Abstract
Biologically active fragments from polymorphonuclear leukocytes (PMN) are simplified systems that can be used to elucidate specific pathways by which cell function is altered. In the current study we have found that cytokineplasts, which are motile fragments derived from the leading front (protopod, lamellipodium) of human PMN, rapidly increase their intracellular free calcium concentration when stimulated by chemotactic formyl peptide or by leukotriene B4, as measured by Quin-2 acetoxymethyl ester fluorescence. As in the parent cell, extracellular EGTA blunts this response only partially. Hence, cytokineplasts retain a mobilizable internal calcium pool, despite a general lack of intracellular organelles. In addition, formyl peptide more than doubles the amount of cytoskeleton-associated (polymerized) actin. In contrast, cytoplasts made by high-speed, discontinuous gradient centrifugation of cytochalasin B-treated leukocytes also increase their intracellular free calcium on stimulation, but cytoskeleton-associated actin increases by only approximately 14%. Thus, defective motile function in the latter cytoplast is associated with compromised effector function (actin polymerization).
Full text
PDF
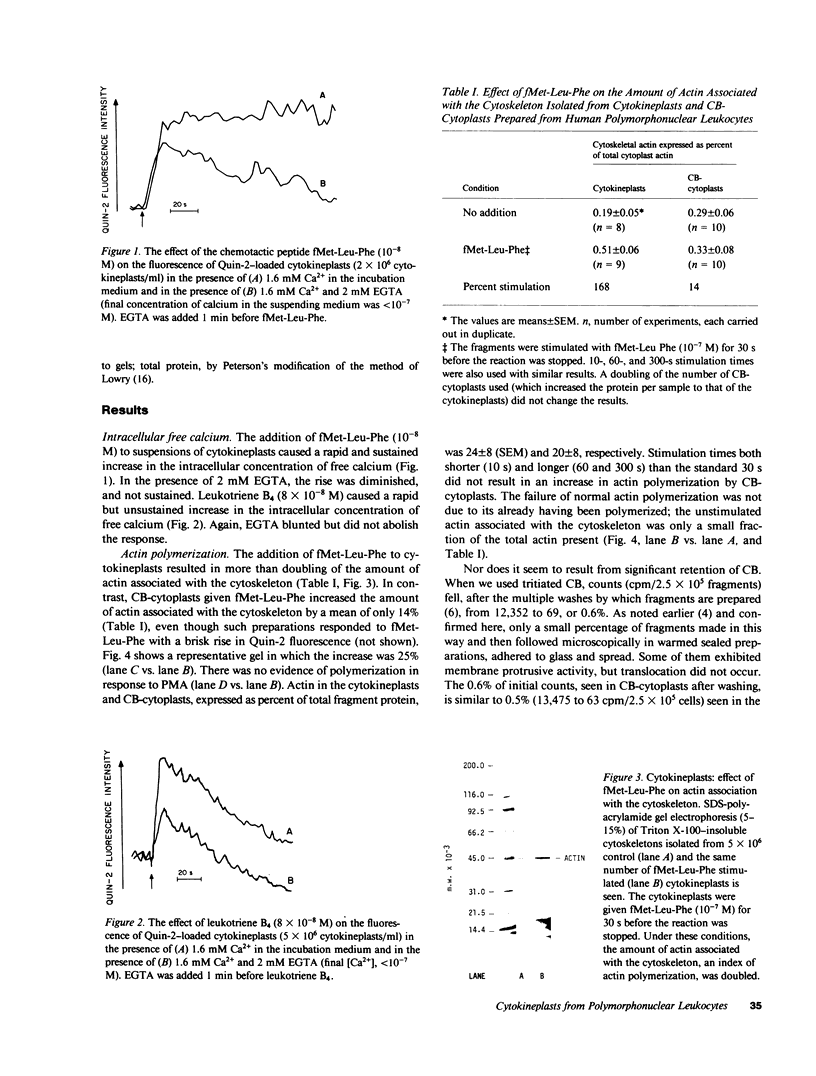
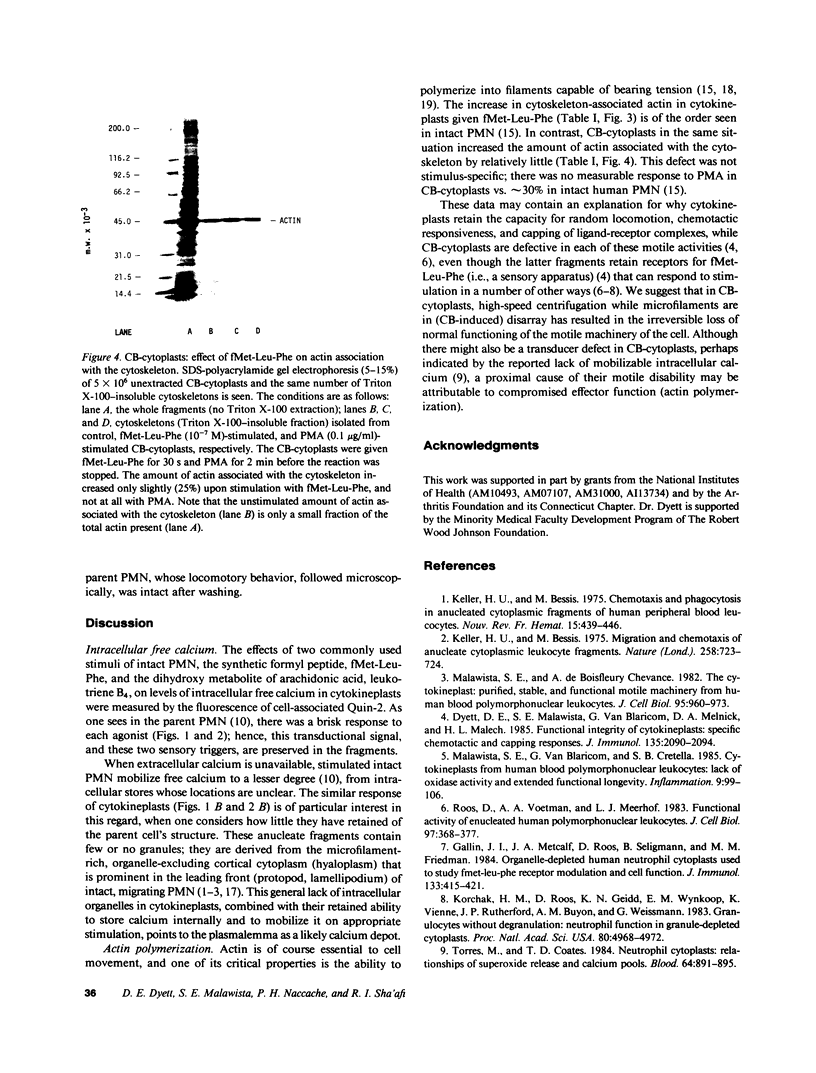

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Dyett D. E., Malawista S. E., Van Blaricom G., Melnick D. A., Malech H. L. Functional integrity of cytokineplasts: specific chemotactic and capping responses. J Immunol. 1985 Sep;135(3):2090–2094. [PubMed] [Google Scholar]
- Gallin J. I., Metcalf J. A., Roos D., Seligmann B., Friedman M. M. Organelle-depleted human neutrophil cytoplasts used to study fmet-leu-phe receptor modulation and cell function. J Immunol. 1984 Jul;133(1):415–421. [PubMed] [Google Scholar]
