Abstract
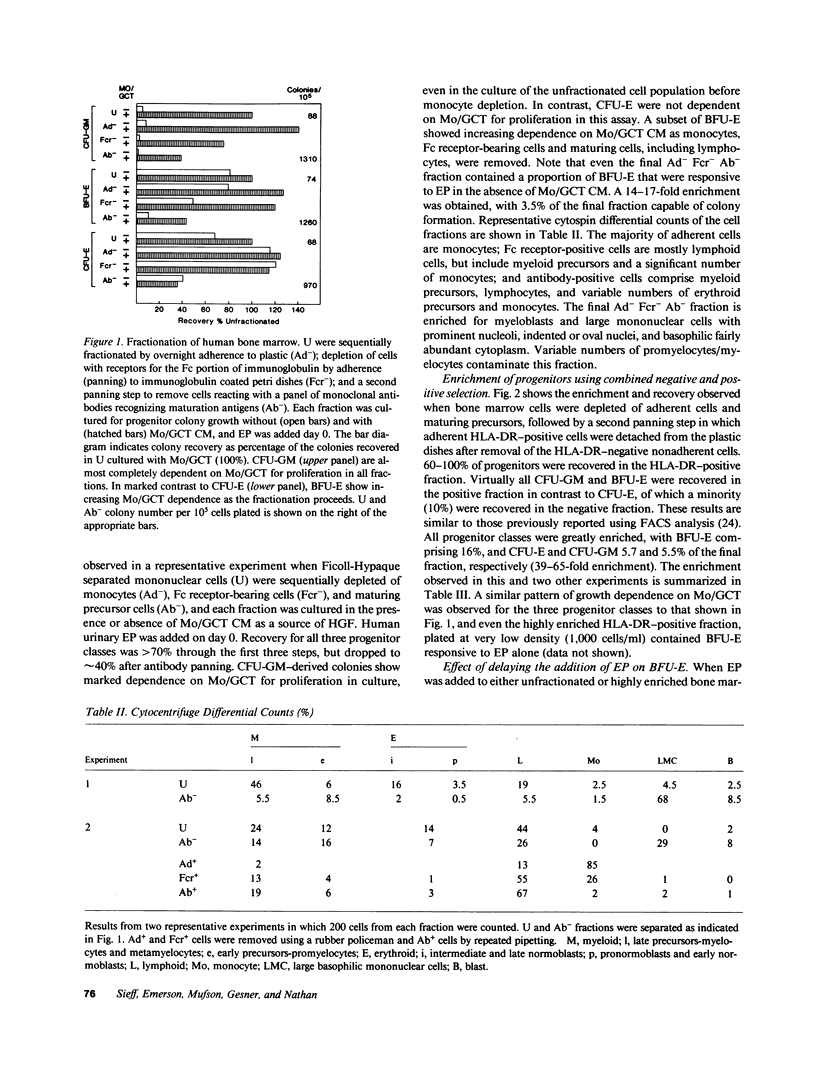
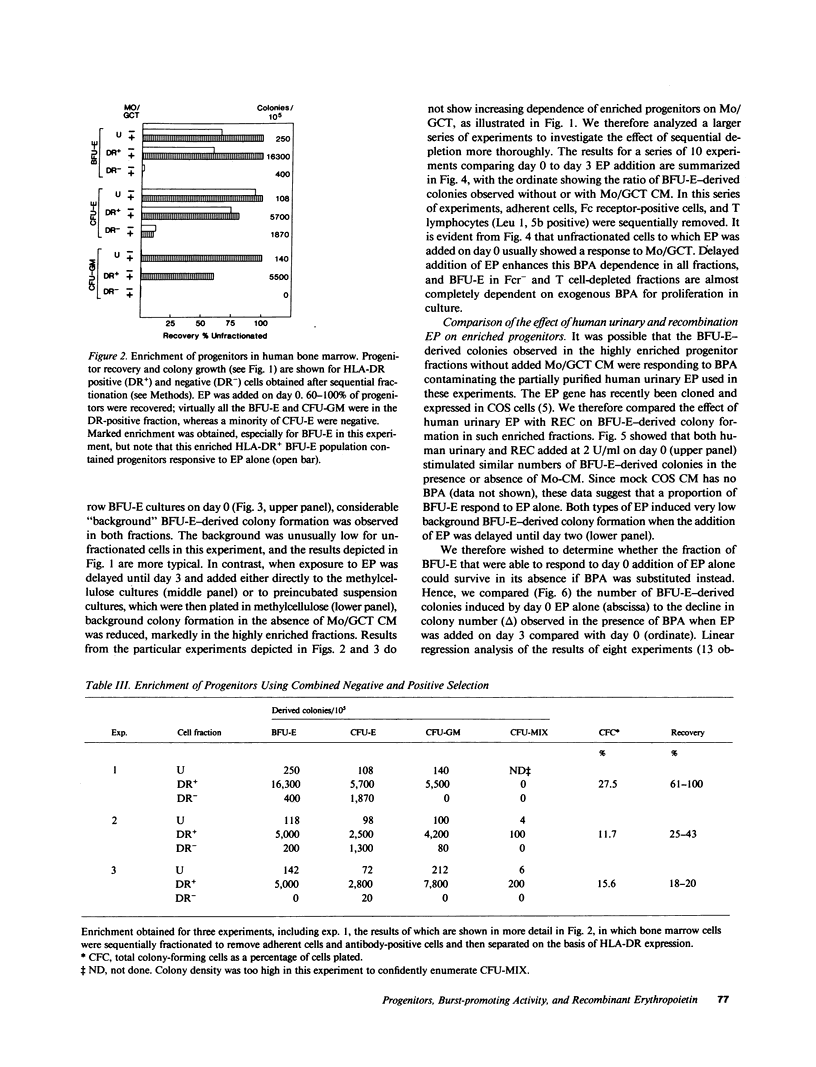
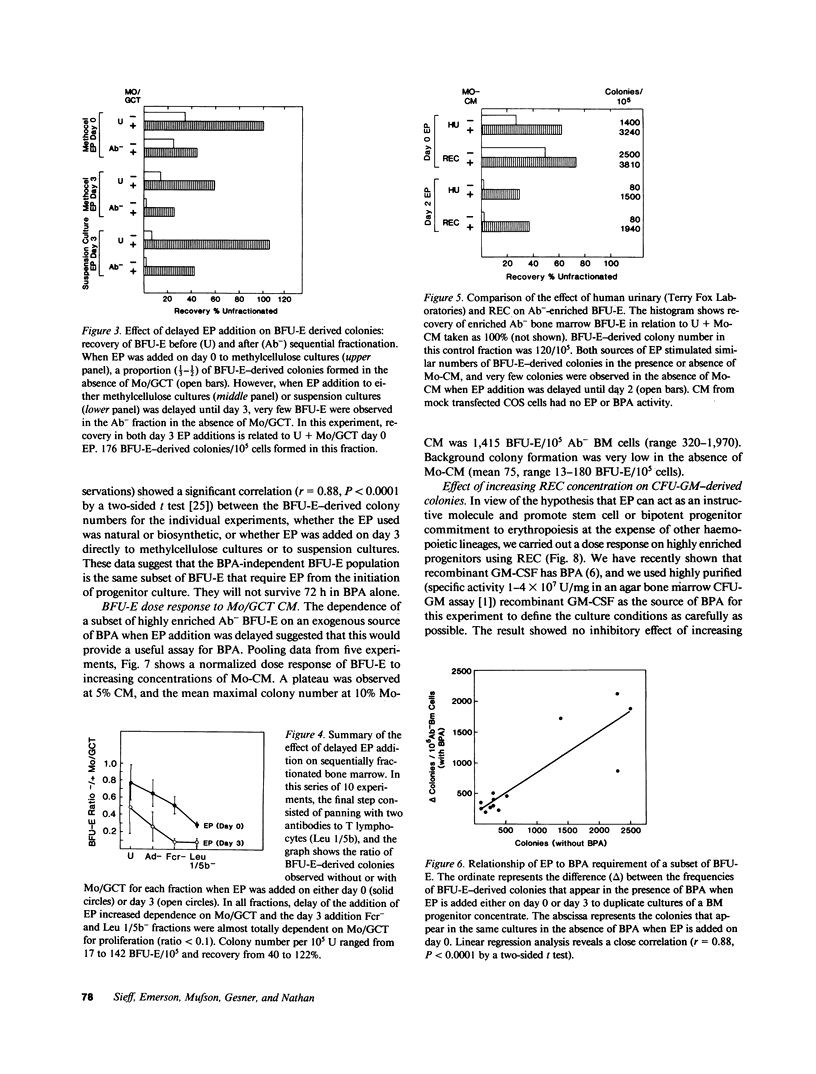
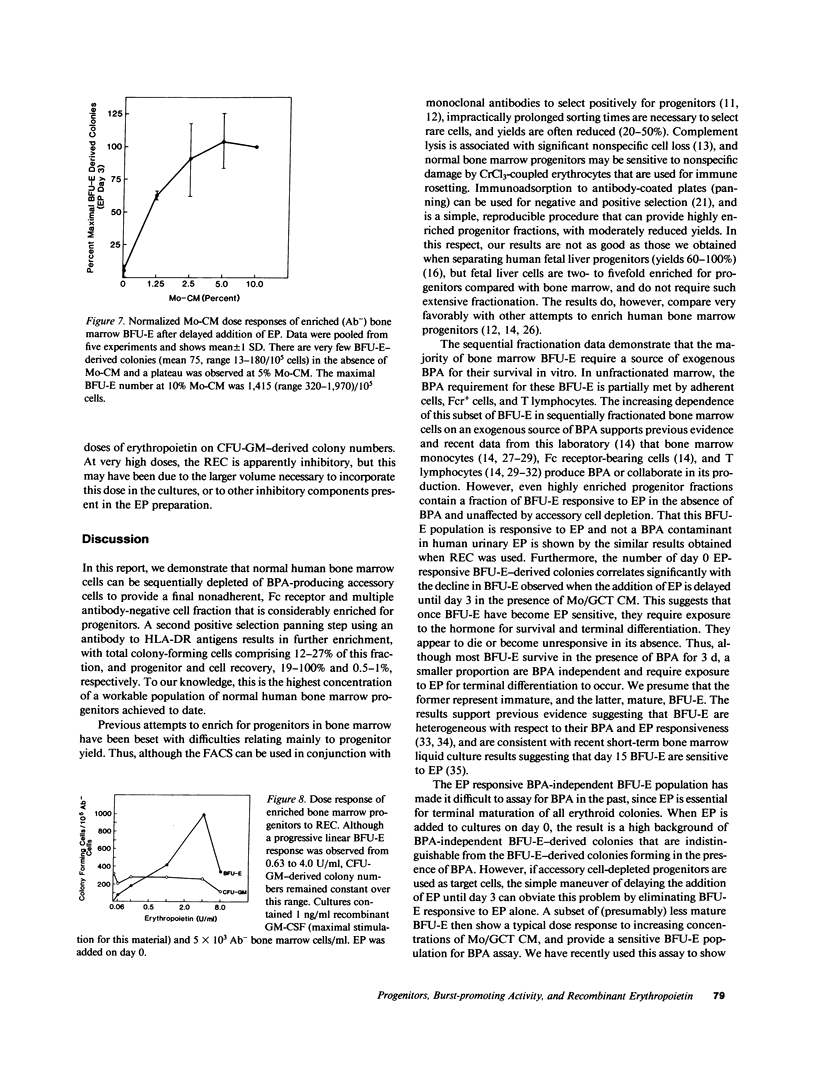
Human bone marrow cells were sequentially fractionated by three negative selection steps to remove adherent cells and Fc receptor-bearing cells, followed by immune adsorption (panning) to deplete maturing cells that react with a panel of monoclonal antibodies. This nonadherent Fc receptor and antibody negative fraction could be further enriched by a positive selection "panning" step, using an antibody to HLA-DR antigen; 12-27% of the cells formed erythroid burst-forming unit (BFU-E), erythroid colony-forming unit, granulocyte-monocyte colony-forming unit, and erythroid and granulocyte and/or monocyte colony-forming unit-derived colonies with recovery of 0.5-1% of the cells and 20-100% of the colony-forming cells. Sequential fractionation resulted in increasing dependence of a subset of BFU-E-derived colonies on exogenous burst-promoting activity (BPA) for proliferation in culture, but the most enriched progenitor fraction still contained a proportion of accessory cell or BPA-independent BFU-E that responded to either natural or biosynthetic erythropoietin when added to cultures on day 0 in the absence of BPA. If the addition of erythropoietin was delayed until day 3, the data suggest that this population of BFU-E either died or became unresponsive to erythropoietin. Delayed addition of erythropoietin to cultures of enriched progenitors provided a sensitive BPA assay, since BPA-independent but erythropoietin-responsive