. Gait parameters were measured using plantar pressure measurement. Maximum isometric forces of the hip muscles were measured using a handheld dynamometer. Differences between the fractured and contralateral leg were calculated. Groups were compared using univariate analysis.
Results
Of 248 internal fixation patients (median age 72 years), salvage arthroplasty was performed in 68 patients (27%). Salvage arthroplasty patients had a significantly lower WOMAC score (median 73 versus 90, P=0.016) than patients who healed uneventfully after internal fixation. Health-related QOL (SF-12) and patient independency did not differ significantly between the groups. Gait analysis showed a significantly impaired progression of the center of pressure in the salvage surgery patients (median ratio −8.9 versus 0.4, P=0.013) and a significant greater loss of abduction strength (median −25.4 versus −20.4 N, P=0.025).
Conclusion
Despite a similar level of dependency and QOL, salvage arthroplasty patients have inferior functional outcome than patients who heal after internal fixation of a femoral neck fracture.
Keywords: femoral neck fracture, hip fracture, Internal fixation, salvage arthroplasty, functional outcome
Introduction
The optimal surgical treatment of femoral neck fractures remains unclear.1–5 Treatment options are internal fixation, arthroplasty, and in specific cases conservative treatment. Revision surgery rates of approximately 35% have been reported after internal fixation failure.1, 3–5 It has been argued that salvage arthroplasty is a safe procedure if internal fixation fails, and that surgical outcome of salvage arthroplasty is satisfactory.6–8 However, little is known about the functional outcome after salvage arthroplasty for failed internal fixation of a femoral neck fracture. Few studies have focused on functional outcome, and have only recorded general function such as walking ability and pain or general health-related quality of life scores.8–12 To the best of our knowledge, a disease-specific functional score was used only in two studies.10, 13 Objective functional outcome parameters such as muscle strength or gait are not available, even though they are important factors influencing walking ability and quality of life. Gait analysis may add information to the results from functional outcome scores like the Western Ontario McMaster Osteoarthritis Index (WOMAC).14 Its value has been proven in clinical studies of other surgical interventions, such as hip arthroplasty for degenerative osteoarthritis.15
The aim of this study was to determine traditional outcome parameters such as patient independency and health-related quality of life (QOL) as well as disease-specific QOL, gait pattern, and muscle strength in patients after salvage arthroplasty for failed internal fixation of patients with a femoral neck fracture. The study was performed as a secondary cohort study to the Dutch sample of an international randomized controlled trial, the FAITH trial. Results of salvage arthroplasty patients were compared with those of patients that did not receive a salvage arthroplasty. We hypothesized that patients after salvage arthroplasty would have worse functional outcome and QOL than patients that did not receive a salvage arthroplasty.
Patients and Methods
Population
This study (clinical trial registration number, NL32419.078.10) was a secondary cohort study to the Dutch sample of an international randomized controlled trial, the FAITH trial (Fixation using Alternative Implants for the Treatment of Hip fractures, NCT00761813). The primary objective of the FAITH trial was to assess the impact of internal fixation implants (sliding hip screw versus multiple cancellous screws) on rates of revision surgery at two years in elderly patients with femoral neck fractures. In the Netherlands 14 hospitals participated and randomized 250 patients between February 2008 and August 2009. These patients were adults aged >50 years, who were ambulatory and not cognitively impaired pre-fracture. Patients had an undisplaced fracture or a displaced fracture (in ASA 1–2 patients, aged 50–80 years, with a fracture that could be reduced closed).16 Surgeries were performed or supervised by a senior surgeon. All patients were allowed weight bearing as tolerated after initial surgery.17
In the current study, all Dutch FAITH patients who received a salvage arthroplasty (for any reason, e.g., avascular necrosis, non-union, internal fixation break-out, or persisting pain) were compared with patients who healed after internal fixation (control group). The decision to plan a re-operation was left to the discretion of the treating surgeon. Surgeons used their preferred approach and type of prosthesis, which therefore varied (both unipolar and bipolar). In a sub-study gait pattern and muscle strength were measured. Patients were included in the gait analysis at least one year after their initial internal fixation surgery.
Exclusion criteria were:
Primary conversion to arthroplasty
Not capable of walking several meters independently
Lower limb abnormalities that could be expected to influence gait pattern
Previous internal fixation or arthroplasty of the contralateral (control) hip.
Salvage surgery patients in the gait analysis were compared with a control sample of patients from the Dutch FAITH population who did not have salvage arthroplasty, but healed after internal fixation. Gait pattern and muscle strength in the control group had been measured in a previously published study, using the same selection criteria and study protocol.18 The study was approved by the Medical Research Ethics Committee (MEC-2010-164).
Data and measurements
Patient and fracture characteristics at the time of the fracture, and surgical characteristics, rehabilitation data, Western Ontario McMaster Osteoarthritis Index (WOMAC) and Short Form-12 (SF-12) scores at two years follow-up were available from the FAITH trial.19, 20 SF-12 scores were converted to a norm-based score and compared with general population norms of the United States (1998), as weighing factors for the Dutch population were not available.
Measurements of gait pattern and muscle strength were performed during a single visit to the outpatient clinic, following the same protocol applied previously.18 Gait analysis was performed using a pressure plate (Footscan®, RSscan International, Olen, Belgium; 2.0 × 0.4m, 125 Hz). Patients were instructed to walk barefoot across the pressure plate at their usual, preferred speed, starting several steps before and ending several steps after the pressure plate. Five measurements were performed per patient. The combination of at least three gait measurements that were most representative for each patient were selected based upon the coefficient of variation, and used for analysis. The following temporospatial gait parameters were analyzed: gait velocity, duration of stance phase, single and double support phase, step length, foot axis, and progression of the center of pressure in the walking direction (COP ΔY). Data of the fractured leg were compared with the contralateral side. The difference was computed using the formula: Parameter fractured leg – Parameter contralateral leg.
