Abstract
Purpose
Many common health conditions arise due to a combination of genetic factors and lifestyle-related behaviors. People’s understanding of the multifactorial nature of health conditions has implications for their receptivity to health messages regarding genomics and medicine, and may be related to their adoption of protective health behaviors. Although past work has investigated aspects of either genetic or behavioral causal beliefs, multifactorial beliefs have not been evaluated systematically.
Methods
Utilizing nationally-representative cross-sectional data from the Health Information National Trends Survey (HINTS), we examined the prevalence of multifactorial beliefs regarding the etiology of cancer, obesity, diabetes, heart disease, and hypertension, as well as associations between such beliefs and demographic, health history, and health behavior variables in the U.S. population.
Results
Among 3,630 participants, the vast majority (64.2-78.6%) endorsed multifactorial beliefs. The number of statistically significant associations were limited. Trends suggest that endorsement of multifactorial beliefs may differ by demographic and health history characteristics. Beliefs about the multifactorial etiology of cancer were associated with cancer screening behaviors. Multifactorial beliefs about other common health conditions were associated with few health promotion behaviors.
Conclusion
These findings and recommendations for future research provide preliminary guidance for developing and targeting genomics-related health messages and communications.
Keywords: causal beliefs, genetics, health behavior, cancer, chronic disease
INTRODUCTION
The successful translation of genomic science into improved clinical and public health outcomes requires that information about the genetic basis for disease be communicated to the public in a way that enables them to understand and use the information when making health decisions.1-5 However, limitations in genomic literacy, defined as “the capacity to obtain, process, understand, and use genomic information for health-related decision making”2 have the potential to reduce the impact of genomics research.2,3
A key starting point for effective communication is to identify what beliefs the audience already holds about the topic prior to implementing communication efforts.3,6,7 Beliefs regarding the causes of a health condition are particularly critical because they influence the behaviors that people adopt.4,8,9 For example, breast cancer survivors who believed that their cancer was caused by poor diet or lack of exercise were more likely to improve their eating habits or physical activity, respectively.9 Conversely, conditions viewed as genetic can be perceived as less controllable and less responsive to behavioral changes, but more responsive to biologically-based therapies such as lipid lowering medication.4 Once pre-existing causal beliefs are identified, health messages that promote healthful decisions and behaviors can be created that either correct erroneous beliefs or support existing beliefs (see 4,6 for details about developing corrective and supportive health messages).
Understanding the fact that most common and chronic health conditions have genetic, behavioral, and environmental risk factors1 is an important component of genomic literacy.2 Individuals who hold erroneous beliefs regarding the multifactorial etiology of common health conditions may have difficulty engaging with future prevention and screening recommendations that are developed based on a multifactorial model of disease causation.3,4,6 Yet, the extent to which the general public holds such multifactorial causal beliefs is unknown. Some research suggests that multifactorial beliefs may be relatively common,10-13 but these beliefs are challenging to examine systematically because laypeople have difficulty articulating the basic concept.14
Several studies have approached the problem by assessing genetic and behavioral causal beliefs as separate constructs. Analyzing endorsement of “singular” beliefs revealed that participants endorse genetics as a causal factor for obesity, diabetes, heart disease, and several different cancers, but the strength of that endorsement and the degree to which they also endorse behavioral factors varies widely.10-12,15-19 Past work has also identified demographic and health history correlates of genetic and behavioral causal beliefs of common diseases.4,8,10-12,15,16,18,20,21 However, the direction and significance of these relationships have also been inconsistent across studies. For example, older adults were more likely to endorse genetics as a risk factor for cancer in one study,15 but older women were less likely to endorse heredity as a cause of breast and colorectal cancer in another.12 The inconsistency of associations between singular causal beliefs and demographic and health history characteristics is likely due to differences among studies in terms of the diseases studied, sample sizes and populations, and methodologies used. These differences preclude drawing overarching conclusions about the direction and magnitude of the relationships. In addition, no studies have reported the association between endorsing both genetic and behavioral causal beliefs with demographic and health history variables, or between these multifactorial beliefs and health behaviors.
The present study builds on and addresses gaps in past work and contributes to the long-term goal of improving genomics-related health messaging and communication for common health conditions. It examines several aspects of the public’s multifactorial causal beliefs, which are defined as the simultaneous endorsement of both genetic and behavioral risk factors for health conditions. It uses data from a population-based, nationally representative survey22 to explore the prevalence of multifactorial beliefs in the U.S., as well as their demographic, health history, and behavioral correlates. Contrary to studies that have examined only one or two health outcomes in the same sample (for exceptions see 7,8), which makes it difficult to identify overarching patterns in interrelationships among causal beliefs and other variables, this study examines multifactorial beliefs about five chronic conditions: cancer, obesity, diabetes, heart disease, and hypertension.
