Abstract
Biomechanics – the study of the relationship between forces and function in living organisms – is thought to play a critical role in a significant number of ophthalmic disorders. This is not surprising, as the eye is a pressure vessel that requires a delicate balance of forces to maintain its homeostasis. Over the past few decades, basic science research in ophthalmology mostly confirmed that ocular biomechanics could explain in part the mechanisms involved in almost all major ophthalmic disorders such as optic nerve head neuropathies, angle closure, ametropia, presbyopia, cataract, corneal pathologies, retinal detachment, and macular degeneration. Translational biomechanics in ophthalmology, however, is still in its infancy. It is believed that its use could make significant advances in diagnosis and treatment. Several translational biomechanics strategies are already emerging, such as corneal stiffening for the treatment of keratoconus, and more are likely to follow. This review aims to cultivate the idea that biomechanics plays a major role in ophthalmology and that its clinical translation, lead by collaborative teams of clinicians and biomedical engineers, will benefit our patients. Specifically, recent advances and future prospects in corneal, iris, trabecular meshwork, crystalline lens, scleral and lamina cribrosa biomechanics are discussed.
Keywords: Translational biomechanics, Ocular Biomechanics, Personalised Medicine, Optical Coherence Tomography, Brillouin Microscopy, Ophthalmic Pathologies, Intraocular Pressure
Introduction
Biomechanics is the science concerned with the origin and effects of forces that act within and upon living organisms at the molecular, cellular, tissue, organ, and body level[1]. Biomechanics considerably enhances our knowledge of human physiology and pathophysiology, allowing us to understand and predict the alterations, remodelling, and failures of certain tissues and organs, and can permit the development of novel clinical and personalized strategies for the management, diagnosis, prognosis, and treatment of biomechanically-related pathologies. The fields of cardiovascular research, orthopaedics and rehabilitation, represent excellent examples in which biomechanics has made a translational impact in current healthcare systems. Biomechanical solutions in these areas are used routinely in clinical practice for 1) diagnosis such as arterial pulse-wave analysis for the diagnosis of hypertension, and 2) therapy such as stent implantation for treating atherosclerosis and aneurysms, prosthetic heart valves for valvular heart disease, and knee replacement implants for osteoarthritis.
Surprisingly, the extent of translational advances in biomechanics in the field of ophthalmology is relatively limited. While the eye is more commonly thought of as an optical rather than a mechanical system, biomechanics does indeed play a critical role in a significant number of ophthalmic pathologies. Despite the fact that the eye represents a challenge for biomechanical research due to its size, we have still witnessed, over the past two decades, the emergence of multiple areas of research related to ocular biomechanics. For instance, scleral and lamina cribrosa (LC) biomechanics contribute to our understanding of myopia[2] and open-angle glaucoma[3] (OAG); iris and trabecular meshwork (TM) biomechanics to that of angle-closure glaucoma[4, 5]; vitreous biomechanics to that of retinal detachment and ocular drug delivery[6]; corneal biomechanics to that of keratoconus [7]; and lens capsule biomechanics to that of cataract[8]. Although, the majority of these endeavors have been limited to basic science research, many of them may be on the cusp of translational impact.
This review aims to discuss how recent knowledge of ocular biomechanics could be translated into clinical practice for the benefit of patients. It offers a general overview of recent advances in corneal, iris, TM, crystalline lens, scleral and LC biomechanics and discusses how engineers and clinicians can collaborate to effectively bring ocular biomechanics to the clinic. This review summarises the Special Interest Group session conducted during the 2013 annual meeting of the Association for Research in Vision and Ophthalmology in Seattle, USA. Ocular biomechanics is a rapidly growing field and other areas of ocular biomechanics (retina, choroid, lens capsule, vitreous and extra-ocular muscle biomechanics as well as ocular blood flow) are not covered in this review.
Corneal Biomechanics
The cornea boasts one of nature's most explicit relationships between structure and function. The relationship between corneal shape and retinal image quality is the basis for an entire field of surgical practice - keratorefractive surgery - and is the mediator of vision loss in disorders such as keratoconus and post-refractive surgery ectasia. The cornea's geometry is, in turn, a product of its constitutive elements, their mechanical properties, and a host of biological processes responsible for maintenance, repair, and disease. Due to this high level of structure-function integration and the accessibility of the tissue, the cornea provides an excellent medium for pursuing the goals of personalized risk assessment and treatment optimization through biomechanical characterization and structural simulation.
Corneal Biomechanics and Associated Disease
The normal corneal structure confers the critical optical property of transparency while providing the mechanical integrity required to conserve anterior corneal curvature over a wide range of loads and hydration levels.[9] Alterations of this structural equilibrium have direct visual consequences. The stroma and its anterior condensation, Bowman layer, are the chief collagenous layers of the cornea and provide the bulk of the cornea's strength. Within a matrix that is approximately 78% water by weight,[10] hundreds of collagen lamellae traverse the cornea. Peripheral disruption of these fibers produces an in-axis flattening effect responsible for the refractive effects of astigmatic keratotomy and hyperopic shift in photoablative keratectomy.[11] A predominantly circumferential population of fibrils in the corneal periphery[12] conserves limbal dimensions[13] and, when incised, leads to a more generalized central flattening response such as that seen after radial keratotomy. In the anterior stroma, more collagen interweaving[14-16] and greater numbers of transverse fiber elements[17, 18] lead to a logarithmic increase in elastic strength from the posterior to anterior stroma[17-19] that has important implications for the depth-dependent biomechanical response of the cornea to refractive surgery. Interlamellar cohesive strength, which derives in part from the presence of crossing fibers, increases as a function of age,[19] varies by meridian,[20] and is lowest in the inferior cornea where the corneal steepening in ectasia most often manifests.[20]
Findings in keratoconus explants suggest that loss of the preferred collagen orientation,[21] fragmentation of Bowman layer, and absence of the typical transverse collagen insertions into Bowman layer[22] may contribute to decreased elastic strength[23] and abnormal shear behavior such as lamellar “slippage.”[21, 24, 25] Similar findings have been observed in histological and ultrastructural studies of corneas that manifested ectasia after refractive surgery.[24] The current state of the art for assessing ectasia risk in refractive surgery candidates[26] highlights 5 readily measurable risk factors for post-LASIK ectasia: young age—a surrogate for corneal elastic strength, which tends to increase in an age-dependent manner,[27] high myopic ablation, topographic abnormalities, and low central corneal residual stromal bed thickness.[26] However, such screening tools suffer from a dependence on retrospective regression models culled from incomplete representations of the cornea's complex 3-dimensional structure and equally complex surgical geometries.[28] Furthermore, no risk models account explicitly for corneal biomechanical properties, which presumably are the final common pathway for development of ectasia.[29]
In vivo Measurement of Corneal Biomechanical Properties
For the reasons summarized above, the capability to measure mechanical properties in a clinical setting is a major translational priority. At the time of writing, the only commercially available device specifically approved for measurement of corneal properties is the Ocular Response Analyzer (ORA, Reichert, Inc.). This modification of the pneumotonometry principle quantifies the dynamics of corneal deformation and recovery during a variable air-puff mediated deformation and provides a measure of corneal hysteresis (CH).[30] CH is the difference between the ingoing and outgoing applanation pressures and represents the energy loss due to viscous damping in the cornea and extracorneal structures.[31] Both CH and a closely related variable, the corneal resistance factor (CRF), are reduced in eyes affected by keratoconus,[30] suggesting a reduced capacity for viscous damping in ectatic disease. The magnitude of this decrease is correlated to keratoconus severity,[32] but the sensitivity and specificity of CH and CRF to differentiate normal eyes from those with low-grade or forme fruste keratoconus are low.[33] Further analysis of waveform signal features and their diagnostic performance is an active area of current investigation that is improving the sensitivity and specificity of the ORA.[34-36] The CorvisST (Oculus), currently approved only for intraocular pressure (IOP) measurement, employs a similar deformation technique but with a non-varying maximum air pressure and monitors one cross-section of the deforming horizontal meridian with a high-speed Scheimpflug camera. Analysis of the deformation characteristics of the cornea from these images is ongoing and may allow for more direct inferences of biomechanical behavior than applanation monitoring alone. However, neither technique is suited for spatial characterization of properties.
Emerging techniques that have demonstrated potential for clinical implementation and the possibility of 3D characterization of corneal properties include supersonic shear imaging,[37] corneal optical coherence elastography (Figure 1),[38, 39] and Brillouin light scattering microscopy.[40, 41] With continued development, these and other methods for non-destructive mapping of corneal material properties are likely to significantly increase the sensitivity of keratoconus diagnosis by allowing detection of elastic property minima and other abnormalities of property distribution. Such data are also likely to enhance the fidelity of patient-specific predictive models and shift risk assessment and surgical planning paradigms from generalized empirical models to a more personalized, deterministic approach.
Figure 1.
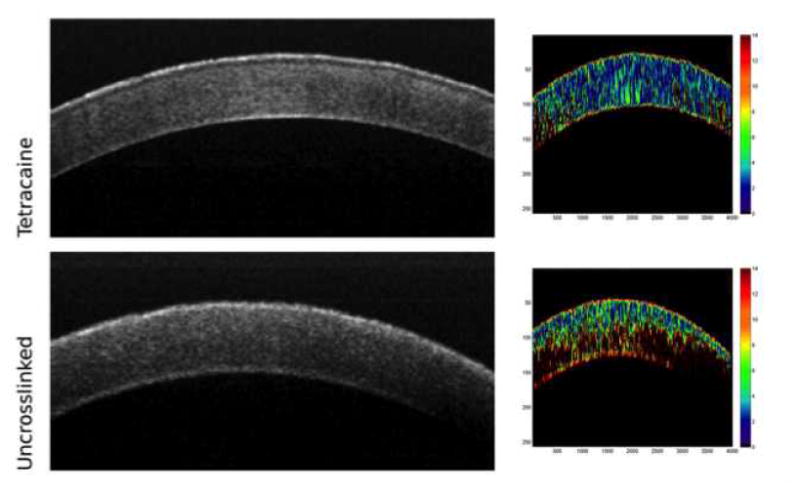
Optical coherence elastograms of control and crosslinked rabbit corneas. Cooler colors (blues) indicate lower strains and correspond to greater relative stiffness. The uncrosslinked cornea (bottom) demonstrates a depth-dependent gradient of properties with greatest stiffness in the anterior stroma. A cornea treated with transepithelial UVA-riboflavin crosslinking with tetracaine as an irritative adjunct (top) shows greater stiffness than the control (from experiments described in [39]).
Toward Simulation-Based Treatment of Corneal Disease and Refractive Error
The finite element method has been used to simulate the effects of radial keratotomy,[42-45] astigmatic keratotomy,[46-48], cataract incisions[49], phototherapeutic keratectomy,[50, 51] PRK[52, 53] and LASIK,[53-57] intracorneal ring segments,[58] and keratoconus[55, 59, 60] on the corneal structure. A more recent emphasis on use of patient-specific geometries obtained from clinical imaging devices has begun to bridge the gap between generalized results and more direct clinical validation of models, an important step toward use of simulation in clinical practice [7, 29, 49, 54, 61].
An example of the utility of patient-specific models that could ultimately help in guiding interventions and improving clinical outcomes can be found in a computational study of collagen crosslinking in morphologically different patient-derived keratoconic geometries.[29] With simulations of the standard broad-zone treatment parameters, observed reductions in curvature were similar to those reported in most clinical series. Through parametric variations and the simulation of smaller, cone-centered treatments, much greater reductions in cone steepness and higher order corneal aberrations were observed (Figure 2). Similar modifications are now being explored in clinical studies. In a collaboration with industry (Avedro, Inc. Waltham, MA), the same group has demonstrated the potential for patterned crosslinking to be used as a standalone procedure [62] or as an adjunct to treat astigmatism and other refractive errors.[63]
Figure 2.