- Keller H. U., Bessis M. Chemotaxis and phagocytosis in anucleated cytoplasmic fragments of human peripheral blood leucocytes. Nouv Rev Fr Hematol. 1975 Jul-Aug;15(4):439–446. [PubMed] [Google Scholar]
- Keller H. U., Bessis M. Migration and chemotaxis of anucleate cytoplasmic leukocyte fragments. Nature. 1975 Dec 25;258(5537):723–724. doi: 10.1038/258723a0. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Roos D., Giedd K. N., Wynkoop E. M., Vienne K., Rutherford L. E., Buyon J. P., Rich A. M., Weissmann G. Granulocytes without degranulation: neutrophil function in granule-depleted cytoplasts. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4968–4972. doi: 10.1073/pnas.80.16.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., De Boisfleury Chevance A. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982 Dec;95(3):960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G., Cretella S. B. Cytokineplasts from human blood polymorphonuclear leukocytes. Lack of oxidase activity and extended functional longevity. Inflammation. 1985 Mar;9(1):99–106. doi: 10.1007/BF00915416. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Voetman A. A., Meerhof L. J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983 Aug;97(2):368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H., Yin H. L., Southwick F. S., Zaner K. S. The motor of leukocytes. Fed Proc. 1984 Sep;43(12):2760–2763. [PubMed] [Google Scholar]
- Torres M., Coates T. D. Neutrophil cytoplasts: relationships of superoxide release and calcium pools. Blood. 1984 Oct;64(4):891–895. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]
- Yassin R., Shefcyk J., White J. R., Tao W., Volpi M., Molski T. F., Naccache P. H., Feinstein M. B., Sha'afi R. I. Effects of chemotactic factors and other agents on the amounts of actin and a 65,000-mol-wt protein associated with the cytoskeleton of rabbit and human neutrophils. J Cell Biol. 1985 Jul;101(1):182–188. doi: 10.1083/jcb.101.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]