Abstract
We have assessed the immune-regulatory and adjuvant activities of a synthetic glycolipid, ABX196, a novel analog of the parental compound α-GalCer. As expected, ABX196 demonstrated a measurable and significant adjuvant effect in mice and monkeys with no appreciable toxicity at the doses used to promote immune responses. We performed a phase I/II dose escalation study of ABX196 in healthy volunteers, with the objectives to evaluate its safety profile, as well as its ability to be utilized as an adjuvant in the context of a prophylactic vaccine against hepatitis B. ABX196 was administered at three doses: 0.2, 0.4, and 2.0µg, in fourty-four subjects. In all individuals injected with ABX196, peripheral blood NKT cells displayed hallmarks of activation, and 45% of them had measurable circulating IFN-γ 24 hours after the first administration. More importantly, the addition of ABX196 to the very poorly immunogenic HBs antigen resulted in protective anti-HBs antibody responses in a majority of patients, demonstrating the adjuvant properties of ABX196 in human. Further analysis of the cohort of subjects receiving ABX196 with HBs antigen also indicates that a single injection appears sufficient to provide protection. A limited set of adverse events linked to the systemic delivery of ABX196 and access to the liver, is discussed in the context of formulation and the need to limit transport of ABX196 to secondary lymphoid tissues for maximal efficacy (Eudra-CT 2012-001566-15).
Keywords: NKT cells, adjuvant, glycolipid, α-GalCer, ABX196
Introduction
Antigen-specific activation or inhibition of particular T cell subsets has been one of the many goals of immunotherapy. The poor pharmacological properties of peptides have limited the applications of this approach in vivo. It appears that T cells that can be activated by glycolipids are one exception, as glycolipids have very well-defined transport, uptake and cellular distribution properties [1, 2]. A family of these glycolipids based on the α-galactosylceramide (α-GalCer) chemistry binds efficiently to CD1d molecules and stimulates specifically a small subset of regulatory lymphocytes called NKT cells. NKT cells are powerful adjuvants of immunity that are recruited rapidly at the site of injury (reviewed in [3]). The main mediators of that sequence of events are IFNγ and IL-4 that NKT cells secrete in large quantities upon activation; subsequent IL12 secretion from DCs, and upregulation of CD40/CD40L on NKT, DC, and B cells sustain the priming reaction.
Preclinical studies in mice showed that ABX196, a novel analog of the parental compound α-GalCer, had a very similar profile to α-GalCer with respect to in vitro and in vivo activation of NKT cells. However, ABX196 was more potent than α-GalCer and induced a cytokine release comparable to the one obtained with the superagonist PBS-57.
The toxicity profile of ABX196 was excellent in mice and monkeys. At very high doses, liver toxicity was seen only in mice with a moderate elevation of hepatic enzymes but not in monkeys. Preclinical studies demonstrated induction of specific cellular and humoral responses at very low doses of ABX196 in the mouse model of prophylactic vaccination to HBV and supported the initiation of a phase I/II study of prophylactic vaccination against hepatitis B in healthy volunteers. Beyond the evaluation of the safety profile of ABX196, the study was also intended to provide preliminary evaluation of single dose vaccination with adjuvant, an approach that would be extremely valuable for a disease like hepatitis B that currently requires three injections and is for that reason poorly amenable to some high-risk populations and developing countries.
Material and Methods
Subjects
The study was approved by the Ethics committee (Pharma-Ethics, South Africa). Healthy male subjects between 18 to 45 years of age, with a body mass index (BMI) calculated as weight in kg/(height in m2) from 18 to 30 kg/m2, not previously vaccinated for Hepatitis B, with NKT percentages in blood lower than 0.3 %, were selected as test population. A positive laboratory test for Hepatitis B surface antigen (HBsAg), HIV 1 and 2 antibodies, HCV antibody, a positive test for urine drug screening, and clinical signs of acute or chronic disease as well current intake of drugs known to affect hepatic metabolism were criteria of exclusion. Written informed consent was obtained from all subjects.
Study Design
This study was a randomized double-blinded dose-escalation study. The aims of the study were to evaluate the safety profile of ABX196 and to determine ABX196 activity based on NKT activation and induction of specific anti-HBsAg responses. Subjects who met the inclusion criteria were assigned to receive either 20 µg HBsAg alone, or 20 µg HBsAg with alum (Heberbiovac HB®), or 20 µg HBsAg with increasing doses of WT1096 intramuscularly (0.2, 0.4, and 2.0µg) (supplementary Figure 1) in 4 successive cohorts; each group was intended to be injected twice at a four week interval. An independent clinical research organization (Keyrus Biopharma, Belgium) performed the computer-generated randomization.
Clinical and Laboratory Evaluations
Inclusion screen included medical history collection, recording of gender, ethnic origin, age, height, weight, and BMI, physical examination, electrocardiogram, blood chemistry and immunology panels, urine drug screen, urinalysis, alcohol breath test, and serology for hepatitis B, C and HIV. Physical examination, urinary drug screen and blood sample collection for laboratory test was performed before each treatment and at day 15, 43, and 56 (end of study). Additional blood samples for laboratory tests were collected on day 5 (cohorts C and D). Following each vaccine dose, participants were kept in observation for 24 hours in order to monitor the possible occurrence of adverse reactions.
Vaccines
ABX196 (lots ABX19600411-A and ABX19600411-A) was supplied as a liposomal suspension at 1.0mg/ml ABX196 (Polymun Scientific, Vienna, Austria). Pre-conditioned vials were stored at 2–8°C. HBsAg (lot S180HBsAg003) was conditioned at 40µg/ml. HBsAg was mixed with either saline solution or WT1096 at the time of injection. Heberbiovac HB® (lots 36/30.1/.358, 36/30.1/.359 and 36/30.1/.360) was conditioned in 1ml doses each containing 20µg of HBsAg adsorbed onto aluminum hydroxide (HeberBiotec HB, Havana, Cuba). All vaccines were administered intramuscularly in the deltoid area using needles microlance BD (21G, 40mm).