The maximum isometric forces of the hip muscles were measured using a handheld dynamometer (MicroFET®, Biometrics BV, Almere, the Netherlands). Flexion, extension, abduction, and adduction strength were measured in a supine position. The means of triplicate measurements were calculated, and the differences between the affected extremity and control side were computed.
Finally, leg length was measured during the visit, using a direct tape measure method. The distance between the anterior superior iliac spine and the medial malleolus was measured twice. The average value was used for analysis. This strategy has an acceptable validity and reliability.21 Patients were also asked if they felt they had a leg length discrepancy. If so, patients completed a Visual Analog Scale (VAS) to indicate how much they felt hampered due to the discrepancy. The VAS ranged from zero (free of complaints) to ten (very much hampered). Use of a heel lift to correct a leg length discrepancy was also recorded. Finally, patient satisfaction with their gait pattern was measured using a VAS, ranging from zero (extremely dissatisfied) to ten (completely satisfied).
Data analysis
Analyses were performed using SPSS (version 16.0, SPSS Inc., Chicago, IL, USA). Because this was an explorative cohort study in a restricted sample of patients, statistical analysis was confined to univariate comparison of patients who received salvage arthroplasty with patients who healed after internal fixation (control group). For continuous variables the Mann-Whitney U-test was used, and the Chi-squared test or Fisher’s exact test for categorical variables. Results with P<0.05 (two-sided test) were regarded as statistically significant. Continuous variables, which were all non-parametric, are presented as medians with interquartile ranges. Categorical variables are presented as numbers and percentages.
Results
Patient, fracture, and treatment characteristics
Of the initial group of 250 randomized patients, two patients could not be followed; one patient turned out not to have a femoral neck fracture and one patient withdrew consent immediately after randomization. Patient, fracture, and treatment characteristics of the remaining 248 patients are shown in Table 1. The study group had a median age of 72 years (P25-P75 62–78). Patients were relatively healthy and independent pre-fracture. Prior to the fracture only 3% of the patients were institutionalized and 13% used an aid for mobilization. Thirteen percent had severe comorbidities (ASA3). The median follow-up was 26 months (P25-P75 25–28) after the initial surgery.
Table 1.
Patient, fracture, and treatment characteristics
| Salvage arthroplasty (HA/THA) (N=68) | Internal Fixation (N=164) | P-value | |
|---|---|---|---|
| Age (years)1 | 72 (66–79) | 70 (62–78) | 0.301 |
| Males2 | 21 (31) | 73 (45) | 0.058 |
| BMI (kg/m2)1 | 24 (22–27) | 24 (22–26) | 0.151 |
| ASA score 32 | 8 (12) | 23 (14) | 0.329 |
| Institutionalized pre-fracture2 | 4 (6) | 3 (2) | 0.199 |
| Pre-fracture use of walking aids2 | 11 (16) | 21 (13) | 0.533 |
| Displaced fracture (Garden III–IV/AO 31-B2-3)2 | 42 (62) | 57 (35) | <0.001 |
| Pauwels 32 | 35 (52) | 42 (26) | <0.001 |
| Implant removed2 | N.A. | 38 (23) | N.A. |
| Revision to THA2 | 45 (66) | N.A. | N.A. |
| Time since last surgery (months)1* | 21 (15–24) | 25 (24–28) | <0.001 |
| Follow-up duration (months)1 | 26 (25–28) | 26 (25–28) | 0.762 |
HA, Hemiarthroplasty; THA, Total Hip Arthroplasty; BMI, Body Mass Index; ASA, American Society of Anesthesiologists; N.A., not applicable.
Differences between groups were tested with the Mann-Whitney U-test for continuous variables, and with the Chi-squared test or Fisher’s exact test for categorical variables.
Data are presented as median with P25-P75 given between brackets.
Data are presented as number with percentages.
This parameter reflects the time since the last surgery (i.e., either the primary internal fixation, the implant removal, or the salvage arthroplasty procedure).
Salvage arthroplasty was performed in 68 patients (27%), of whom 45 (66%) received a total hip arthroplasty. Patients who received a salvage total hip arthroplasty were significantly younger than patients who received a salvage hemiarthroplasty (median age 70 versus 76 years, P=0.035). The total hip arthroplasty patients were also more independent in their functioning pre-fracture (0% versus 17% living institutionalised, P=0.011, 9% versus 30% use of walking aid, P=0.036)
Of the 180 patients who healed after internal fixation 38 patients (21%) had their implant removed during the follow-up, mainly because of painful hardware. Taking all revision surgeries into account, there was a significantly shorter time between last surgery and final follow-up in the salvage arthroplasty patients than in the patients who healed after internal fixation (median 21 versus 25 months, P<0.001).
Salvage arthroplasty was performed more frequently after a displaced fracture (Garden III–IV/AO 31-B2-3); 62% in the salvage arthroplasty group versus 35% in the healed after internal fixation group; P=0.001) or a Pauwels III fracture (52% versus 26%, P=<0.001).22 Of all undisplaced fractures (Garden I–II/AO 31-B1) 20% failed, whereas 42% of all displaced fractures failed. Other characteristics were similar in both groups (Table 1).