Conceptual Framework and Hypotheses
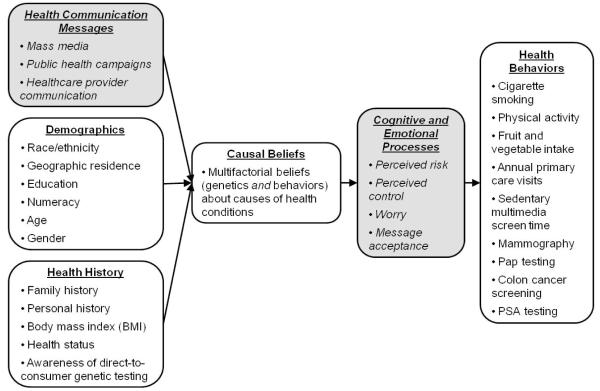
This study is guided by a conceptual framework (Figure 1) that draws upon theoretical and empirical work from psychology, public health, and genomic medicine.1,4,5,8,9,23,24 It describes how multiple factors can influence individuals’ causal beliefs about a disease, which in turn can affect their thoughts, feelings, and health-related behaviors. The basic premise is that a multifactorial model of disease causation represents a more complex set of beliefs than a single-factor model comprised of only genetic or behavioral causes.2,14 This complex information may be difficult to understand for populations that already experience limitations in health knowledge. Therefore, we hypothesize that multifactorial causal beliefs will be more common among individuals who are non-Hispanic white,25,26 live in urban (versus rural) geographic areas,27 and have more education28 and higher numeracy (the ability to use numerical information to make effective health decisions).29
Figure 1.
Conceptual framework. This framework describes how multiple factors can influence causal beliefs. These beliefs in turn can affect health cognitions, emotions, and subsequent health behaviors. Concepts examined in the present study are shown in the white boxes. The shaded boxes include additional relevant factors that provide a context for these processes. We do not examine these additional factors in the current research, but they could serve as targets for future research.
In addition, because involvement with the healthcare system can result in improved health knowledge,30 health history characteristics that lead to more engagement with the healthcare system may be associated with multifactorial beliefs. Thus, we hypothesize that having a family or personal history of a health condition, being overweight or obese, and having lower self-reported health status will be associated with higher endorsement of multifactorial causal beliefs. Lastly, because the marketing practices of direct-to-consumer (DTC) genomic tests emphasize the interactive roles of genetics and behavior, we hypothesize that awareness of DTC testing will be associated with the endorsement of multifactorial beliefs.
Given that no previous work has examined how multifactorial causal beliefs may be associated with health behaviors, our examination of these relationships is exploratory in nature and no directional hypotheses are specified. We examine health promotion behaviors relevant to the common health conditions of interest: cigarette smoking, physical activity, fruit and vegetable intake, annual primary care visits, and multimedia screen time (sedentary leisure time spent watching television or movies, searching the Internet, and playing computer games). We also examined how multifactorial beliefs may be associated with cancer detection behaviors including mammography, Pap testing, colorectal cancer screening, and prostate-specific antigen (PSA) testing.
MATERIALS AND METHODS
Data Source and Participants
Data were obtained from the Health Information National Trends Survey (HINTS 4, Cycle 2), which was administered through mailed self-report questionnaires from October 2012 through January 2013. HINTS is a population-based, nationally representative survey of the civilian non-institutionalized population of the U.S. Detailed information about its methodology and response rate is available at hints.cancer.gov/docs/HINTS_4_Cycle2_Methods_Report.pdf. Participants were included in the present analyses if they were age 18 or older and, for analyses regarding health behaviors, were the appropriate age and gender for the behavior of interest.
Measures
Causal beliefs were assessed with two questions for each condition: “How much do you think health behaviors like diet, exercise and smoking determine whether or not a person will develop each of the following conditions [cancer, obesity, diabetes, heart disease, hypertension]?”, and “How much do you think genetics, that is characteristics passed from one generation to the next, determine whether or not a person will develop each of the following conditions?” The response options were: Not at all; A little; Somewhat; A lot.
Demographic variables were age, sex, education, race/ethnicity, and rural/urban geographic residence. The numeracy variable was: “As far as you know, who has a greater chance of getting cancer—a person with a 1 in 1,000 chance of getting cancer, or a person with a 1 in 100 chance?”
Health history variables included self-reported health status; family history of cancer; personal diagnosis of cancer, heart disease, diabetes, or hypertension; and body mass index (BMI). Awareness of direct-to-consumer (DTC) genetic testing was also examined. HINTS did not assess family history of heart disease, diabetes, or hypertension.
Health behaviors included cigarette smoking status, adherence to the American College of Sports Medicine’s recommendation of obtaining at least 150 minutes per week of physical activity, minutes per day of sedentary screen time, fruit and vegetable intake (as analyzed by Wang and Coups20), and obtaining annual primary care visits. Adherence to recommendations for mammography and Pap screening,31 and use of colorectal cancer screening and prostate-specific antigen (PSA) testing were also assessed.
Analysis Plan
Data were analyzed using SAS 9.3 in compliance with HINTS analytic recommendations.22 Five dichotomous variables were created to represent multifactorial beliefs for each health condition: cancer, obesity, diabetes, heart disease, and hypertension. Participants were defined as endorsing multifactorial beliefs for a given condition if they responded “A lot” or “Somewhat” to both genetic and behavior causal beliefs items for that condition. Individuals who responded “A lot” or “Somewhat” to genetics and “A little” or “Not at all” to health behaviors were categorized as endorsing mostly genetics. Individuals who responded “A lot” or “Somewhat” to health behaviors and “A little” or “Not at all” to genetics were categorized as endorsing mostly behavior. Individuals who responded “A little” or “Not at all” to both genetics and health behaviors were categorized as endorsing neither. The multifactorial beliefs items were then dichotomized to represent the endorsement or non-endorsement of multifactorial beliefs for a given health condition.
Participant characteristics and behaviors were examined using weighted descriptive statistics.22 Associations between demographic and health history characteristics and multifactorial beliefs were analyzed through five separate multivariable logistic regressions. For each regression a separate multifactorial belief variable was the outcome. The demographic and health history variables were the predictors. Family history of cancer and personal history of each condition were included only in those models for which they were conceptually consistent. For example, personal history of cancer was included only for analyses of multifactorial beliefs about cancer. Family history of cancer was included in the analyses for beliefs about heart disease and hypertension in addition to beliefs about cancer because bivariate analyses indicated it was a potential confounder.