Immunomonitoring
All samples from individual subjects were tested simultaneously on blinded samples. For NKT measurements, ten ml of heparinized peripheral blood were collected before and 24 hours following each treatment, and at day 56 (end of study). PBMCs were isolated by Ficoll density gradient centrifugation and cryopreserved in liquid nitrogen until use. For staining, 1×106 PBMCs were incubated with α-GalCer or vehicle loaded CD1d-tetramers (ProImmune, UK) for 30 min at 4°C, then with anti-CD3 antibody (Becton–Dickinson Biosciences) for 30 min at 4 °C. Analysis was performed on a FACSCanto using CELLQuest software (BD Biosciences).
Cytokine was measured before, 4 and 24h after the first and second injections and at the end of study using a multiplex electrochemiluminescense assay according to the manufacturer’s instructions (MSD) and analyzed using the Discovery Workbench MSD software.
Anti-HBsAg antibody measurements
Anti-HBsAg antibody measurements were performed on serum samples collected before each treatment and at day 15, 43, and 56 using commercially available assays (Architect®Abbott).
The architect anti-HBs assay is a two steps immune assay, using Chemiluminescent Microparticle Immunoassay (CMIA) technology, for the quantitative determination of anti-HBs in human serum. In the first step, sample and recombinant HBsAg (rHBsAg) coated paramagnetic microparticles are combined. Anti-HBs present in the sample binds to the rHBsAg coated microparticles. After washing, acridinium-labeled rHBsAg conjugate is added in the second step. Following another wash cycle, Pre Trigger and Trigger solutions are added to the reaction mixture. The resulted chemiluminescent reaction is measured as relative lights units (RLUs). A direct relationship exists between the amount of anti-HBs in the sample and the RLUs detected by the ARCHITECT immunoassay system optics. The concentration of anti-HBs is determined using a previously generated ARCHITECT anti-HBs calibration curve. If the concentration of the specimen is less than 10.0 mIU/mL, the specimen is considered non-reactive by the architect anti-HBs assay. Samples with anti-HBs concentration greater than or equal to 10.0 mIU/mL are considered reactive.
Statistical analysis
Statistical analyses were performed using SAS® software (version 9.1). Data from the HBsAg and commercial vaccine treatment groups were pooled across their respective cohorts. Data from 0.4µg ABX196 treatment were pooled across cohorts A and D.
Results
Preclinical studies
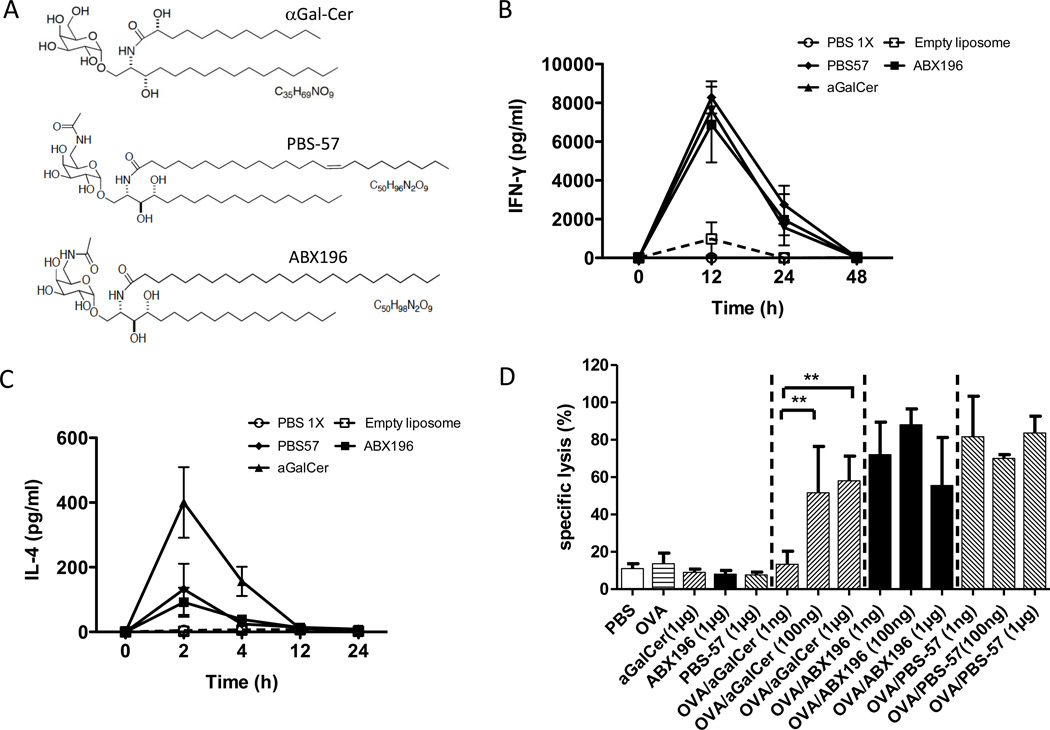
A large number of α-GalCer variants were tested in mice and compared to α-GalCer and known agonists of NKT cells such as PBS-57 [4]. Our final choice was ABX196, based on its capacity to activate NKT cells in vitro and in vivo with a profile very comparable to PBS-57. The structures of α-Galcer, PBS-57 and ABX196 are shown in Figure 1A. The GMP synthesis of ABX196 was accomplished using a synthetic pathway based on the published C6”-modified glycolipids [5]. ABX196 was synthesized as reported for related compounds with small molecules appended at C6 on galactose via amides, except that acetic anhydride was used to form the acetamide group, and lignoceric acid was used in the synthesis of the ceramide portion of the molecule. The synthetic scheme of ABX196 is shown in supplementary Figure 2. The structure of ABX196 was confirmed by proton and carbon NMR spectroscopy and mass spectrometry. Its administration in mice induced the systemic production of large quantities of IFNγ comparable to those induced by PBS-57 (Figure 1B). However, as compared to α-GalCer, ABX196 induced significant reduced quantities of IL-4, indicating a Th1 bias (Figure 1C). In vitro experiments showed that NKT cells from adult PBMC proliferate in response to α-GalCer (KRN 7000) stimulation (100ng/ml) in the presence of IL-2, but the expansion was significantly lower when compared to ABX196 or PBS-57 (not shown). The adjuvant properties of ABX196 were demonstrated for antigen-specific cytolytic CD8 T cell responses when compared to the administration of the antigen or glycolipid alone (Figure 1D). Cytotoxic activity of CD8+ T cells was assessed by VITAL assay on blood samples collected one day after injection of the SIINFEKL-loaded targets. As shown, ABX196 and PBS-57 exhibited similar cytotoxic activity at low and high doses, while at very low doses (1ng/mouse), the cytotoxicity induced by α-GalCer was significantly lower; these results confirmed the known ad juvant properties of NKT agonists [6], and also demonstrated that ABX196 was a potent as PBS-57, and more potent than α-GalCer.