Patient independency, health-related and disease-specific quality of life (QOL)
Health-related quality of life and patient independency did not differ significantly between the patients who healed after internal fixation and the salvage arthroplasty patients. There was no significant difference in SF-12 score, rates of institutionalization, the ability to walk independently, or the use of physical therapy at two years follow-up (Table 2). However, the salvage arthroplasty patients reported significantly lower median WOMAC scores at two years follow-up than the patients that healed after internal fixation (73 versus 90 points, P=0.016). This difference was mainly seen in the functional domain of the questionnaire, and to a lesser extent in the pain and stiffness domain. The salvage arthroplasty patients also reported a significant longer total use of physical therapy (median 26 weeks versus 11 weeks in the group healed after internal fixation; P=0.002). No significant differences in independency and QOL scores were found when comparing hemiarthroplasty patients with total hip arthroplasty patients in the salvage group.
Table 2.
Patient independency, health-related and disease-specific quality of life (QOL)
| Salvage arthroplasty (HA/THA) (N=68) | Internal Fixation (N=164) | P-value | |
|---|---|---|---|
| SF-12 score1 | 93 (82–109) | 99 (86–109) | 0.347 |
| WOMAC score1 | 73 (56–94) | 90 (71–97) | 0.016 |
| Currently institutionalized2 | 10 (18) | 18 (12) | 0.550 |
| Currently using walking aids2 | 29 (52) | 58 (39) | 0.113 |
| Currently receiving physical therapy2 | 12 (21) | 26 (19) | 0.546 |
| Duration of physical therapy (weeks)1a | 26 (12–55) | 11 (6–28) | 0.002 |
HA, Hemiarthroplasty; THA, Total Hip Arthroplasty; SF-12, Short Form 12; WOMAC, Western Ontario McMaster Osteoarthritis Index
Differences between groups were tested with the Mann-Whitney U-test for continuous variables, and with the Chi-squared test or Fisher’s exact test for categorical variables.
Data are presented as median with P25-P75 given between brackets.
Data are presented as number with percentages.
Data on the duration of the physical therapy were only collected in the 96 patients that participated in the gait analysis study
Gait analysis, muscle strength and leg length discrepancy
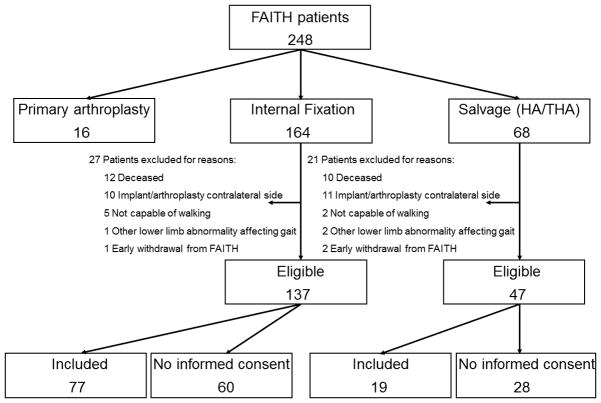
Of the 68 salvage arthroplasty patients, 47 were eligible to study gait pattern and muscle strength, following the inclusion and exclusion criteria for this study (Figure 1). Nineteen patients gave informed consent. The patient characteristics of the 28 patients that did not want to participate (i.e., age, ASA-score and pre-fracture use of aids) did not differ significantly from those in the included population. The included patients were compared with a control group of 77 patients who healed after internal fixation (Figure 1). Characteristics of these two subgroups of 19 and 77 patients were similar as the characteristics summarized in Table 1 for the total groups.
Figure 1. Flowchart of salvage arthroplasty patients participating in the gait analysis study.
* The 77 patients in the control group (i.e., patients who healed after internal fixation) were selected and included from this subgroup.
The gait parameters did not differ statistically significantly between the groups, except for the progression of the center of pressure in the walking direction (COP ΔY; Table 3). The COP is a parameter indicating the degree and direction of roll-off of the foot. The progression of the COP reflects the transfer of load from the left to the right limb and vice versa. The COP progression in the walking direction was significantly decreased for the fractured leg in the salvage arthroplasty patients, whereas an increase was noted in the patients who healed after internal fixation (median ratio −8.9 versus 0.4, P=0.013). Median gait velocity was 1.1 m/s in both groups. Patient scored their satisfaction with gait pattern a median of 7.4 on a VAS, which did not differ significantly between the groups.
Table 3.
Gait analysis, muscle strength, and leg length discrepancy
| Salvage arthroplasty (HA/THA) (N=19) | Internal Fixation (N=77) | P-value | |
|---|---|---|---|
| Gait velocity (m/s)1 | 1.0 (0.6–1.2) | 1.2 (1.1–1.5) | 0.413 |
| Stance time (% of gait cycle)1§b | −1.8 (−5.2–0.1) | −1.6 (−3.8– −0.1) | 0.446 |
| Single support phase (% of gait cycle)1§b | −2.2 (−4.0– −0.2) | −0.5 (−4.4–1.0) | 0.554 |
| Double support phase (% of gait cycle)1§b | −0.3 (−1.7–1.1) | 0.2 (−2.1–2.6) | 0.545 |
| Step length (cm)1§ | 1.8 (−1.5–4.1) | 0.0 (−3.2–3.8) | 0.249 |
| Foot axis (°)1§ | −2.3 (−10.2–9.0) | 0.6 (−5.1–4.9) | 0.402 |
| COP ΔY (cm)1§ | −8.9 (−13.0– −1.8) | 0.4 (−8.1–6.8) | 0.013 |
| VAS score satisfaction with gait pattern1 | 7.1 (4.7–8.5) | 7.4 (5.0–8.7) | 0.847 |
| Flexion (N)1§ | −18.6 (−41.1–9.3) | −1.3 (−13.5–4.1) | 0.108 |
| Extension (N)1§ | −14.1 (−37.5–6.2) | −3.5 (−26.9–13.2) | 0.226 |
| Adduction (N)1§ | −6.9 (−26.0–11.6) | −2.8 (−29.3–19.0) | 0.713 |
| Abduction (N)1§ | −25.4 (−67.5– −17.8) | −20.4 (−35.0–0.7) | 0.025 |
| LLD (cm)1 | 0.0 (−0.8–1.0) | 0.8 (0.3–1.8) | 0.001 |
| Feeling of LLD2 | 3 (16) | 31 (40) | 0.061 |
| VAS score complaints LLD1a | 4.9 (2.6–6.0) | 4.0 (1.5–7.2) | 0.813 |
| Heel lift use2 | 1 (5) | 23 (30) | 0.036 |
HA, Hemiarthroplasty; THA, Total Hip Arthroplasty; LLD, Leg Length Discrepancy; VAS, Visual Analog Scale; COP, Center of Pressure line
Differences between groups were tested with the Mann-Whitney U test for continuous variables, and with the Chi-squared test or Fisher’s exact test for categorical variables.