BFU-E were eliminated. The surviving BFU-E that were dependent for their proliferation on the presence of both BPA and erythropoietin showed a characteristic dose response to increasing BPA concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Dessypris E. N., Krantz S. B. Effect of pure erythropoietin on DNA-synthesis by human marrow day 15 erythroid burst forming units in short-term liquid culture. Br J Haematol. 1984 Feb;56(2):295–306. doi: 10.1111/j.1365-2141.1984.tb03957.x. [DOI] [PubMed] [Google Scholar]
- DiPersio J. F., Brennan J. K., Lichtman M. A., Abboud C. N., Kirkpatrick F. H. The fractionation, characterization, and subcellular localization of colony-stimulating activities released by the human monocyte-like cell line, GCT. Blood. 1980 Oct;56(4):717–727. [PubMed] [Google Scholar]
- Eaves C. J., Eaves A. C. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978 Dec;52(6):1196–1210. [PubMed] [Google Scholar]
- Edwards P. A. Monoclonal antibodies that bind to the human erythrocyte-membrane glycoproteins glycophorin A and Band 3 [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):334–335. doi: 10.1042/bst0080334. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Sieff C. A., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Purification of fetal hematopoietic progenitors and demonstration of recombinant multipotential colony-stimulating activity. J Clin Invest. 1985 Sep;76(3):1286–1290. doi: 10.1172/JCI112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Golde D. W., Kaufman S. E., Westbrook C. A., Hewick R. M., Kaufman R. J., Wong G. G., Temple P. A., Leary A. C., Brown E. L. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. 1985 Jun 27-Jul 3Nature. 315(6022):768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. I., Miller W. J., Branda R. F., Zanjani E. D., Jacob H. S. Regulation of erythroid colony formation by bone marrow macrophages. Blood. 1980 Jun;55(6):1047–1050. [PubMed] [Google Scholar]
- Greenberg P. L., Baker S., Link M., Minowada J. Immunologic selection of hemopoietic precursor cells utilizing antibody-mediated plate binding ("panning"). Blood. 1985 Jan;65(1):190–197. [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G., Bright S., Chumbley G., Hoang T., Metcalf D., Munro A. J., Waldmann H. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood. 1983 Oct;62(4):873–882. [PubMed] [Google Scholar]
- Hoang T., Gilmore D., Metcalf D., Cobbold S., Watt S., Clark M., Furth M., Waldmann H. Separation of hemopoietic cells from adult mouse marrow by use of monoclonal antibodies. Blood. 1983 Mar;61(3):580–588. [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Lipton J. M., Nathan D. G. Identification of three accessory cell populations in human bone marrow with erythroid burst-promoting properties. J Clin Invest. 1985 Apr;75(4):1278–1284. doi: 10.1172/JCI111827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton J. M., Kudisch M., Nathan D. G. Response of three classes of human erythroid progenitors to the absence of erythropoietin in vitro as a measure of progenitor maturity. Exp Hematol. 1981 Nov;9(10):1035–1041. [PubMed] [Google Scholar]