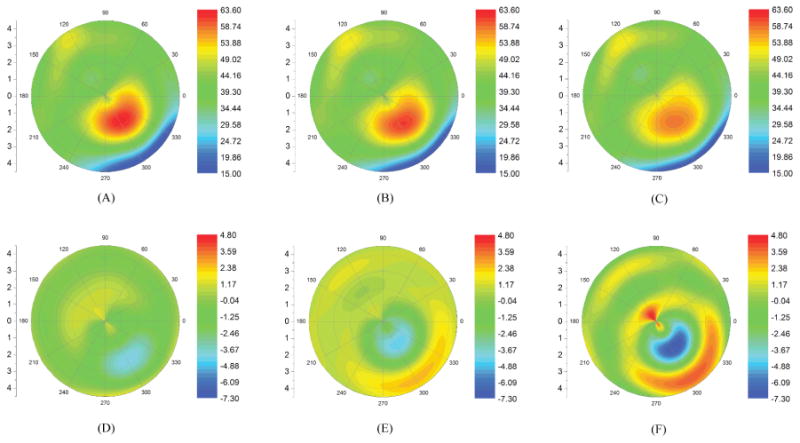
Finite element analysis comparing the topographic effects of A) a standard 9mm collagen crosslinking treatment, B) a more graduated UV treatment profile with a smaller effective diameter, and C) a graduated treatment centered on the cone. Tangential curvature maps (D-F) show the change from pre-to-post crosslinking state. Topographic flattening was greatest with the cone-centered simulation.[29]
These are merely examples of a growing movement towards using simulation for understanding corneal disease mechanisms and driving rational changes to our approach to treatment. Advances in corneal biomechanical property measurement and patient-specific modeling have the potential to enhance early keratoconus diagnosis, enable personalized, procedure-specific ectasia risk assessment through simulation, and drive optimized treatment design for a variety of corneal refractive conditions.
Anterior Segment Biomechanics (Iris and Trabecular Meshwork)
Until recently, interest in the subject of anterior segment biomechanics was confined to cornea, with iris and TM biomechanics being rarely discussed in the literature. In recent years, an interest in iris biomechanics has been stimulated by a renewed appreciation the role of iris dynamics in the pathogenesis of angle closure[64] combined with the increasing burden of angle closure glaucoma (particularly coupled to the ageing population in Asia). Similarly, a proliferation of glaucoma surgical interventions directed at the angle, most of which are classified under the umbrella term of ‘micro-invasive glaucoma surgery’ (MIGS) has stimulated interest in TM biomechanics [65].
Iris biomechanics
Iris thickness has been found to be different among ethnicities[66] and increased iris thickness is associated with angle closure[67]. Changes in iris dynamics may contribute greater to raised IOP upon dilation in angle closure patients than in open angle patients[64]. The interaction of iris, lens and TM in the pathogenesis of angle closure and the long-term implications in relation to the development of peripheral anterior synechiae are poorly understood. Recent clinical imaging studies suggest that iris ‘stretch’ amplitude may be slower[68] and loss of iris volume less in angle closure patients upon dilation[64], indicating that the iris biomechanical response to physiological or pathological stimuli may contribute to the pathogenesis of acute or chronic episodes of angle closure.
In vitro testing of iris biomechanics has, to date, been limited. Wyatt constructed a mathematical model for non-linear iris ‘stretch’ to explain “minimum wear and tear” of iris tissue in spite of the constant strain it undergoes with pupil dilation throughout life[69]. Barocas and colleagues examined porcine iris tissue using microindentation experiments and found that the posterior layer (pigmented epithelium, sphincter, dilator components) was stiffer than the anterior stromal layer indicating complex biomechanical behavior[70]. The ‘sponge-like’ properties of the iris are presumed to account for the variability of iris volume amongst different pathological states[64], however this remains to be demonstrated.
Current imaging tools and computing speeds allow us to acquire a three-dimensional view of the iris in vivo. It is possible that further enhancement in imaging resolution and software modeling, will enable the measurement of iris biomechanics in vivo[4, 71]. This may have clinical translational impact as it may lead to an improved understanding of angle closure pathogenesis and furthermore may lead to improvements in materials for iris-replacement devices.
TM biomechanics
While glaucoma is defined as an optic neuropathy, the main pathology in accounting for elevated IOP is identified as TM outflow facility resistance. Further, any modification in IOP to slow glaucoma progression is commonly performed using medications or angle surgeries that target the TM. Accumulation of glycosaminoglycans in the extracellular matrix and thickening of TM beams with loss of trabecular spaces combined with chronic inflammatory changes have been found to be the hallmark in primary glaucomas[72]. These cytoskeletal changes can be assumed to alter biomechanical properties of this specialized tissue in the eye. Evidence for this is found in atomic force microscopic measurements on TM cells and ex-vivo uniaxial testing on TM tissues showing stiffer properties in glaucoma patients[73]. Alteration in the biophysical attributes of TM can contribute to outflow facility changes, and thus influence onset and progression of glaucoma[74].
Ethier and colleagues, studying F-actin architecture in Schlemm's canal endothelial cells, found that the presence or absence of these proteins depended on shear stress forces of aqueous humor flow and may alter the caliber of the canal, underlining the need for studying such forces in detail to explain pathological processes and how it can possibly be modified pharmacologically or mechanically[75]. Zeng and colleagues studied Young's modulus of cultured Schlemm's canal using magnetic pulling cytometry and finite element modeling and compared it with imaging studies with pressure loading on primate Schlemm's canal. They concluded that increasing IOP appears to increase the modulus[76]. Measurement of such properties in vivo can be of immense utility in examining the outflow facility and formulating strategies to alter glaucomatous pathological processes using appropriate drugs[77] or in improving angle surgical techniques. It can be envisaged that such measurements can be used as clinical biomarkers for pathological processes and prognostication of disease severity.
Limitations of anterior segment biomechanics research include accurate application of load to impart stress on the target tissue in vivo and lack of resolution in current imaging technologies to provide strain values using appropriate software modeling. Further, due to the closeness of iris and TM to the surrounding tissues, it may be important to find ways to filter the biomechanical properties using rigorous modeling methods. Techniques such as ophthalmodynamometry and ultrasound are being investigated as the method of loading, while improvement in resolution is possible in future using e.g. micro-OCT imaging[78]. Recently, Kagemann and colleagues have shown that the conventional outflow pathway, including Schlemm's canal, can be imaged in vivo using clinically available optical coherence tomography (OCT) devices[79], and that the technology is sensitive enough to allow detection of acute effects of IOP [80].
In vivo anterior segment biomechanics research is clearly in its nascent stages, however, it is likely that it will become of paramount importance to push the frontiers of glaucoma management further.
Crystalline Lens Biomechanics
Crystalline Lens Biomechanics and Associated Disease
The primary function of the crystalline lens is focusing the light coming from an object to form its image on the retina. The ‘tunability’ or accommodation of the lens is a biomechanical process, involving the ciliary muscle that is connected to the capsule of the lens via the ciliary body and the zonular fibers (Figure 3a) [81]. Distance vision is achieved when the ciliary muscle is relaxed, which increases the tension and stretches the lens to have a longer focal length. For near vision, the ciliary muscle contracts, relieving the tension, and the lens elastically restores its intrinsic rounder shape with a shorter focal length [82].
Figure 3.
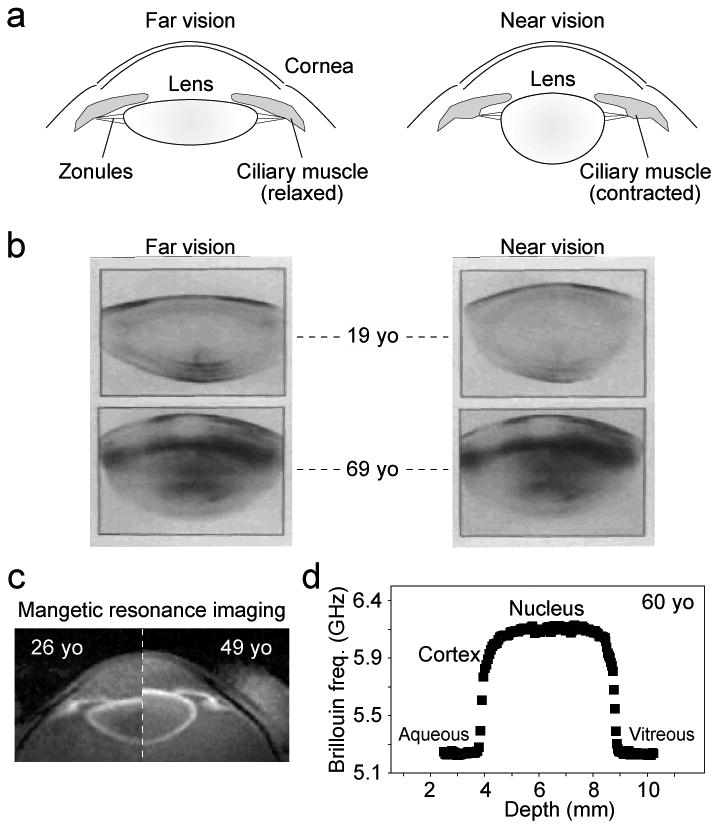
(a) Schematic of the eye during accommodation. (b) Scheimpflug images of 19-year-old (top) and 69-year-old (bottom) humans in unaccomodated (left, far vision) vs. accommodated (right, near vision) states. The stretching of the lens by the tension of the zonules is apparent in the young but not in the old lens. Images were reproduced and modified with permission from Koretz and Handelman, Sci. Am. 259, p.92, 1988. (c) MRI images of a 26-year-old vs. a 49-year-old subject in the unaccomodated state. The difference in size is apparent. Images were reproduced and modified with permission from Strenk et al., Progr. Eye Ret. Reas. 24, p.379, 2005. (d) The Brillouin frequency shift, which is directly related to the hypersonic longitudinal modulus, along the optics axis of a 60-year-old normal volunteer (measured at an optical wavelength of 780 nm).
Unfortunately, the accommodative ability of the human eye decreases continuously with aging. By the age of 50, almost everyone experiences the onset of presbyopia, with the associated difficulty in focussing on near objects [83]. Why does this occur?
From a biomechanical point of view, it has long been suspected that the ciliary muscle loses its contraction power with age. Although it sounds plausible, experimental data indicated that the function of the ciliary muscle actually remains largely intact even after the accommodative power has been considerably compromised [84]. The age-dependent change of the capsule and zonular fibers were found to be too small to account for the decrease in accommodation power [85, 86]. The human lens continuously grows in size with age, and thus its optical and mechanical properties change [87-90]. It has been widely accepted that the increased stiffness of the crystalline lens with age is the primary cause of presbyopia; as age progresses, the lens tissue loses its elasticity gradually, which restricts the accommodation range [91, 92] (Figure 3b).
Current methods for characterizing crystalline lens biomechanics in vivo
Several studies have demonstrated age-related stiffening of excised human and animal lenses by using various testing tools, such as a spinning cup [93], mechanical stretchers [91, 94], stress-strain equipment [95, 96], and bubble-based acoustic radiation force [97]. Ultrasound has also been used to measure the spatial variation of packing density inside the lens ex vivo [98].