Data are presented as median with P25-P75 given between brackets.
Data are presented as number with percentages.
The VAS score for complaints as a result of a LLD was only measured in the 34 patients that indicated having the feeling of a LLD.
These variables had >10% missing data, because they require a completely measured gait cycle for both legs, which was often not feasible (Stance Time 14% missing and Single/Double Support Phase 54%).
The values displayed for these variables represent the difference between the two legs (Parameter fractured leg – Parameter contralateral leg).
A negative value therefore represents a decrease in the fractured leg, a positive value an increase.
Salvage arthroplasty patients had a significantly greater loss of abduction strength in the fractured leg than patients who healed after internal fixation did (median −25.4 versus −20.4 N, P=0.025; Table 3). Finally, the leg length discrepancy was less in the salvage arthroplasty patients than in patients who healed after internal fixation (median 0.0 versus 0.8 cm, P=0.001). Consequently, they used a heel lift less often (5% versus 30%, P=0.036).
Discussion
Salvage arthroplasty resulted in inferior disease-specific functional outcome scores (WOMAC) than successful internal fixation did. Twenty seven percent of patients required salvage arthroplasty after internal fixation of a femoral neck fracture. This is in line with previously published data, both for the percentage failure in displaced fractures (37%) and undisplaced fractures (19%).1, 4, 5 To the best of our knowledge, functional outcome of salvage surgery patients has never previously been compared with outcome of patients who healed uneventfully after internal fixation. However, Blomfeldt et al. showed a worse functional outcome of salvage arthroplasty after failed internal fixation compared with primary arthroplasty.10
The observed inferior disease-specific functional outcome scores did not lead to a difference in health-related quality of life. With a median SF-12 score of 93 points, salvage arthroplasty patients seemed to have a good health-related quality of life. This may reflect a good coping mechanism of the relatively young and healthy femoral neck fracture study population. It also demonstrates that functional outcome after hip surgery should be tested with a disease specific questionnaire, because generic questionnaires like the SF-12 may not be specific enough.
A more deviant gait pattern may contribute to the inferior functional outcome in patients after salvage arthroplasty. In our study group, salvage arthroplasty patients had a more impaired progression of the center of pressure in the fractured leg, indicating an impaired transfer of load underneath the affected limb. This could be the effect of impaired balance, or, as indicated by the univariate analysis, an overall impaired muscle strength of the hip abductor muscles in the affected limb.23 None of the other individual gait parameters reached statistical significance when comparing the groups. Perhaps with increasing numbers, more significant alterations in gait pattern may be measured in the salvage arthroplasty patients. Moreover, although the left-right differences in gait parameters seem small, research in patients after total hip arthroplasty for osteoarthritis has indicated that these subtle difference have clinical relevance.24
Another contributing factor to the inferior functional outcome in patients after salvage arthroplasty is a greater loss of abductor muscle strength. The median loss of 25 N can be expected to have clinical relevance. This greater loss of strength in the salvage arthroplasty patients can be explained by the need to recover from multiple surgeries and an additional incision and exposure for the arthroplasty (which is more extensive than for internal fixation, depending on the type of prostheses and the surgical approach). This extra surgery causes more damage to the underlying tissue, mainly the abductor muscles. Furthermore, these patients have often suffered from a period of pain and limping, and have been hampered in their rehabilitation process preceding the salvage surgery, mainly caused by the primary reason of the salvage arthroplasty (usually avascular necrosis or non-union/implant break-out). Our results show that the re-operation cannot salvage the functional level following a long period with a suboptimal internal fixation. In accordance, salvage surgery patients may benefit from more specific rehabilitation programs aimed at improving hip muscle strength (e.g. gait assisted functional electro stimulation).
The inferior functional outcome of salvage arthroplasty patients in the current study and in the study by Blomfeldt et al. suggests that patients receiving internal fixation of a femoral neck fracture should be selected very carefully. The notion that salvage arthroplasty is a safe procedure if internal fixation fails, should perhaps be reconsidered with caution. This aspect should receive more attention as previous studies suggest little difference in functional outcome.6, 8 In the current study, patients receiving a salvage arthroplasty more frequently had a displaced fracture classification (both Garden and Pauwels). As such, our data suggest that surgeons could more liberally consider a primary arthroplasty for patients with displaced (Garden III–IV), sheer (Pauwels 3) femoral neck fractures.16 However, further research comparing functional outcome in patients after primary and salvage arthroplasty should render more evidence on this matter.
Our data do not suggest superiority of any type of arthroplasty over the other, as patients treated with salvage hemiarthroplasty and total hip arthroplasty had similar patient independency and quality of life scores. Surgeons do seem to take patient characteristics into account when deciding on type of arthroplasty, as salvage total hip arthroplasty patients were significantly younger and more independent in their functioning pre-fracture.
