Abstract
Background
Phosphoinositide 3-kinases (PI3Ks) are relatively conserved and important intracellular lipid kinases involved in signalling and other biological pathways. In the free-living nematode Caenorhabditis elegans, the heterodimeric form of PI3K consists of catalytic (AGE-1) and regulatory (AAP-1) subunits. These subunits are key components of the insulin-like signalling pathway and play roles in the regulation of the entry into and exit from dauer. Although, in parasitic nematodes, similar components are proposed to regulate the transition from free-living or arrested stages to parasitic larvae, nothing is known about PI3Ks in relation to the transition of third-stage larvae (L3s) to parasitism in Haemonchus contortus.
Methods
An integrated molecular approach was used to investigate age-1 and aap-1 of H. contortus (Hc-age-1 and Hc-aap-1) in C. elegans.
Results
The two genes Hc-age-1 and Hc-aap-1 were transcribed in all life stages, with the highest levels in the egg, infective L3 and adult female of H. contortus. The expression of these genes was localized to the intestine, contrasting the pattern of their orthologues in C. elegans (where they are expressed in both head neurons and the intestine). The yeast two-hybrid analysis demonstrated that the adaptor-binding domain of Hc-AGE-1 interacted strongly with the Hc-AAP-1; however, this complex did not rescue the function of its orthologue in age-1-deficient C. elegans.
Conclusions
This is the first time that the PI3K-encoding genes have been characterized from a strongylid parasitic nematode. The findings provide insights into the role of the PI3K heterodimer represented by Hc-age-1 and Hc-aap-1 in the developmental biology of H. contortus.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-014-0498-2) contains supplementary material, which is available to authorized users.
Keywords: Parasitic nematode, Haemonchus contortus, age-1, aap-1, Development, Transgenesis
Background
Phosphoinositide 3-kinases (PI3Ks) are relatively conserved and important intracellular lipid kinases that synthesize lipid second messengers by phosphorylating the 3-hydroxyl group of the inositol head-group in the phosphatidylinositol and phosphoinositides [1,2]. This phosphorylation process gives rise to the activation of a series of downstream effector proteins, such as protein kinase, regulators of small GTPases and/or scaffolding proteins [3,4], which play key roles in lipid and cell signalling as well as membrane trafficking. In multicellular organisms, including the Caenorhabditis elegans (free-living nematode), Drosophila melanogaster (vinegar fly) and Mus musculus (mouse), PI3Ks play key roles in the regulation of cellular metabolism and/or growth [5-9]. Additionally, PI3Ks are involved in the regulation of human maladies, such as heart disease and cancer, and are thus considered possible therapeutic targets [10].
Based on sequence homology and lipid substrate preference, PI3Ks are classified into classes I to III [11]. In C. elegans, PI3K (class I) is a heterodimer consisting of two subunits encoded by age-1 (orthologue of the p110 catalytic subunit) and aap-1 (orthologue of the p50/p55-like regulatory subunit) [12,13]. This AGE-1/AAP-1 heterodimer is a key component of the canonical insulin-PI3K signalling pathway, which plays an important role in dauer formation in C. elegans [14]; genetic screens have shown that PI3K is downstream of the insulin-like receptor DAF-2 [15-17] and upstream of PDK-1 [18,19], AKT-1/AKT-2 kinases [20] and the fork-head transcriptional factor DAF-16 [21-23]. In C. elegans, loss-of-function mutations in the gene age-1 lead to dauer constitutive phenotypes [6]. Similarly, C. elegans lacking AAP-1 have phenotypes that are similar to age-1 mutants [13]. Upstream signalling via DAF-2 activates PI3K by phosphorylation, which, in turn, negatively regulates DAF-16, a crucial regulator of dauer formation, with its characteristics of developmental arrest, longevity and stress-resistance [24,25].
Although advances have been made in understanding the mechanisms involved in the entry into and exit from dauer in C. elegans, very little is known about such mechanisms in parasitic nematodes. Some workers [26-29] have proposed that infective third-stage larvae (L3s) of parasitic nematodes represent the same or a similar developmental state to dauer in C. elegans, with comparable features (including an inability to feed, resistance to stress/environment and extended lifespan). In addition, there are indications that mechanisms controlling the transition of free-living to invasive L3s in parasitic nematodes are similar to those that regulate the recovery from dauer in C. elegans. For instance, recently, two orthologues of Ss-age-1 and Ss-aap-1 were identified in the parasitic nematode Strongyloides stercoralis [30]. The proteins and anatomical expression patterns of AGE-1s from C. elegans and S. stercoralis were shown to be similar. Additionally, LY294002 (a specific inhibitor of PI3-kinase) was shown to block L3 activation in vitro, with a cessation of feeding in treated L3s [30,31]. These findings suggested that insulin-like signalling is conserved and plays a critical role in activating L3s of parasitic nematodes during their invasion of the animal host.
The nuclear genomes and transcriptomes of Haemonchus contortus [32,33] provide a foundation to begin to explore mechanisms of key molecules involved in developmental processes. However, a lack of effective in vitro-culture and genetic methods for parasitic nematodes has hampered the study of the developmental biology of these nematodes [28,34-36]. In contrast, C. elegans provides a powerful surrogate system to shed light on gene function in parasitic nematodes, such as Ancylostoma caninum [37,38], H. contortus [39,40] and S. stercoralis [41,42].
In the present study, we isolated and characterized the C. elegans age-1 and aap-1 orthologues of H. contortus (Hc-age-1 and Hc-aap-1). Using in vivo and in vitro techniques, we characterized and compared the transcriptional profiles and expression patterns of Hc-age-1 and Hc-aap-1 in different developmental stages of the parasite. To investigate the proposed orthology of Hc-AGE-1 and Ce-AGE-1, we tested whether the adaptor-binding domains of Hc-AGE-1 and Hc-AAP-1 interacted with each other as a heterodimer in a yeast two-hybrid assay, and then assessed function using constructs containing the Ce-age-1 and Hc-age-1 regulatory and coding regions by attempting heterologous rescue of an age-1 mutant strain of C. elegans.
Methods
Ethics statement
All the experimental animals used were treated strictly in accordance with the recommendations in the Guide for the Regulation for the Administration of Affairs Concerning Experimental Animals of P. R. China. The protocol employed was approved by the Animal Ethics Committee of Hubei Province (permit no. SYXK-0029). The care and maintenance of animals were in accordance with government guidelines.
Worm strains and their maintenance
The N2 strain of C. elegans was obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, USA) and maintained using standard methods [43]. C. elegans mutant strain CY246 [age-1 (mg44); sqt-1 (sc13)/mnC1 dpy-10 (e128); unc-52 (e444) II], was maintained by picking wild-type phenotypes. The age-1-/- F1 progeny of this strain, which are marked in cis with the sqt-1 roller phenotype and exhibit maternal rescue, produce F2 progeny that constitutively form roller dauers [30]. The Haecon-5 strain of H. contortus was maintained in goats (raised helminth-free), which were infected intra-ruminally with 8,000 L3. Eggs, L1s, L2s and L3s were harvested from the faeces from infected goats, as described previously [40]. For the collection of L4s and adults, infected goats were euthanized with an overdose of pentobarbitone sodium (Lethobarb, Virbac) at 8 and 30 days of infection, respectively. These two developmental stages were collected from abomasa at necropsy, washed extensively in physiological saline to remove debris, and male and female worms separated prior to snap freezing in liquid nitrogen and subsequent storage at -80°C.