Figure 1. ABX196 demonstrates an agonist and adjuvant activity in mice.
(A) α-Galcer, PBS-57 and ABX196 [(2S, 3S, 4R)-2-tetracosanoylamino-1-O-(6-Acetamido-α-D galactopyranosyl) octadecane-1,3,4-triol] structures.
(B) Mice (3 per group) received by I.M. injection PBS, or empty liposome or α-Galcer (1µg) or PBS-57 (1µg) or ABX196 (1µg). Sera were collected before and 12, 24 and 48 hours post injection. IFN-γ level was assessed by cytometric bead array (CBA).
(C) Mice (3 per group) received by I.M. injection PBS, or empty liposome or α-Galcer (1µg) or PBS-57 (1µg) or ABX196 (1µg). Sera were collected before and 2, 4, 12 and 24 hours post injection. IL-4 level was assessed by cytometric bead array (CBA).
(D) Mice (6 per group) received by I.M. injection, PBS, OVA (50µg) or α-Galcer (KRN7000) (1µg) or ABX196 (1µg) or PBS-57 (1µg) or OVA (50µg) mix with α-Galcer or with PBS-57 or with ABX196 at three doses (1ng, 100ng and 1µg). Fourteen days later, mice received (I.V. injection) a mix of irrelevant peptide and SIINFEKL pulsed splenocytes differentially labelled with CFSE. The specific lysis was assessed in the blood 24h later by flow cytometry.
Toxicity studies
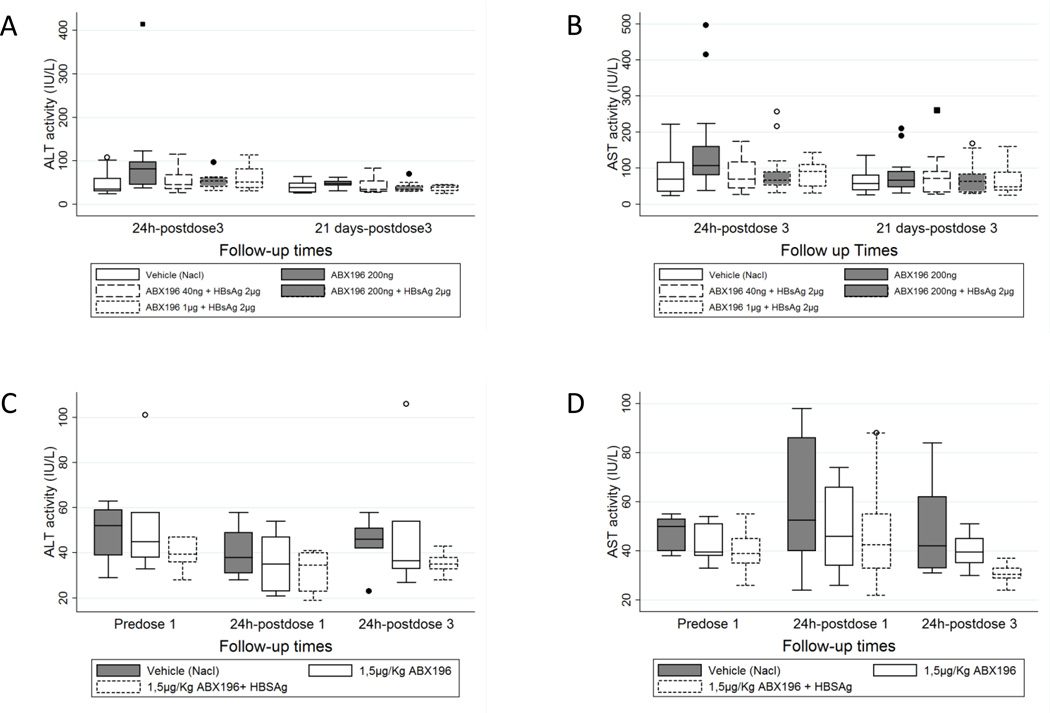
Pre-clinical toxicity studies were carried out in mice and monkeys. The potential liver toxicity of NKT cell activation is well known, and even determinant in hepatitis models such as the one induced by the injection of concanavalin A [7]. Consequently, we examined the level of blood aminotransferases (both alanine and aspartate aminotransferases) in preliminary toxicities studies and in a co-administration study of HBsAg and ABX196 in mice and monkeys. Preliminary toxicity studies were performed using PBS-57 and ABX196. In the mouse, increase in transaminase activities was not observed with the compounds at all doses (up to 10 µg/mouse) as measured two weeks after the last injection. In monkeys, the increase in transaminases was found in one animal out of four at the highest dose tested (100µg/Kg) and only with compound PBS-57 (not shown). In the regulatory toxicology study of co-administration of HBsAg and ABX196, modification of the liver biology was not observed. Monkeys were tested to the maximal dose of 1.5 µg per kg without adverse effects (Figure 2).
Figure 2. Toxicity profile of ABX196 in mice and monkeys.
For mice experiments, ABX196 was repeatedly administered intramuscularly to Swiss Crl CD1 mice of 9 weeks at three doses levels (40, 200 and 1000ng) alone or combined to the antigen HBsAg (2µg/mouse). The three intramuscular administrations were separated by 2 weeks and followed by a 1-day or 14-days observation period. Group 1 animals (controls) (solid line empty box) received the vehicle, Group 2 received ABX196 alone (200ng) (solid line filled box), Group 3 (dash line empty box), 4 (short dash line empty box) and 5 (short dash line filled box) received ABX196 at a dose level combined to the antigen. Each group consisted of 20 mice. Half of the mice were euthanized 24 hours and the other half 21 days after the last dosing. Results are shown for ALT (A) and AST (B). Isolated increase when compared to the control group was noted for ALT and AST activities in one animal of group 2 (496 IU/L and 415 IU/L respectively). In the absence of relevant increased activity of these enzymes in other animals from this group and in the higher dose groups, and in the absence of pre-test evaluation, it could not be concluded whether this observation was already present in this animal before treatment or was due to individual responsiveness.