Associations between multifactorial beliefs and behaviors were examined using multivariable logistic and linear regression for dichotomous and continuous outcomes, respectively. Behavioral variables were the outcomes and the multifactorial belief variables were the predictors. These analyses also included the demographic and conceptually consistent health history variables as covariates. Cancer screening behaviors were relevant only for women aged 40+ (breast), women aged 21-65 (cervical), men and women aged 50+ (colon), and men aged 50+ (prostate).31
RESULTS
Participant characteristics (n=3,630) are described in Table 1. Weighted analyses indicated that the participants were approximately equal in gender, primarily white, primarily resided in an urban location, and were on average 46.6 years of age. A majority of participants had at least some college education, and most answered the numeracy item correctly. Slightly fewer than half of participants reported being in “excellent” or “very good” health. Reports of any family history or a personal history of cancer were greater than available national estimates.32,33 Self-reported personal history of diabetes, heart disease, or hypertension were approximately equivalent to national estimates.34-36 Fewer participants reported being obese compared to national estimates.37 Most participants were never smokers, and only 33.9% were adherent to physical activity guidelines. Participants reported on average approximately 3.5 hours of sedentary leisure screen time per day. The vast majority of eligible women were adherent with Pap and mammography guidelines. Between half and two-thirds of eligible participants reported having ever been screened for colon cancer or having undergone PSA testing.
Table 1.
Participant Characteristics (N=3,630)
| Participant Characteristics | n (unweighted) | % (weighted) |
|---|---|---|
| Demographics a | ||
| Age (Weighted mean, SE)b | 46.6 | 0.07 |
| Sex | ||
| Female | 2214 | 51.4 |
| Male | 1416 | 48.6 |
| Educational attainment | ||
| Less than high school | 339 | 13.4 |
| High school/GED | 797 | 20.4 |
| Vocational/Technical, Some college | 1085 | 37.6 |
| College or Postgraduate | 1409 | 28.6 |
| Race/Ethnicity | ||
| Non-Hispanic White | 2233 | 66.4 |
| All Others | 1397 | 33.6 |
| Geographic Residence | ||
| Urban | 3087 | 83.7 |
| Rural | 543 | 16.3 |
| Numeracyc | ||
| Correct | 3023 | 85.7 |
| Incorrect | 607 | 14.3 |
| Health History a | ||
| Self-reported Health Status | ||
| Excellent | 382 | 11.2 |
| Very good | 1232 | 35.9 |
| Good | 1277 | 34.1 |
| Fair/Poor | 632 | 15.9 |
| Missing | 107 | 2.9 |
| Family History of Cancer | ||
| Yes | 2412 | 65.0 |
| No | 870 | 25.3 |
| Unknown/Missing | 348 | 9.6 |
| Personal History of Cancer | ||
| Yes | 468 | 8.2 |
| No | 3162 | 91.8 |
| Personal History of Heart Disease | ||
| Yes | 359 | 6.9 |
| No | 3140 | 89.6 |
| Missing | 131 | 3.5 |
| Personal History of Diabetes | ||
| Yes | 659 | 14.1 |
| No | 2827 | 82.3 |
| Missing | 144 | 3.5 |
| Personal History of Hypertension | ||
| Yes | 1499 | 33.9 |
| No | 1998 | 62.5 |
| Missing | 133 | 3.6 |
| Body Mass Index (BMI) | ||
| Underweight/Normal | 1168 | 34.3 |
| Overweight | 1168 | 31.4 |
| Obese | 1027 | 26.9 |
| Missing | 267 | 7.4 |
| Aware of Direct-to-Consumer (DTC) Genetic Tests | ||
| Yes | 1745 | 48.4 |
| No | 1826 | 50.6 |
| Missing | 59 | 0.9 |
| Health Behaviors | ||
| Smoker Status | ||
| Current | 586 | 18.7 |
| Former | 939 | 22.7 |
| Never | 2052 | 58.6 |
| Missing | 53 | 1.0 |
| Annual Primary Care Visits | ||
| Adherent | 2544 | 65.9 |
| Non-adherent | 1043 | 33.4 |
| Missing | 43 | 0.7 |
| Fruit/Vegetable Intake Indexd (Weighted mean, SE) |
5.0 | 0.1 |
| Exercise (at least 150 minutes per week)eR> | ||
| Yes | 1220 | 33.9 |
| No | 2095 | 55.5 |
| Missing | 315 | 10.6 |
| Screen Time (Minutes per day) (Weighted mean, SE) |
218.7 | 6.4 |
| Pap Screening Adherence | ||
| Yes | 1342 | 81.4 |
| No | 286 | 16.4 |
| Missing | 42 | 2.2 |
| Mammography Adherence | ||
| Yes | 1175 | 72.4 |
| No | 408 | 24.6 |
| Missing | 54 | 2.9 |
| Ever Had Colon Cancer Screening | ||
| Yes | 1483 | 69.1 |
| No | 562 | 28.5 |
| Missing | 58 | 2.4 |
| Ever Had PSA Testing | ||
| Yes | 614 | 67.4 |
| No | 260 | 30.8 |
| Missing | 29 | 1.8 |
Missing values were imputed for Age (n=118), Sex (n=68), Educational attainment (n=89), Race/Ethnicity (n=492), and Personal history of cancer (n=31).
