Abstract
Background
Parenteral opioids are used for pain relief in labour in many countries throughout the world.
Objectives
To assess the acceptability, effectiveness and safety of different types, doses and modes of administration of parenteral opioids given to women in labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 April 2011) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials examining the use of intramuscular or intravenous opioids (including patient controlled analgesia) for women in labour. We looked at studies comparing an opioid with another opioid, placebo, other non-pharmacological interventions (TENS) or inhaled analgesia.
Data collection and analysis
At least two review authors independently assessed study eligibility, collected data and assessed risk of bias.
Main results
We included 57 studies involving more than 7000 women that compared an opioid with placebo, another opioid administered intramuscularly or intravenously or compared with TENS to the back. The 57 studies reported on 29 different comparisons, and for many outcomes only one study contributed data. Overall, the evidence was of poor quality regarding the analgesic effect of opioids, satisfaction with analgesia, adverse effects and harm to women and babies. There were few statistically significant results. Many of the studies had small sample sizes, and low statistical power. Overall findings indicated that parenteral opioids provided some pain relief and moderate satisfaction with analgesia in labour, although up to two-thirds of women who received opioids reported moderate or severe pain and/or poor or moderate pain relief one or two hours after administration. Opioid drugs were associated with maternal nausea, vomiting and drowsiness, although different opioid drugs were associated with different adverse effects. There was no clear evidence of adverse effects of opioids on the newborn. We did not have sufficient evidence to assess which opioid drug provided the best pain relief with the least adverse effects.
Authors’ conclusions
Parenteral opioids provide some relief from pain in labour but are associated with adverse effects. Maternal satisfaction with opioid analgesia was largely unreported but appeared moderate at best. This review needs to be examined alongside related Cochrane reviews examining pain management in labour. More research is needed to determine which analgesic intervention is most effective, and provides greatest satisfaction to women with acceptable adverse effects for mothers and their newborn.
Medical Subject Headings (MeSH): Analgesia, Obstetrical [*methods]; Analgesics, Opioid [*administration & dosage; adverse effects]; Injections, Intramuscular; Injections, Intravenous; Labor Pain [*drug therapy]; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
This review is one in a series of Cochrane reviews examining pain management in labour. These reviews contribute to an overview of systematic reviews of pain management for women in labour (Jones 2011b) and share a generic protocol (Jones 2011a).
Description of the condition
Pain during labour is normal, being one of the few examples of pain which does not signal pathology or harm. This does not make the experience of pain any less, but it may alter the way pain is perceived, both by the woman in labour and those providing care.
Characteristics of labour pain
Pain during labour is intermittent, accompanying uterine contractions (Findley 1999). Characteristically the pain intensifies as the contraction increases, reaching a peak when the contraction is at its strongest, then diminishing as the uterus relaxes. Between contractions the uterus is at rest and there is usually no associated pain. As labour progresses the uterine contractions grow stronger, more frequent and longer lasting; at the same time they become more painful. Typically the strongest, most frequent, and most painful, uterine contractions occur at the end of the first stage of labour as the cervix reaches full dilatation. While the vast majority of women will describe at least some stages of labour as painful, the severity of reported pain varies considerably (Findley 1999).
Pain relief in labour - physiology and pain perceptions
Labour pain as perceived by women is a unique, subjective and complex neuro-hormonal phenomenon, which involves the interaction of physiological and psychological factors (Genesi 1998a; Genesi 1998b; Trout 2004). Several factors have been shown to reduce pain experienced by women in labour. These include continuous support of a caregiver, attendance of a birth companion and a relaxed birth environment (Hodnett 2002). Two other key determinants that may influence the pain level that a woman experiences are feeling in control of her behaviour, and the care she receives. The extent to which a woman can actively participate in negotiating the care she receives has also been linked to overall maternal satisfaction with the childbirth experience (Green 2003; Hodnett 2002). The degree to which a woman is satisfied with the birth experience is not, therefore, solely associated with the pain felt. Having more control will foster a woman’s sense of self-belief and confidence in her capacity to labour and give birth, which will also affect her pain perception (Lowe 1993; Lowe 1996). From the clinical point of view, the management of pain during labour involves much more than simply the provision of a pharmacological intervention. Related Cochrane reviews have demonstrated the value of continuous support, midwifery models and non-pharmacological approaches to managing pain in labour (Barragán 2011; Cluett 2009; Dowswell 2009; Hatem 2008; Hodnett 2007; Hunter 2007).