The main limitation of this study is the restricted number of included patients in the secondary gait analysis study. Multivariable analyses were not feasible. Selection bias seems unlikely, as the patient characteristics of the 28 patients that did not participate did not differ significantly from those in the included population. Due to a limited number of patients in the salvage arthroplasty group it was not possible to perform subgroup analyses by surgical approach or type of prosthesis. A larger sample size is needed in order to perform more detailed analyses on the factors that contribute to the inferior functional outcome of salvage surgery patients.
A second limitation is the difference in time since last surgery between the study groups, indicating that the study groups may not have been completely comparable. However, the median time since last surgery was >20 months in both groups. The functional progression that can be expected after that time period is limited. This difference will therefore probably have only very limited influence on the results of this study.
The population in the current study consisted of relatively young and healthy persons; demented patients and patients unsuitable for internal fixation were excluded. The results of this study should therefore not be generalized to all hip fracture patients,
In conclusion, patients requiring salvage arthroplasty after initial internal fixation of a femoral neck fracture have inferior functional outcome than patients who healed after internal fixation. A greater loss of muscle strength and a more deviant gait pattern may have contributed to this. Despite lower functional outcome scores, these patients do not have a worse health-related quality of life, probably caused by an adequate coping mechanism of our relatively young and healthy study population. When considering IF for fitter FNF patients the possibility of a salvage arthroplasty must be acknowledged and patients can be informed about slightly lesser functional outcome.
Acknowledgments
Source of funding
Members of the research team received a grant from Fonds NutsOhra (grant number T-0602-43), The Netherlands Organization for Health Research and Development (ZonMw; grant number 171102008), Stichting Coolsingel (grant number 79), Physicians’ Services Incorporated Foundation (grant number 08-18), Canadian Institutes of Health Research (grant number 177466) and National Institutes of Health (grant number 1R01-AR055267-01A1). The funding agencies were not involved in the study design, data collection, data analysis, manuscript preparations or publication decisions for this manuscript.
FAITH trial study group
Steering Committee: Mohit Bhandari (Chair), Marc Swiontkowski, Philip J. Devereaux, Gordon Guyatt, Martin J. Heetveld, Kyle Jeray, Susan Liew, Emil H. Schemitsch, Lehana Thabane, Stephen Walter
Global Methods Centre: Mohit Bhandari (Principal Investigator); Sheila Sprague (Research Program Manager); Taryn Scott, Marilyn Swinton, Helena Viveiros (Research Coordination); Diane Heels-Ansdell, Qi Zhou (Statistical Analysis); Lisa Buckingham, Aravin Duraikannan (Data Management); Deborah Maddock (Grants Manager) (McMaster University)
US Methods Centre: Marc Swiontkowski (Principal Investigator); Julie Agel (Research Coordination) (University of Minnesota)
Netherlands Method Centre: Martin J. Heetveld (Principal Investigator); Esther M.M. Van Lieshout (Research Coordination); Stephanie M. Zielinski (Trial Coordination) (Erasmus MC, University Medical Center Rotterdam)
UK Methods Centre: Amar Rangan (Principal Investigator); Birgit Hanusch (Research Coordination) (The James Cook University Hospital)
Central Adjudication Committee: Gregory J Della Rocca (Chair), Robert Haverlag, Susan Liew, Gerard Slobogean
Data Safety Monitoring Board: Jeffrey Katz (Chair), Brenda Gillespie, Gail A. Greendale, Pierre Guy, Curtis Hartman, Craig Rubin, James Waddell
Clinical Site Investigators
The following persons participated in the FAITH Study:
Canada
Robert McCormack, Kelly Apostle, Dory Boyer, Farhad Moola, Bertrand Perey, Trevor Stone, Darius Viskontas, H. Michael Lemke, Mauri Zomar, Karyn Moon, Raely Moon, Amber Oatt (Royal Columbian Hospital); Richard E. Buckley, Paul Duffy, Robert Korley, Shannon Puloski, Kelly Johnston, James Powell, Kimberly Carcary (Foothills Medical Centre); David Sanders, Abdel Lawendy, Christina Tieszer (London Health Sciences Centre); David Stephen, Hans Kreder, Richard Jenkinson, Markku Nousiainen, Terry Axelrod, John Murnaghan, Diane Nam, Robin Richards,. Sebastian Rodriguez-Elizalde, Veronica Wadey, Albert Yee, Katrine Milner, Monica Kunz, Melanie MacNevin, Ria Cagaanan (Sunnybrook Health Sciences Centre); Ryan Bicknell, Jeff Yach, Davide Bardana, Gavin Wood, Mark Harrison, David Yen, Sue Lambert, Fiona Howells, Angela