One observation for age equaled 176 years and was recoded as a missing observation.
Missing observations (n=249) were coded as “Incorrect”.
Possible scores for fruit/vegetable intake ranged from 0-12.
The American College of Sports Medicine Physical Activity Guidelines recommend at least 150 minutes of moderate activity per week.
The vast majority of participants endorsed multifactorial causal beliefs about common health conditions (see Table 2). However, participants endorsed multifactorial beliefs slightly less often for cancer and obesity (approximately 64% of participants) than for the other conditions (76-78%). Most participants who did not endorse multifactorial beliefs endorsed behavioral explanations for all conditions except for cancer, which was more frequently perceived as being caused by genetics.
Table 2.
Multifactorial beliefs (N=3,630)
| n (unweighted) | % (weighted) | |
|---|---|---|
| Cancer | ||
| Multifactorial beliefs | 2391 | 64.3 |
| Mostly genetics | 437 | 14.2 |
| Mostly behavior | 406 | 11.7 |
| Neither | 248 | 6.6 |
| Missing | 148 | 3.3 |
| Obesity | ||
| Multifactorial beliefs | 2378 | 64.1 |
| Mostly genetics | 108 | 3.6 |
| Mostly behavior | 864 | 25.4 |
| Neither | 111 | 3.1 |
| Missing | 169 | 3.8 |
| Diabetes | ||
| Multifactorial beliefs | 2856 | 78.6 |
| Mostly genetics | 170 | 4.9 |
| Mostly behavior | 364 | 10.2 |
| Neither | 113 | 3.4 |
| Missing | 127 | 2.8 |
| Heart Disease | ||
| Multifactorial beliefs | 2862 | 77.8 |
| Mostly genetics | 160 | 6.1 |
| Mostly behavior | 337 | 9.4 |
| Neither | 97 | 2.8 |
| Missing | 174 | 3.9 |
| Hypertension | ||
| Multifactorial beliefs | 2790 | 76.4 |
| Mostly genetics | 154 | 5.7 |
| Mostly behavior | 432 | 11.4 |
| Neither | 117 | 3.4 |
| Missing | 137 | 3.1 |
Few of the associations between multifactorial beliefs and demographic and health history characteristics were statistically significant at p<.05 (see Table 3). In addition, with the exception of obesity, there was no consistent pattern of statistically significant associations according to the condition of interest or participant characteristic. However, several nonsignificant trends were consistent across all five health conditions. The odds of endorsing multifactorial beliefs trended higher for women than men, among college graduates than those with less than a high school education, among racial/ethnic minorities than non-minorities, among rural than urban residents, and among individuals who correctly answered the numeracy item. Multifactorial beliefs also trended higher among those aware of DTC genetic testing.
Table 3.
Multivariable analysis of demographic and health history characteristics associated with multifactorial causal beliefsa
| Participant Characteristics | Cancer (“Endorses”) OR, 95% CI |
Obesity (“Endorses”) OR, 95% CI |
Diabetes (“Endorses”) OR, 95% CI |
Heart Disease (“Endorses”) OR, 95% CI |
Hypertension (“Endorses”) OR, 95% CI |
|---|---|---|---|---|---|
| Age | 1.01, 1.00-1.02 | 1.01, 1.00-1.02 | 0.99, 0.98-1.00 | 1.00, 0.99-1.01 | 0.99, 0.98-1.00 |
| Sex | |||||
| Female | 1.28, 0.95-1.74 | 1.24, 0.94-1.62 | 1.33, 0.95-1.86 | 1.32, 0.90-1.93 | 1.28, 0.91-1.79 |
| Male | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Educational Attainment | |||||
| Less than high school | 0.64, 0.38-1.05 | 0.81, 0.43-1.53 | 0.63, 0.38-1.03 | 0.35, 0.19-0.62 | 0.64, 0.33-1.24 |
| High school/GED | 0.84, 0.55-1.29 | 1.07, 0.71-1.62 | 0.92, 0.59-1.44 | 0.76, 0.40-1.41 | 0.96, 0.59-1.57 |
| Vo-Tec/Some college | 0.91, 0.66-1.26 | 1.05, 0.75-1.47 | 0.81 0.53-1.22 | 0.83, 0.53-1.29 | 0.94, 0.64-1.39 |
| College/Postgraduate | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| All Others | 1.52, 1.05-2.18 | 1.21, 0.92-1.58 | 1.26, 0.86-1.85 | 1.04, 0.69-1.57 | 1.19, 0.83-1.71 |
| Geographic Residence | |||||
| Urban | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Rural | 1.44, 1.00-2.06 | 1.34, 0.91-1.96 | 1.36, 0.85-2.17 | 1.33, 0.87-2.02 | 1.14, 0.78-1.66 |
| Numeracy | |||||
| Correct | 1.40, 0.99-2.00 | 1.28, 0.85-1.91 | 1.67, 1.10-2.53 | 1.38, 0.85-2.26 | 1.44, 0.92-2.25 |
| Incorrect | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Self-reported Health Status | |||||
| Excellent | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Very Good | 1.02, 0.58-1.79 | 0.94, 0.58-1.51 | 0.98, 0.50-1.95 | 1.05, 0.51-2.13 | 1.20, 0.66-2.18 |
| Good | 1.10, 0.62-1.96 | 1.03, 0.66-1.60 | 1.04, 0.51-2.14 | 1.29, 0.58-2.85 | 1.40, 0.74-2.66 |
| Fair/Poor | 1.06, 0.52-2.15 | 0.99, 0.53-1.83 | 0.81, 0.36-1.82 | 1.12, 0.46-2.75 | 1.05, 0.48-2.27 |
| BMI | |||||