A caregiver’s perception of a woman’s labour pain may be different from what the woman is actually experiencing (Callister 1995). A large UK survey that collected maternal and midwifery assessments of pain relief found that midwives rated pethidine more positively than the women who received it (Chamberlain 1993). Practitioners’ attitudes to maternal pain vary (Leap 2004), wherein some adopt a rescue position to relieve the pain and recommend the use of analgesia, whilst others facilitate the woman to optimise coping mechanisms, using strategies involving breathing and/or relaxation techniques and positions that offer her more comfort. Women’s attitudes towards, and preferences for, intrapartum pain relief vary widely. Whilst some women prefer to labour without the use of pharmacological analgesia, others opt, for example, to use epidural analgesia throughout labour. Good communication and sensitive support from caregivers improves a woman’s experience of labour, and her overall satisfaction with care, regardless of her choice of pain relief or levels of reported pain (Hodnett 2002). It is important that decisions for coping with the pain of labour are based on informed choice (Green 2003; Hawkins 2003).
Pain relief in labour - the use of opioids
The use of pain-relieving drugs during labour is now standard care in many countries throughout the world (Findley 1999; Reynolds 2000). The extent of usage of parenteral opioids during labour is unclear; however, most obstetric units in developed countries offer intramuscular opioids, along with facilities for epidural analgesia. Opioids are relatively inexpensive, and use of the opioid drugs pethidine, meptazinol or diamorphine during labour is common midwifery and obstetric practice in some countries. In other parts of the world, parenteral opioids commonly used in labour include morphine, nalbuphine, fentanyl and more recently remifentanil (Evron 2007). Worldwide, pethidine is the most commonly used opioid (Bricker 2002). In the UK, a midwife can take responsibility for giving a woman an intramuscular injection of either pethidine or diamorphine, without a prescription from a medical practitioner, whether she is working in the hospital or community care setting (MHRA 2007).
In the UK, estimates for opioid use showed that 34% of women overall used pethidine or another opioid during labour, with variation between NHS Hospital Trusts between 5% and 66% (Healthcare Commission 2007). A survey of 4800 women reported that 32.9% used pethidine or another opioid, and 10.5% of these women also had an epidural (Redshaw 2007). The use of an opioid varied by parity, with more nulliparae reporting use (with or without an epidural) compared with multiparous women. Use of pethidine in the UK has declined from 42% in 1995 to 33% in 2006, yet the proportion of women who received an epidural has changed little over this time period: 27% in 1995 and 28% in 2006 (Redshaw 2007). In the USA, 39% to 56% of women received an opioid during labour (Hawkins 1999). Studies in New Zealand and the UK have revealed that more than 95% of hospitals surveyed routinely offered intramuscular pethidine (Lee 2004; Saravanakumar 2007). In the UK study, approximately half (49%) of the units surveyed offered patient-controlled intravenous opioid analgesia for use in labour (Saravanakumar 2007).
Some maternity practitioners have voiced concerns about the use of parenteral opioid analgesia during labour. These centre on doubt about analgesic effectiveness, and anxiety about the sedative effects on women and babies. Concerns relating to maternal outcomes include an impaired capacity to engage in decision making about care, nausea and/or vomiting, and the slowing down of gastric emptying, which increases the risk of inhalation of gastric contents should a general anaesthetic be required in an emergency situation. If a woman feels drowsy or sedated, she is less likely to mobilise and adopt an upright position, and as a result this may lengthen her labour, and make it more painful (Lawrence 2009).
Effects on the baby
Opioids readily cross the placenta by passive diffusion. It is estimated that it can take a newborn three to six days to eliminate pethidine, and its metabolite, norpethidine, from its system (Hogg 1977). Pethidine has been shown to significantly affect fetal heart rate variability, accelerations and decelerations during labour (Sekhavat 2009; Solt 2002). Changes in normal fetal heart indices have consequences for the woman. She will be required to have electronic fetal heart rate monitoring (EFM) if she is in hospital, and transfer to hospital if she is in a community setting. Results from observational studies have reported effects of opioids on the newborn that include inhibited sucking at the breast and decreased alertness, resulting in delayed effective breastfeeding (Nissen 1995; Ransjo-Arvidson 2001; Righard 1990).