DNA and RNA preparation
Genomic DNA was extracted from mixed-stage C. elegans (N2 strain) using the EasyPure Genomic DNA Kit (TransGen Biotech, China); total RNA was isolated using the TRIzol Plus Purification kit (Life Technologies, USA). Genomic DNA of H. contortus was extracted from cultured L3s, and total RNA was isolated from various stages, including eggs, first-stage larvae (L1), second-stage larvae (L2), infective L3s (iL3s), females and males of fourth-stage (L4) and adults using the same kits. RNA integrity and yields were verified by electrophoresis and spectrophotometric analysis (NanoDrop Technologies), respectively. RNA was treated with RQ1-RNase-Free DNase (Promega, USA). Nucleic acids were frozen and stored at -80°C.
Isolation of genes and cDNAs
Guided by transcriptomic and genomic datasets for H. contortus [32,33], we isolated full-length Hc-age-1 and Hc-aap-1 cDNAs and genes. Coding regions were obtained by 5’- and 3’-rapid amplification of cDNA ends (RACE) (SMARTer RACE cDNA Amplification Kit, Clontech, USA) and cloned separately into the plasmid vector pMD-19 T (Takara, Japan) and sequenced using the primer pairs Hc-age-1 F/Hc-age-1R or Hc-aap-1 F/Hc-aap-1R (Additional file 1). Gene sequences were available from contigs [33], and exon-intron junctions were identified using the program Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). Genomic sequences upstream of the genes Hc-age-1 and Hc-aap-1 were sequenced as described previously [40].
Bioinformatic analyses
Nucleotide sequences were assembled using the program CAP3 (http://seq.cs.iastate.edu/cap3.html) and compared with those in non-redundant databases using the BLAST v.2.0 suite of programs from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/BLAST), the Wellcome Sanger Centre (www.sanger.ac.uk) and the Parasite Genome (www.ebi.ac.uk) databases to verify their identity. The conceptual translation of individual cDNAs into amino acid sequences was performed using the selection “translate”, available at http://bioinformatics.org/. Protein motifs were identified by scanning the databases Pfam (www.sanger.ac.uk/Software/Pfam) and Motif Scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan). Signal sequences were predicted using SignalP v.2.0 [44] available via the Center for Biological Sequence Analysis (www.cbs.dtu.dk/services/SignalP). Amino acid sequence alignments were carried out using the program Clustal W [45] and adjusted manually. Promoter elements in the 5’-untranslated region (UTR) were predicted using the transcription element search system available at http://www.dna.affrc.go.jp/PLACE/signalscan.html.
Amino acid sequences of Hc-AGE-1 and Hc-AAP-1 (determined in this study) and homologous sequences from other invertebrates (nematodes, Drosophila melanogaster and Saccharomyces cerevisiae) and vertebrates (human and mouse) were used for phylogenetic analyses. Sequences were aligned (as described above), and analyses conducted using the neighbor joining (NJ), maximum parsimony (MP) and maximum likelihood (ML) methods, based on the Jones-Taylor-Thornton (JTT) model [46]. Confidence limits were assessed using 1000 pseudo-replicates for NJ, MP and ML trees, and other settings were obtained using the default values using the program MEGA v.5.0 [46]. A 50% cut-off value was implemented for the consensus tree.
Transcript abundance based on RNA-seq analysis
Transcriptomic data for different developmental stages of H. contortus (Haecon 5 strain, Australia), including eggs, L1s, L2s, L3s, L4 males, L4 females, adult males and adult females, were generated previously by RNA-seq [33] and made publicly available. The abundances of relevant transcripts were established as described previously [33].
Construction of plasmids
Two constructs Ce-age-1p::Ce-age-1 (102 bp)::gfp::Ce-age-1 t and Hc-age-1p::Hc-age-1 (45 bp)::gfp::Ce-age-1 t (designated pL-Cagep and pL-Hagep; Additional file 2) were made. Briefly, the 1,480 bp region 5’ to Ce-age-1 and 1,900 bp region 5’ to Hc-age-1 were each PCR-amplified from C. elegans and H. contortus genomic DNAs, respectively. The gfp-coding region, with introns, was amplified from the pPD95.75 vector, and the Ce-age-1 t sequence was amplified from C. elegans genomic DNA. Then, the different elements were fused by overlap-extension PCR [30,47] employing primer pairs Ce-age-gfp-F/Ce-age-gfp-R or Hc-age-gfp-5F/Hc-age-gfp-5R (Additional file 1). The PCR products were cloned into the pGEM-T-Easy vector (Promega) and sequenced.
Using a similar strategy, two plasmid constructs Ce-aap-1p 1515 bp::Ce-aap-1 (24 bp)::gfp::Ce-unc-54 t and Hc-aap-1p 2524 bp::Hc-aap-1 (36 bp)::gfp::Ce-unc-54 t (designated pL-Caapp and pL-Haapp) containing the promoters, the gfp reporter and Ce-unc-54 t (terminator) were made. In brief, the 1,515 bp region 5’ to Ce-aap-1 and 2,524 bp region 5’ to Hc-aap-1 were PCR-amplified from C. elegans and H. contortus genomic DNAs, respectively. The gfp and Ce-unc-54 t fragments were amplified from the pPD95.75 vector. Then, the promoter, gfp and terminator were fused by overlap-extension PCR using primer pair Ce-aap-gfp-F/Ce-aap-gfp-R or Hc-aap-gfp-F/Hc-aap-gfp-R (Additional file 1). Finally, the amplicons were cloned into pGEM-T-Easy and sequenced.
For the gene rescue assay, the constructs Ce-age-1p::Ce-age-1 (3,549 bp)::Ce-unc-54 t (designated pL-Cage1), Ce-age-1p::Hc-age-1 (3,471 bp)::Ce-unc-54 t (designated pL-Hage1) and Ce-aap-1p::Hc-aap-1 (1,284 bp)::Ce-unc-54 t (designated pL-Haap1) were made (Additional file 2). The inferred promoters of Ce-age-1 (1,275 bp) and Ce-aap-1 (1,515 bp) were amplified from genomic DNA of C. elegans and sequenced; Ce-unc-54 t was from vector pPV238; the coding sequences of Ce-age-1, Hc-age-1 & Hc-aap-1 were amplified from C. elegans and H. contortus cDNAs, respectively. Then, three fragments, including the Ce-age-1 promoter, Ce-age-1 coding sequence and Ce-unc-54 t were fused to create pL-Cage1 by overlap-extension PCR. The pL-Hage1 plasmid was made from the C. elegans vector pPV238. Briefly, pPV238 was cut at the PstI and BstZ17I restriction sites, and the 5’ region of Ce-age-1 added, with the Mlu1 restriction site upstream of BstZ171 being inserted. Then, the Hc-age-1 coding region with MluI and BstZ17I was inserted to create pL-Hage1 using the primer pairs Ce-age-pstF/Ce-age-mlbstR and Hc-age-mluF/Hc-age-bstzR (Additional file 1). Similarly, vector pPV199 was cut at the endonuclease sites BamHI, AgeI or MluI, and the Ce-aap-1 promoter and Hc-aap-1 coding regions were inserted, in turn, to create the pL-Haap1 using the PCR primer pairs Ce-aap-pbamF/Ce-aap-pageR and Hc-aap-ageF/Hc-aap-mluR, respectively (Additional file 1).
