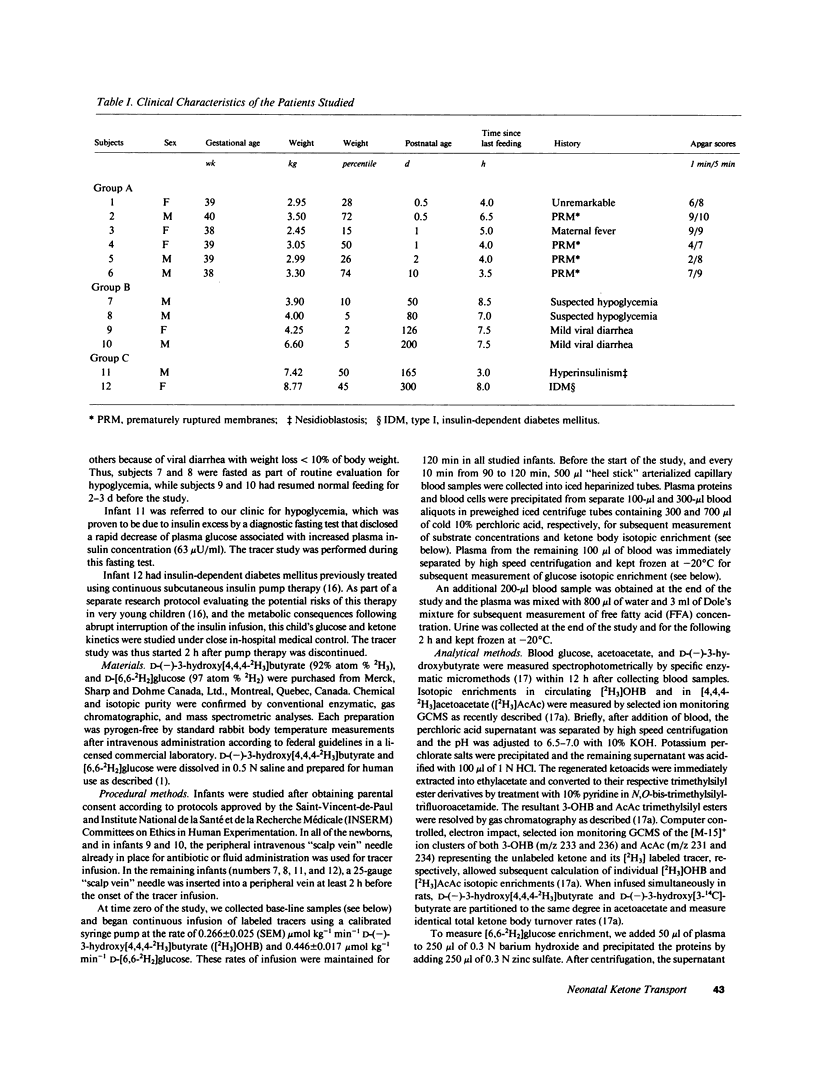
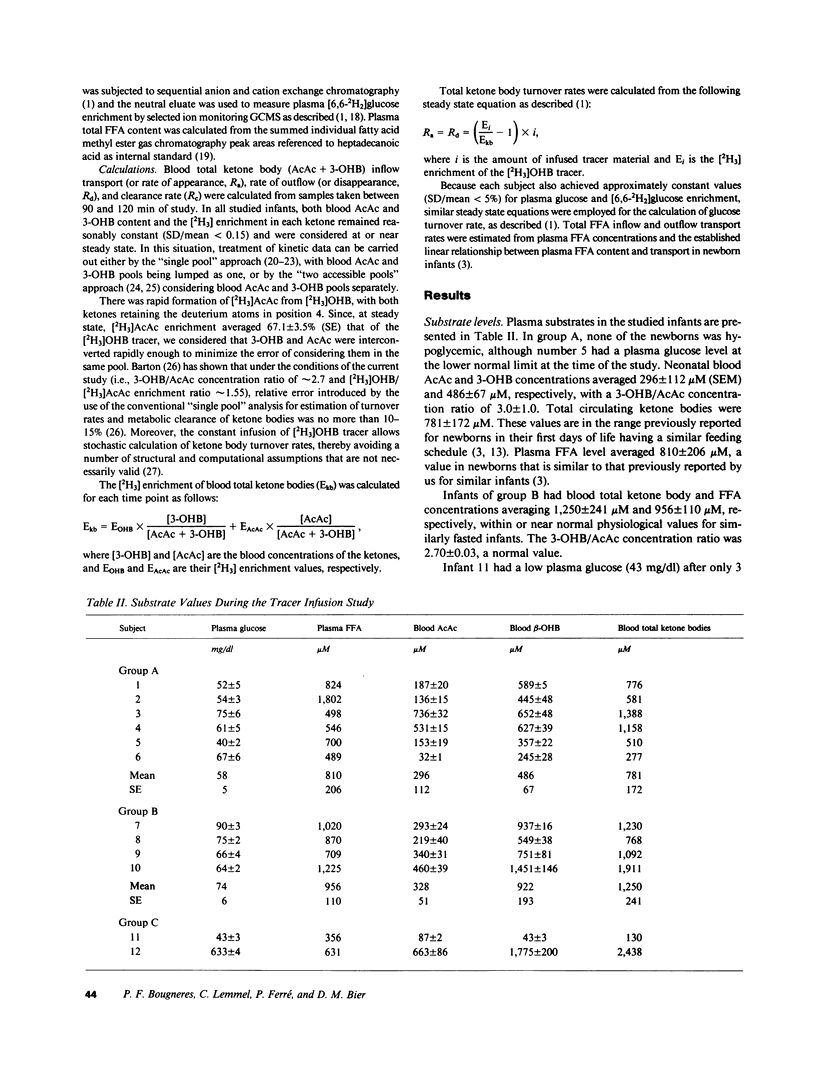
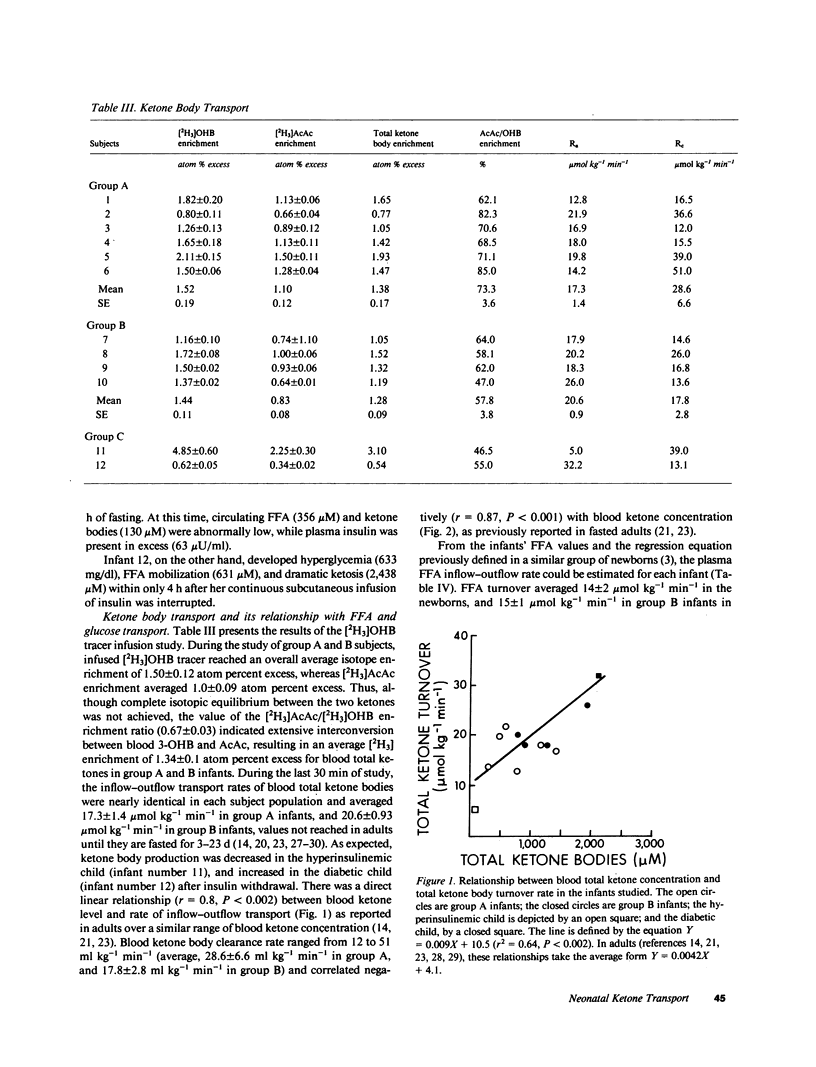
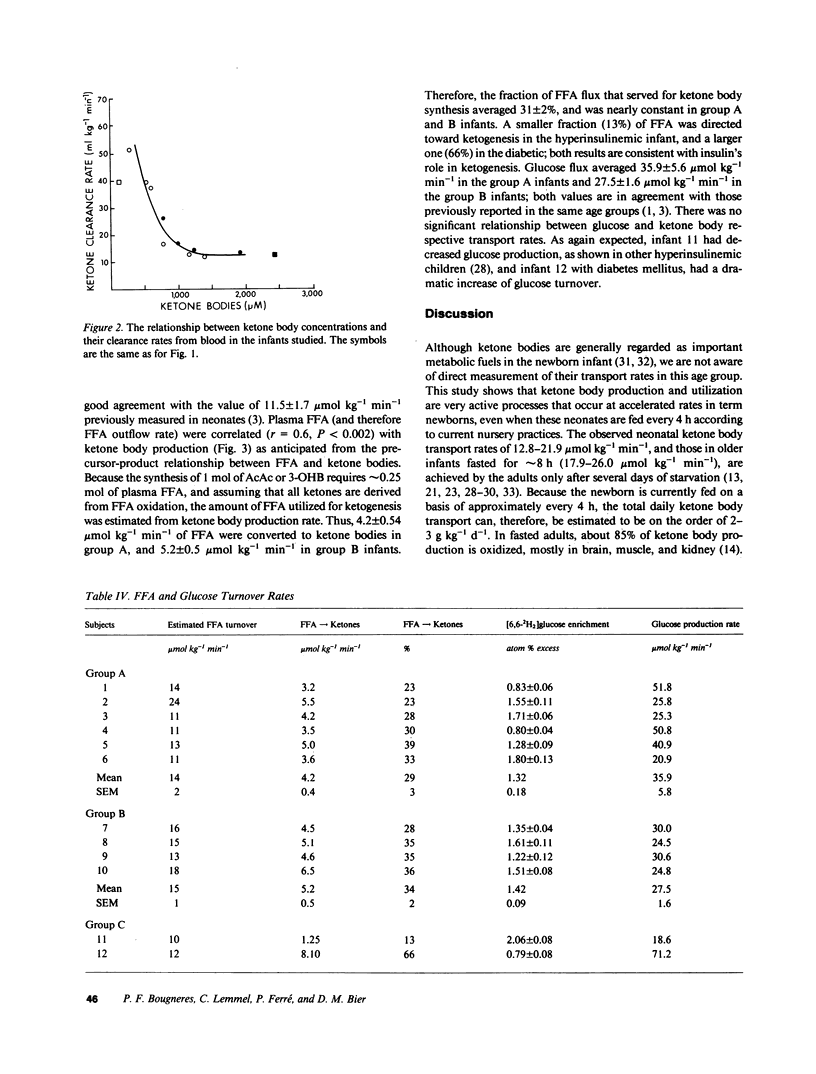
Abstract
Using a continuous intravenous infusion of D-(-)-3-hydroxy[4,4,4-2H3]butyrate tracer, we measured total ketone body transport in 12 infants: six newborns, four 1-6-mo-olds, one diabetic, and one hyperinsulinemic infant. Ketone body inflow-outflow transport (flux) averaged 17.3 +/- 1.4 mumol kg-1 min-1 in the neonates, a value not different from that of 20.6 +/- 0.9 mumol kg-1 min-1 measured in the older infants. This rate was accelerated to 32.2 mumol kg-1 min-1 in the diabetic and slowed to 5.0 mumol kg-1 min-1 in the hyperinsulinemic child. As in the adult, ketone turnover was directly proportional to free fatty acid and ketone body concentrations, while ketone clearance declined as the circulatory content of ketone bodies increased. Compared with the adult, however, ketone body turnover rates of 12.8-21.9 mumol kg-1 min-1 in newborns fasted for less than 8 h, and rates of 17.9-26.0 mumol kg-1 min-1 in older infants fasted for less than 10 h, were in a range found in adults only after several days of total fasting. If the bulk of transported ketone body fuels are oxidized in the infant as they are in the adult, ketone bodies could account for as much as 25% of the neonate's basal energy requirements in the first several days of life. These studies demonstrate active ketogenesis and quantitatively important ketone body fuel transport in the human infant. Furthermore, the qualitatively similar