For monkey experiments, ABX196 was repeatedly administered intramuscularly on days 0, 21 and 42 to Cynomolgus monkeys (Macaca fascicularis) of 36 to 45 months (weighing from 2.2 to 2.9 kg) at 1, 5µg/kg alone or combined to the antigen HBsAg (2µg/Kg). Group 1 animals (controls) (solid line filled box) received the vehicle, Group 2 received ABX196 alone (solid line empty box) and Group 3 ABX196 combined to the antigen (short dash line empty box). Each group consisted of 6 mice. Blood samples were collected pretest (predose), the day after the first administration (24 hours post dose1) and at the end of the treatment period (24 hours post dose3). Results are shown for ALT (C) and AST (D)
Study population
One hundred and thirty six subjects underwent pre-inclusion screening (Supplementary Table 1). Reasons for exclusion were: presence of serum anti-HBs antibodies (50%), NKT cell levels > 0.3 % (22%), prohibited drug substance use (12 %), withdrawal of consent, and other causes (2%) (use of prohibited medication, positivity for Hepatitis C, HIV, anti-HB core antibody, high BMI, history of TB). Among the 44 participants in the intention-to-treat population, the treatment groups were similar in demographic characteristics (Supplementary Table 2). Most subjects were mainly mixed ethnicity. The mean age of the volunteers was 27 years, and the average BMI was 24 kg/m2.
NKT cells characterization
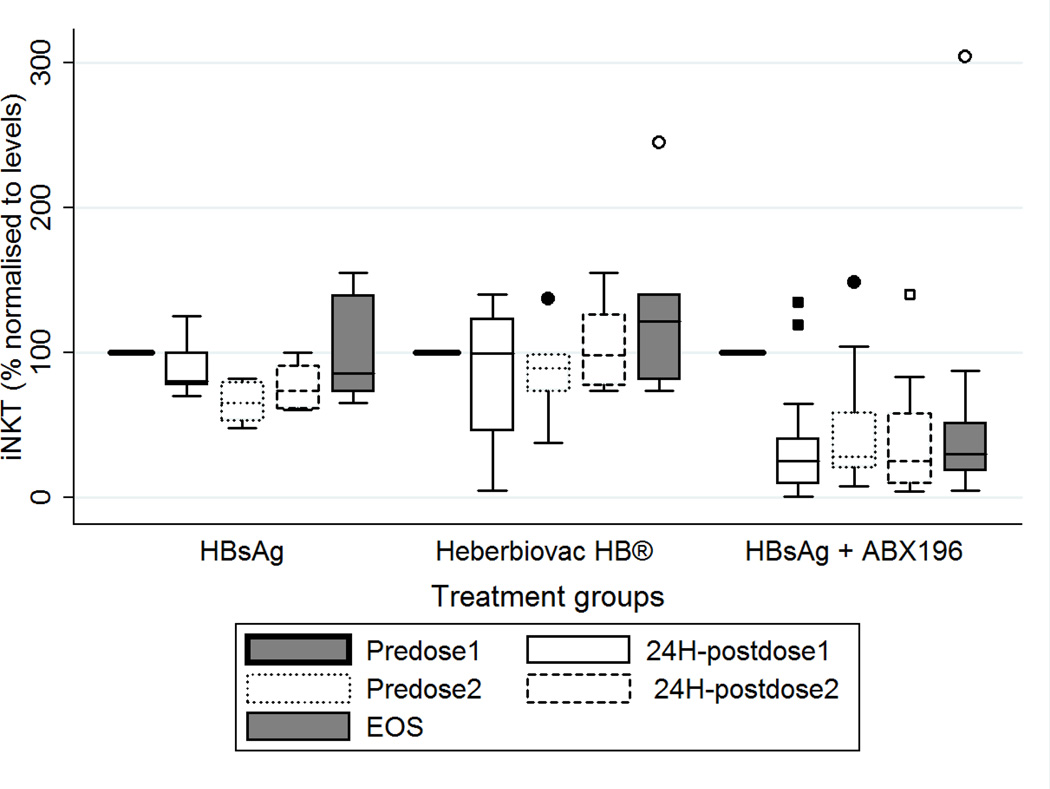
All subjects analyzed received at least one vaccination. NKT cell numbers determined before the first injection defined the 100% level for each individual subject. Representative plots of 3 subjects administered with HBsAg + saline or Heberbiovac HB® or HBsAg + ABX196 (dose 0,2µg) are shown in Supplementary Figure 3. As shown in Figure 3, when compared to this reference, 24h post-injection NKT numbers dropped to very low levels in all subjects receiving ABX196 at all three doses (32.6% mean (20.2 to 45%) whereas subjects receiving Heberbiovac HB® or HBsAg remained at starting levels with means of 87.3 (43.5 to 131%) and 88.7% (67.3 to 110%), respectively. In ABX196-treated groups, recovery of starting NKT cell numbers was not observed. At the end of study, the average number was 43.8% (22.3 to 65.3%) for these latter groups, whereas Heberbiovac HB®-receiving subjects were at 124.2% (69.4 to 179%), and HBsAg-receiving subjects at 98.6% (66.6 to 130.6%).
Figure 3. Systemic response of NKT cells to ABX196.
Sequential blood samples were taken prior to and 24 hours following each administration, and at the end of study (EOS, on day 56). NKT numbers were defined as percentage of α-GalCer-CD1d tetramer positive cells in the CD3-positive cell population. Graph shows data normalized to the respective frequencies measured before dosing (pre dose1) in healthy volunteers who received either HBsAg (HBsAg: n= 7 subjects), Heberbiovac HB® (n= 8) or ABX196 + HBsAg (n=29 subjects): pre-dose 1 (Solid line), 24h post dose 1 (solid line empty box), pre-dose 2 (day 28) (dash empty box), 24 hours post-dose 2 (short-dash empty box), end of study (Day 56) (solid line filled box). Statistical analysis using a Fisher test showed no difference of NKT cell numbers between Heberbiovac HB® and HBsAg-receiving groups, and a highly significant differe nce between those two groups and the ABX196-treated groups (p = 0.006 for HBsAg versus ABX196; p = 0.0001 for Heberbiovac HB® versus ABX196).