Yeast two-hybrid assay
A yeast two-hybrid system (Gal4-based Matchmaker Gold, Clontech) was used to test interactions between the adaptor-binding domain of Hc-AGE-1 and Hc-AAP-1 [48,49]. Translational fusion to the Gal4-activation domain (AD) was conducted as follows: a full-length Hc-aap-1 cDNA was PCR-amplified from H. contortus using the primer pair Hc-aap-NdeF/Hc-aap-BamR (Additional file 1) and cloned into the vector pGADT7 via the NdeI and BamHI restriction sites. To construct a translational fusion to the Gal4-binding domain (BD), a cDNA fragment encoding the adaptor-binding domain of Hc-age-1 PCR-amplified from H. contortus using primer pair Hc-age-NdeF/Hc-age-BamR was cloned into the vector pGBKT7 via NdeI and BamHI sites.
The constructs fused to Hc-aap-1 or Hc-age-1 were sequenced to verify their reading frames and then transformed into yeast strains Y2HGold and Y187. Interaction trap analysis of diploids, resulting from the mating of Y2HGold and Y187 strains, was carried out using the manufacturer’s protocol (Clontech) employing minimal medium agar plates lacking leucine, tryptophan (plasmid selection marker) and either histidine (low stringency for protein-protein interaction), histidine (in the presence of 1.5 μM 3-amino-triazole; medium stringency) or histidine plus adenine (high stringency). Colonies were assessed after 5 days of incubation at 30°C. The yeast-two hybrid assay was repeated twice.
Transformation of C. elegans
The N2 or CY246 (wild-type) strain of adult hermaphrodites of C. elegans were transformed using a gonad microinjection method [50]. In brief, to study the expression patterns of Ce-age-1, Hc-age-1, Hc-aap-1 and Ce-aap-1 promoters, each of the constructs was microinjected with 20 ng/μl for pL-Cagep, pL-Hagep, pL-Caapp or pL-Haapp, together with 80 ng/μl PRF4 containing the rol-6 marker gene. Injected worms were transferred to nematode growth medium (NGM) plates with Escherichia coli (OP50) lawns and incubated at 20°C. Transformed worms were identified among F1 progeny based on a “right-roller” and the green fluorescence protein (GFP) phenotype. Then, transformants were anaesthetized using 10 mM levamisole and immobilized on a 2% agar pad, and examined using a stereoscopic and compound fluorescence microscope, equipped with differential interference contrast optics (DIC) and a camera (Olympus BX51, Japan).
Gene rescue assay in the mutant strain CY246 of C. elegans
To assess the ability of Hc-age-1 to rescue age-1 in C. elegans (mutant strain CY246), three plasmids (pL-Cage1, pL-Hage1 and pL-Haap1; Additional file 2) were constructed. Each of the plasmids was injected with 20 ng/μl of test plasmid and co-injected with 2 ng/μl of the marker Ce-myo-2 p::mCherry::Ce-unc-54 t (pCFJ90) and 78 ng/μl of pUC19. Additionally, worms were injected with the same amount of the marker pCFJ90 and 98 ng/μl of pUC19 alone, as controls. Injected worms were transferred to NGM + OP50 plates, and transformants identified by fluorescence microscopy. Lines were established from single F2 transformants. The pL-Cage1 and pL-Hage1 lines were assessed for gene rescue based on the presence of progeny from F1 rollers and the ability of F2 rollers to passage for ≥10 generations.
Results
Characterization of cDNAs and phylogenetic analysis of amino acid sequence data
The Hc-age-1 cDNA was 3,471 bp in length and encoded a protein (Hc-AGE-1) of 1,156 amino acids. Hc-AGE-1 had 31-44% amino acid sequence similarity to homologues from Ascaris suum, C. elegans, Loa loa, Parastrongyloides trichosuri and S. stercoralis as well as Homo sapiens and Mus musculus. Hc-AGE-1 consists of five conserved domains, including an adaptor-binding domain proposed to interact with a regulatory subunit (a Ras-binding domain that mediates activation by the small GTPase Ras) and the C2-like, phosphatidylinositol kinase and catalytic domains (Figure 1a). By contrast, the Hc-aap-1 cDNA was 1,284 bp in length and encoded a protein (Hc-AAP-1) of 427 amino acids. Hc-AAP-1 had 29-40% similarity to homologues from Brugia malayi, C. brenneri, C. elegans and L. loa. The Hc-AAP-1 sequence included N-terminal SH2 (Src-homolog 2), C-terminal SH2 domains and an inter-SH2 domain required for binding to the catalytic subunit [51] (Figure 1b).
Figure 1.
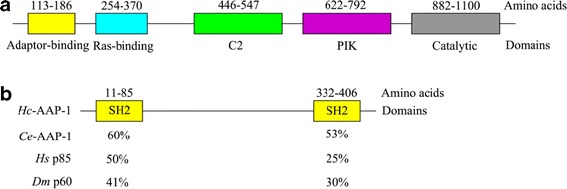
Main functional domains of phosphoinositide 3-kinase (PI3K) catalytic subunit Hc -AGE-1 and the regulatory subunit Hc -AAP-1 of Haemonchus contortus . (a) Colours represent different functional domains: adaptor-binding domain (yellow), Ras-binding domain (light blue), C2 domain (green), PIK domain (purple) and the catalytic domain (grey). (b) N-terminal and C-terminal SH2 domains (yellow). Numbers represent amino acid positions. Percentage under the amino and carboxylic terminal SH2 domain shows the similarity to the SH2 domains of homologues from various eukaryotes, including Caenorhabditis elegans, Drosophila melanogaster and Homo sapiens.
The Hc-AGE-1 sequence was aligned with 14 AGE-1 homologues (from six nematodes and three eukaryotes) and subjected to phylogenetic analyses (Figure 2a). There was concordance in topology among the MP, ML and NJ trees, which all displayed three strongly supported clusters representing classes I, II and III, respectively. Hc-AGE-1 had the closest relationship to class I homologs from C. elegans and C. briggsae. The predicted amino acid sequence of Hc-AAP-1 was aligned with ten AAP-1 homologues (from eight nematodes and two other metazoans) and subjected to phylogenetic analyses (Figure 2b). Again, there was concordance in topology among the NJ, MP and ML trees which showed that Hc-AAP-1 has its closest relationship with Caenorhabditis homologs, to the exclusion of AAP-1 s from S. stercoralis as well as A. suum, B. malayi and L. loa, with the AAP-1 s from the latter three species grouping together with absolute nodal support (100%). Interestingly, AAP-1 of Trichinella spiralis grouped with D. melanogaster and H. sapiens homologues, with 97% support (Figure 2b).
Figure 2.