However, the reported mechanical properties of the crystalline lens are highly variable. Indentation tests measured age-related lens hardening and found the cortex to be softer than the center [99]. Shear rheometry on frozen lenses revealed a massive age-related increase in lens modulus but found the nucleus to be softer than the cortex in young lenses [100-102]. Shear rheometry studies on fresh lenses found small modulus changes between the ages 20 and 40 and high regional uniformity [103], whereas a recent spinning test indicated high regional variation between the cortex and nucleus [104]. It remains unclear exactly how much the lens stiffness changes with age and to what extent the specific spatial profile of the lens modulus contributes to accommodation [105].
Currently, there are no established non-invasive methods to measure the elastic properties of the lens in vivo. Imaging instruments, such as magnetic resonance imaging (Figure 3c) and OCT, can visualize the 3-dimensional shape of the lens during accommodation, but they do not measure the biomechanical properties of the lens. Elastography and ultrasound have low spatial resolution and measurement sensitivity [106]. A recently developed Brillouin microscopy method is a promising technique for high-resolution in vivo measurement [107, 108]. Brillouin microscopy has revealed a dramatic age-dependent increase in the longitudinal modulus of the murine lens in vivo [109]. The hypersonic modulus of the human lens exhibits a bell-shaped axial profile (Figure 3d), with the peak modulus in the nucleus appearing to have little difference with age [110].
Translating crystalline lens biomechanics into clinical practice
The economic cost associated with presbyopia is significant in the modern society as the age of the population in the workforce steadily grows [111]. Current treatment options for presbyopia (e.g. monovision correction, multifocal spectacles) only improve single-distance vision but do not restore the active change of dioptric power of the young eye [112-114].
Improved understanding of the biomechanics of the lens is beneficial for the development of novel treatment strategies, such as drug-induced disruption of the chemical bonds leading to lens stiffening [115], laser-induced softening of the lens [116], and lens tissue replacement with biocompatible polymer material with the mechanical properties similar to young human lenses [117, 118].
Finally, the biomechanical properties of the lens may play a role in age-related nuclear cataracts. The pathogenesis of cataracts is not fully understood but has been linked with various molecular processes including the reduced transport of small molecules, such as anti-oxidants, in the lens, increased viscoelastic modulus [119, 120], protein crosslinking [121], protein-membrane binding [122]. Improved understanding of the regional variation of lens elasticity may provide an insight into the underlying molecular processes, such as protein expression and micro-structural changes.
Scleral Biomechanics
Importance of Scleral Biomechanics in Glaucoma
The hallmark of glaucoma is excavation of the optic nerve head (ONH)[123], in parallel with progressive loss of function due to death of retinal ganglion cells (RGC), whose axons pass through the ONH. Both mean IOP[124] and IOP fluctuation[125] are closely associated with incident human glaucoma and its progressive worsening. The risk factor for the development and progression of glaucoma is the level of IOP, rather than whether or not this exceeds statistically ‘normal’ limits. IOP lowering slows the progressive RGC loss[126-128], whether baseline IOP is above normal or not.
IOP-generated stress could contribute to glaucoma injury at the ONH and is potentially amenable to therapeutic intervention. The evidence that scleral connective tissues mediate glaucoma damage is convincing. The ONH zones in which physical deformation is greatest are those that suffer more RGC axon injury[129]. Axial myopes are more susceptible to open angle glaucoma (OAG)[130], probably due to the mechanical disadvantage of larger globe diameter and thinner sclera. Corneal hysteresis is a risk factor for OAG progression[131], and scleral rigidity in OAG eyes is increased in vivo[132, 133], and in vitro[134].
Current Methods for Characterizing Scleral Biomechanics and its Association with Axonal Loss
Studies of peripapillary sclera are presently more feasible than study of the ONH itself[135]. Biomechanical models[136, 137] suggest that scleral behavior drives the IOP-induced mechanical strain transmitted to the ONH[138, 139], and scleral responses to IOP could be both detrimental and beneficial to RGC survival. Mouse, rat and monkey IOP elevation models generate data relevant to human glaucoma[140-143]. Astrocytes in the mouse ONH resemble the structure of the collagenous primate lamina cribrosa[144] and mouse sclera has similar molecular structure to human sclera[145, 146]. The sclera's extracellular macromolecules include predominately type 1 collagen and successive collagen lamellae alternate in orientation much like a basket-weave. In the peripapillary sclera, collagen and elastin fibrils run circumferentially around the ONH[123, 147-152]. The ONH and peripapillary sclera undergo dramatic stretching, deepening and widening in glaucoma.
Recently, IOP-generated stress and strain in posterior sclera have been studied in vitro in mouse[153], tree shrew[154], monkey[139, 155, 156], and human eyes[134, 157]. The greatest scleral strain is in the peripapillary region[158]. Human glaucoma eyes with RGC loss are measurably stiffer in peripapillary sclera than controls, as are experimental mouse and monkey glaucoma eyes[134]. But, we do not know whether human eyes would be more or less susceptible to glaucoma damage if they were stiffer at baseline.
Quigley and colleagues found that experimental glaucoma models produce more damage in some strains of mice than in others[159]. They identified differences in scleral structure and response to mouse glaucoma that are associated with susceptibility. The CD1 mice strain (albino) were more susceptible to RGC loss than the B6 strain[160] (black) and a mouse mutated in collagen 8α2 (Aca23) was even less susceptible than B6[161]. Large eye size alone was not the most important factor in susceptibility, since Aca23 had the biggest baseline axial lengths and least damage. Interestingly, resistance to damage was associated with reduced axial elongation after IOP elevation. Young B6 eyes increased axial length significantly more than older B6 and were more susceptible to RGC loss[161]. Likewise, young CD1 mice (which lose more RGC) increased axial length with glaucoma more than young B6 mice[162]. Aca23 mutant eyes elongated less than controls and were less sensitive to RGC loss[161]. The less susceptible B6 mouse strain had thicker peripapillary sclera at baseline and did not undergo uniform scleral thinning with glaucoma as did the more susceptible CD1 mice. The number of fibroblast-containing and antero-posteriorly oriented lamellae increased in glaucoma eyes. Second harmonic generation imaging showed that the normal circumferential pattern of collagen fibrils in the peripapillary sclera was widened in significantly damaged glaucoma eyes. After glaucoma, there were more small diameter collagen fibrils [163].
Inflation tests of enucleated mouse eyes found the most resistant Aca23 strain had the stiffest sclera, while the most susceptible CD1 mice had greater meridional peripapillary strain than B6 and greater meridional anisotropy of the inflation response. In all strains, chronic IOP elevation caused steeper pressure-strain responses.
In B6, CD1, and Aca23 mice, Quigley and colleagues measured the diffusion of fluorescein isothiocyanate-dextran into a photo-bleached zone of excised sclera by confocal microscopy. Scleral diffusivity was significantly greater in Aca23 and B6 mice than in CD1 mice, matching their relative susceptibility to glaucoma injury. Glaucoma caused decreased diffusivity, with greater decreases in the vital peripapillary area than elsewhere [164]. The difference in thickness between fresh and fixed sclera was nearly 68% in control mice, but differed by only 10% between fresh and fixed glaucoma sclera. The data suggest significant loss of non-fibrillar, soluble components of the sclera in glaucoma.
Scleral fibroblasts of the sclera make up 15% of its thickness in histological measurements. In experimental glaucoma, there is an expansion of the cell layers in thickness and a 6-fold increase in dividing cells 1 week after IOP elevation [165]. Furthermore, labeling for α-smooth muscle actin, actinin, thrombospondin, paxillin and integrins were increased in glaucoma scleral fibroblasts. Proteomic studies of sclera in glaucoma mice found increases in molecules important for scleral maintenance and remodeling, including thrombospondins 1 and 4, myosins fibromodulin, and heparin sulfate proteoglycan. There was upregulation in canonical pathways for integrin-linked kinase and actin microskeleton signaling. This combined anatomical and proteomic evidence is consistent with a transition to the myofibroblast phenotype among scleral cells, as seen also in experimental myopia.[2, 166]. Thus, it is likely that therapeutic targets to alter susceptibility to glaucoma damage may exist in pathways related to scleral remodeling[167, 168].
Altering Scleral Biomechanics as a Potential Approach to Glaucoma Therapy
While the evidence does not definitively support a specific treatment, decreased susceptibility seems associated with greater stiffness at baseline, retention of scleral fibrillar component thickness, and resistance to elongation under elevated IOP. If we wish to produce eyes with a steeper stress—strain relationship, increased cross-linking of the collagen, as already performed in the cornea for keratoconus would be a suitable approach[169]. Ideally, this would be application of a cross-linking agent that does not require ultraviolet light activation, delivered by subconjunctival injection or sustained release format in an outpatient setting. Side effects would be less with local application compared to systemic therapy.
Further animal research is needed to show that this proposed method would protect RGC. For example, reducing peripapillary scleral strain by cross-linking, instead of protecting the eye, might intensify the translaminar pressure gradient, making the eye less resistant to glaucoma damage. Second, the method must avoid off-target effects, such as damage to eye muscles or major ocular blood vessels that traverse the sclera, or toxic exposure of RGCs. Third, the precise degree of cross-linking needed may vary from person to person, requiring a method to estimate the extent of treatment, or multiple small treatments. Methods are needed to estimate the mechanical state of the sclera in the living eye, probably using imaging technology with induced perturbations in IOP [170-172]. If a beneficial effect on sclera would actually be achieved by producing a less stiff response, agents that affect collagens or non-collagenous elements could include enzymatic digestion with collagenase, elastase, chondroitinase, or hyaluronidase. The feasibility of such an approach has recently been demonstrated in vitro [173].
The second potential treatment area would be to modulate the scleral fibroblast response to glaucoma. In Marfan syndrome, the mutated site in fibrillin-1 acts by activating transforming growth factor β (TGFβ)[174, 175], leading to aortic dissection, ocular lens dislocation, and high myopia. Both gene expression and protein levels of TGFβ are elevated in OAG eyes in their human TM[176] and ONH[177, 178], and our proteomic analyses in mouse glaucoma show increases in thrombospondins, which are activators of TGFβ. A TGFβ antagonist, losartan, halts the clinical abnormality of the aorta in a mouse Marfan model[179, 180]. TGFβ is involved in scleral remodeling in experimental myopia in tree shrews[181]. Abnormal activation/inhibition of TGFβ in the sclera and ONH could increase susceptibility to IOP-induced stress and potentiate OAG damage. A losartan-type treatment may beneficially modulate of the scleral response in glaucoma.
A new therapeutic approach to glaucoma is proposed that involves reduction in IOP-generated mechanical insult at the ONH through alteration of the sclera to block initial injury to RGC axons at the earliest stage of RGC dysfunction. This research area could identify candidate genes related to glaucoma damage and to myopia.