In conclusion, at all doses tested and irrespective of the starting blood NKT cell percentages, ABX196 induced a down regulation of T cell receptor level on NKT cells, a hallmark of NKT cell activation [8].
Cytokine responses
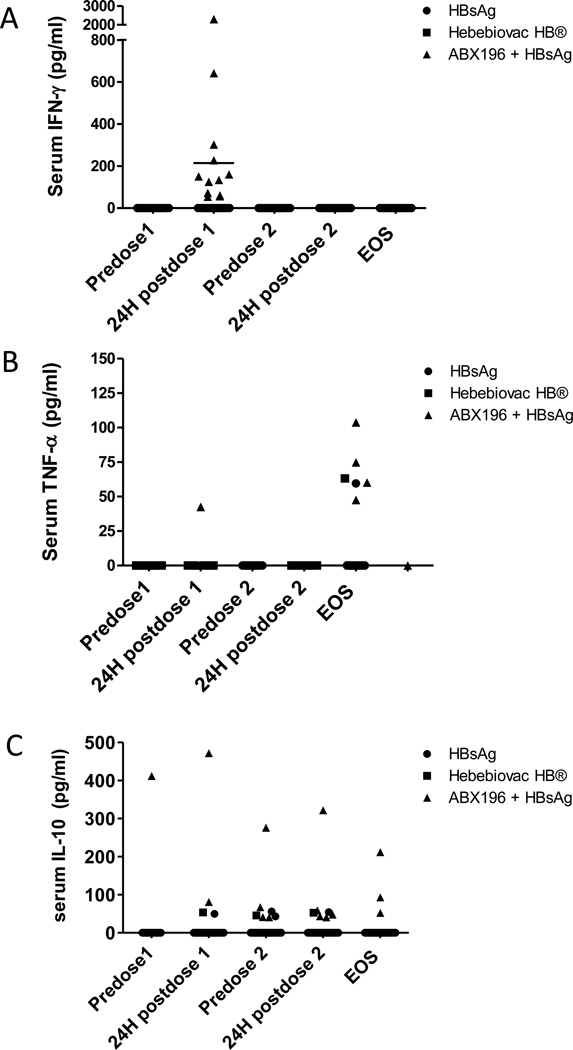
To follow and monitor possible systemic effects of the vaccination, measurements of circulating cytokines were carried out at each visit. This study revealed two significant features. Firstly, both TNFα and IL-10 were detected in a percentage of all groups, consistent with the activation of inflammatory pathways (TNFα) and anti-inflammatory regulatory loops (IL-10) (Figure 4). An interesting feature of the TNFα secretion is that it was detectable only at the end of study and never at an earlier point, even in the occurrence of a serious side effect with the exception of one patient. To the opposite, IL-10 was detectable mostly following the first injection and was consistent with a resolving phase. The second most important observation was that ABX196 induced systemic detectable IFNγ 24 hours after the first administration in 45% of subjects (13/29) (Figure 4). Noticeably, IFNγ secretion was measurable only after the first injection. The disappearance of this cytokine following the second injection, and the concurrent diminution of NKT cell numbers demonstrated the direct relationship between NKT cells and IFNγ secretion, and suggested strongly that following a single injection of agonist, NKT cells were anergic or hyporesponsive as observed in mice [9].
Figure 4. Cytokine profile following vaccines administration in healthy subjects.
Serum samples were collected prior to and 24 hours following each administration (on day 0 and day 28), and at the end of study (EOS, on day 56) for cytokines measurements (IL-1β, IL-2, IL-6, IL-10, IL-12p70, IFN-γ, GM-CSF, and TNF-α) in HBsAg (plain round), Heberbiovac HB® (plain square) and ABX196 + HBsAg (plain triangles). Data represent individual subject concentration (pg/ml) for IFN-γ (A), TNF-α (B) and IL-10 (C). TNFα was detected in 1/7 (14%), 2/8 (25%) and 5/29 (17%) in the HBsAg alone, Heberbiovac HB® and ABX196-receiving groups, respectively. IL-10 was detected in 2/7 (28%), 1/8 (12.5%) and 10/29 (34%) in the HBsAg alone, Heberbiovac HB®, and ABX196-receiving groups, respectively. Detectable levels of IFNγ were never observed in the subjects vaccinated with HBsAg alone or Heberbiovac HB®. All other cytokines were detected only sporadically or not at all (IL-1β and IL-2). For instance, GMCSF was detected in only one subject with no apparent correlation to vaccination, whilst IL12p70 was detected only in the subject that developed the most severe adverse event following the injection of 2.0µg ABX196, and IL-6 was detected one time following a second injection of 2.0µg ABX196.