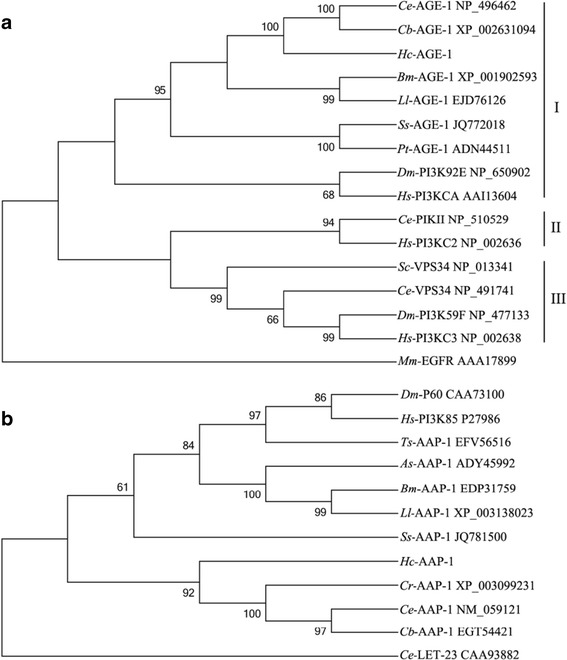
Neighbor joining trees showing the relationship of Haemonchus contortus phosphoinositide 3-kinase (PI3K) catalytic subunit Hc -AGE-1 and regulatory subunit Hc -AAP-1. The trees were calculated using the Jones-Taylor-Thornton (JTT) model in the MEGA program version 5.0. Bootstrap values above or below the branches (1,000 iterations) are shown for robust clades (>50 %). (a) The species used in the analysis include six nematodes (Strongyloides stercoralis, Ss-AGE-1; Parastrongyloides trichosuri, Pt-AGE-1; Brugia malayi, Bm-AGE-1; Loa loa, Ll-AGE-1; Caenorhabditis elegans, Ce-AGE-1, Ce-PIKII and Ce-VPS34; C. briggsae, Cb-AGE-1) and three non-nematodes (Drosophila melanogaster, Dm-PI3K92E and Dm-PI3K59F; Homo sapiens, Hs-PI3KCA, Hs-PI3KC2 and Hs-PI3KC3; Saccharomyces cerevisiae, Sc-VPS34). Mus musculus EGFR AAA17899 was used as an outgroup. (b) AAP-1 used in the analysis included Caenorhabditis elegans, Ce-AAP-1; C. briggsae, Cb-AAP-1; C. remani, Cr-AAP-1; Strongyloides stercoralis, Ss-AAP-1; Ascaris sum, As-AAP-1; Brugia malayi, Bm-AAP-1; Loa loa, Ll-AAP-1; Trichinella spiralis, Ts-AAP-1; Drosophila melanogaster, Dm-P60; Homo sapiens, Hs-PI3K85). C. elegans Ce-LET-23 CAA93882 was used as an outgroup. GenBank accession numbers are listed on the right of each species.
Genomic organization and putative promoter elements
We located the full-length genes of Hc-age-1 and Hc-aap-1 in the H. contortus genome [33]. The Hc-age-1 gene was 12,896 bp in length and had 29 exons (75-198 bp); the Hc-aap-1 gene was 6,172 bp in length and contained 11 exons (69-179 bp) (Figure 3). All exon/intron boundaries abided by the GT-AG rule in Hc-aap-1 (Additional file 3), with the exception of the first and fifth exons in Hc-age-1 (Additional file 4). Hc-age-1 and Hc-aap-1 had more exons and introns compared with orthologous genes in C. elegans [12,13] and S. stercoralis [30]. The promoter regions predicted for Hc-age-1 and Hc-aap-1 were 1,900 bp and 2,524 bp in length, respectively. In the promoter regions of both genes, E- (CANNTG), inverse GATA (TTATC), inverse CAAT (ATTGG), CAAT (CCAAT) and TATA boxes were identified.
Figure 3.
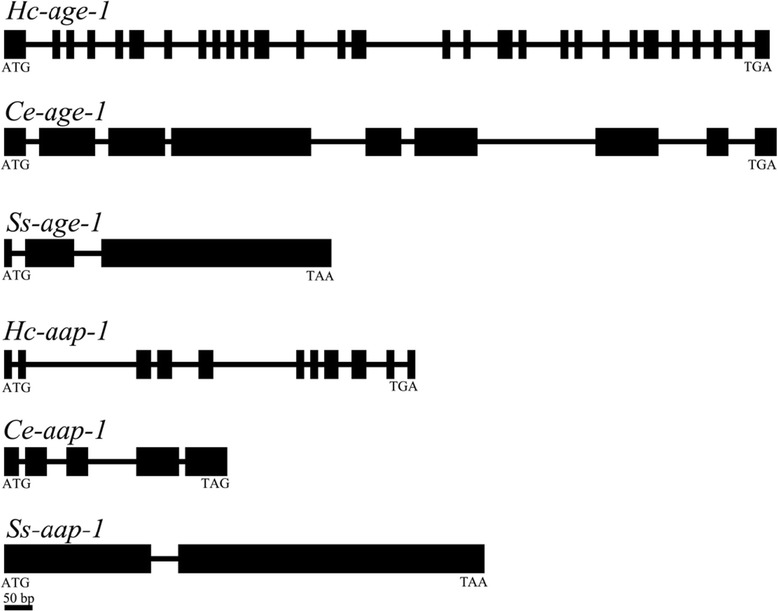
Schematic diagram displaying the genomic organization of age-1 and aap-1 from Haemonchus contortus ( Hc-age-1 and Hc-aap-1 ), Caenorhabditis elegans ( Ce-age-1 and Ce-aap-1 ) and Strongyloides stercoralis ( Ss-age-1 and Ss-aap-1 ). Black boxes represent exons. Lines between the exons represent introns. Start (ATG) or stop (TAG/TGA/TAA) codons are indicated.
Transcriptional profiles in H. contortus, and localization of expression in transgenic C. elegans
Hc-age-1 and Hc-aap-1 were transcribed in all developmental stages of H. contortus. Highest transcription was measured in eggs, L3s and female adults (Hc-age-1), or eggs, L3s, female L4s and female adults (Hc-aap-1) (Figure 4). Expression was localized in C. elegans (N2) transformed with plasmid pL-Hagep containing the 1,900 bp-predicted promoter region of Hc-age-1 or plasmid pL-Haapp containing the 2,524 bp-predicted promoter region of Hc-aap-1, using pRF4 as a marker. In addition, C. elegans (N2) transformed with gfp fused to each Ce-age-1 and Ce-aap-1 constructs were used as controls. The transgenic lines were screened for the roller phenotype and GFP expression. GFP expression driven by the Ce-age-1 promoter was detected in amphidial neurons and the intestine, consistent with previous reports for S. stercoralis [30]. GFP expression driven by the Hc-age-1 promoter was detected in the entire intestine at L1 and L2 stages, but concentrated in the anterior intestine of the L3 stage (Figure 5c-f). Similarly, GFP expression driven by the Ce-aap-1 promoter was observed in intestine and amphidial/phasmidial neurons (Figure 5g-h) in accordance with previous report [13]. The expression of Hc-aap-1 was also predominantly present in anterior intestine, which is highly analogous to that of Hc-age-1 (Figure 5i-j). Despite some variation in expression among individual larvae, C. elegans containing Ce-age-1 p::gfp and Ce-aap-1 p::gfp showed a similar expression profile (amphidial/head neurons and intestine), as did C. elegans containing Hc-age-1 p::gfp and Hc-aap-1 p::gfp (anterior intestine). Based on their apparent co-location, Hc-AGE-1 and Hc-AAP-1 might interact as a heterodimer [52].
Figure 4.
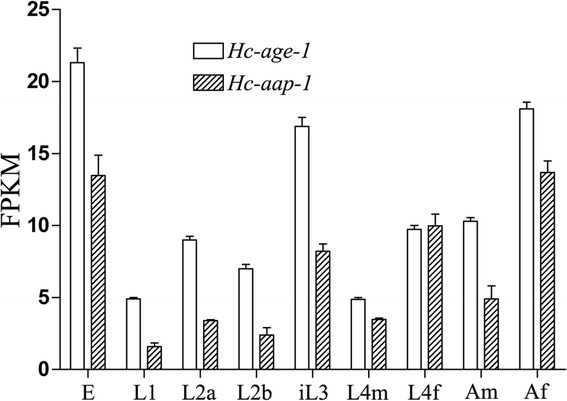
Developmental profile of transcriptional abundance for Hc-age-1 (white bar) and Hc-aap-1 (striped bar) in different developmental stages of Haemonchus contortus . Transcript abundance were compared among eight developmental stages, each in biological triplicate (n =3); eggs (E), the first-stage larvae (L1), the second-stage larvae (L2), the infective L3 (iL3), the fourth-stage males (L4m), the fourth-stage females (L4f), adult males (Am) and adult females (Af). Transcript abundances were counted as fragments per kilobase of coding exon per million mapped reads (FPKM). Error bars represent 95 % confidence intervals.