| Underweight/Normal | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Overweight | 1.27, 0.97-1.67 | 1.04, 0.79-1.38 | 1.23, 0.89-1.71 | 1.19, 0.84-1.69 | 1.20, 0.91-1.58 |
| Obese | 1.04, 0.71-1.52 | 1.58, 1.09-2.31 | 1.73, 1.08-2.79 | 1.99, 1.24-3.21 | 1.84, 1.17-2.89 |
| Aware DTC Genetic Tests | |||||
| Yes | 1.23, 0.92-1.63 | 1.27, 0.95-1.69 | 1.49, 1.03, 2.16 | 1.42, 0.98-2.05 | 1.48, 1.10-1.99 |
| No | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Family History-Cancerb | |||||
| Yes | 1.75, 1.28-2.40 | 1.19, 0.74-1.93 | 1.29, 0.85-1.95 | ||
| No | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Personal History-Cancer | |||||
| Yes | 1.11, 0.77-1.59 | ||||
| No | 1.00 (ref) | ||||
| Personal History-Heart Disease | |||||
| Yes | 0.87, 0.53-1.43 | ||||
| No | 1.00 (ref) | ||||
| Personal History-Diabetes | |||||
| Yes | 1.21, 0.71-2.06 | ||||
| No | 1.00 (ref) | ||||
| Personal History-Hypertension | |||||
| Yes | 1.16, 0.86-1.58 | ||||
| No | 1.00 (ref) |
Bolded text denotes associations where p<.05. Italicized text indicates relationships for which odds ratios are in the same direction across all five diseases.
All conceptually consistent demographic and health history variables were included in all models. Shaded areas indicate analyses that were not conducted because the relationships were conceptually inconsistent. Because family history of cancer was significantly associated with multifactorial beliefs about heart disease and hypertension in bivariate analyses, family history was included in those models as a covariate.
The behavioral analyses yielded more complex findings (see Table 4). Endorsing multifactorial beliefs about cancer was statistically significantly (p<.05) associated with having ever undergone colon cancer screening, being adherent to mammography and Pap screening guidelines, and having ever undergone PSA testing. However, there were very few statistically significant findings for the remaining behaviors. Nevertheless, there were consistent nonsignificant trends indicating that endorsing multifactorial beliefs may be associated with higher odds of obtaining annual primary care visits and more screen time per day.
Table 4.
Multivariable analysis of the association between multifactorial causal beliefs and engagement in health behaviorsa
| Participant Characteristics |
Cancer (“Endorses”) OR, 95% CI |
Obesity (“Endorses”) OR, 95% CI |
Diabetes (“Endorses”) OR, 95% CI |
Heart Disease (“Endorses”) OR, 95% CI |
Hypertension (“Endorses”) OR, 95% CI |
|---|---|---|---|---|---|
| Smoking | |||||
| Nonsmoker | 0.78 (0.51-1.17) | 1.29 (0.76-2.19) | 0.95 (0.59-1.61) | 0.85 (0.47-1.52) | 1.11 (0.66-1.90) |
| Former | 0.82 (0.55-1.22) | 1.17 (0.74-1.87) | 0.92 (0.52-1.62) | 0.76 (0.43-1.34) | 0.97 (0.60-1.56) |
| Current | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
|
Annual Primary Care
Visit |
|||||
| Yes | 1.11 (0.84-1.47) | 1.02 (0.79-1.32) | 1.04 (0.73-1.47) | 1.18 (0.90-1.55) | 1.43 (1.08-2.05) |
| No | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Adherent to Exercise Guidelines (150 mins/wk) |
|||||
| Yes | 1.00 (0.71-1.4) | 1.07 (0.86-1.33) | 0.85 (0.63-1.43) | 0.94 (0.68-1.29) | 1.15 (0.84-1.57) |
| No | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
|
Pap Test Adherence
(Women) |
|||||
| Yes | 2.00 (1.22-3.27) | ||||
| No | 1.00 (ref) | ||||
|
Mammogram Adherence
(Women) |
|||||
| Yes | 1.64 (1.08-2.50) | ||||
| No | 1.00 (ref) | ||||
| Ever had PSA Test (Men) | |||||
| Yes | 1.84 (1.08-3.12) | ||||
| No | 1.00 (ref) | ||||
| Colon Cancer Screening | |||||
| Yes | 1.43 (1.05-1.94) | ||||
| No | 1.00 (ref) | ||||
|
|
|||||
| b, 95% CI | b, 95% CI | b, 95% CI | b, 95% CI | b, 95% CI | |
|
|
|||||
| Fruit/Vegetable Indexb | .04 (−0.29-0.36) | 0.06 (−0.26-0.39) | −0.13 (−0.65-0.38) | 0.02 (−0.43-0.47) | −0.03 (−0.41-0.36) |
|
Screen time (minutes per
day) b |
21.68 (0.13-43.23) | 19.7 (−4.81-44.17) | 12.55 (−12.64-37.74) | 27.10 (4.54-49.65) | 20.19 (−2.3-42.67) |
Bolded text denotes associations where p<.05. Italicized text indicates relationships for which odds ratios are in the same direction across all five diseases. All conceptually consistent demographic and health history variables were included in all models. Shaded areas indicate analyses that were not conducted because the relationships were conceptually inconsistent. Because family history of cancer was significantly associated with multifactorial beliefs about heart disease and hypertension in bivariate analyses, family history was included in those models as a covariate.