Lamina Cribrosa Biomechanics
Lamina Cribrosa Biomechanics and Associated Disease
Elevated IOP is the primary risk factor for the development and progression of glaucoma, and lowering IOP remains the only proven intervention to decrease the risk of vision loss.[182] The mechanisms by which elevated IOP affects the tissues of the ONH, and the LC within, are poorly understood.[183] The ONH is often described as a weak spot on the posterior pole, mainly due to the lower density of connective tissue relative to the sclera.[184] The LC is thought to provide structural and nutritional support to the RGC axons. Since the glaucomatous deterioration of RGC axons is believed to initiate at the level of the LC[182], the hypothesis has arisen that inadequate LC support would trigger events that contribute to RGC axon damage. [183] Such insufficient support would present as an altered LC biomechanical environment produced by an altered elevated IOP or by a highly sensitive “frail” LC. The difficulty of visualising the LC in vivo for experimentation has meant that much of what is known about the effects of IOP on the LC has been learned indirectly through the use of numerical modeling and ex-vivo studies. [184]
Modeling and Ex-vivo Methods for characterizing Lamina Cribrosa Biomechanics
Modeling studies have shown that the LC and sclera are not biomechanically independent. Instead, they form a system in which the LC sensitivity to IOP is determined by a complex interaction of multiple factors, including tissue anatomy and mechanical properties.[185, 186] These studies have shown that LC biomechanics is highly sensitive to the properties of the sclera, especially those of the peripapillary sclera.[137, 138, 156, 187, 188] These results have several implications of interest to the translation of ocular biomechanics into clinical practice. First, sensitivity to IOP, and perhaps susceptibility to glaucoma, is affected by variations in tissue properties, both anatomy and composition, that arise for example with aging[155] or disease[139]. Second, the complex factor interactions mean that determining whether a particular patient is sensitive to IOP might require measurements of multiple parameters, some of which may be measurable in the clinic. This, in turn, will require validated methods to estimate those parameters that cannot be measured directly. This has been done mainly through inverse modeling schemes, which have been used to estimate LC stiffness or the biomechanical role of the pore and beam architecture characteristic of the LC.[189, 190] Clinical practice could be augmented with user friendly methods that do not require complex simulation for characterisation of LC biomechanics [170].
The LC has also been studied ex-vivo. A common approach is the use of histomorphometry, often focused on measuring changes induced by acute increases in IOP (e.g., LC displacement),[191] or identifying LC characteristics that are abnormal in individuals with glaucoma (e.g., LC thickened early in the disease or thinner later on),[192] or in individuals at increased risk of glaucoma (e.g., a thinner LC in individuals with myopia).[193] Tissue sectioning for histology, while powerful, is subject to limitations and potential confounders (discussed in [194]). Hence, methods have also been developed to study LC biomechanics and architecture ex-vivo without the need to section the tissue, for example using second harmonic generated images[195]. These have enabled measurement of eye-specific displacements and deformations of the human LC induced by acute increases in IOP. The deformations measured were substantial and of magnitudes that have been shown to be of biological significance (Figure 4). Studies of LC microstructure ex-vivo have suggested that ocular hypertension results in remodeling of the LC, including recruitment of retrolaminar septae[196] and posterior migration of the LC insertion into the pia mater [197, 198].
Figure 4.
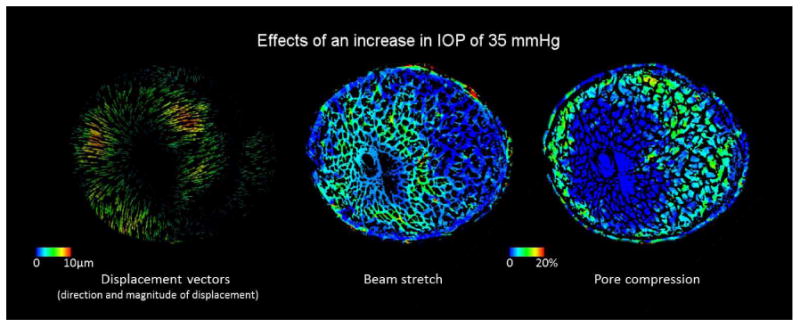
Biomechanical effects on the LC of a 79 year old donor eye to an acute increase in IOP of 35 mmHg (from 10 to 45 mmHg). The effects were computed analyzing second harmonic generated images acquired ex-vivo.[195]
In-vivo Measurements of Lamina Cribrosa Biomechanics and Clinical Relevance
Even though knowledge acquired through modeling and ex-vivo studies can impact clinical practice, widespread integration of ocular biomechanics calls for methods for in-vivo measurement. For many years the most detailed in vivo information on posterior pole biomechanics was obtained using scanning laser ophthalmoscopy (mainly the HRT), even though the device was incapable of measuring the LC.[183, 184]
In recent years, in-vivo study of LC structure and biomechanics has been boosted thanks to the advent of OCT imaging, which allows visualization deep within the ONH, including the LC.[199-202] Early studies using OCT have found that acute increases in IOP do not cause displacement of the LC[203], whereas a more recent study has found significant anterior LC displacements following trabeculectomy at 1-week post-surgery[204]. These studies emphasize that LC mechanical behavior is still far from being understood. It is well accepted that to study LC biomechanics, it is necessary to image the LC, and that it can be misleading to solely rely on surrogate measures.[184] The LC research community is therefore now carefully assessing the extent to which OCT is able to image the posterior LC surface,[205] and to be used for mapping the in vivo deformation of the LC[171, 206]. OCT technology and methods continue to mature and LC morphometry is increasingly carried out this way. Current developments for improving the capabilities of an OCT to characterize the LC in-vivo include adaptive optics (Figure 5),[207] swept source[200], longer wavelengths, and techniques for enhanced depth imaging or image compensation.[199, 205, 208] These have enabled another important development of recent years, namely the imaging and characterization of the LC microstructure in vivo, using OCT[200, 209, 210] SLO[211] or a combination of both[212]. Application of these tools to better assess the LC structure and biomechanics holds great promise to produce patient-specific knowledge that may be translated to the clinic and to help understand glaucomatous neuropathy.
Figure 5.
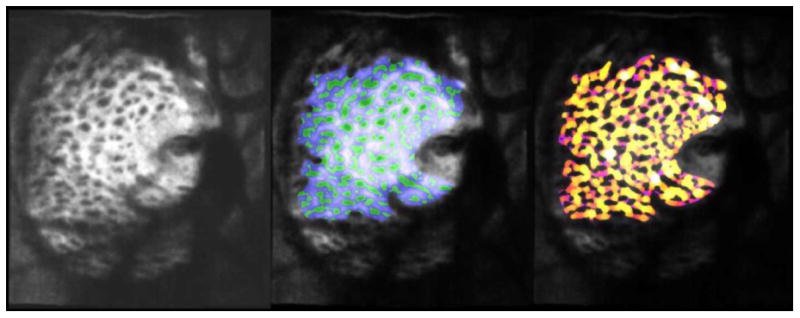
C-mode section at the level of the LC through an Adaptive optics OCT scan of a glaucomatous eye acquired in-vivo (left). The beams (blue) and pores (green) were identified using a semi-automated segmentation technique (middle). Beam thickness was then measured at every voxel, where hotter colors represent thicker beams (right). (Courtesy of the Glaucoma Imaging Group, University of Pittsburgh)[207]
Translating knowledge of biomechanics into clinical practice will require a much better understanding and characterization of the critical load-bearing tissues, such as the sclera and LC. Validation of the models needs to take a central place. More advanced models, as well as better experimental techniques will help lead to the needed improved understanding of the place of the individual within the population (e.g., identifying whether a particular patient presents with a robust or frail posterior pole and LC[213]) and how these are expected to vary with and without intervention. A solid understanding of the relationship between cross-sectional and longitudinal data will help make the translation to the clinic faster and more effective. Integrating biomechanics into the clinic also necessitates a better understanding of mechanotransduction, at both the short and long time scales. It is well recognized that patients vary substantially in the ill effects of mechanical stimulation.[183, 184]
Conclusion
Ocular biomechanics is a rapidly growing area of research interest and one that, at a superficial level at least, has yet to make a substantial translational impact. In reality, the success of many pre-existing surgical techniques (particularly those relating to corneal refractive surgery and keratoconus) is greatly influenced by the biomechanics and remodelling of the targeted tissues. Recognizing the many different ways in which current clinical practice - whether it relates to refractive surgery, glaucoma management, angle closure, cataract surgery or presbyopia - is influenced by ocular biomechanics will help foster a closer collaboration between clinicians, clinician-scientists, basic scientists and biomedical engineers. It is this improvement in communication between different, but related, disciplines that will allow the valley between basic science ocular biomechanics research and true clinical translation to be breached.
At a simple level, the most pressing concern for those engaged in ocular biomechanics research is to demonstrate that biomechanical properties for individual ocular tissues are measurable in vivo. With ever increasing improvements in imaging technology, as well as imaging processing techniques, these objectives should be realized. Achieving this will have the dual benefit of enabling the development of validated numerical models and allow biomechanical testing to become part of the battery of clinical investigations available in practice. This review has highlighted a number of areas in which this is close to realization (LC imaging, corneal imaging and iris imaging).
The Holy Grail, however, is to utilize the knowledge gleaned from biomechanical theory and testing to generate therapeutic strategies for multiple ophthalmic conditions. As is clear from this review, whatever therapeutic approach is adopted it is likely that a ‘bespoke’ personalized treatment will be required. The most likely area in which this will first become a reality is in corneal and refractive surgery. In corneal cross-linking for keratoconus, there is already a therapeutic treatment that directly influences the biomechanical properties of the cornea to retard the progression of a pathological process. In addition, laser-refractive procedures are already individualized in order to correct a patient's refractive error and other optical aberrations (if the procedure is wavefront-guided). Given the current volume of refractive procedures undertaken, and the costs involved, it is likely that biomechanical ‘enhancements’ might be adopted if they could be demonstrated to improve outcomes or reduce the risk of complications such as ectasia. By the same token, presbyopia and high myopia are two conditions whose high prevalence might stimulate (and once again, this may be financially motivated) a more rapid crossover of biomechanics research into translational therapeutics. Open angle glaucoma, on the other hand, remains a little more enigmatic. This review has detailed a number of elegant experiments that have explained the rationale for proposing a protective role for the alteration of scleral biomechanics. The ability to clinically measure scleral and LC biomechanics in vivo will likely need to ‘catch-up’ with this experimental work before a serious attempt is made to pursue this as a therapeutic strategy in humans.
In conclusion, ocular biomechanics has an impact in many areas of ophthalmic pathology, a number of which have been discussed in this review. It is hoped that increased awareness and interest in this relatively new field of research will stimulate other scientists and clinicians to join and effectively push the discipline further so that it can go on to eventually improve the quality of life of our patients.
Acknowledgments
MJAG acknowledges support from the Singapore Ministry of Education, Academic Research Fund, Tier 1 and from a NUS Young Investigator Award (NUSYIA_FY13_P03); Acknowledgment is also made to the donors of the NGR, a program of the BrightFocus Foundation (formerly American Health Assistance Foundation or AHAF). NGS acknowledges a proportion of his financial support from the Department of Health through the award made by the UK National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health. IAS acknowledges support from the National Institutes of Health grants R01-EY023966 and P30-EY008098. SHY and GS acknowledge support (in part) by NIH P41-EB015903, R21EY023043, K25EB015885, and the Harvard Clinical and Translational Science Center (NIH UL1-RR025758). WJD acknowledges support in part by NIH R01 EY023381, the National Keratoconus Foundation/Discovery Eye Foundation, Unrestricted and Challenge Grants from Research to Prevent Blindness to the Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University. WJD is a recipient of a Research to Prevent Blindness Career Development Award.
Footnotes
Financial Disclosure: SHY and GS are listed as inventors on intellectual property held by Massachusetts General Hospital related to biomechanical measurement. WJD is listed as an inventor on intellectual property held by Cleveland Clinic related to biomechanical measurement and modeling and has received research funding and royalties related to use of IP from Avedro, Zeiss and Topcon. WJD is a consultant to Ziemer.