Why it is important to do this review
This review evaluates effects of parenteral opioids for analgesia in labour. The use of intramuscular injection of opioid analgesia in labour became a traditional part of midwifery practice without evidence from randomised controlled trials for its analgesic effectiveness, impact on labour outcomes or acceptability to women. It is thought its perceived analgesic efficacy may be due, at least in part, to its sedative effects rather than a true reduction in perceived pain (NICE 2007). There remains uncertainty amongst practitioners as to which opioid provides the most effective pain relief, and whether opioids used during labour are acceptable to women. The most effective and acceptable mode of administration also remains unknown. In addition, there are concerns about the potential adverse effects associated with the use of opioids in labour, particularly the effects on the newborn in relation to infant feeding.
At present, the choice of opioid for analgesia in labour depends on what is available in different hospitals. However, no matter what facilities and drugs are available, women often have no choice as to which drug is used, and healthcare professionals have little information to guide decision-making. Whilst there have been previous reviews on this topic (Bricker 2002; Elbourne 2006) this review provides an up-to-date summary of existing knowledge. We aim to provide best evidence to facilitate discussions between maternity practitioners and women to enable them to make informed decisions about their choice of analgesia during labour.
OBJECTIVES
To assess the effectiveness, safety and acceptability to women of different types, doses and modes of administration of parenteral opioid analgesia in labour. A second objective is to assess the effects of opioids in labour on the baby in terms of safety, condition at birth and early feeding.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. We did not include quasi-randomised trials. We included studies presented only in abstracts provided that there was enough information to allow us to assess eligibility and risk of bias; if there was insufficient information we attempted to contact study authors.
Types of participants
Women in labour. We have excluded studies focusing specifically and exclusively on women in high-risk groups, or women in premature labour (before 37 weeks’ gestation), but have included studies which include such women as part of a broader sample.
Types of interventions
Parenteral opioids (intramuscular and intravenous drugs, including patient controlled analgesia).
Drugs for comparison include pethidine or meperidine, nalbuphine, butorphanol, diamorphine, buprenorphine, meptazinol, pentazocine, tramadol, alfentanil, sufentanil, remifentanil and fentanyl.
The following comparisons were eligible for the review.
An opioid versus placebo using the same route of administration.
An opioid versus another opioid using the same route of administration.
An opioid plus an add-on drug versus another opioid plus the same add-on drug using the same route of administration.
One opioid versus the same opioid but a different dose.
We planned to use trialists’ definitions of higher and lower doses of the same drugs, as high and low doses are different for different opioids.
Where different doses of the same drug were compared with the same comparator (e.g. 40 mg pethidine versus placebo, and 80 mg pethidine versus placebo), we planned to use subgroup analyses to examine findings.
This review is one in a series of Cochrane reviews examining pain management in labour. These reviews contribute to an overview of systematic reviews of interventions for pain management in labour (Jones 2011b), and share a generic protocol (Jones 2011a). To avoid duplication, the different methods of pain management have been listed in a specific order, from one to 15. Individual reviews focusing on particular interventions include comparisons with only the intervention above it on the list. Methods of pain management identified in the future will be added to the end of the list. The current list is as follows.
Placebo/no treatment
Hypnosis
Biofeedback (Barragán 2011)
Intracutaneous or subcutaneous sterile water injection (Derry 2011)
Immersion in water (Cluett 2009)
Aromatherapy (Smith 2011b)
Relaxation techniques (yoga, music, audio)
Acupuncture or acupressure (Smith 2011a)
Manual methods (massage, reflexology)
Transcutaneous electrical nerve stimulation (TENS) (Dowswell 2009)
Inhaled analgesia
Opioids (this review)
Non-opioid drugs (Othman 2011)
Local anaesthetic nerve blocks (Novikova 2011)
Epidural (including combined spinal epidural) (Anim-Somuah 2005; Simmons 2007)
Accordingly, this review includes comparisons of an opioid with: 1. placebo/no treatment; 2. hypnosis; 3. biofeedback; 4. intracutaneous or subcutaneous sterile water injection; 5. immersion in water; 6. aromatherapy; 7. relaxation techniques (yoga, music, audio); 8. acupuncture or acupressure; 9. manual methods (massage, reflexology); 10. TENS; 11. inhaled analgesia; or 12. another opioid (as specified above).