Figure 5.
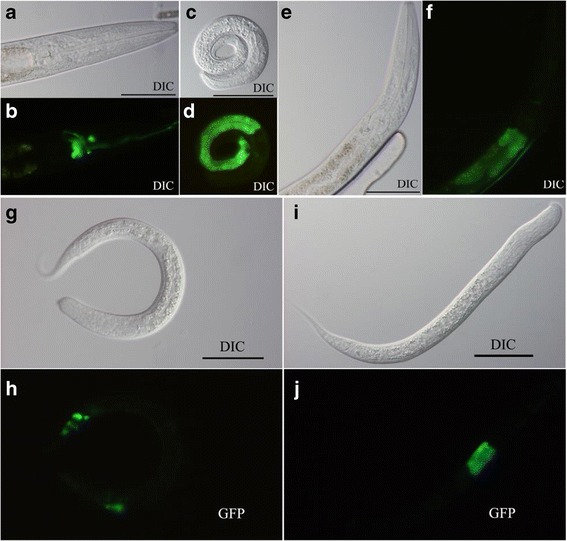
Expression patterns of Hc-age-1 and Hc-aap-1 . Representative expression patterns of Ce-age-1, Hc-age-1, Ce-aap-1 and Hc-aap-1 displayed in Caenorhabditis elegans transformed by either of the four green fluorescent protein (GFP) constructs pL-Cagep, pL-Hagep, pL-Caapp and pL-Haapp (Additional file 2). Three transgenic lines were produced for each of the four plasmids. (a and b) Differential interference contrast (DIC) (a) and fluorescence (b) images of transgenic third-stage larva (L3) of C. elegans expressing Ce-age-1p::gfp (pL-Cagep). (c and d) DIC (c) and fluorescence (d) images showing the expression pattern of Hc-age-1 p::gfp (pL-Hagep) in first-stage larva (L1). (e and f) DIC (e) and fluorescence (f) images, showing the expression pattern of Hc-age-1p::gfp (pL-Hagep) in L3. (g and h) DIC (g) and fluorescence (h) images showing expression pattern of Ce-aap-1 p::gfp (pL-Caapp) in L3. (I and J) DIC (i) and fluorescence (j) images showing expression pattern of Hc-aap-1 p::gfp (pL-Haapp) in L3. Scale bars =50 μm.
Assessing the interaction of AAP-1 with AGE-1
In multicellular organisms, binding by the adaptor subunit (Ce-AAP-1) can stabilize and activate the PI3K catalytic subunit (Ce-AGE-1) [11,13,51]. The interaction of these two subunits is governed by the NH2 terminus of the catalytic subunit and the inter-SH2 domain of the adaptor subunit [51]. To assess whether there was an interaction between Hc-AAP-1 and the adaptor-binding domain of Hc-AGE-1, several fusion constructs in the vectors pGADT7 and pGBKT7, respectively, were prepared for analysis in the yeast two-hybrid system. Co-expression of the full-length gene Hc-aap-1 fused to pGADT7 (GAL4 AD-Hc-AAP-1) with the adaptor-binding domain of Hc-age-1 fused to pGBKT7 (GAL4 BD-Hc-AGE-1) resulted in high β-galactosidase expression activity (Additional file 5). The co-expression of pGADT7 or pGBKT7 fused to the large T-antigen or murine p53 (pGADT7-T and pGBKT7-53, respectively; positive controls) displayed β-galactosidase expression (Additional file 5). In contrast, the co-expression of pGADT7-Hc-aap-1 with pGBKT7-53 or pGBKT7-Hc-age-1 with pGADT7-T did not show such expression (Additional file 5). These findings indicated that the adaptor-binding domain of Hc-AGE-1 was capable of interacting with Hc-AAP-1 in the yeast two-hybrid system.
Attempts of cross-species complementation in C. elegans CY246 with Hc-age-1 and Hc-aap-1
C. elegans mutant strain CY246 [age-1 (mg44) mutant] could be fully rescued by the homologous gene in pL-Cage1 (Additional file 2). Rescue was evidenced based on the presence of F1 roller progeny, and F2 rollers were passaged for ≥10 generations. This result suggests that the main regulatory and coding regions are functional in age-1 (mg44) mutants, thus providing a reliable basis for comparative studies of Hc-AGE-1.
To attempt to establish the functional characteristics of the Hc-age-1 gene, the construct comprising the Ce-age-1 promoter and the Hc-age-1 coding region (pL-Hage1, Additional file 2) was transformed into the CY246 mutant strain. Hc-age-1 mRNA was detected in all lines of the CY246 strain transformed with the rescuing construct. Three lines were screened, and gene rescue was attempted. However, no individuals from the progeny of age-1-/-C. elegans transformed with pL-Hage1 were rescued.
Although the Ce-age-1 promoter was functional in the positive control, Hc-age-1 did not rescue the mutant strain. A possible explanation is that the heterodimer was processed incorrectly, either by the thermally labile AGE-1 subunit or by the inhibition of catalytic activity by a conformational disorder. To address this proposal, the plasmid (pL-Haap1) containing the Hc-aap-1 coding sequence, under the control of the Ce-aap-1 promoter, was constructed (Additional file 2). Although Hc-AAP-1 conferred a strong interaction with the Hc-AGE-1 catalytic subunit in a yeast-two hybrid assay, the transgenic lines transformed with both the pL-Haap1 and pL-Hage1 constructs did not rescue C. elegans age-1 (mg44) mutants.
Discussion
In C. elegans, signalling through the phosphoinositide 3-kinases heterodimer is a key regulator of the switch from dauer to continuous development [5,13]. PI3Ks, which are relatively conserved enzymes from unicellular eukaryotes to multicellular organisms, play important roles in intracellular trafficking, cell metabolism, differentiation and/or survival [53-56]. Recently, components of insulin-like signalling have been identified in parasitic nematodes, including A. caninum [37,38], H. contortus [39,40] and S. stercoralis [30,41,42,57], suggesting that similar signalling is utilized by both free-living and parasitic nematodes. In the present study, two PI3K genes were identified in H. contortus using available data [33], including a Hc-age-1 encoding a p110 catalytic subunit and a Hc-aap-1 encoding a p50/p55-like regulatory subunit, consistent with those in C. elegans and S. stercoralis [12,13,30].