References
- 1.Fung YC. Biomechanics: Mechanical properties of living tissues. 2nd. New York, NY: Springer-Verlag; 1993. [Google Scholar]
- 2.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86(1):E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 3.Sigal IA, Roberts MD, Girard MJA, Burgoyne CF, Downs JC. Ocular disease: mechanisms and management. New York: Elvesier; 2009. Biomechanical changes of the optic disc. [Google Scholar]
- 4.Amini R, Whitcomb JE, Al-Qaisi MK, Akkin T, Jouzdani S, Dorairaj S, et al. The posterior location of the dilator muscle induces anterior iris bowing during dilation, even in the absence of pupillary block. Invest Ophthalmol Vis Sci. 2012;53(3):1188–94. doi: 10.1167/iovs.11-8408. Epub 2012/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camras LJ, Stamer WD, Epstein D, Gonzalez P, Yuan F. Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Invest Ophthalmol Vis Sci. 2012;53(9):5242–50. doi: 10.1167/iovs.12-9825. Epub 2012/07/13. [DOI] [PubMed] [Google Scholar]
- 6.Repetto R, Siggers JH, Stocchino A. Mathematical model of flow in the vitreous humor induced by saccadic eye rotations: effect of geometry. Biomech Model Mechanobiol. 2010;9(1):65–76. doi: 10.1007/s10237-009-0159-0. Epub 2009/05/28. [DOI] [PubMed] [Google Scholar]
- 7.Sinha Roy A, Rocha KM, Randleman JB, Stulting RD, Dupps WJ., Jr Inverse computational analysis of in vivo corneal elastic modulus change after collagen crosslinking for keratoconus. Exp Eye Res. 2013;113:92–104. doi: 10.1016/j.exer.2013.04.010. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedrigi RM, Dziezyc J, Humphrey JD. Altered mechanical behavior and properties of the human anterior lens capsule after cataract surgery. Exp Eye Res. 2009;89(4):575–80. doi: 10.1016/j.exer.2009.06.001. Epub 2009/06/16. [DOI] [PubMed] [Google Scholar]
- 9.Rom ME, Keller WB, Meyer CJ, Meisler DM, Chern KC, Lowder CY, et al. Relationship between corneal edema and topography. CLAO J. 1995;21(3):191–4. [PubMed] [Google Scholar]
- 10.Maurice DM. The cornea and sclera. In: Davson H, editor. The Eye. Orlando, FL: Academic Press; 1984. pp. 1–158. [Google Scholar]
- 11.Dupps WJ, Jr, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg. 2001;17(6):658–69. doi: 10.3928/1081-597X-20011101-05. [DOI] [PubMed] [Google Scholar]
- 12.Meek KM, Newton RH. Organization of collagen fibrils in the corneal stroma in relation to mechanical properties and surgical practice. J Refract Surg. 1999;15(6):695–9. doi: 10.3928/1081-597X-19991101-18. [DOI] [PubMed] [Google Scholar]
- 13.Edmund C. Corneal topography and elasticity in normal and keratoconic eyes. A methodological study concerning the pathogenesis of keratoconus Acta Ophthalmol Suppl. 1989;193:1–36. [PubMed] [Google Scholar]
- 14.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Investigative Ophthalmology and Visual Science. 1991;32(8):2244–58. [PubMed] [Google Scholar]
- 15.Polack FM. Morphology of the cornea. I. Study with silver stains. Am J Ophthalmol. 1961;51:1051–6. doi: 10.1016/0002-9394(61)91794-9. [DOI] [PubMed] [Google Scholar]
- 16.Smolek MK, McCarey BE. Interlamellar adhesive strength in human eyebank corneas. Investigative Ophthalmology and Visual Science. 1990;31(6):1087–95. [PubMed] [Google Scholar]
- 17.Winkler M, Chai D, Kriling S, Nien CJ, Brown DJ, Jester B, et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci. 2011;52(12):8818–27. doi: 10.1167/iovs.11-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler M, Shoa G, Xie Y, Petsche SJ, Pinsky PM, Juhasz T, et al. Three-Dimensional Distribution of Transverse Collagen Fibers in the Anterior Human Corneal Stroma. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.13-13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF. Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008;24(1):S85–9. doi: 10.3928/1081597X-20080101-15. [DOI] [PubMed] [Google Scholar]
- 20.Smolek MK. Interlamellar cohesive strength in the vertical meridian of human eye bank corneas. Investigative Ophthalmology and Visual Science. 1993;34(10):2962–9. [PubMed] [Google Scholar]
- 21.Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46(6):1948–56. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 22.Morishige N, Wahlert AJ, Kenney MC, Brown DJ, Kawamoto K, Chikama T, et al. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest Ophthalmol Vis Sci. 2007;48(3):1087–94. doi: 10.1167/iovs.06-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31(4):435–41. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 24.Dawson DG, Randleman JB, Grossniklaus HE, O'Brien TP, Dubovy SR, Schmack I, et al. Corneal ectasia after excimer laser keratorefractive surgery: histopathology, ultrastructure, and pathophysiology. Ophthalmology. 2008;115(12):2181–91 e1. doi: 10.1016/j.ophtha.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006 doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 27.Elsheikh A, Anderson K. Comparative study of corneal strip extensometry and inflation tests. J R Soc Interface. 2005;2(3):177–85. doi: 10.1098/rsif.2005.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupps WJ., Jr Ectasia risk: barriers to understanding. J Cataract Refract Surg. 2012;38(5):735–6. doi: 10.1016/j.jcrs.2012.03.018. Epub 2012/04/24. [DOI] [PubMed] [Google Scholar]
- 29.Roy AS, Dupps WJ., Jr Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci. 2011;52(12):9174–87. doi: 10.1167/iovs.11-7395. Epub 2011/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31(1):156–62. doi: 10.1016/j.jcrs.2004.10.044. Epub 2005/02/22. [DOI] [PubMed] [Google Scholar]
- 31.Kling S, Marcos S. Contributing factors to corneal deformation in air puff measurements. Invest Ophthalmol Vis Sci. 2013;54(7):5078–85. doi: 10.1167/iovs.13-12509. [DOI] [PubMed] [Google Scholar]
- 32.Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48(7):3026–31. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- 33.Kirwan C, O'Malley D, O'Keefe M. Corneal hysteresis and corneal resistance factor in keratoectasia: findings using the Reichert ocular response analyzer. Ophthalmologica. 2008;222(5):334–7. doi: 10.1159/000145333. Epub 2008/07/17. [DOI] [PubMed] [Google Scholar]
- 34.Schweitzer C, Roberts CJ, Mahmoud AM, Colin J, Maurice-Tison S, Kerautret J. Screening of forme fruste keratoconus with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2010;51(5):2403–10. doi: 10.1167/iovs.09-3689. [DOI] [PubMed] [Google Scholar]
- 35.Mikielewicz M, Kotliar K, Barraquer RI, Michael R. Air-pulse corneal applanation signal curve parameters for the characterisation of keratoconus. Br J Ophthalmol. 2011;95(6):793–8. doi: 10.1136/bjo.2010.188300. [DOI] [PubMed] [Google Scholar]
- 36.Hallahan KM, Roy AS, Ambrosio R, Salomao M, Dupps JWJ. Discriminant value of custom ocular response analyzer waveform derivatives in keratoconus. Ophthalmol. 2013 doi: 10.1016/j.ophtha.2013.09.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanter M, Touboul D, Gennisson JL, Bercoff J, Fink M. High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE transactions on medical imaging. 2009;28(12):1881–93. doi: 10.1109/TMI.2009.2021471. Epub 2009/05/09. [DOI] [PubMed] [Google Scholar]
- 38.Ford MR, Dupps WJ, Jr, Rollins AM, Roy AS, Hu Z. Method for optical coherence elastography of the cornea. J Biomed Opt. 2011;16(1):016005. doi: 10.1117/1.3526701. Epub 2011/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong BK, Lin MP, Ford MR, Santhiago MR, Singh V, Grossman GH, et al. Biological and biomechanical responses to traditional epithelium-off and transepithelial riboflavin-UVA CXL techniques in rabbits. J Refract Surg. 2013;29(5):332–41. doi: 10.3928/1081597X-20130415-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarcelli G, Yun SH. In vivo Brillouin optical microscopy of the human eye. Opt Express. 2012;20(8):9197–202. doi: 10.1364/OE.20.009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54(2):1418–25. doi: 10.1167/iovs.12-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna KD, Jouve FE, Waring GO. Preliminary computer simulation of the effects of radial keratotomy. Arch Ophthalmol. 1989;107(6):911–8. doi: 10.1001/archopht.1989.01070010933044. [DOI] [PubMed] [Google Scholar]
- 43.Vito RP, Shin TJ, McCarey BE. A mechanical model of the cornea: the effects of physiological and surgical factors on radial keratotomy surgery. Refract Corneal Surg. 1989;5(2):82–8. [PubMed] [Google Scholar]
- 44.Pinsky PM, Datye DV. A microstructurally-based finite element model of the incised human cornea. J Biomech. 1991;24(10):907–22. doi: 10.1016/0021-9290(91)90169-n. [DOI] [PubMed] [Google Scholar]
- 45.Velinsky SA, Bryant MR. On the computer-aided and optimal design of keratorefractive surgery. Refract Corneal Surg. 1992;8(2):173–82. [PubMed] [Google Scholar]
- 46.Hanna KD, Jouve FE, Waring GO, Ciarlet PG. Computer simulation of arcuate and radial incisions involving the corneoscleral limbus. Eye. 1989;3(Pt 2):227–39. doi: 10.1038/eye.1989.32. [DOI] [PubMed] [Google Scholar]
- 47.Pinsky PM, Datye DV. Numerical modeling of radial, astigmatic, and hexagonal keratotomy. Refract Corneal Surg. 1992;8(2):164–72. [PubMed] [Google Scholar]
- 48.Hanna KD, Jouve FE, Waring GO, Ciarlet PG. Computer simulation of arcuate keratotomy for astigmatism. Refract Corneal Surg. 1992;8(2):152–63. [PubMed] [Google Scholar]
- 49.Studer HP, Riedwyl H, Amstutz CA, Hanson JV, Buchler P. Patient-specific finite-element simulation of the human cornea: a clinical validation study on cataract surgery. J Biomech. 2013;46(4):751–8. doi: 10.1016/j.jbiomech.2012.11.018. Epub 2012/12/19. [DOI] [PubMed] [Google Scholar]
- 50.Bryant MRFD, Campos M, McDonnell PJ. Finite element analysis of corneal topographic changes after excimer laser phototherapeutic keratectomy. Invest Ophthalmol Vis Sci. 1993;31(Suppl):804. [Google Scholar]
- 51.Katsube N, Wang R, Okuma E, Roberts C. Biomechanical response of the cornea to phototherapeutic keratectomy when treated as a fluid-filled porous material. J Refract Surg. 2002;18(5):S593–7. doi: 10.3928/1081-597X-20020901-19. [DOI] [PubMed] [Google Scholar]
- 52.Uchio E, Watanabe Y, Kadonosono K, Matsuoka Y, Goto S. Simulation of airbag impact on eyes after photorefractive keratectomy by finite element analysis method. Graefes Arch Clin Exp Ophthalmol. 2003;241(6):497–504. doi: 10.1007/s00417-003-0679-8. [DOI] [PubMed] [Google Scholar]
- 53.Alastrue V, Calvo B, Pena E, Doblare M. Biomechanical modeling of refractive corneal surgery. J Biomech Eng. 2006;128(1):150–60. doi: 10.1115/1.2132368. [DOI] [PubMed] [Google Scholar]
- 54.Deenadayalu C, Mobasher B, Rajan SD, Hall GW. Refractive change induced by the LASIK flap in a biomechanical finite element model. Journal of refractive surgery (Thorofare, NJ : 1995) 2006;22(3):286–92. doi: 10.3928/1081-597X-20060301-15. Epub 2006/04/11. [DOI] [PubMed] [Google Scholar]
- 55.Pandolfi A, Manganiello F. A model for the human cornea: constitutive formulation and numerical analysis. Biomechan Model Mechanobiol. 2006;(5):237246. doi: 10.1007/s10237-005-0014-x. [DOI] [PubMed] [Google Scholar]
- 56.Roy AS, Dupps WJ., Jr Effects of altered corneal stiffness on native and postoperative LASIK corneal biomechanical behavior: A whole-eye finite element analysis. J Refract Surg. 2009;25(10):875–87. doi: 10.3928/1081597X-20090917-09. Epub 2009/10/20. [DOI] [PubMed] [Google Scholar]