Regression estimates are unstandardized for the fruit and vegetable index and screen time.
DISCUSSION
Holding a multifactorial etiological model of disease is an important part of genomic health literacy.2 Results from this study indicate that the vast majority of people in the U.S. attribute five common health conditions to a combination of genetic and behavioral risk factors. The extent to which people hold such multifactorial beliefs varies slightly across conditions, but in no case does the prevalence fall below 64%. This widespread endorsement of multifactorial beliefs provides population-based evidence that is consistent with findings from local and clinic-based studies.10-13,22 Because health messages are more likely to be accepted, understood, and acted upon when crafted in a way that is consistent with causal beliefs, health messages that describe a genetic link to a common health condition should also emphasize the role of behavior and, when possible, the process by which genes and behavior interact to affect the disease process.4,6
Another notable finding is that, whereas most individuals who did not endorse cancer multifactorial beliefs endorsed “mostly genetic” causation, those not endorsing a multifactorial etiology for the other four conditions were more likely to endorse “mostly behavior” causal beliefs (see Table 2). This difference could be because: 1) the portrayal of cancer in the news and popular media focuses more on genetics, 2) people are unaware that multiple behaviors contribute to carcinogenesis, or 3) people perceive cancer as less controllable than other conditions. Future research should explore each of these possibilities when crafting health messages.
In contrast to our hypotheses, very few demographic or health history characteristics were associated with multifactorial causal beliefs at the traditional criterion for statistical significance (p<.05). The relative dearth of statistically significant associations in the present research is consistent with some research that reported no or limited associations between demographic factors and singular causal beliefs,11 but in contrast to several other studies.10,12,15,20 However, the limited degree of consistency in statistical significance according to health condition or participant characteristic raises concerns about possible spurious associations. The only exception was that being obese was significantly associated with holding multifactorial beliefs for 4 of the 5 health conditions: obesity, diabetes, heart disease, and hypertension. An elevated BMI defines obesity and is a risk factor for these conditions. It is possible that obese participants’ endorsement of multifactorial beliefs is the result of greater exposure to educational health messages regarding their personal disease risks.
A closer examination of the data reveals many nonsignificant trends in which the direction of the odds ratios is in the same direction across all five diseases. This occurs for 7 of 9 demographic and health history characteristics. Although no definitive statements can be made at this time without conducting additional research, the general trends suggest that multifactorial beliefs may be more common among women, college graduates, racial/ethnic minorities, rural residents, people with higher numeracy, and those who are aware of DTC genetic tests. The education and numeracy findings are consistent with our hypotheses and established literature.28,29 Numeracy is likely a critical skill for interpreting messages regarding the relative and combined contribution of genetic and lifestyle factors in disease etiology,38 and numeracy and education are positively correlated. 29 These findings highlight the need to further explore how numeracy and other aspects of health and genomic literacy may be associated with people’s causal beliefs regarding the multifactorial etiology of common health conditions.
The trends in associations between multifactorial beliefs and being a woman or having a rural residence were unexpected and deserve more detailed examination. It is also unclear why racial/ethnic minorities may be more likely to endorse multifactorial beliefs, but this finding is consistent with reports11 that ethnically-diverse participants who endorsed genetic causes of common diseases were more likely to also endorse lifestyle causes of disease than were those who did not endorse genetic causes. The trends suggesting that awareness of DTC genetic testing may be associated with multifactorial beliefs may reflect greater experiences with marketing and messages about the interplay between genetic and behavioral factors in the development of common health conditions. However, this speculation should be confirmed in studies designed specifically for that purpose.
Our exploration of the links between multifactorial beliefs and health behaviors yielded mixed findings. Endorsing multifactorial beliefs about cancer was associated with statistically significantly higher engagement in cancer screening behaviors. However, the relationship with health promotion behaviors was less clear. Very few of the remaining statistical tests yielded statistically significant results, and again there was no consistency across health conditions or health promotion behaviors. Furthermore, the direction of the odds ratios and linear regression estimates was inconsistent for smoking status, exercise, and fruit and vegetable intake. This strongly suggests that multifactorial beliefs may not be related to these variables. In contrast, the odds ratios and linear estimates were consistent for obtaining an annual primary care visit and screen time.
One explanation may be that cancer screening, primary care visits, and screen time represent opportunities for encountering information that increase understanding of the multifactorial nature of common health conditions. That is, physicians may share information about the multiple causes of disease during cancer screening and primary care visits. Screen time may reflect exposure to health messages delivered through television or the Internet. Another possibility is that endorsing multifactorial beliefs for cancer buffers the tendency for people to draw mental associations between “cancer” and “death.” Such cancer-death associations are linked to avoiding healthcare providers.39 Thus, people who do not endorse multifactorial beliefs about cancer may have stronger cancer-death associations, which may lead to avoiding healthcare providers and decreased cancer screening opportunities. These hypotheses are purely speculative and require further investigation. The differential relevance of multifactorial beliefs for detection and promotion behaviors is also worth exploring.