Ward (Human Mobility Research Centre, Queen’s University and Kingston General Hospital); Chad Coles, Ross Leighton, Michael Biddulph, David Johnston, Mark Glazebrook, David Alexander, Cathy Coady, Michael Dunbar, Kelly Trask, Shelley MacDonald, Gwen Dobbin (Queen Elizabeth II Health Sciences Centre); Emil H. Schemitsch, Henry Ahn, Jeremy A Hall, Michael D McKee, Daniel B Whelan, Aaron Nauth, Milena Vicente, Lisa Wild, Ryan Khan, and Jennifer Hidy (St. Michael’s Hospital); Paul Zalzal, Heather Brien, V. Naumetz, Brad Weening, Nicole Simunovic (Oakville Trafalgar Memorial Hospital); Eugene K. Wai, Steve Papp, Wade T. Gofton, Allen Liew, Stephen P. Kingwell, Darren M. Roffey, Vivian Borsella (Ottawa Hospital); Victoria Avram (Juravinski Hospital and Cancer Centre)
United States
Todd M. Oliver, Vicki Jones (Boone Hospital Center – Columbia Orthopaedic Group); Clifford Jones, James Ringler, Terrence Endres, Debra L. Sietsema (Orthopaedics Associates of Michigan); Kyle J. Jeray, J. Scott Broderick, David R. Goetz, Thomas B. Pace, Thomas M. Schaller, Scott E. Porter, Stephanie L. Tanner, Rebecca G. Snider, Lauren A. Nastoff, Shea A. Bielby (Greenville Hospital System); Andrew J Marcantonio, Richard Iorio, John Garfi (Lahey Clinic); Michael J. Prayson, Richard Laughlin, Joseph Rubino, Jedediah May, Geoffrey Ryan Rieser, Liz Dulaney-Cripe, Chris Gayton (Miami Valley Hospital); Julie A. Switzer, Peter A. Cole, Sarah A. Anderson, Paul M. Lafferty, Mengnai Li, Thuan V. Ly, Scott B. Marston, Amy L. Foley, Sandy Vang, David M. Wright (Regions Hospital-University of Minnesota); Heather A. Vallier, Andrea Dolenc, Chalitha Robinson (MetroHealth Medical Center); John T. Gorczyca, Jonathan M. Gross, Catherine A. Humphrey, Stephen Kates, Krista Noble, Allison W McIntyre, Kaili Pecorella (University of Rochester Medical Center); James Shaer, Tyson Schrickel, Barbara Hileman (St. Elizabeth Health Center); Craig A. Davis, Stewart Weinerman, Peter Weingarten, Philip Stull, Stephen Lindenbaum, Michael Hewitt, John Schwappach, Janell K. Baker (Colorado Orthopedic Consultants); Samir Mehta, John Esterhai, Jaimo Ahn, Annamarie D. Horan, Kelly McGinnis, Christine A. Kaminski, Brynn N. Kowalski (University of Pennsylvania); Lisa K. Cannada, David Karges, Leslie Hill (St. Louis University Hospital); Ivan Tarkin, Peter Siska, Gary Gruen, Andrew Evans, Dana J. Farrell, James Irrgang, Arlene Luther (University of Pittsburgh Medical Center); Jonathan P. Keeve, Christopher G. Anderson, Michael D. McDonald, Jodi M. Hoffman (Northwest Orthopaedic Specialists); Mark Jenkins, Jules Dumais, Amanda W. Romero (Texas Tech University Health Sciences Center – Lubbock); Joseph R. Hsu, James Ficke, Michael Charlton, Matthew Napierala, Mary Fan (US Army Institute of Surgical Research); William W. Cross III, Joseph R. Cass, Stephen A. Sems, Michael E. Torchia, Tyson Scrabeck (Mayo Clinic); Carlos A. Sagebien, Mark S. Butler, James T. Monica, Patricia Seuffert (University Orthopaedic Associates, LLC); Michael L. Brennan, Robert Probe, Evelyn Kile, Kelli Mills, Lydia Clipper, Michelle Yu, Katie Erwin (Scott and White Memorial Hospital); Paul Tornetta III, Hope Carlisle, Heather Silva (Boston University Medical Center); Michael Archdeacon, Ryan Finnan, Toan Le, John Wyrick, Shelley Hess (UC Health/University of Cincinnati Medical Center); Jessica McBeth (Santa Clara Valley Medical Center); Kamran Aurang, Gary Zohman, Brett Peterson, Roger B. Huff, (Kaiser Permanente); Joseph Baele, Timothy Weber, Matt Edison (OrthoIndy); Andrew H. Schmidt, Jerald R. Westberg (Hennepin County Medical Center); Charles J. DePaolo, Rachel Alosky, Leslie E. Shell, Lynne Hampton, Stephanie Shepard, Tracy Nanney, Claudine Cuento (Mission Hospital Research Institute); Karl Shively, Janos P. Ertl, Brian Mullis, J. Andrew Parr, Ripley Worman, Valda Frizzell, Molly M. Moore, Erin Tobias, Emily Thomas (Indiana University – Wishard Health Services); Robert V. Cantu, Eric R. Henderson, Linda S. Eickhoff (Dartmouth-Hitchcock Medical Center); David P. Zamorano, Deeba Pourmand, Deanna Lawson (University of California Irvine Medical Center); E. Mark Hammerberg, Philip Stahel, David Hak, Cyril Mauffrey, Douglas Gibula, Hannah Gissel, Corey Henderson (Denver Health Medical Center); Gregory J. Della Rocca, Brett D. Crist, Yvonne M. Murtha, Melinda McPherson, Linda K. Anderson (University of Missouri Health Care); Michael P. Dohm, Abby Zellar (Western Slope Study Group); Colleen Linehan, Lindsey Pilling (Covenant Healthcare of Saginaw) Daniel Horwitz, Kent Strohecker (Geisinger Medical Center); Courtland G. Lewis, Stephanie Caminiti, Raymond J. Sullivan, Elizabeth Roper (University of Connecticut – Hartford Hospital); William Obremskey, Philip Kregor, Justin E. Richards, Kenya String fellow (Vanderbuilt University Medical Center)