relationships between the newborn and the adult relative to free fatty acid concentration and ketone inflow, and with regard to ketone concentration and clearance rate, suggest that intrahepatic and extrahepatic regulatory systems controlling ketone body metabolism are well established by early postnatal life in humans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., Räihä N., Rahiala E. L., Kekomäki M. Oxidation of glucose and D-B-OH-butyrate by the early human fetal brain. Acta Paediatr Scand. 1975 Jan;64(1):17–24. doi: 10.1111/j.1651-2227.1975.tb04375.x. [DOI] [PubMed] [Google Scholar]
- Anday E. K., Stanley C. A., Baker L., Delivoria-Papadopoulos M. Plasma ketones in newborn infants: absence of suckling ketosis. J Pediatr. 1981 Apr;98(4):628–630. doi: 10.1016/s0022-3476(81)80781-0. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Fery F., Neef M. A. Changes induced by exercise in rates of turnover and oxidation of ketone bodies in fasting man. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jan;44(1):5–11. doi: 10.1152/jappl.1978.44.1.5. [DOI] [PubMed] [Google Scholar]
- Balasse E. O. Kinetics of ketone body metabolism in fasting humans. Metabolism. 1979 Jan;28(1):41–50. doi: 10.1016/0026-0495(79)90166-5. [DOI] [PubMed] [Google Scholar]
- Barton R. N. Isotopic study of ketone body kinetics: invalidity of calculations based upon specific radioactivity of total ketone bodies. Metabolism. 