Sequence and structural analyses revealed that Hc-AGE-1 and Hc-AAP-1 are relatively conserved among H. contortus, C. elegans and other nematodes. Hc-AGE-1 has representative structural domains, including an N-terminal adaptor-binding domain, inferred to interact with the adaptor regulatory subunit, a Ras-binding domain predicted to regulate activation by the small GTPase Ras, a C2 domain, a phosphatidylinositol kinase homology (PIK) domain and a C-terminal catalytic domain (cf. Figure 1). Hc-AAP-1 has a common structural domain possessing an inter-SH2 domain, flanked by both Src-homology 2 domains (SH2) and necessary for binding to the catalytic subunit (cf. Figure 1). In bovine PI3K, although the inter-SH2 of the regulatory subunit confers a strong interaction with the catalytic subunit, more than one SH2 domain, in combination with inter-SH2 domain, are required for the conformational changes required to activate the p110 subunit through phosphotyrosine peptide binding [51]. Hence, it was reasonable to hypothesize that the Hc-AAP-1 regulatory subunit can bind the Hc-AGE-1 catalytic subunit to assemble the PI3K heterodimer. This hypothesis was tested using the yeast two-hybrid assay, revealing that the adaptor-binding domain of Hc-AGE-1 readily and strongly interacts with the Hc-AAP-1 regulatory subunit, analogous to orthologous molecules in C. elegans and D. melanogaster [13,58]. Phylogenetic analysis of inferred amino acid sequences showed that Hc-AGE-1 and Hc-AAP-1 grouped with the orthologues Ce-AGE-1 and Cb-AGE-1 and Cr-AAP-1 (free-living nematodes), respectively. In addition, AGE-1 and AAP-1 orthologues are present in various parasitic nematodes, including A. suum, B. malayi and S. stercoralis. Therefore, these findings suggest that the Hc-AGE-1/Hc-AAP-1PI3 heteromer kinase is generally conserved and might, thus, have similar functional characteristics in various nematodes.
Some genes are transcriptionally regulated during the key transition associated with the substantial growth and development in nematodes, such as the switch from the free-living to parasitic stages for parasitic nematodes and the transition from the arrested to the developmentally active state in C. elegans [30,33]. Overall, an assessment of transcription showed that Hc-age-1 and Hc-aap-1 were transcribed at a low level throughout the life cycle of H. contortus, like Ce-age-1 and Ss-age-1, which is in accordance with the hypothesis that the insulin-like signalling is regulated post translationally by AGE-1, rather than at the transcriptional level. Furthermore, the Hc-age-1 and Hc-aap-1 were transcriptionally up-regulated in eggs, infective L3s and female adults of H. contortus, which suggests the PI3K might be involved in nematode development and/or reproduction. Significantly, up-regulation in iL3, which is akin to a developmentally arrested stage, and has a significantly reduced metabolic rate and transcription [33], hints to PI3K having a functional role in recovery from developmental arrest.
The expression of Hc-age-1 was mainly localised to the intestine in the larval stages of C. elegans, which is consistent with the expression pattern of the Ce-age-1, gleaned from high throughput analyses in C. elegans (see WormAtlas: http://gfpweb.aecom.yu.edu/strain?name=BC10837), where the Ce-age-1 promoter::gfp-fusion construct is expressed in the intestine and amphidial/head neurons. Similarly, the Hc-aap-1 promoter::gfp-fusion construct was concentrated in the anterior intestine, which is in accord with the Ce-aap-1 promoter [13], where the expression of Ce-aap-1 promoter::gfp-fusion plasmid was in intestine and head/tail neurons. The expression of Hc-age-1 and Hc-aap-1 showed a strong spatio-temporal relationship with a promoter-promoter interaction [52], which may indicate a functional correlation. Indeed, Hc-AGE-1 and Hc-AAP-1 can bind tightly to form a PI3K heterodimer, as confirmed by yeast two-hybrid analysis and as predicted also for a multicellular organism, including bovine, C. elegans and mouse [13,51,59]. To some extent, the heterologous expression pattern might enable us to mine for protein-protein interaction information by determining in which tissues or cells these proteins are co-expressed. In comparison with C. elegans, the expression of Hc-age-1 and Hc-aap-1 was mainly concentrated in the intestine rather than the nervous systems, which might suggest that PI3K plays a crucial role in feeding in parasitic nematodes. Thus, like in A. caninum, A. ceylanicum and S. stercoralis, the PI3K specific inhibitor LY294002 inhibits the resumption of feeding [30,31].
Based on our findings, we suggest that Hc-age-1 is a functional orthologue of Ce-age-1, and could be activated by upstream growth factor receptor tyrosine kinases (RTKs), and negatively regulates the Foxo-class transcription factor DAF-16. To test this proposal, heterologous genetic complementation was applied in C. elegans. The C. elegans mutant strain CY246 was fully rescued by the Ce-age-1 construct, indicating that the Ce-age-1 promoter can direct suitable expression and appropriate heterodimer binding to enable signalling. However, rescue was not achieved when the Hc-age-1 construct was introduced into the CY246 strain. A possible explanation is that the exogenous Hc-AGE-1 subunit cannot bind the endogenous Ce-AAP-1 subunit in vivo to constitute the functional PI3K. It is proposed that Hc-AGE-1 and Hc-AAP-1 bind to each other to constitute a functional PI3K in transgenic C. elegans, rather than a single thermally labile AGE-1 subunit [59]. However, we were unable to detect any transgenic lines (transformed with two plasmids containing Hc-age-1 and Hc-aap-1 together) that could be passaged.
In strongylid nematodes, the resumption of feeding has been used as a phenotypic marker in in vitro-activation assays [30,31,60,61], and the PI3 kinase-specific inhibitor LY294002 effectively blocks the resumption of feeding of exsheathed L3s [30,31], which infers that PI3K plays a key role in the activation of L3s of parasitic nematodes, as it does the exit from dauer in C. elegans. Regarding the failure of Hc-age-1 to complement the C. elegans age-1 (mg44) mutant strain, a possible explanation is that the proposed orthologues of Hc-age-1 and Hc-aap-1 are unable to assemble the functional heterodimer and/or attain their native conformation, because of a lack of molecular chaperones, such as calnexin and calreticulin [62,63], even though they bind tightly to each other in the yeast two-hybrid system. Additionally, the evolution of gene regulation might relate to variation in the temporal and spatial control of gene expression pattern in tissues [64]. We observed that the expression patterns of Hc-age-1 and Hc-aap-1 tend to be in the intestine of C. elegans compared with those of Ce-age-1 and Ce-aap-1, with expression mainly in head neurons. This difference might suggest some functional variation of PI3K between free-living and parasitic nematodes.
The “dauer hypothesis” proposes that similar molecular signalling pathways control the entry into and exit from arrested development in C. elegans and in parasitic nematodes [65,66]. Heterologous complementation showed that the main components of insulin-like signalling, including daf-16, daf-2, age-1 and daf-12 in parasitic nematodes [37,39-41,67], have similar functions to C. elegans orthologues. However, it is, nonetheless, interesting to note that the roles of PI3K from H. contortus appear to be divergent. Determining the lipid and protein kinase activities of PI3K in H. contortus might help establish whether PI3Ks or PI3K signalling is conserved or not. Some new heritable, transgenic elements or tools applied in the “model nematodes” S. stercoralis and P. trichosuri, including piggyback [68], might help address this and other questions relating to the insulin-like signalling pathway.
Conclusions
In conclusion, we investigated the PI3K-encoding genes Hc-age-1 and Hc-aap-1 in the parasitic nematode H. contortus. We characterized their cDNAs, genomic DNAs and promoter sequences, and determined their transcription profiles in various developmental stages. Then, we confirmed the interaction of Hc-age-1 and Hc-aap-1 by yeast two-hybrid analysis. Finally, we predicted function by gene expression location and complementation in heterologous C. elegans. Taken together, the present results provide important insights into the characteristics of PI3K in a strongylid nematode, particularly in relation to developmental processes.