Strengths, Limitations, and Additional Future Directions
This study represents one of the first attempts to evaluate beliefs about the multifactorial etiology of common health conditions in the U.S. population. It benefits from the use of a large, population-based survey that resulted in nationally-representative estimates of the prevalence and correlates of multifactorial beliefs.22 These findings, regardless of statistical significance, suggest several novel research questions for future investigations. Most importantly, to what extent do multifactorial beliefs motivate behavior prospectively over time? The cross-sectional nature of this study precludes determining the directionality or causality of the relationships. Another question is whether screen time is associated with exposure to genomics-related health messages. If so, does such exposure facilitate the development of multifactorial beliefs? Rural residents have been severely underrepresented in causal beliefs research. Mixed methods research should attempt to clarify the content of their beliefs, how the content is developed, and how their beliefs may differ from their urban counterparts. Research should also examine whether it is more effective to target messages that emphasize the multifactorial nature of common conditions to the specific groups that may be in more need, such as those with low education or urban residents, or to simply disseminate the same message to all segments of the population. In addition, how important is it to include details about the extent to which the condition is heritable – that is, the actual balance of genetic versus non-genetic contribution? Future research should also investigate whether multifactorial beliefs vary according to cancer site. The answers to these and other research questions could begin to guide decisions about what content to include in genomics-related health messages, which demographic groups should be targeted by which messages, and which communication channels should be used to convey genomics information about common health conditions.
These findings should be evaluated in the following context. First, we developed a conceptual framework and posited tentative hypotheses for the demographic and health history variables. However, because the literature on multifactorial causal beliefs was so sparse in comparison to research on singular genetic and causal beliefs, we approached this study from a largely exploratory orientation. For that reason, we did not correct for multiple statistical comparisons. This exploratory orientation is also reflected in our interpretation of the results. We combined the conventional criterion for statistical significance with an evaluation of the extent to which the direction of the odds ratios and linear regression estimates were consistent across all the health conditions. This strategy is consistent with recent recommendations that researchers focus more on estimation and confidence intervals than on null hypothesis significance testing.40 Thus, these results should be seen as preliminary and hypothesis-generating, not definitive. Another limitation is that the measure of multifactorial beliefs addressed beliefs regarding the role of genetics and behavior only broadly. Future studies should assess perceptions of other risk factors, such as environmental toxins or psychological stress. Other limitations of HINTS and many surveys include using single-item measures and self-reported data.
Conclusion
Despite the broad scope of this research, its exploratory nature, and the complexity of its results, we report three take-home points: 1) multifactorial beliefs about the etiology of common health conditions are highly prevalent in the U.S. population; 2) the extent to which these beliefs are endorsed may vary according to the specific health condition and demographic and health history characteristics; and 3) the association between endorsing multifactorial beliefs and engagement in health behavior may be stronger for cancer detection behaviors than health promotion behaviors. We have identified several hypotheses and research questions that follow directly from these findings. We have also indicated how the results of such studies could be used to guide the development, targeting, and dissemination of genomics-related health messages and communications. Consequently, this research represents a small and preliminary, yet important, step in the translation of basic genomics research to improved public health outcomes.
ACKNOWLEDGEMENTS
We thank Siobhan Sutcliffe, ScD, for her statistical expertise and recommendations.
Funding: This research was supported by the American Cancer Society: “Communicating multifactorial genetic risks to underserved populations,” 121164-MRSG-11-214-01-CPPB (PI: Erika Waters) and the Barnes Jewish Hospital Foundation (EAW, JM). JGH was supported in part by the National Cancer Institute Cancer Prevention Fellowship Program.
Footnotes
Conflict of Interest The authors have no conflicts of interest.
REFERENCES
- 1.Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature. 2003;422(6934):835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- 2.Hurle B, Citrin T, Jenkins JF, et al. What does it mean to be genomically literate?: National Human Genome Research Institute Meeting Report. Genet Med. 2013;15(8):658–663. doi: 10.1038/gim.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanie AD, Epstein Jayartne T, Sheldon JP, et al. Exploring the public understanding of basic genetic concepts. J Genet Couns. 2004;13(4):305–319. doi: 10.1023/b:jogc.0000035524.66944.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research. Soc Sci Med. 2006;62:1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Hesse BW, Arora NK, Khoury MJ. Implications of Internet availability of genomic information for public health practice. Public Health Genomics. 2012;15(3-4):201–208. doi: 10.1159/000335892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron LD, Marteau TM, Brown PM, Klein WM, Sherman KA. Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: The role of coherence. J Behav Med. 2012;35(3):286–298. doi: 10.1007/s10865-011-9361-5. [DOI] [PubMed] [Google Scholar]
- 7.Richards M, Ponder M. Lay understanding of genetics: A test of a hypothesis. J Med Genet. 1996;33(12):1032–1036. doi: 10.1136/jmg.33.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. Routledge; New York, NY: 2003. pp. 42–65. [Google Scholar]