- 57.Sinha Roy A, Dupps WJ., Jr Patient-specific modeling of corneal refractive surgery outcomes and inverse estimation of elastic property changes. J Biomech Eng. 2011;133(1):011002. doi: 10.1115/1.4002934. Epub 2010/12/29. [DOI] [PubMed] [Google Scholar]
- 58.Kling S, Marcos S. Finite-element modeling of intrastromal ring segment implantation into a hyperelastic cornea. Invest Ophthalmol Vis Sci. 2013;54(1):881–9. doi: 10.1167/iovs.12-10852. [DOI] [PubMed] [Google Scholar]
- 59.Gefen A, Shalom R, Elad D, Mandel Y. Biomechanical analysis of the keratoconic cornea. Journal of the mechanical behavior of biomedical materials. 2009;2(3):224–36. doi: 10.1016/j.jmbbm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho LA, Prado M, Cunha RH, Costa Neto A, Paranhos A, Jr, Schor P, et al. Keratoconus prediction using a finite element model of the cornea with local biomechanical properties. Arq Bras Oftalmol. 2009;72(2):139–45. doi: 10.1590/s0004-27492009000200002. Epub 2009/05/26. [DOI] [PubMed] [Google Scholar]
- 61.Roy AS, Dupps WJ., Jr Patient-specific modeling of corneal refractive surgery outcomes and inverse estimation of elastic property changes. J Biomech Eng. 2011;133(1):011002. doi: 10.1115/1.4002934. Epub 2010/12/29. [DOI] [PubMed] [Google Scholar]
- 62.Seven I, Sinha Roy A, Dupps WJ. Patterned corneal collagen crosslinking for astigmatism: A computational modeling study. J Cataract Refract Surg. 2014 doi: 10.1016/j.jcrs.2014.03.019. Accepted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seven I, Dupps JWJ. Patient-specific finite element simulations of standard incisional astigmatism surgery and a novel patterned collagen crosslinking approach to astigmatism treatment. J Med Dev. 2013 doi: 10.1115/1.4025980. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quigley HA. The iris is a sponge: a cause of angle closure. Ophthalmology. 2010;117(1):1–2. doi: 10.1016/j.ophtha.2009.11.002. Epub 2010/02/02. [DOI] [PubMed] [Google Scholar]
- 65.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi: 10.1097/ICU.0b013e32834ff1e7. Epub 2012/01/18. [DOI] [PubMed] [Google Scholar]
- 66.Lee RY, Huang G, Porco TC, Chen YC, He M, Lin SC. Differences in iris thickness among african americans, caucasian americans, Hispanic americans, chinese americans, and filipino-americans. J Glaucoma. 2013;22(9):673–8. doi: 10.1097/IJG.0b013e318264ba68. Epub 2012/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang BS, Narayanaswamy A, Amerasinghe N, Zheng C, He M, Chan YH, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95(1):46–50. doi: 10.1136/bjo.2009.178129. Epub 2010/06/10. [DOI] [PubMed] [Google Scholar]
- 68.Zheng C, Cheung CY, Aung T, Narayanaswamy A, Ong SH, Friedman DS, et al. In vivo analysis of vectors involved in pupil constriction in Chinese subjects with angle closure. Invest Ophthalmol Vis Sci. 2012;53(11):6756–62. doi: 10.1167/iovs.12-10415. Epub 2012/08/30. [DOI] [PubMed] [Google Scholar]
- 69.Wyatt HJ. A ‘minimum-wear-and-tear’ meshwork for the iris. Vision Res. 2000;40(16):2167–76. doi: 10.1016/s0042-6989(00)00068-7. Epub 2000/07/06. [DOI] [PubMed] [Google Scholar]
- 70.Whitcomb JE, Amini R, Simha NK, Barocas VH. Anterior-posterior asymmetry in iris mechanics measured by indentation. Exp Eye Res. 2011;93(4):475–81. doi: 10.1016/j.exer.2011.06.009. Epub 2011/07/27. [DOI] [PubMed] [Google Scholar]
- 71.Amini R, Barocas VH. Anterior chamber angle opening during corneoscleral indentation: the mechanism of whole eye globe deformation and the importance of the limbus. Invest Ophthalmol Vis Sci. 2009;50(11):5288–94. doi: 10.1167/iovs.08-2890. Epub 2009/06/26. [DOI] [PubMed] [Google Scholar]
- 72.Sihota R, Goyal A, Kaur J, Gupta V, Nag TC. Scanning electron microscopy of the trabecular meshwork: understanding the pathogenesis of primary angle closure glaucoma. Indian journal of ophthalmology. 2012;60(3):183–8. doi: 10.4103/0301-4738.95868. Epub 2012/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52(5):2147–52. doi: 10.1167/iovs.10-6342. Epub 2011/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKee CT, Wood JA, Shah NM, Fischer ME, Reilly CM, Murphy CJ, et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32(9):2417–23. doi: 10.1016/j.biomaterials.2010.11.071. Epub 2011/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ethier CR, Read AT, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J. 2004;87(4):2828–37. doi: 10.1529/biophysj.103.038133. Epub 2004/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng D, Juzkiw T, Read AT, Chan DW, Glucksberg MR, Ethier CR, et al. Young's modulus of elasticity of Schlemm's canal endothelial cells. Biomech Model Mechanobiol. 2010;9(1):19–33. doi: 10.1007/s10237-009-0156-3. Epub 2009/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.WuDunn D. Mechanobiology of trabecular meshwork cells. Exp Eye Res. 2009;88(4):718–23. doi: 10.1016/j.exer.2008.11.008. Epub 2008/12/17. [DOI] [PubMed] [Google Scholar]
- 78.Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nature medicine. 2011;17(8):1010–4. doi: 10.1038/nm.2409. Epub 2011/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kagemann L, Wollstein G, Ishikawa H, Nadler Z, Sigal IA, Folio LS, et al. Visualization of the conventional outflow pathway in the living human eye. Ophthalmology. 2012;119(8):1563–8. doi: 10.1016/j.ophtha.2012.02.032. Epub 2012/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kagemann L. IOP elevation reduces Schlemm's canal cross-sectional area. IOVS. 2013 doi: 10.1167/iovs.13-13264. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glasser A. Restoration of accommodation: surgical options for correction of presbyopia. Clinical and Experimental Optometry. 2008;91(3):279–95. doi: 10.1111/j.1444-0938.2008.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.von Helmholz HH. Handbuch der Physiologishen. Optik. 1909 [Google Scholar]
- 83.Murphy SL, Xu J, KD K. Deaths: Preliminary Data for 2010. National Vital Statistics Reports. 2012;60:30. [PubMed] [Google Scholar]
- 84.Ostrin LA, Glasser A. Edinger-Westphal and pharmacologically stimulated accommodative refractive changes and lens and ciliary process movements in rhesus monkeys. Experimental Eye Research. 2007;84(2):302–13. doi: 10.1016/j.exer.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brian PD, Melinda KD. The lens capsule. Experimental Eye Research. 2009;88 doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fisher R. Elastic constants of the human lens capsule. The Journal of physiology. 1969;201(1):1–20. doi: 10.1113/jphysiol.1969.sp008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McLeod SD. The challenge of presbyopia. Archives of Ophthalmology. 2002;120(11):1572–4. doi: 10.1001/archopht.120.11.1572. [DOI] [PubMed] [Google Scholar]
- 88.Schachar R, Pierscionek B. Lens hardness not related to the age-related decline of accommodative amplitude. Molecular vision. 2007;13:1010–1. [PMC free article] [PubMed] [Google Scholar]
- 89.Belaidi A, Pierscionek B. Modeling internal stress distributions in the human lens: can opponent theories coexist? Journal of vision. 2007;7(11):1–13. doi: 10.1167/7.11.1. [DOI] [PubMed] [Google Scholar]
- 90.Coleman DJ, Fish SK. Presbyopia, accommodation, and the mature catenary. Ophthalmology. 2001;108(9):1544–51. doi: 10.1016/s0161-6420(01)00691-1. [DOI] [PubMed] [Google Scholar]
- 91.Glasser A, Campbell M. Presbyopia and the optical changes in the human crystalline lens with age. Vision research. 1998;38(2):209–38. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 92.Glasser A, Campbell M. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision research. 1999;39(11):1991–4006. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- 93.Fisher RF. Elastic constants of human lens. Journal of Physiology-London. 1971;212(1):147–80. doi: 10.1113/jphysiol.1971.sp009315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manns F, Parel JM, Denham D, Billotte C, Ziebarth N, Borja D, et al. Optomechanical response of human and monkey lenses in a lens stretcher. Investigative ophthalmology & visual science. 2007;48(7):3260–8. doi: 10.1167/iovs.06-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weeber HA, Eckert G, Pechhold W, van der Heijde RGL. Stiffness gradient in the crystalline lens. Graefes Archive for Clinical and Experimental Ophthalmology. 2007;245(9):1357–66. doi: 10.1007/s00417-007-0537-1. [DOI] [PubMed] [Google Scholar]
- 96.Baradia H, Nikand N, Glasser A. Mouse lens stiffness measurements. Experimental Eye Research. 2010;91(2):300–7. doi: 10.1016/j.exer.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Erpelding TN, Hollman KW, O'Donnell M. Mapping age-related elasticity changes in porcine lenses using bubble-based acoustic radiation force. Experimental Eye Research. 2007;84(2):332–41. doi: 10.1016/j.exer.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dekorte CL, Vandersteen AFW, Thijssen JM, Duindam JJ, Otto C, Puppels GJ. Relation between local acoustic parameters and protein distribution in human and procine eye lenses. Experimental Eye Research. 1994;59(5):617–27. doi: 10.1006/exer.1994.1147. [DOI] [PubMed] [Google Scholar]
- 99.Pau H, Kranz J. The increasing sclerosis of the human lens with age and its relevance to accommodation and presbyopia. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie. 1991;229(3):294–300. doi: 10.1007/BF00167888. [DOI] [PubMed] [Google Scholar]
- 100.Heys K, Cram S, Truscott R. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Molecular vision. 2004;10:956–1019. [PubMed] [Google Scholar]
- 101.Weeber H, Eckert G, Pechhold W, van der Heijde R. Stiffness gradient in the crystalline lens. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie. 2007;245(9):1357–423. doi: 10.1007/s00417-007-0537-1. [DOI] [PubMed] [Google Scholar]
- 102.Hollman K, O'Donnell M, Erpelding T. Mapping elasticity in human lenses using bubble-based acoustic radiation force. Experimental eye research. 2007;85(6):890–3. doi: 10.1016/j.exer.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schachar R, Chan R, Fu M. Viscoelastic properties of fresh human lenses under 40 years of age: implications for the aetiology of presbyopia. The British journal of ophthalmology. 2011;95(7):1010–3. doi: 10.1136/bjo.2011.202895. [DOI] [PubMed] [Google Scholar]