The Netherlands
J. Carel Goslings, Robert Haverlag, Kees Jan Ponsen. (Academic Medical Center); Maarten W.G.A. Bronkhorst, Onno R. Guicherit (Bronovo Ziekenhuis); Peter Patka, Martin G. Eversdijk, Rolf Peters, Dennis Den Hartog, Oscar J.F. Van Waes, Pim Oprel (Erasmus MC, University Medical Center Rotterdam); Piet A.R. de Rijcke, Cees L. Koppert, Steven E. Buijk, Richard P.R. Groenendijk, Imro Dawson, Geert W.M. Tetteroo, Milko M.M. Bruijninckx, Pascal G. Doornebosch, Eelco J.R. de Graaf (IJsselland Ziekenhuis); Martin J. Heetveld, Gijs A. Visser, Heyn Stockmann, Rob Silvis, Jaap P. Snellen, Bram Rijbroek, Joris J.G. Scheepers, Erik G.J. Vermeulen, Michiel P.C. Siroen, Ronald Vuylsteke, Hans L.F. Brom, Herman Rijna (Kennemer Gasthuis); Gert R Roukema, Hong Josaputra, Paul Keller, Peter D. de Rooij, Hans Kuiken, Han Boxma, Berry I. Cleffken, Ronald Liem (Maasstad Ziekenhuis); Steven J. Rhemrev, Coks H.R. Bosman, Alexander de Mol van Otterloo, Jochem Hoogendoorn, Alexander C. de Vries, Sven A.G. Meylaerts (Medisch Centrum Haaglanden); Rudolf W. Poolman, Maarten P. Simons, Frank H.W.M. van der Heijden, W. Jaap Willems, Frank R.A.J. de Meulemeester, Cor P. van der Hart, Kahn Turckan, Sebastiaan Festen, Frank de Nies, Robert Haverlag, Nico J.M. Out, Jan Bosma (Onze Lieve Vrouwe Gasthuis); Maarten van der Elst, Carmen C. van der Pol, Martijne van ’t Riet, Tom M. Karsten, Mark R. de Vries, Laurents P.S. Stassen, Niels W.L. Schep, G. Ben Schmidt, W.H. Hoffman (Reinier de Graaf Gasthuis); Michiel J.M. Segers, Jacco A.C. Zijl, Bart Verhoeven, Anke B. Smits, Jean Paul P.M. de Vries, Bram Fioole, Henk van der Hoeven, Evert B.M. Theunissen, Tammo S. de Vries Reilingh, Lonneke Govaert, Philippe Wittich, Maurits de Brauw, Jan Wille, Peter M.N.Y.M. Go, Ewan D. Ritchie, Ronald N. Wessel, Eric R. Hammacher (St. Antonius Ziekenhuis); Michiel H.J. Verhofstad, Joost Meijer, Teun van Egmond, Frank H.W.M. van der Heijden, Igor van der Brand (St. Elisabeth Ziekenhuis); Harm M van der Vis, Martin Campo, Ronald Verhagen, G.H. Robert Albers, Arthur W. Zurcher (Tergooi Ziekenhuizen); Albert van Kampen, Jan Biert, Arie B. van Vugt, Michael J.R. Edwards, Taco J. Blokhuis, Jan Paul M. Frölke, Leo M.G. Geeraedts, Jean W.M. Gardeniers, Edward T.C.H. Tan, Lodewijk M.S.J. Poelhekke, Maarten C. de Waal Malefijt, Bart Schreurs (University Medical Center St. Radboud); Rogier K.J. Simmermacher, Jeroen van Mulken, Karlijn van Wessem, Taco J. Blokhuis, Steven M. van Gaalen, Luke P.H. Leenen (University Medical Center Utrecht)
International
Susan Liew, Harvinder Bedi, Ashley Carr, Andrew Chia, Steve Csongvay, Hamish Curry, Stephen Doig, Craig Donohue, Elton Edwards, Greg Etherington, Andrew Gong, Arvind Jain, Doug Li, Russell Miller, Ash Moaveni, Matthias Russ, Lu Ton, Otis Wang, Zoe Murdoch, Claire Sage (The Alfred, Australia); Frede Frihagen, John Clarke-Jenssen, Geir Hjorthaug, Torben Ianssen, Asgeir Amundsen, Jan Egil Brattgjerd, Tor Borch, Berthe Bøe, Bernhard Flatøy, Sondre Hasselund, Knut Jørgen Haug, Kim Hemlock, Tor Magne Hoseth, Geir Jomaas, Thomas Kibsgård, Bjørn Kristiansen, Tarjei Lona, Gilbert Moatshe, Oliver Müller, Marius Molund, Tor Nicolaisen, Fredrik Nilsen, Jonas Rydinge, Morten Smedsrud, Are Stødle, Axel Trommer, Stein Ugland, Elise Berg Vesterhus, Anne Christine Brekke (Ulleval University Hospital, Norway); Ateet Sharma, Amir Sanghavi (Satellite Orthopaedic Hospital and Research Centre, India); Kevin Tetsworth, Donald Geoff, Patrick Weinrach, Paul Pincus, Steven Yang, Brett Halliday, Trevor Gervais, Michael Holt, Annette Flynn (Royal Brisbane and Women’s Hospital, Australia); Amal Shankar Prasad, Vimlesh Mishra (Madhuraj Nursing Home, India); Ajay Gupta, Niraj Jain (Nirmal Hospital, India); Mahesh Bhatia, Vinod Arora, Mahesh Bhatia (RLB Hospital and Research Centre, India); D.C. Sundaresh, Angshuman Khanna (M.S. Rammaiah Medical College & Hospital, India); Anil Rai, Subash (Highway Hospital, India); Marinis Pirpiris, David Love, Andrew Bucknill, Richard J Farrugia (Royal Melbourne Hospital, Australia); Akhil Dadi, Naveen Palla (Sunshine Hospital, India); B. Sachidananda Rai, Janakiraman Rajakumar (Unity Health Complex, India); Joe Joseph Cherian, Davy J Olakkengil, Gaurav Sharma (St John’s Medical College Hospital, India)
Footnotes
Level of Evidence: Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
References
- 1.Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85-A(9):1673–1681. doi: 10.2106/00004623-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari M, Devereaux PJ, Tornetta P, 3rd, et al. Operative management of displaced femoral neck fractures in elderly patients. An international survey. J Bone Joint Surg Am. 2005;87(9):2122–2130. doi: 10.2106/JBJS.E.00535. [DOI] [PubMed] [Google Scholar]