- Mangan K. F., Chikkappa G., Bieler L. Z., Scharfman W. B., Parkinson D. R. Regulation of human blood erythroid burst-forming unit (BFU-E) proliferation by T-lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. Blood. 1982 May;59(5):990–996. [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Moore M. A., Williams N., Metcalf D. Purification and characterisation of the in vitro colony forming cell in monkey hemopoietic tissue. J Cell Physiol. 1972 Apr;79(2):283–292. doi: 10.1002/jcp.1040790213. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Nicola N. A., Metcalf D. Purification of hemopoietic progenitor cells from human marrow using a fucose-binding lectin and cell sorting. Blood. 1980 Nov;56(5):798–805. [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., von Melchner H., Burgess A. W. Isolation of murine fetal hemopoietic progenitor cells and selective fractionation of various erythroid precursors. Blood. 1981 Aug;58(2):376–386. [PubMed] [Google Scholar]
- Nijhof W., Wierenga P. K. Isolation and characterization of the erythroid progenitor cell: CFU-E. J Cell Biol. 1983 Feb;96(2):386–392. doi: 10.1083/jcb.96.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid C. D., Baptista L. C., Chanarin I. Erythroid colony growth in vitro from human peripheral blood null cells: evidence for regulation by T-lymphocytes and monocytes. Br J Haematol. 1981 May;48(1):155–164. doi: 10.1111/j.1365-2141.1981.00155.x. [DOI] [PubMed] [Google Scholar]