Adverse events
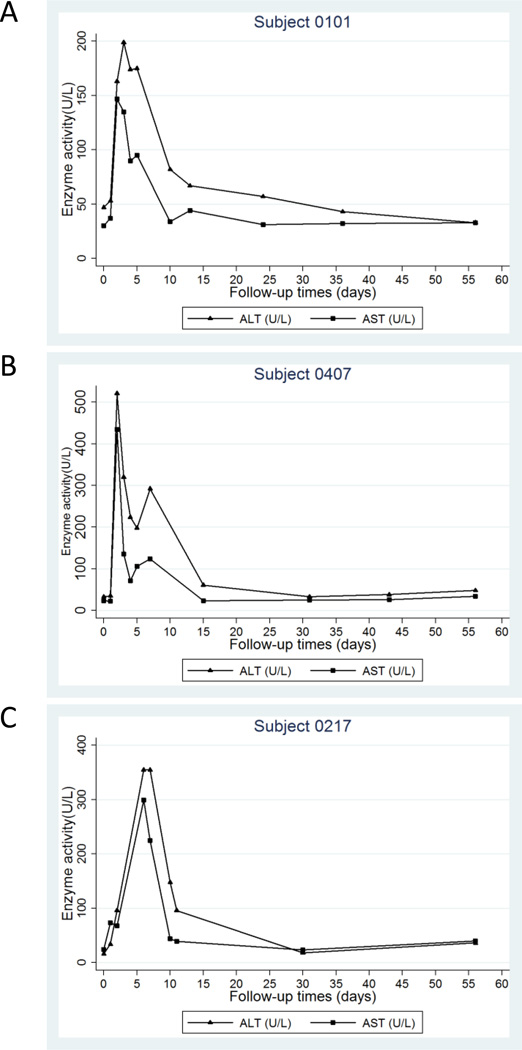
A total of 293 adverse events (AEs) were recorded and 251 of them could be associated with one of the injected ABX196 vaccines (Table 1). As anticipated most AEs were mild or moderate and included the most subjective manifestations such as headaches and asthenia. Three subjects receiving HBsAg in conjunction with ABX196 experienced severe AEs. In these three instances, systemic IFNγ (at level of 72, 302 and 2303 pg/ml respectively) was accompanied by a transitory increase in hepatic transaminases levels (AST and ALT) (Figure 5). None of the individuals of the HBsAg group, and only one from the Heberbiovac HB® group showed an elevation of ALT and AST at day 5 (46 and 53 UI/L, respectively (supplementary Figure 4). At that same sampling time, 33% (2/6) and 50% (3/6) subjects receiving HBsAg with 0.2µg and 0.4µg ABX196, respectively, showed a slight elevation of ALT while a slight elevation was noted at 24 hours post dose in 42% (5/12) subjects receiving HBsAg with 2µg ABX196 (supplementary Figure 5); all the subjects in the 0.2µg ABX196 group had enzymes level that remained below the three-fold range that would have triggered their exclusion from treatment (supplementary Table 3). To the opposite, two subjects from the 0.4µg ABX196 group and one from the 2.0µg ABX196 group had ALT levels that justified their withdrawal; these patients were the SAEs. In all three cases, the liver biology was altered only transiently and to moderate levels (Figure 5). In any case, it appears reasonable to associate IFNγ secretion, liver toxicity and dose of ABX196 with the occurrence of SAEs.
Table I.
Adverse Events
| HBsAg + ABX196 | ||||||
|---|---|---|---|---|---|---|
| HBsAg n = 7 |
Heberbiovac n = 8 |
HBsAg + ABX196 0.2 µg n = 6 |
HBsAg + ABX196 0.4 µg n = 11 |
HBsAg + ABX196 2 µg n = 12 |
||
| TEAE | Number | 24 | 18 | 26 | 115 | 110 |
| % Subjects | 85.7% | 87.5% | 66.7% | 100.0% | 100.0% | |
| Moderate and Severe TEAE | Number | 8 | 1 | 10 | 36 | 37 |
| % Subjects | 42.9% | 12.5% | 33.3% | 90.9% | 91.7% |
TEAE: Treatment Emergent Adverse Event
Figure 5. Liver enzymes in three subjects with SAE.
Serum ALT (plain triangles) and AST (plain squares) concentrations for subjects 0101 and 0407 (treated with ABX196 (0.4µg) + HBsAg (A and B), and 0217 (treated with ABX196 (2µg) (C). The participants were followed until normalization to the baseline values.
Antibody response
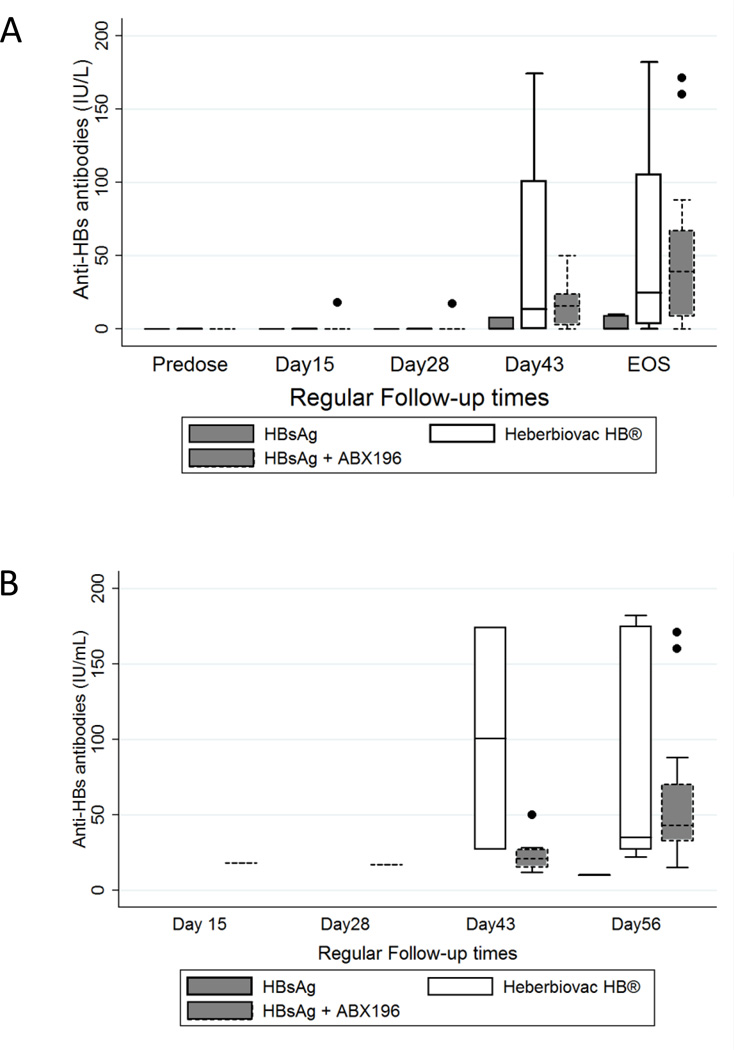
The success of hepatitis B vaccination is based on anti-HBsAg antibody titers at the end of immunization; a titer higher than 10 international units constitutes the hallmark of a successful protective response. As shown in Figure 6A, at the end of study 17% (1/6), 50% (3/6), and 79% (15/19) of individuals receiving HBsAg alone, Heberbiovac HB®, and HBsAg with ABX196 (all doses), respectively, developed protective immunity. In the Heberbiovac HB® and HBsAg alone groups, protective antibodies responses were not seen before the second injection (day 43), whereas titers of 18 IU/ml were seen at day 15 in patient 407 who received a single injection of HBsAg with 0.4µg ABX196 and developed an SAE, and titers of 17 IU/mL were seen at day 28 in one subject administered with HBsAg and 2.0µg ABX196. Amongst the four highest responders (titers >100IU/L), two were immunized with HBsAg and 2.0µg ABX196 (anti-HBsAg titers of 160IU and 171IU/L) and the other two with Heberbiovac HB® (anti-HBsAg titers of 175IU and 182IU/L). Figure 6B shows the antibodies titers in seroprotected subjects. It is important to also notice that five out of six subjects (83%) who received a single injection of HBsAg with 0.4µg ABX196 developed protective anti-HBsAg immunity, a number comparable to the subjects who received two doses of the same vaccine formulation (75%, 3 out of 4 subjects). Altogether, these results demonstrated the adjuvant effect of ABX196 in man for the development of protective anti-HBsAg immunity, and suggested that this effect was observed after a single injection of the vaccine when it combined the antigen and ABX196.