- 9.Costanzo ES, Lutgendorf SK, Roeder SL. Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer. Psychooncology. 2011;20(1):53–61. doi: 10.1002/pon.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashida S, Goodman M, Pandya C, et al. Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics. 2011;14(4-5):307–316. doi: 10.1159/000316234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderson SC, Diefenbach MA, Streicher SA, et al. Genetic and lifestyle causal beliefs about obesity and associated diseases among ethnically diverse patients: A structured interview study. Public Health Genomics. 2013;16(3):83–93. doi: 10.1159/000343793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Miller SM, Egleston BL, Hay JL, Weinberg DS. Beliefs about the causes of breast and colorectal cancer among women in the general population. Cancer Causes & Control. 2010;21(1):99–107. doi: 10.1007/s10552-009-9439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condit CM. Public attitudes and beliefs about genetics. Annu Rev Genomics Hum Genet. 2010;11:339–359. doi: 10.1146/annurev-genom-082509-141740. [DOI] [PubMed] [Google Scholar]
- 14.Condit CM, Gronnvoll M, Landau J, Shen L, Wright L, Harris TM. Believing in both genetic determinism and behavioral action: A materialist framework and implications. Public Understanding of Science. 2009;18(6):730–746. [Google Scholar]
- 15.Sanderson SC, Waller J, Humphries SE, Wardle J. Public awareness of genetic influence on chronic disease risk: Are genetic and lifestyle causal beliefs compatible? Public Health Genomics. 2011;14(4-5):290–297. doi: 10.1159/000294280. [DOI] [PubMed] [Google Scholar]
- 16.Parrott RL, Silk KJ, Condit C. Diversity in lay perceptions of the sources of human traits: Genes, environments, and personal behaviors. Soc Sci Med. 2003;56(5):1099–1109. doi: 10.1016/s0277-9536(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill SC, McBride CM, Alford SH, Kaphingst KA. Preferences for genetic and behavioral health information: The impact of risk factors and disease attributions. Ann Behav Med. 2010;40(2):127–137. doi: 10.1007/s12160-010-9197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wold KS, Byers T, Crane LA, Ahnen D. What do cancer survivors believe causes cancer? (United States) Cancer causes & control. 2005 Mar;16(2):115–123. doi: 10.1007/s10552-004-2414-0. [DOI] [PubMed] [Google Scholar]
- 19.Claassen L, Henneman L, Nijpels G, Dekker J, Marteau T, Timmermans D. Causal beliefs and perceptions of risk for diabetes and cardiovascular disease, The Netherlands, 2007. Prev Chronic Dis. 2011 Nov;8(6):A130. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Coups EJ. Causal beliefs about obesity and associated health behaviors: results from a population-based survey. Int J Behav Nutr Phys Act. 2010;7:19. doi: 10.1186/1479-5868-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condit CM. Public understandings of genetics and health. Clin Genet. 2010;77(1):1–9. doi: 10.1111/j.1399-0004.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute [Accessed September 27, 2013];Health Information National Trends Survey 4 (HINTS 4) Cycle 2 Analytic Recommendations. 2013 http://hints.cancer.gov/
- 23.McBride CM, Hensley-Alford S, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Putting science over supposition in the arena of personalized genomics. Nat Genet. 2008;40(8):939–942. doi: 10.1038/ng0808-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services [Accessed Feb 27, 2012];Healthy People 2020. 2012 http://www.healthypeople.gov/2020/default.aspx.
- 25.Oh A, Shaikh A, Waters EA, Atienza A, Moser R, Perna F. Health disparities in awareness of physical activity and cancer prevention: Findings from the National Cancer Institute’s 2007 Health Information National Trends Survey (HINTS) J Health Commun. 2010;15(Suppl 3):60–77. doi: 10.1080/10810730.2010.522694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez AS, Rutten LJ, Oh A, et al. Perceptions of cancer controllability and cancer risk knowledge: the moderating role of race, ethnicity, and acculturation. J Cancer Educ. 2013 Jun;28(2):254–261. doi: 10.1007/s13187-013-0450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenberg NE, Studts CR, Hatcher-Keller J, Buelt E, Adams E. Patterns and determinants of breast and cervical cancer non-screening among Appalachian women. Women & health. 2013;53(6):552–571. doi: 10.1080/03630242.2013.809400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanath K, Breen N, Meissner H, et al. Cancer knowledge and disparities in the information age. J Health Commun. 2006;11(Suppl 1):1–17. doi: 10.1080/10810730600637426. [DOI] [PubMed] [Google Scholar]
- 29.Nelson WL, Moser RP, Han PK. Exploring objective and subjective numeracy at a population level: findings from the 2007 Health Information National Trends Survey (HINTS) J Health Commun. 2013;18(2):192–205. doi: 10.1080/10810730.2012.688450. [DOI] [PubMed] [Google Scholar]
- 30.Osborne RH, Elsworth GR, Whitfield K. The Health Education Impact Questionnaire (heiQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns. 2007 May;66(2):192–201. doi: 10.1016/j.pec.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.American Cancer Society [Accessed September 27, 2013];American Cancer Society Guidelines for the Early Detection of Cancer. 2013 http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.
- 32.Surveillance Epidemiology and End Results (SEER) Program [Accessed November 1, 2013];Prevalence database: “US Estimated Complete Prevalence Counts on 1/1/2010”. 2013 based on the November 2012 SEER data submission. www.seer.gov.
- 33.Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet Med. 2010 Nov;12(11):726–735. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control [Accessed September 30, 2013];2011 National Diabetes Fact Sheet. 2011 May 23; http://www.cdc.gov/diabetes/pubs/estimates11.htm#2.
- 35.Centers for Disease Control Vital Signs: Awareness and Treatment of Uncontrolled Hypertension Among Adults - United States, 2003-2010. MMWR. 2012 Sep 4;61 [PubMed] [Google Scholar]
- 36.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS data brief. 2012 Jan;(82):1–8. [PubMed] [Google Scholar]
- 38.Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Communicating genetic and genomic information: health literacy and numeracy considerations. Public health genomics. 2011;14(4-5):279–289. doi: 10.1159/000294191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser RP, Arndt J, Han P, Waters EA, Amsellem M, Hesse BW. Perceptions of Cancer as a Death Sentence: Prevalence and Consequences. J Health Psychol. doi: 10.1177/1359105313494924. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cumming G. The new statistics: why and how. Psychol Sci. 2014 Jan;25(1):7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]