- 104.Wilde GS, Burd HJ, Judge SJ. Shear modulus data for the human lens determined from a spinning lens test. Experimental Eye Research. 2012;97(1):36–48. doi: 10.1016/j.exer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weeber H, van der Heijde R. On the relationship between lens stiffness and accommodative amplitude. Experimental eye research. 2007;85(5):602–9. doi: 10.1016/j.exer.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 106.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annual Review of Biomedical Engineering. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 107.Scarcelli G, Yun SH. Brillouin Confocal Microscopy for three-dimensional mechanical imaging. Nature Photonics. 2008;2:39–43. doi: 10.1038/nphoton.2007.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scarcelli G, Yun SH. In vivo Brillouin optical microscopy of the human eye. Optics Express. 2012;20:9197. doi: 10.1364/OE.20.009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scarcelli G, Kim P, Yun S. In vivo measurement of age-related stiffening in the crystalline lens by brillouin optical microscopy. Biophysical journal. 2011;101(6):1539–84. doi: 10.1016/j.bpj.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bailey S, Twa M, Gump J, Venkiteshwar M, Bullimore M, Sooryakumar R. Light-scattering study of the normal human eye lens: elastic properties and age dependence. IEEE transactions on bio-medical engineering. 2010;57(12):2910–7. doi: 10.1109/TBME.2010.2052393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.NIA. NIH; 2007. Growing Older in America: The Health and Retirement Study. http://wwwnianihgov/sites/default/files/HRS_Text_WEBpdf [Internet] [Google Scholar]
- 112.Reggiani Mello G, Krueger R. Femtosecond laser photodisruption of the crystalline lens for restoring accommodation. International ophthalmology clinics. 2011;51(2):87–182. doi: 10.1097/IIO.0b013e31820f8844. [DOI] [PubMed] [Google Scholar]
- 113.Lichtinger A, Rootman DS. Intraocular lenses for presbyopia correction: past, present, and future. Current Opinion in Ophthalmology. 2012;23(1):40–6. doi: 10.1097/ICU.0b013e32834cd5be. [DOI] [PubMed] [Google Scholar]
- 114.Sheppard AL, Bashir A, Wolffsohn JS, Davies LN. Accommodating intraocular lenses: a review of design concepts, usage and assessment methods. Clinical and Experimental Optometry. 2010;93(6):441–52. doi: 10.1111/j.1444-0938.2010.00532.x. [DOI] [PubMed] [Google Scholar]
- 115.Blum RD, Burns WR, Till JS. Presbyopia treatment by lens alteration. 2012 inventors. [Google Scholar]
- 116.Krueger RR, Kuszak J, Lubatschowski H, Myers RI, Ripken T, Heisterkamp A. First safety study of femtosecond laser photodisruption in animal lenses: Tissue morphology and cataractogenesis. Journal of Cataract and Refractive Surgery. 2005;31(12):2386–94. doi: 10.1016/j.jcrs.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 117.Kessler J. EXPERIMENTS IN REFILLING LENS. Archives of Ophthalmology. 1964;71(3):412–&. doi: 10.1001/archopht.1964.00970010428021. [DOI] [PubMed] [Google Scholar]
- 118.Parel JM, Gelender H, Trefers WF, Norton EWD. PHACO-ERSATZ - CATARACT-SURGERY DESIGNED TO PRESERVE ACCOMMODATION. Graefes Archive for Clinical and Experimental Ophthalmology. 1986;224(2):165–73. doi: 10.1007/BF02141492. [DOI] [PubMed] [Google Scholar]
- 119.McGinty SJ, Truscott RJW. Presbyopia: The first stage of nuclear cataract? Ophthalmic Research. 2006;38(3):137–48. doi: 10.1159/000090645. [DOI] [PubMed] [Google Scholar]
- 120.Heys KR, Truscott RJW. The stiffness of human cataract lenses is a function of both age and the type of cataract. Experimental Eye Research. 2008;86(4):701–3. doi: 10.1016/j.exer.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: Does deamidation contribute to crystallin insolubility? Journal of Proteome Research. 2006;5(10):2554–66. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Friedrich MG, Truscott RJW. Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life. Investigative ophthalmology & visual science. 2009;50(10):4786–93. doi: 10.1167/iovs.09-3588. [DOI] [PubMed] [Google Scholar]
- 123.Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95(5):673–91. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- 124.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefe's Arch Clin Exper Ophthalmol. 2005;243:513–8. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- 125.Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–35. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 126.Morrison JC, Nylander KB, Lauer AK, Cepurna WO, Johnson E. Glaucoma drops control intraocular pressure and protect optic nerves in a rat model of glaucoma. Invest Ophthalmol Vis Sci. 1998;39(3):526–31. Epub 1998/03/21. [PubMed] [Google Scholar]
- 127.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–79. doi: 10.1001/archopht.120.10.1268. Epub 2002/10/09. [DOI] [PubMed] [Google Scholar]
- 128.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13. doi: 10.1001/archopht.120.6.701. discussion 829-30. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 129.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99(1):137–43. doi: 10.1001/archopht.1981.03930010139020. Epub 1981/01/01. [DOI] [PubMed] [Google Scholar]
- 130.Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16(4):406–18. doi: 10.1097/IJG.0b013e31806540a1. Epub 2007/06/16. [DOI] [PubMed] [Google Scholar]
- 131.Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141(5):868–75. doi: 10.1016/j.ajo.2005.12.007. Epub 2006/03/11. [DOI] [PubMed] [Google Scholar]
- 132.Ebneter A, Wagels B, Zinkernagel MS. Non-invasive biometric assessment of ocular rigidity in glaucoma patients and controls. Eye (Lond) 2009;23(3):606–11. doi: 10.1038/eye.2008.47. Epub 2008/03/01. [DOI] [PubMed] [Google Scholar]
- 133.Hommer A, Fuchsjaeger-Mayrl G, Resch H, Vass C, Garhofer G, Schmetterer L. Estimation of ocular rigidity based on pneumotonometric measurement of pulse amplitude and laser interferometric measurement of fundus pulse in patients with primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.07-1342. [DOI] [PubMed] [Google Scholar]
- 134.Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53(4):1714–28. doi: 10.1167/iovs.11-8009. Epub 2012/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, et al. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci. 2012;52(10):7109–21. doi: 10.1167/iovs.11-7448. Epub 2011/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci. 2000;41(10):2991–3000. [PubMed] [Google Scholar]
- 137.Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5500. Epub 2010/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46(11):4189–99. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- 139.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52(8):5656–69. doi: 10.1167/iovs.10-6927. Epub 2011/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morrison JC, Dorman-Pease ME, Dunkelberger GR, Quigley HA. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990;108(7):1020–4. doi: 10.1001/archopht.1990.01070090122053. Epub 1990/07/01. [DOI] [PubMed] [Google Scholar]
- 141.Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64(1):85–96. doi: 10.1006/exer.1996.0184. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 142.Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest Ophthalmol Vis Sci. 2011;52(1):434–41. doi: 10.1167/iovs.10-5856. Epub 2010/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wong AA, Brown RE. A neurobehavioral analysis of the prevention of visual impairment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2012;53(9):5956–66. doi: 10.1167/iovs.12-10020. Epub 2012/08/04. [DOI] [PubMed] [Google Scholar]
- 144.Sun D, Lye-Barthel M, Masland RH, Jakobs TC. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. The Journal of comparative neurology. 2009;516(1):1–19. doi: 10.1002/cne.22058. Epub 2009/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou J, Rappaport EF, Tobias JW, Young TL. Differential gene expression in mouse sclera during ocular development. Invest Ophthalmol Vis Sci. 2006;47(5):1794–802. doi: 10.1167/iovs.05-0759. Epub 2006/04/28. [DOI] [PubMed] [Google Scholar]
- 146.Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 1998;125(2):237–41. doi: 10.1016/s0002-9394(99)80096-8. [DOI] [PubMed] [Google Scholar]
- 147.Hernandez MR, Andrzejewska WM, Neufeld AH. Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am J Ophthalmol. 1990;109(2):180–8. doi: 10.1016/s0002-9394(14)75984-7. [DOI] [PubMed] [Google Scholar]
- 148.Quigley HA, Dorman-Pease ME, Brown AE. Quantitative study of collagen and elastin of the optic nerve head and sclera in human and experimental monkey glaucoma. Curr Eye Res. 1991;10(9):877–88. doi: 10.3109/02713689109013884. [DOI] [PubMed] [Google Scholar]
- 149.Quigley HA, Brown A, Dorman-Pease ME. Alterations in elastin of the optic nerve head in human and experimental glaucoma. Br J Ophthalmol. 1991;75(9):552–7. doi: 10.1136/bjo.75.9.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yan D, McPheeters S, Johnson G, Utzinger U, Vande Geest JP. Microstructural differences in the human posterior sclera as a function of age and race. Invest Ophthalmol Vis Sci. 2011;52(2):821–9. doi: 10.1167/iovs.09-4651. Epub 2010/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pijanka JK, Coudrillier B, Ziegler K, Sorensen T, Meek KM, Nguyen TD, et al. Quantitative mapping of collagen fiber orientation in non-glaucoma and glaucoma posterior human sclerae. Invest Ophthalmol Vis Sci. 2012;53(9):5258–70. doi: 10.1167/iovs.12-9705. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Girard MJ, Dahlmann-Noor A, Rayapureddi S, Bechara JA, Bertin BM, Jones H, et al. Quantitative mapping of scleral fiber orientation in normal rat eyes. Invest Ophthalmol Vis Sci. 2012;52(13):9684–93. doi: 10.1167/iovs.11-7894. Epub 2011/11/15. [DOI] [PubMed] [Google Scholar]
- 153.Myers KM, Cone FE, Quigley HA, Gelman S, Pease ME, Nguyen TD. The in vitro inflation response of mouse sclera. Exp Eye Res. 2010;91(6):866–75. doi: 10.1016/j.exer.2010.09.009. Epub 2010/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000;41(8):2028–34. Epub 2000/07/13. [PubMed] [Google Scholar]
- 155.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009;50(11):5226–37. doi: 10.1167/iovs.08-3363. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Girard MJ, Downs JC, Bottlang M, Burgoyne CF, Suh JK. Peripapillary and Posterior Scleral Mechanics-Part II: Experimental and Inverse Finite Element Characterization. J Biomech Eng. 2009;131(5):051012. doi: 10.1115/1.3113683. Epub 2009/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Grytz R, Fazio MA, Girard MJ, Libertiaux V, Bruno L, Gardiner S, et al. Material properties of the posterior human sclera. J Mech Behav Biomed Mater. 2013 doi: 10.1016/j.jmbbm.2013.03.027. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fazio MA, Grytz R, Bruno L, Girard MJ, Gardiner S, Girkin CA, et al. Regional Variations in Mechanical Strain in the Posterior Human Sclera. Invest Ophthalmol Vis Sci. 2012 doi: 10.1167/iovs.12-9668. Epub 2012/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp Eye Res. 2012;99:27–35. doi: 10.1016/j.exer.2012.04.006. Epub 2012/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res. 2010;91(3):415–24. doi: 10.1016/j.exer.2010.06.018. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Steinhart MR, Cone FE, Nguyen C, Nguyen TD, Pease ME, Puk O, et al. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Molecular vision. 2012;18:1093–106. Epub 2012/06/16. [PMC free article] [PubMed] [Google Scholar]
- 162.Steinhart MR, Cone-Kimball E, Nguyen C, Nguyen TD, Pease ME, Chakravarti S, et al. Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res. 2014;119:54–60. doi: 10.1016/j.exer.2013.12.008. Epub 2013/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cone-Kimball E, Nguyen C, Oglesby EN, Pease ME, Steinhart MR, Quigley HA. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Molecular vision. 2013;19:2023–39. Epub 2013/10/23. [PMC free article] [PubMed] [Google Scholar]