Types of outcome measures
Primary outcomes
Maternal satisfaction with analgesia measured during labour.
Maternal satisfaction with analgesia in labour measured during the postnatal period.
Secondary outcomes
For women
Maternal pain score or pain measured in labour.
Additional analgesia required: epidural.
Maternal sleepiness during labour.
Nausea and vomiting in labour.
Caesarean section.
Assisted vaginal birth.
Postpartum haemorrhage (as defined by the trial authors).
Breastfeeding at discharge.
Breastfeeding in the postnatal period (four to six weeks).
Sense of control in labour (as defined by trialists).
Satisfaction with childbirth experience (as defined by trialists).
Effect (negative) on mother/baby interaction.
For babies
Fetal heart rate changes in labour (persistent decelerations or tachycardia).
Naloxone administration.
Neonatal resuscitation.
Apgar score less than seven at one minute.
Apgar score less than seven at five minutes.
Apgar score less than seven at ten minutes.
Admission to special care baby unit/neonatal intensive care unit (as defined by trialists).
Newborn neuro-behavioural scores.
Neurodevelopment outcomes during infancy.
Other
Cost (as defined by trialists).
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 April 2011).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of background review articles and the reference lists of papers retrieved by the search described above. We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Four review authors (R Ullman, T Dowswell, L Smith, E Burns) independently assessed for inclusion all the studies identified as a result of the search strategy. Two authors assessed each report and we resolved any disagreement through discussion or, if required, we consulted a third author.
Data extraction and management
We designed a form to collect data. For each report, two review authors independently collected the data using the agreed form (all review authors were involved in data collection). We resolved discrepancies through discussion or, if required, we consulted a third author. We entered data into Review Manager software (RevMan 2011) and checked them for accuracy.
When information in trial reports was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each included study using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and outlined below. We resolved any disagreement by discussion, or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non-random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated” analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias, for example was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data-dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We aimed to explore the impact of the level of bias through undertaking sensitivity analyses - see Sensitivity analysis.
Measures of treatment effect
We carried out statistical analysis using Review Manager software (RevMan 2011). We used fixed-effect meta-analysis for combining data where trials examined the same intervention, and where we judged the trials’ populations and methods to be sufficiently similar. Where we suspected clinical or statistical heterogeneity between studies, sufficient to suggest that treatment effects might differ between trials, we carried out random-effects meta-analysis.
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way (e.g. using the same pain scale) between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different scales.
Unit of analysis issues
Cluster-randomised trials
We intended to include cluster-randomised trials in the analyses along with individually randomised trials using the methods described in the Handbook (Higgins 2011). Their sample sizes would be adjusted using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from another source. If we used ICCs from other sources, we would report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster-randomised trials and individually randomised trials, we planned to synthesise the relevant information. We would consider it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Crossover trials
We did not include crossover trials.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data (more than 10% for outcomes where data were collected in labour) in the overall assessment of treatment effect by using sensitivity analyses.
Where data were not reported for some outcomes or groups, we attempted to contact the study authors for further information. We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we attempted to restore them to the correct group.
Assessment of heterogeneity
We examined heterogeneity between trials using the T2 and I2 statistics. If we identified heterogeneity among the trials, we planned to explore it by prespecified subgroup analysis provided data were available to do this, and by performing sensitivity analysis. Where we thought that an average treatment effect was clinically meaningful, we used a random-effects model for meta-analysis in the presence of moderate or high levels of heterogeneity (I2 greater than 30%), and for these outcomes we have reported I2, T2, the P value for the Chi2 test for heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see ’Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data. We were not able to explore possible publication bias by using funnel plots, as too few studies were included in each comparison.
Subgroup analysis and investigation of heterogeneity
We intended to conduct planned subgroup analysis using the methods described by Deeks 2001 and set out in the Cochrane Handbook for Systematic Reviews (Higgins 2011).
We had planned to carry out the following subgroup analyses.