1980 Apr;29(4):392–396. doi: 10.1016/0026-0495(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Barton R. N. The interconversion and disposal of ketone bodies in untreated and injured post-absorptive rats. Biochem J. 1973 Nov;136(3):531–543. doi: 10.1042/bj1360531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. Kinetic analysis of turnover data. Prog Biochem Pharmacol. 1979;15:67–108. [PubMed] [Google Scholar]
- Bier D. M., Leake R. D., Haymond M. W., Arnold K. J., Gruenke L. D., Sperling M. A., Kipnis D. M. Measurement of "true" glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977 Nov;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- Bougnères P. F., Ferré P., Chaussain J. L., Job J. C. Glucose metabolism in hyperinsulinemic infants: the effects of fasting and sodium DL-beta-hydroxybutyrate on glucose production and utilization rates. J Clin Endocrinol Metab. 1983 Nov;57(5):1054–1060. doi: 10.1210/jcem-57-5-1054. [DOI] [PubMed] [Google Scholar]
- Bougnères P. F., Karl I. E., Hillman L. S., Bier D. M. Lipid transport in the human newborn. Palmitate and glycerol turnover and the contribution of glycerol to neonatal hepatic glucose output. J Clin Invest. 1982 Aug;70(2):262–270. doi: 10.1172/JCI110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnères P. F., Landier F., Lemmel C., Mensire A., Chaussain J. L. Insulin pump therapy in young children with type 1 diabetes. J Pediatr. 1984 Aug;105(2):212–217. doi: 10.1016/s0022-3476(84)80115-8. [DOI] [PubMed] [Google Scholar]
- Dubowitz L. M., Dubowitz V., Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970 Jul;77(1):1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- Féry F., Balasse E. O. Ketone body production and disposal in diabetic ketosis. A comparison with fasting ketosis. Diabetes. 1985 Apr;34(4):326–332. doi: 10.2337/diab.34.4.326. [DOI] [PubMed] [Google Scholar]