Figure 6. Anti-HBs antibody responses in clinical cohort.
(A) Blood samples were taken prior to each administration (pre-dose and on day 28 (pre dose2), on day 15, day 43 and at the end of study (EOS, on day 56). Data are shown for HBsAg (n= 7 subjects, solid line filled box), Heberbiovac HB® (n= 8 subjects, solid line empty box) and ABX196 + HBsAg (n=29 subjects, short dash filled box). Protective levels of antibodies responses (18 IU/mL) was noted from day 15 following the first administration in one subject in ABX196 at dose level 0.4 µg and from 28 following the first administration (17 IU/mL) in another subject in ABX196 at dose level 0.4 µg. The majority of the subjects developed protective antibody titers from day 43.
(B) Antibodies titers in seroprotected subjects vaccinated with HBsAg (short-dashed line); Heberbiovac HB® (solid line empty box) and ABX196 + HBsAg (solid line filled whisker box).
Post-clinical studies
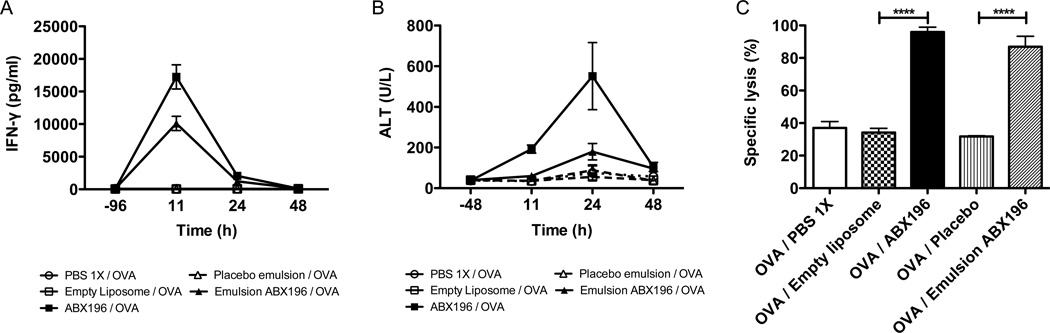
It is very obvious from this first clinical trial that the concept of using NKT cell agonists as adjuvants of immunity is validated. However, it is also obvious that serious side effects linked to the systemic delivery of the compound to the liver and the subsequent activation of the liver-resident NKT cells and production of IFNγ, need to be eliminated to allow the use of ABX196 or related molecules in the formulation of prophylactic vaccines. In order to do so, we have decided to explore new formulations that could alter the systemic delivery of ABX196 in mice. The validation of this approach is shown in Figure 7 where the classical liposomal formulation is compared to an emulsion containing ABX196, oil, solvent and/or surfactant. In this particular case, both IFN-γ secretion and the level of blood ALT are greatly diminished when ABX196 is formulated in an emulsion as compared to the liposomal delivery (Figure 7 A and B). However, the cytotoxic activity as assessed by VITAL assay showed that the emulsion formulation did not impact on the antigen, as OVA specific cytotoxic responses were induced following immunization with the emulsion formulation (Figure 7C). These data demonstrate that alternate formulations could modify the systemic effects of ABX196. Consequently, it is reasonable to assume that the potential liver toxicity of NKT agonists can be lessened by formulations that limit transport to the liver.
Figure 7. Formulation changes the toxicity of ABX196.
Mice (9 per group) received by I.M. injection, PBS (empty round), empty liposome (1µg) (empty square), emulsion placebo (empty placebo), ABX196 (1µg) formulated in liposome (plain square) or in emulsion (plain triangle). Sera were collected before (−96 or −48) and 11, 24 and 48 hours post injection for INF-γ (A) and ALT (B) measurements. Fourteen days later, mice received (I.V. injection) a mix of irrelevant peptide and SIINFEKL pulsed splenocytes differentially labelled with CFSE. The specific lysis was assessed in the blood 24h later by flow cytometry (C).
Discussion
Earlier studies have used NKT agonists in man in the context of advanced cancers for which traditional chemotherapies had failed. In all instances, series were very small and αGalcer, the only NKT agonist used, was either injected I.V. directly into the tumor [10] or loaded in autologous dendritic cells before reinfusion in patients [11–13]. Most of the patients receiving these experimental treatments had very compromised immune systems and low numbers of NKT cells. Consequently, the effects of the therapy, and the side effects, were difficult to appreciate in such heterogeneous groups. Our study is the first of its kind as it meant to evaluate the effects of a stronger agonist of NKT cells than αGalcer in healthy subjects and in the context of a prophylactic vaccine. As expected, the N KT cell agonist ABX196 demonstrated a measurable and significant adjuvant effect in mice and monkeys with no appreciable toxicity at the doses used to promote immune responses. The first surprise of the current clinical trial is the maximal effective dose of ABX196 in man as compared to mice and monkeys. Neither animal model was predictive of the maximal effective dose in human, highlighting the known limitations of pre-clinical studies in animals [14, 15]. This discrepancy highlights the differences of lipid composition and transport of human and animal sera. The precise molecular basis for this difference is currently unknown but lipoprotein abundance and distribution between omnivorous and rodent species should be explored first [16]. The cellular uptake and catabolism of NKT agonists could also be different between species [2, 17].