By parity (nulliparous versus multiparous women).
By spontaneous versus induced or augmented labour.
Term versus preterm birth.
Continuous support in labour versus no continuous support.
Where different doses of the same drug were examined (e.g. pethidine 40 mg or pethidine 80 mg versus a placebo), we separated analyses into subgroups to examine the impact of different doses. For fixed-effect and random-effects meta-analyses we planned to assess differences between subgroups by inspection of the subgroups’ confidence intervals: non-overlapping confidence intervals indicating a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality for important outcomes in the review. Where there was risk of bias associated with a particular aspect of study quality (e.g. inadequate allocation concealment), we have explored this by sensitivity analyses.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Using the search strategy, in an earlier version of this review, we identified 165 papers representing 138 studies. For this updated version we identified a further five studies (Castro 2004; El-Kerdawy 2010; Solek-Pastuszka 2009; Tawfik 1982; Thakur 2004) and an additional published report for a study which was ongoing when the earlier review was completed (Douma 2010). Following the updated search we excluded three of these studies (Castro 2004; El-Kerdawy 2010; Solek-Pastuszka 2009) and included three (Douma 2010; Tawfik 1982; Thakur 2004). In this update we have included 57 studies and excluded 86.
Included studies
We included 57 studies involving more than 7000 women (see Characteristics of included studies).
Most of the studies included in the review examined an opioid drug administered intramuscularly (IM) and compared either an opioid with placebo, or with another opioid. A smaller number of studies examined opioid drugs administered intravenously (IV), sometimes with a degree of patient control over the amount of drug infused (PCA). None of the included studies examined subcutaneous administration of opioids. Some of the studies compared opioids with other non-pharmacological interventions such as TENS (three studies). Trials with more than two arms may be included in more than one comparison.
IM comparisons
IM pethidine versus IM placebo (three studies) (Kamyabi 2003; Sliom 1970; Tsui 2004).
IM meptazinol versus IM pethidine (eight studies) (De Boer 1987; Jackson 1983; Morrison 1987; Nel 1981; Nicholas 1982; Osler 1987; Sheikh 1986; Wheble 1988). (In the studies by De Boer 1987 and Jackson 1983, women in both study groups also received add-on drugs.).
IM meptazinol versus IM pethidine PCA administration (one study) (Li 1988).
IM diamorphine versus IM pethidine (both groups had prochlorperazine) (Fairlie 1999).
IM tramadol versus IM pethidine (seven studies) (Bitsch 1980; Fieni 2000; Husslein 1987; Keskin 2003; Khooshideh 2009; Prasertsawat 1986; Viegas 1993).
In an additional study comparing tramadol with pethidine, both groups also had triflupromazine (Kainz 1992).
IM dihydrocodeine versus IM pethidine (Sliom 1970).
IM pentazocine versus IM pethidine (six studies) (Borglin 1971; Duncan 1969; Levy 1971; Moore 1970; Mowat 1970; Refstad 1980).
IM Pentazocine + promazine versus IM pethidine + promazine (Refstad 1980).
IM nalbuphine versus IM pethidine (four studies) (Lardizabal 1999; Lisboa 1997; Mitterschiffthaler 1991; Wilson 1986).
IM phenazocine versus IM pethidine (Grant 1970).
IM morphine versus pethidine (one study) (Prasertsawat 1986).
IM butorphanol versus IM pethidine (Maduska 1978).
IM tramadol versus no treatment (one study) (Li 1994).
One study compared a spasmolytic drug (Avacan ®) with IM pentazocine (Hamann 1972).
IM pentazocine versus IM Pethilorphan® (O’Dwyer 1971).
We were unable to include data from the following comparisons because of a lack of information in the reports of the studies. IM buprenorphine versus IM pethidine (Tharamas 1999).
A four-arm trial by Wahab 1988 compared nalbuphine, butorphanol, pentazocine and a placebo.
IV comparisons
-
17
IV fentanyl versus IV pethidine (one study) (Rayburn 1989).
-
18
IV nalbuphine versus IV pethidine (one study) (Giannina 1995).
-
19
IV phenazocine versus IV pethidine (one study) (Olson 1964).
-
20
IV butorphanol versus IV pethidine (three studies) (Hodgkinson 1979; Nelson 2005; Quilligan 1980).