- Galster A. D., Clutter W. E., Cryer P. E., Collins J. A., Bier D. M. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981 Jun;67(6):1729–1738. doi: 10.1172/JCI110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull D. Storage and supply of fatty acids before and after birth. Br Med Bull. 1975 Jan;31(1):32–36. doi: 10.1093/oxfordjournals.bmb.a071238. [DOI] [PubMed] [Google Scholar]
- Keller U., Sonnenberg G. E., Stauffacher W. Validation of a tracer technique to determine nonsteady-state ketone body turnover rates in man. Am J Physiol. 1981 Mar;240(3):E253–E262. doi: 10.1152/ajpendo.1981.240.3.E253. [DOI] [PubMed] [Google Scholar]
- Kraus H., Schlenker S., Schwedesky D. Developmental changes of cerebral ketone body utilization in human infants. Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):164–170. doi: 10.1515/bchm2.1974.355.1.164. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Guest M. J., Foster D. W. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382–4390. [PubMed] [Google Scholar]
- Melichar V., Drahota Z., Hahn P. Ketone bodies in the blood of full term newborns, premature and dysmature infants and infants of diabetic mothers. Biol Neonat. 1967;11(1):23–28. doi: 10.1159/000240051. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S., Johnson C. A., Rajan R., Owen O. E. The metabolism of ketone bodies in developing human brain: development of ketone-body-utilizing enzymes and ketone bodies as precursors for lipid synthesis. J Neurochem. 1975 Dec;25(6):905–908. doi: 10.1111/j.1471-4159.1975.tb04428.x. [DOI] [PubMed] [Google Scholar]