Acknowledgements
This study was supported by “National Natural Science Foundation of China (NSFC)” (Grant no. 31172310), the National Key Basic Research Program (973 program) of China (Grant No. 2015CB150300) and “Special Fund for Agro-scientific Research in the Public Interest, China” (Grant no. 201303037) to MH, by a grant from The National Institutes of Health (NIH), USA (AI-50688) to JBL, and by funds from the National Health and Medical Research Council (NHMRC) and the Australian Research Council (ARC) of Australia to RBG. This study was also supported by a Victorian Life Sciences Computation Initiative (VLSCI), Australia, grant number VR0007 on its Peak Computing Facility at the University of Melbourne, an initiative of the Victorian Government, Australia (RBG).
Abbreviations
- DIC
Differential interference contrast optics
- GFP
Green fluorescence protein
- JTT
Jones-Taylor-Thornton model
- L3s
The third-stage larvae
- ML
Maximum likelihood
- MP
Maximum parsimony
- NGM
Nematode growth medium
- NJ
Neighbour-joining
- PCR
Polymerase chain reaction
- RACE
Rapid amplification of cDNA ends
- UTR
Untranslated region
Additional files
Primers used to isolate Hc-age-1 and Hc-aap-1 genes of Haemonchus contortus and to make constructs for green fluorescent protein (GFP) localization in Caenorhabditis elegans and the heterologous genetic complementation assay.
Cloning strategy for reporter and rescuing constructs. The constructs containing the Caenorhabditis elegans Ce-age-1 and Ce-aap-1 promoters (pL-Cagep and pL-Caapp) and the Haemonchus contortus Hc-age-1 and Hc-aap-1 promoters (pL-Hagep and pL-Haapp) were made based on pPD95.75 by the overlapping extension PCR. The rescuing constructs (pL-Cage1, pL-Hage1 and pL-Haap1) containing the Ce-age-1, Hc-age-1 and Hc-aap-1 coding regions, respectively, were made by removing the gfp coding sequence from pPV238 or pPV199 (cf. 57) and linking the appropriate cDNA. A, B, M and P represent the restriction sites for AgeI, BstZ17I, MluI and PstI, respectively.
The length of exon and intron and splice donor sequences for each exon and intron of Hc-aap-1 gene. The dinucleotide consensus sequence at the splice site is italicized and underlined. All nucleotide sequences are 5’ to 3’.
The length of exon and intron and splice donor sequences for each exon and intron of Hc-age-1 gene. The dinucleotide consensus sequence at the splice site is italicized and underlined. All nucleotide sequences are 5’ to 3’.
The adaptor-binding domain of Hc -AGE-1 interacted with Hc -AAP-1 in a yeast two-hybrid assay. (A) The positive control showed that pGBKT7-53 can interact with pGADT7-T; (B) The negative control showed that pGBKT7-Lam and pGADT7-T did not interact; (C) The test group pGBKT7-Hc-age-1 and pGADT7-Hc-aap-1 did interact with QDO/X/A; (D) pGBKT7-Lam and pGADT7-Hc-aap-1 did not interact with QDO/X/A; (E) pGBKT7-Hc-age-1 and pGADT7-T did not interact with QDO/X/A.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MH, JBL and FCL conceived the project. FCL and YFW carried out laboratory work. FCL, PKK, YFW and MH performed the data analyses. FCL and MH interpreted the data. RBG, JBL, FYY, LH, RZ, JLZ contributed reagents/materials/analysis tools. FCL, RBG, JBL and MH wrote the manuscript, and all authors read and approved the final manuscript.
Contributor Information
Fa-Cai Li, Email: li78561270@163.com.
Robin B Gasser, Email: robinbg@unimelb.edu.au.
James B Lok, Email: jlok@vet.upenn.edu.
Pasi K Korhonen, Email: pasi.korhonen@unimelb.edu.au.
Yi-Fan Wang, Email: wangyifan_123456@163.com.
Fang-Yuan Yin, Email: yinfangyuan96@126.com.
Li He, Email: 463771946@qq.com.
Rui Zhou, Email: rzhou@mail.hzau.edu.cn.
Jun-Long Zhao, Email: zhaojunlong@mail.hzau.edu.cn.
Min Hu, Email: mhu@mail.hzau.edu.cn.
References
- 1.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 2.Downes CP, Carter AN. Phosphoinositide 3-kinase: a new effector in signal transduction? Cell Signal. 1991;3:501–513. doi: 10.1016/0898-6568(91)90027-R. [DOI] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 7.Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/S0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 8.Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110 alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 10.Stein RC, Waterfield MD. PI3-kinase inhibition: a target for drug development? Mol Med Today. 2000;6:347–357. doi: 10.1016/S1357-4310(00)01770-6. [DOI] [PubMed] [Google Scholar]
- 11.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 12.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 13.Wolkow CA, Munoz MJ, Riddle DL, Ruvkun G. Insulin receptor substrate and p55 orthologous adaptor proteins function in the Caenorhabditis elegans daf-2/insulin-like signaling pathway. J Biol Chem. 2002;277:49591–49597. doi: 10.1074/jbc.M207866200. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/S0968-0004(97)01061-X. [DOI] [PubMed] [Google Scholar]
- 15.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 16.Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/S0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- 17.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 19.Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 22.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 23.Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol cell. 1998;2:887–893. doi: 10.1016/S1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- 24.Yen K, Narasimhan SD, Tissenbaum HA. DAF-16/Forkhead box O transcription factor: many paths to a single Fork(head) in the road. Antioxid Redox Signal. 2011;14:623–634. doi: 10.1089/ars.2010.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay A, Oh SW, Tissenbaum HA. Worming pathways to and from DAF-16/FOXO. Exp Gerontol. 2006;41:928–934. doi: 10.1016/j.exger.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Blaxter M. Caenorhabditis elegans is a nematode. Science. 1998;282:2041–2046. doi: 10.1126/science.282.5396.2041. [DOI] [PubMed] [Google Scholar]
- 27.Hashmi S, Tawe W, Lustigman S. Caenorhabditis elegans and the study of gene function in parasites. Trends Parasitol. 2001;17:387–393. doi: 10.1016/S1471-4922(01)01986-9. [DOI] [PubMed] [Google Scholar]
- 28.Gilleard JS. The use of Caenorhabditis elegans in parasitic nematode research. Parasitology. 2004;128:S49–S70. doi: 10.1017/S003118200400647X. [DOI] [PubMed] [Google Scholar]