- 164.Pease ME, Oglesby EN, Cone-Kimball E, Jefferys JL, Steinhart MR, Kim AJ, et al. Scleral Permeability Varies by Mouse Strain and Is Decreased by Chronic Experimental Glaucoma. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.13-13327. Epub 2014/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oglesby EN, Steinhart MR, Tezel G, Cone-Kimball E, Jefferys JL, Pease ME, et al. Scleral Fibroblast Response to Experimental Glaucoma in Mice. IOVS. 2014 Acpected for publication. [PMC free article] [PubMed] [Google Scholar]
- 166.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82(2):185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 167.Strouthidis NG, Girard MJA. Altering the way the optic nerve head responds to intraocular pressure—a potential approach to glaucoma therapy. Current Opinion in Pharmacology. 2012 doi: 10.1016/j.coph.2012.09.001. In Press. [DOI] [PubMed] [Google Scholar]
- 168.Quigley HA, Cone FE. Development of diagnostic and treatment strategies for glaucoma through understanding and modification of scleral and lamina cribrosa connective tissue. Cell Tissue Res. 2013;353(2):231–44. doi: 10.1007/s00441-013-1603-0. Epub 2013/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7. doi: 10.1016/s0002-9394(02)02220-1. Epub 2003/04/30. [DOI] [PubMed] [Google Scholar]
- 170.Sigal IA, Grimm JL, Schuman JS, Kagemann L, Ishikawa H, Wollstein G. A Method to Estimate Biomechanics and Mechanical Properties of Optic Nerve Head Tissues From Parameters Measurable Using Optical Coherence Tomography. IEEE Transactions on Medical Imaging. 2014 doi: 10.1109/TMI.2014.2312133. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Girard MJ, Strouthidis NG, Desjardins A, Mari JM, Ethier CR. In vivo optic nerve head biomechanics: performance testing of a three-dimensional tracking algorithm. J R Soc Interface. 2013;10(87):20130459. doi: 10.1098/rsif.2013.0459. Epub 2013/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tang J, Liu J. Ultrasonic measurement of scleral cross-sectional strains during elevations of intraocular pressure: method validation and initial results in posterior porcine sclera. J Biomech Eng. 2012;134(9):091007. doi: 10.1115/1.4007365. Epub 2012/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murienne BJ, Nguyen TD. Proteoglycan Contribution to the Mechanical Behavior of the Porcine Posterior Sclera. Amer Soc Mech Engin. 2012 Paper No. SBC2012-80821:427-8. [Google Scholar]
- 174.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nature genetics. 2003;33(3):407–11. doi: 10.1038/ng1116. Epub 2003/02/25. [DOI] [PubMed] [Google Scholar]
- 175.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. The Journal of clinical investigation. 2004;114(11):1586–92. doi: 10.1172/JCI22715. Epub 2004/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(8):5240–50. doi: 10.1167/iovs.11-7287. Epub 2011/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2007;48(7):3161–77. doi: 10.1167/iovs.06-1282. Epub 2007/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR. Transforming growth factor beta isoforms in human optic nerve heads. Br J Ophthalmol. 1999;83(2):209–18. doi: 10.1136/bjo.83.2.209. Epub 1999/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332(6027):361–5. doi: 10.1126/science.1192152. Epub 2011/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–21. doi: 10.1126/science.1124287. Epub 2006/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jobling AI, Nguyen M, Gentle A, McBrien NA. Isoform-specific changes in scleral transforming growth factor-beta expression and the regulation of collagen synthesis during myopia progression. The Journal of biological chemistry. 2004;279(18):18121–6. doi: 10.1074/jbc.M400381200. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 182.Quigley HA. Glaucoma: macrocosm to microcosm the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2005;46(8):2662–70. doi: 10.1167/iovs.04-1070. [DOI] [PubMed] [Google Scholar]
- 183.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 184.Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88(4):799–807. doi: 10.1016/j.exer.2009.02.003. Epub 2009/02/17. [DOI] [PubMed] [Google Scholar]
- 185.Sigal IA. Interactions between geometry and mechanical properties on the optic nerve head. Invest Ophthalmol Vis Sci. 2009;50(6):2785–95. doi: 10.1167/iovs.08-3095. Epub 2009/01/27. [DOI] [PubMed] [Google Scholar]
- 186.Roberts MD, Liang Y, Sigal IA, Grimm J, Reynaud J, Bellezza A, et al. Correlation between Local Stress and Strain and Lamina Cribrosa Connective Tissue Volume Fraction in Normal Monkey Eyes. Invest Ophthalmol Vis Sci. 2010;51(1):295–307. doi: 10.1167/iovs.09-4016. Epub 2009/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2011;93(1):4–12. doi: 10.1016/j.exer.2010.09.014. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 188.Girard MJ, Downs JC, Burgoyne CF, Suh JK. Peripapillary and posterior scleral mechanics-part I: development of an anisotropic hyperelastic constitutive model. J Biomech Eng. 2009;131(5):051011. doi: 10.1115/1.3113682. Epub 2009/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral Biomechanics in the Aging Monkey Eye. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-3363. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Grytz R, Sigal IA, Ruberti JW, Meschke G, Downs JC. Lamina Cribrosa Thickening in Early Glaucoma Predicted by a Microstructure Motivated Growth and Remodeling Approach. Mech Mater. 2012;44:99–109. doi: 10.1016/j.mechmat.2011.07.004. Epub 2012/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yan DB, Coloma FM, Metheetrairut A, Trope GE, Heathcote JG, Ethier CR. Deformation of the lamina cribrosa by elevated intraocular pressure. Br J Ophthalmol. 1994;78(8):643–8. doi: 10.1136/bjo.78.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, et al. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci. 2007;48(10):4597–607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004;45(8):2660–5. doi: 10.1167/iovs.03-1363. [DOI] [PubMed] [Google Scholar]
- 194.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. 3D morphometry of the human optic nerve head. Exp Eye Res. 2010;90(1):70–80. doi: 10.1016/j.exer.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 195.Sigal IA, Grimm JL, Jan NJ, Reid K, Minckler DS, Brown DJ. Eye-Specific IOP-Induced Displacements and Deformations of Human Lamina Cribrosa. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.13-12724. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza AJ, Burgoyne CF, et al. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50(2):681–90. doi: 10.1167/iovs.08-1792. Epub 2008/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sigal IA, Flanagan JG, Lathrop KL, Tertinegg I, Bilonick R. Human lamina cribrosa insertion and age. Invest Ophthalmol Vis Sci. 2012;53(11):6870–9. doi: 10.1167/iovs.12-9890. Epub 2012/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, et al. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci. 2011;52(10):7109–21. doi: 10.1167/iovs.11-7448. Epub 2011/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Girard MJ, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011;52(10):7738–48. doi: 10.1167/iovs.10-6925. Epub 2011/05/10. [DOI] [PubMed] [Google Scholar]
- 200.Wang B, Nevins JE, Nadler Z, Wollstein G, Ishikawa H, Bilonick RA, et al. In vivo lamina cribrosa micro-architecture in healthy and glaucomatous eyes as assessed by optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(13):8270–4. doi: 10.1167/iovs.13-13109. Epub 2013/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mari JM, Strouthidis NG, Park SC, Girard MJ. Enhancement of lamina cribrosa visibility in optical coherence tomography images using adaptive compensation. Invest Ophthalmol Vis Sci. 2013;54(3):2238–47. doi: 10.1167/iovs.12-11327. Epub 2013/03/02. [DOI] [PubMed] [Google Scholar]
- 202.Sigal IA, Wang B, Strouthidis NG, Akagi T, Girard MJA. Recent advances in OCT imaging of the lamina cribrosa. British Journal of Ophthalmology. 2014 doi: 10.1136/bjophthalmol-2013-304751. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Agoumi Y, Sharpe GP, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118(1):52–9. doi: 10.1016/j.ophtha.2010.05.016. Epub 2010/07/27. [DOI] [PubMed] [Google Scholar]
- 204.Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012;119(7):1359–66. doi: 10.1016/j.ophtha.2012.01.034. Epub 2012/04/03. [DOI] [PubMed] [Google Scholar]
- 205.Kim TW, Kagemann L, Girard MJ, Strouthidis NG, Sung KR, Leung CK, et al. Imaging of the lamina cribrosa in glaucoma: perspectives of pathogenesis and clinical applications. Curr Eye Res. 2013;38(9):903–9. doi: 10.3109/02713683.2013.800888. Epub 2013/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Girard MJA, Zimmo L, White E, Mari JM, Ethier CR, Strouthidis NG. Towards a Biomechanically-based Diagnosis for Glaucoma: In Vivo Deformation Mapping of the Human Optic Nerve Head. ASME 2012 Summer Bioengineering Conference; June 20-23; Fajardo, Puerto Rico, USA. 2012. [Google Scholar]
- 207.Nadler Z, Wang B, Wollstein G, Nevins JE, Ishikawa H, Kagemann L, et al. Automated lamina cribrosa microstructural segmentation in optical coherence tomography scans of healthy and glaucomatous eyes. Biomedical Optics Express. 2013;4(11):2596–608. doi: 10.1364/BOE.4.002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Foin N, Mari JM, Nijjer S, Sen S, Petraco R, Ghione M, et al. Intracoronary imaging using attenuation-compensated optical coherence tomography allows better visualisation of coronary artery diseases. Cardiovascular revascularization medicine : including molecular interventions. 2013 doi: 10.1016/j.carrev.2013.03.007. Epub 2013/05/02. [DOI] [PubMed] [Google Scholar]
- 209.Akagi T, Hangai M, Takayama K, Nonaka A, Ooto S, Yoshimura N. In vivo imaging of lamina cribrosa pores by adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2012;53(7):4111–9. doi: 10.1167/iovs.11-7536. Epub 2012/06/07. [DOI] [PubMed] [Google Scholar]
- 210.Nadler Z, Wang B, Wollstein G, Nevins JE, Ishikawa H, Kagemann L, et al. Automated lamina cribrosa microstructural segmentation in optical coherence tomography scans of healthy and glaucomatous eyes. Biomedical optics express. 2013;4(11):2596–608. doi: 10.1364/BOE.4.002596. Epub 2013/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ivers KM, Li C, Patel N, Sredar N, Luo X, Queener H, et al. Reproducibility of measuring lamina cribrosa pore geometry in human and nonhuman primates with in vivo adaptive optics imaging. Invest Ophthalmol Vis Sci. 2011;52(8):5473–80. doi: 10.1167/iovs.11-7347. Epub 2011/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sredar N, Ivers KM, Queener HM, Zouridakis G, Porter J. 3D modeling to characterize lamina cribrosa surface and pore geometries using in vivo images from normal and glaucomatous eyes. Biomedical optics express. 2013;4(7):1153–65. doi: 10.1364/BOE.4.001153. Epub 2013/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sigal IA, Bilonick RA, Kagemann L, Wollstein G, Ishikawa H, Schuman JS, et al. The optic nerve head as a robust biomechanical system. Invest Ophthalmol Vis Sci. 2012;53(6):2658–67. doi: 10.1167/iovs.11-9303. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]