- Persson B., Gentz J. The pattern of blood lipids, glycerol and ketone bodies during the neonatal period, infancy and childhood. Acta Paediatr Scand. 1966 Jul;55(4):353–362. doi: 10.1111/j.1651-2227.1966.tb08806.x. [DOI] [PubMed] [Google Scholar]
- Persson B., Settergren G., Dahlquist G. Cerebral arterio-venous difference of acetoacetate and D- -hydroxybutyrate in children. Acta Paediatr Scand. 1972 May;61(3):273–278. doi: 10.1111/j.1651-2227.1972.tb16098.x. [DOI] [PubMed] [Google Scholar]
- Reichard G. A., Jr, Owen O. E., Haff A. C., Paul P., Bortz W. M. Ketone-body production and oxidation in fasting obese humans. J Clin Invest. 1974 Feb;53(2):508–515. doi: 10.1172/JCI107584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno A., Gurpide E. Estimation of average times of residence, recycle and interconversion of blood-borne compounds using tracer methods. J Clin Endocrinol Metab. 1973 Feb;36(2):263–276. doi: 10.1210/jcem-36-2-263. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Settergren G., Lindblad B. S., Persson B. Cerebral blood flow and exchange of oxygen, glucose, ketone bodies, lactate, pyruvate and amino acids in infants. Acta Paediatr Scand. 1976 May;65(3):343–353. doi: 10.1111/j.1651-2227.1976.tb04896.x. [DOI] [PubMed] [Google Scholar]
- VAN DUYNE C. M., HAVEL R. J. Plasma unesterified fatty acid concentration in fetal and neonatal life. Proc Soc Exp Biol Med. 1959 Oct-Dec;102:599–602. doi: 10.3181/00379727-102-25330. [DOI] [PubMed] [Google Scholar]