- 29.Britton C, Murray L. Using Caenorhabditis elegans for functional analysis of genes of parasitic nematodes. Int J Parasitol. 2006;36:651–659. doi: 10.1016/j.ijpara.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Stoltzfus JD, Massey HC, Jr, Nolan TJ, Griffith SD, Lok JB. Strongyloides stercoralis age-1: a potential regulator of infective larval development in a parasitic nematode. PLoS One. 2012;7:e38587. doi: 10.1371/journal.pone.0038587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol. 2004;34:909–914. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Laing R, Kikuchi T, Martinelli A, Tsai IJ, Beech RN, Redman E, Holroyd N, Bartley DJ, Beasley H, Britton C, Curran D, Devaney E, Gilabert A, Hunt M, Jackson F, Johnston SL, Kryukov I, Li K, Morrison AA, Reid AJ, Sargison N, Saunders GI, Wasmuth JD, Wolstenholme A, Berriman M, Gilleard JS, Cotton JA. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013;14:R88. doi: 10.1186/gb-2013-14-8-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz EM, Korhonen PK, Campbell BE, Young ND, Jex AR, Jabbar A, Hall RS, Mondal A, Howe AC, Pell J, Hofmann A, Boag PR, Zhu XQ, Gregory T, Loukas A, Williams BA, Antoshechkin I, Brown C, Sternberg PW, Gasser RB. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013;14:R89. doi: 10.1186/gb-2013-14-8-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalinna BH, Brindley PJ. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends Parasitol. 2007;23:197–204. doi: 10.1016/j.pt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Knox DP, Geldhof P, Visser A, Britton C. RNA interference in parasitic nematodes of animals: a reality check? Trends Parasitol. 2007;23:105–107. doi: 10.1016/j.pt.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Maule AG, McVeigh P, Dalzell JJ, Atkinson L, Mousley A, Marks NJ. An eye on RNAi in nematode parasites. Trends Parasitol. 2011;27:505–513. doi: 10.1016/j.pt.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Gao X, Frank D, Hawdon JM. Molecular cloning and DNA binding characterization of DAF-16 orthologs from Ancylostoma hookworms. Int J Parasitol. 2009;39:407–415. doi: 10.1016/j.ijpara.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelmedin V, Brodigan T, Gao X, Krause M, Wang Z, Hawdon JM. Transgenic C. elegans dauer larvae expressing hookworm phospho null DAF-16/FoxO exit dauer. PLoS One. 2011;6:e25996. doi: 10.1371/journal.pone.0025996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu M, Lok JB, Ranjit N, Massey HC, Jr, Sternberg PW, Gasser RB. Structural and functional characterisation of the fork head transcription factor-encoding gene, Hc-daf-16, from the parasitic nematode Haemonchus contortus (Strongylida) Int J Parasitol. 2010;40:405–415. doi: 10.1016/j.ijpara.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Lok JB, Gasser RB, Korhonen PK, Sandeman MR, Shi D, Zhou R, Li X, Zhou Y, Zhao J, Hu M. Hc-daf-2 encodes an insulin-like receptor kinase in the barber's pole worm, Haemonchus contortus, and restores partial dauer regulation. Int J Parasitol. 2014;44:485–496. doi: 10.1016/j.ijpara.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massey HC, Jr, Bhopale MK, Li X, Castelletto M, Lok JB. The fork head transcription factor FKTF-1b from Strongyloides stercoralis restores DAF-16 developmental function to mutant Caenorhabditis elegans. Int J Parasitol. 2006;36:347–352. doi: 10.1016/j.ijpara.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massey HC, Jr, Nishi M, Chaudhary K, Pakpour N, Lok JB. Structure and developmental expression of Strongyloides stercoralis fktf-1, a proposed ortholog of daf-16 in Caenorhabditis elegans. Int J Parasitol. 2003;33:1537–1544. doi: 10.1016/S0020-7519(03)00205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiernagle T: Maintenance ofC. elegans. In The C. elegans Research Community. Edited by WormBook. ᅟ: ᅟ; ᅟ. Available from: http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html.
- 44.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int J Neural Syst. 1997;8:581–599. doi: 10.1142/S0129065797000537. [DOI] [PubMed] [Google Scholar]
- 45.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson MD, Fitch DH. Overlap extension PCR: an efficient method for transgene construction. Methods Mol Biol. 2011;772:459–470. doi: 10.1007/978-1-61779-228-1_27. [DOI] [PubMed] [Google Scholar]
- 48.Konrad C, Kroner A, Spiliotis M, Zavala-Gongora R, Brehm K. Identification and molecular characterisation of a gene encoding a member of the insulin receptor family in Echinococcus multilocularis. Int J Parasitol. 2003;33:301–312. doi: 10.1016/S0020-7519(02)00265-5. [DOI] [PubMed] [Google Scholar]
- 49.Khayath N, Vicogne J, Ahier A, BenYounes A, Konrad C, Trolet J, Viscogliosi E, Brehm K, Dissous C. Diversification of the insulin receptor family in the helminth parasite Schistosoma mansoni. FEBS J. 2007;274:659–676. doi: 10.1111/j.1742-4658.2006.05610.x. [DOI] [PubMed] [Google Scholar]
- 50.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holt KH, Olson L, Moye-Rowley WS, Pessin JE. Phosphatidylinositol 3-kinase activation is mediated by high-affinity interactions between distinct domains within the p110 and p85 subunits. Mol Cell Biol. 1994;14:42–49. doi: 10.1128/mcb.14.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, Carvunis AR, Pulak R, Shingles J, Reece-Hoyes J, Hunt-Newbury R, Viveiros R, Mohler WA, Tasan M, Roth FR, Le Peuch C, Hope IA, Johnsen R, Moerman DG, Barabási AL, Baillie D, Vidal M. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- 53.Fry MJ. Structure, regulation and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1994;1226:237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 54.Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001;3:304–312. doi: 10.1186/bcr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 56.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 57.Massey HC, Jr, Ranjit N, Stoltzfus JD, Lok JB. Strongyloides stercoralis daf-2 encodes a divergent ortholog of Caenorhabditis elegans DAF-2. Int J Parasitol. 2013;43:515–520. doi: 10.1016/j.ijpara.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinkove D, Leevers SJ, MacDougall LK, Waterfield MD. p60 is an adaptor for the Drosophila phosphoinositide 3-kinase, Dp110. J Biol Chem. 1997;272:14606–14610. doi: 10.1074/jbc.272.23.14606. [DOI] [PubMed] [Google Scholar]
- 59.Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Nat Acad Sci U S A. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hawdon JM, Volk SW, Rose R, Pritchard DI, Behnke JM, Schad GA. Observations on the feeding behaviour of parasitic third-stage hookworm larvae. Parasitology. 1993;106:163–169. doi: 10.1017/S0031182000074953. [DOI] [PubMed] [Google Scholar]
- 61.Cantacessi C, Campbell BE, Young ND, Jex AR, Hall RS, Presidente PJ, Zawadzki JL, Zhong W, Aleman-Meza B, Loukas A, Sternberg PW, Gasser RB. Differences in transcription between free-living and CO2-activated third-stage larvae of Haemonchus contortus. BMC genomics. 2010;11:266. doi: 10.1186/1471-2164-11-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hebert DN, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- 63.Vassilakos A, Cohen-Doyle MF, Peterson PA, Jackson MR, Williams DB. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. EMBO J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- 64.Carroll SB. Endless forms: the evolution of gene regulation and morphological diversity. Cell. 2000;101:577–580. doi: 10.1016/S0092-8674(00)80868-5. [DOI] [PubMed] [Google Scholar]
- 65.Crook M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int J Parasitol. 2014;44:1–8. doi: 10.1016/j.ijpara.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hotez P, Hawdon J, Schad GA. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans Daf-c paradigm. Parasitol Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-D. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Zhou XE, Motola DL, Gao X, Suino-Powell K, Conneely A, Ogata C, Sharma KK, Auchus RJ, Lok JB, Hawdon JM, Kliewer SA, Xu HE, Mangelsdorf DJ. Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Nati Acad Sci U S A. 2009;106:9138–9143. doi: 10.1073/pnas.0904064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao H, Li X, Nolan TJ, Massey HC, Jr, Pearce EJ, Lok JB. Transposon-mediated chromosomal integration of transgenes in the parasitic nematode Strongyloides ratti and establishment of stable transgenic lines. PLoS Pathog. 2012;8:e1002871. doi: 10.1371/journal.ppat.1002871. [DOI] [PMC free article] [PubMed] [Google Scholar]