- 3.Blomfeldt R, Tornkvist H, Ponzer S, et al. Internal fixation versus hemiarthroplasty for displaced fractures of the femoral neck in elderly patients with severe cognitive impairment. J Bone Joint Surg Br. 2005;87(4):523–529. doi: 10.1302/0301-620X.87B4.15764. [DOI] [PubMed] [Google Scholar]
- 4.Heetveld MJ, Rogmark C, Frihagen F, et al. Internal fixation versus arthroplasty for displaced femoral neck fractures: what is the evidence? J Orthop Trauma. 2009;23(6):395–402. doi: 10.1097/BOT.0b013e318176147d. [DOI] [PubMed] [Google Scholar]
- 5.Parker MJ, Gurusamy K. Internal fixation versus arthroplasty for intracapsular proximal femoral fractures in adults. Cochrane Database Syst Rev. 2006;(4):CD001708. doi: 10.1002/14651858.CD001708.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franzen H, Nilsson LT, Stromqvist B, et al. Secondary total hip replacement after fractures of the femoral neck. J Bone Joint Surg Br. 1990;72(5):784–787. doi: 10.1302/0301-620X.72B5.2211756. [DOI] [PubMed] [Google Scholar]
- 7.Mortazavi SM, MRG, Bican O, et al. Total hip arthroplasty after prior surgical treatment of hip fracture is it always challenging? J Arthroplasty. 2012;27(1):31–36. doi: 10.1016/j.arth.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166–169. doi: 10.1097/00005131-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Angelini M, McKee MD, Waddell JP, et al. Salvage of failed hip fracture fixation. J Orthop Trauma. 2009;23(6):471–478. doi: 10.1097/BOT.0b013e3181acfc8c. [DOI] [PubMed] [Google Scholar]
- 10.Blomfeldt R, Tornkvist H, Ponzer S, et al. Displaced femoral neck fracture: comparison of primary total hip replacement with secondary replacement after failed internal fixation: a 2-year follow-up of 84 patients. Acta Orthop. 2006;77(4):638–643. doi: 10.1080/17453670610012728. [DOI] [PubMed] [Google Scholar]
- 11.McKinley JC, Robinson CM. Treatment of displaced intracapsular hip fractures with total hip arthroplasty: comparison of primary arthroplasty with early salvage arthroplasty after failed internal fixation. J Bone Joint Surg Am. 2002;84-A(11):2010–2015. doi: 10.2106/00004623-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson LT, Jalovaara P, Franzen H, et al. Function after primary hemiarthroplasty and secondary total hip arthroplasty in femoral neck fracture. J Arthroplasty. 1994;9(4):369–374. doi: 10.1016/0883-5403(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 13.Archibeck MJ, Carothers JT, Tripuraneni KR, et al. Total hip arthroplasty after failed internal fixation of proximal femoral fractures. J Arthroplasty. 2013;28(1):168–171. doi: 10.1016/j.arth.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Lindemann U, Becker C, Unnewehr I, et al. Gait analysis and WOMAC are complementary in assessing functional outcome in total hip replacement. Clin Rehabil. 2006;20(5):413–420. doi: 10.1191/0269215506cr958oa. [DOI] [PubMed] [Google Scholar]
- 15.Sinha A, Twycross-Lewis R, Small C, et al. Motion analysis as an outcome measure for hip arthroplasty. Surgeon. 2011;9(5):284–291. doi: 10.1016/j.surge.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.NVvH. Definitief concept. May 11, 2007. Richtlijn: Behandeling van de proximale femurfractuur bij de oudere mens (Guideline: Treatment of proximal femur fractures in the elderly patient) (Definitive concept 5-11-2007) [Google Scholar]
- 17.Zielinski SM, Bouwmans CA, Heetveld MJ, et al. The societal costs of femoral neck fracture patients treated with internal fixation. Osteoporos Int. 2013 doi: 10.1007/s00198-013-2487-2. [DOI] [PubMed] [Google Scholar]
- 18.Zielinski SM, Keijsers NL, Praet SF, et al. Femoral neck shortening after internal fixation of a femoral neck fracture. Orthopedics. 2013;36(7):e849–858. doi: 10.3928/01477447-20130624-13. [DOI] [PubMed] [Google Scholar]
- 19.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 20.Roorda LD, Jones CA, Waltz M, et al. Satisfactory cross cultural equivalence of the Dutch WOMAC in patients with hip osteoarthritis waiting for arthroplasty. Ann Rheum Dis. 2004;63(1):36–42. doi: 10.1136/ard.2002.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurney B. Leg length discrepancy. Gait Posture. 2002;15(2):195–206. doi: 10.1016/s0966-6362(01)00148-5. [DOI] [PubMed] [Google Scholar]
- 22.Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1–133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 23.Chen CJ, Chou LS. Center of mass position relative to the ankle during walking: a clinically feasible detection method for gait imbalance. Gait Posture. 2010;31(3):391–393. doi: 10.1016/j.gaitpost.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Benedetti MG, Catani F, Benedetti E, et al. To what extent does leg length discrepancy impair motor activity in patients after total hip arthroplasty? Int Orthop. 2010;34(8):1115–1121. doi: 10.1007/s00264-009-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]