- Robinson J., Sieff C., Delia D., Edwards P. A., Greaves M. Expression of cell-surface HLA-DR, HLA-ABC and glycophorin during erythroid differentiation. Nature. 1981 Jan 1;289(5793):68–71. doi: 10.1038/289068a0. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Emerson S. G., Donahue R. E., Nathan D. G., Wang E. A., Wong G. G., Clark S. C. Human recombinant granulocyte-macrophage colony-stimulating factor: a multilineage hematopoietin. Science. 1985 Dec 6;230(4730):1171–1173. doi: 10.1126/science.3877981. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Torok-Storb B., Martin P. J., Hansen J. A. Regulation of in vitro erythropoiesis by normal T cells: evidence for two T-cell subsets with opposing function. Blood. 1981 Jul;58(1):171–174. [PubMed] [Google Scholar]
- Van Zant G., Goldwasser E. Competition between erythropoietin and colony-stimulating factor for target cells in mouse marrow. Blood. 1979 May;53(5):946–965. [PubMed] [Google Scholar]
- Van Zant G., Goldwasser E. Simultaneous effects of erythropoietin and colony-stimulating factor on bone marrow cells. Science. 1977 Nov 18;198(4318):733–735. doi: 10.1126/science.302986. [DOI] [PubMed] [Google Scholar]
- Westbrook C. A., Gasson J. C., Gerber S. E., Selsted M. E., Golde D. W. Purification and characterization of human T-lymphocyte-derived erythroid-potentiating activity. J Biol Chem. 1984 Aug 25;259(16):9992–9996. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells from cells forming colonies in culture. J Cell Physiol. 1969 Oct;74(2):171–182. doi: 10.1002/jcp.1040740209. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S. Human erythroid burst-forming units. Growth in vitro is dependent on monocytes, but not T lymphocytes. J Clin Invest. 1981 Mar;67(3):702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]