The side effects that we observed in the current study can reasonably be attributed to the transport of ABX196 to the liver and the subsequent activation of the liver NKT cells. This direct activation leads to the secretion of IFNγ by NKT cells and recruited NK cells [18] and hepatocyte damage due to the direct cytokines cytotoxicity. It is also reasonable to assume that if we can limit the systemic delivery of ABX196 to the liver, we should be capable of limiting IFNγ systemic production and side effects. This reasoning is based on experimental results in knockout mice in which glycolipid transport to the liver is limited by the absence of FAAH protein [19]. In this model, αGalcer transport to the liver is greatly compromised, leading to the absence of systemic IFNγ secretion but increased vaccinal responses [19]. It appears reasonable to envisage that formulation could achieve the same result of limiting serum transport and deleterious effects.
Based on the downregulation of TCR on their NKT cells after vaccination and the antibody response that was obtained, it is clear that ABX196 elicited a stimulation of NKT cells in vivo in most subjects. Therefore, the usage of ABX196 to stimulate NKT cells in vivo is validated. Because the activation and antibody responses appeared independent of the basal level of peripheral blood NKT ce lls, it suggests that the adjuvant effects that are seen with NKT agonists are linked to the lymph node-resident NKT cells and not to the circulating NKT cells. The compartmentalization of the NKT cell populations has been well documented in the mouse and is most likely important in man as well [20].
The most important conclusion of our study is that ABX196 is an adjuvant in human. Indeed, the addition of ABX196 to the very poorly immunogenic HBsAg resulted in vigorous anti-HBs antibody responses. The study was underpowered to conclude definitely whether ABX196 with the HBsAg was a superior vaccine than the commercial vaccine or not but the ABX196 groups converted to protective titers in 79% of the subjects whereas only 50% of the subjects receiving the commercial vaccine did. Finally, the current results also suggest that protective immune responses can be obtained after a single injection in a majority of subjects when ABX196 is associated to the antigen.
In conclusion, the adjuvanticity of NKT agonists was demonstrated in man for the vaccination against hepatitis B. The control and reduction of the systemic delivery of the glycolipid adjuvant to the liver by reformulation or new modes of administration will be needed for large scale use of NKT agonists in immunotherapy.
Supplementary Material
Acknowledgments
The authors would like to thank Marie-Louise Michel, Odile Launay and Pierre Vandepapelière for their pertinent advices during the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
V. Serra conceived the study J. Nitcheu Tefit contributed to acquisition, analysis and interpretation of data, and wrote the paper. S Crabe contributed to acquisition, analysis and interpretation of data. Bernard Orlandini contributed to study design and collection of data. Haylene Nell conducted the study. Shenglou Deng prepared the compounds. Paul Savage, Albert Bendelac, Luc Teyton designed the compound and Luc Teyton contributed to interpretation of data and manuscript writing. All authors have approved the final article.
Conflicts of Interest
The authors declare no conflict of interest.
Contributor Information
Josianne Nitcheu Tefit, Email: Josianne.nitcheu@abivax.com.
Sandrine Crabé, Email: sandrine.crabe@abivax.com.
Bernard Orlandini, Email: bernard.orlandini@phinc.fr.
Haylene Nell, Email: haylenenell@ttctrials.co.za.
Albert Bendelac, Email: abendela@bsd.uchicago.edu.
Shenglou Deng, Email: sdeng@chem.byu.edu.
Paul B. Savage, Email: paul_savage@byu.edu.
Luc Teyton, Email: lteyton@scripps.edu.
Vincent Serra, Email: vincent.serra@abivax.com.
REFERENCES
- 1.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nature immunology. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freigang S, Landais E, Zadorozhny V, Kain L, Yoshida K, Liu Y, et al. Scavenger receptors target glycolipids for natural killer T cell activation. The Journal of clinical investigation. 2012;122:3943–3954. doi: 10.1172/JCI62267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson V. Turned on by danger: activation of CD1d-restricted invariant natural killer T cells. Immunology. 2012;137:20–27. doi: 10.1111/j.1365-2567.2012.03612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhou XT, Forestier C, Goff RD, Li C, Teyton L, Bendelac A, et al. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6"-amino-6"-deoxy-galactosylceramides. Org Lett. 2002;4:1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Lalani S, Parekh VV, Wu L, Van Kaer L. Glycolipid ligands of invariant natural killer T cells as vaccine adjuvants. Expert Rev Vaccines. 2008;7:1519–1532. doi: 10.1586/14760584.7.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. The Journal of clinical investigation. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 11.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. The Journal of experimental medicine. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 13.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 14.Knight A. Animal experiments scrutinised: systematic reviews demonstrate poor human clinical and toxicological utility. ALTEX. 2007;24:320–325. doi: 10.14573/altex.2007.4.320. [DOI] [PubMed] [Google Scholar]
- 15.Greek R, Menache A. Systematic reviews of animal models: methodology versus epistemology. Int J Med Sci. 2013;10:206–221. doi: 10.7150/ijms.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin W, Carballo-Jane E, McLaren DG, Mendoza VH, Gagen K, Geoghagen NS, et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. 2012;53:51–65. doi: 10.1194/jlr.M019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freigang S, Kain L, Teyton L. Transport and uptake of immunogenic lipids. Mol Immunol. 2013;55:179–181. doi: 10.1016/j.molimm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. Journal of immunology. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 19.Freigang S, Zadorozhny V, McKinney MK, Krebs P, Herro R, Pawlak J, et al. Fatty acid amide hydrolase shapes NKT cell responses by influencing the serum transport of lipid antigen in mice. The Journal of clinical investigation. 2010;120:1873–1884. doi: 10.1172/JCI40451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. The Journal of experimental medicine. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.