-
21
IV morphine versus IV pethidine (two studies) (Campbell 1961; Olofsson 1996).
-
22
IV alphaprodine (nisentil) versus IV pethidine (one study) (Gillam 1958).
-
23
IV fentanyl versus butorphanol (one study) (Atkinson 1994).
IV pethidine versus no treatment (one study) (Neumark 1978). (We were unable to use data from this study for this comparison in the review. See Characteristics of included studies tables.)
IV/PCA comparisons
-
24
PCA pentazocine versus PCA pethidine (one study) (Erskine 1985).
-
25
PCA remifentanil versus PCA pethidine (three studies) (Blair 2005; Douma 2010; Volikas 2001).
-
26
PCA nalbuphine versus PCA pethidine (one study) (Frank 1987).
-
27
PCA fentanyl versus PCA alfentanil (one study) (Morley-Forster 2000).
-
28
PCA fentanyl versus PCA pethidine (one study) (Douma 2010).
Opioids versus TENs
-
29
IV pethidine (50 mg) versus TENS to lower back (Neumark 1978), IM pethidine (50 mg) versus TENS to back (Tawfik 1982), IM tramadol (100 mg) versus TENS to back (Thakur 2004).
Excluded studies
We have excluded 86 studies (see Characteristics of excluded studies).
Reasons for exclusions (some of the studies were excluded for more than one reason).
In 16 studies the focus was on epidural analgesia (Camann 1992; Evron 2007; Evron 2008; El-Kerdawy 2010; Gambling 1998; Ginosar 2003; Grandjean 1979; McGrath 1992; Morris 1994; Nafisi 2006; Polley 2000, Rabie 2006; Solek-Pastuszka 2009; Volmanen 2008; Wiener 1979; Wong 2005). The use of epidural analgesia for pain management in labour is covered in related Cochrane reviews (Anim-Somuah 2005; Simmons 2007).
In 13 studies, women in both groups received the same opioid and the focus of studies was on add-on drugs; so, for example, both groups received pethidine with one group, in addition, receiving a sedative. The focus of these trials was on the effects of the add-on drug (Aiken 1971; Ballas 1976; De Lamerens 1964; Hodgkinson 1978; Malkasian 1967; McQuitty 1967; Posner 1960; Powe 1962; Ron 1984; Roberts 1960; Spellacy 1966; Wan 1965; Williams 1962).
Eighteen studies were not randomised trials, or it was not clear that there was any random allocation to groups (Balcioglu 2007; Bredow 1992; Brelje 1966; Callaghan 1966; Cincadze 1978; Cullhed 1961; Eliot 1975; MacVicar 1960; Moore 1974; Pandole 2003; Rowley 1963; Savage 1955; Singh 2001; Soontrapa 2002; Suvonnakote 1986; Tripti 2006; Vavrinkova 2005; Volmanen 2005).
In three studies it was not clear that participants were in labour (Chang 1976; Krins 1969; Tomlin 1965).
In the study by Bare 1962 women did not receive an opioid.
In the study by Kaltreider 1967 the focus was on a high-risk group (women in preterm labour) and post-randomisation exclusions meant that results were difficult to interpret.
We excluded two studies as levels of attrition meant that results were at high risk of bias. There were serious methodological problems in the study by Robinson 1980 and complete data were available for only approximately one-third of those randomised. In the study by De Kornfeld 1964, data on pain outcomes were available for less than half the sample at one hour; results from this study were therefore very difficult to interpret.
Five trials were reported in trial registers or in brief abstracts and we were unable to assess risk of bias or extract results. We attempted to contact authors for more information without success (Goodlin 1988, Kalaskar 2007; Morgan 2004; Overton 1992; Taskin 1993).
The focus of four studies was not on pain relief, so women may have received an opioid with the purpose of promoting progress in labour (Sosa 2004; Tournaire 1980; Treisser 1981; Von Vorherr 1963). In one of these studies women were specifically excluded if they complained of pain (Sosa 2004), and in another, women in the two groups also received oxytocin with each study group receiving a different dose (Von Vorherr 1963). A further two studies did not focus on pain relief but rather on newborn serum bilirubin (McDonald 1964) or platelet function (Greer 1988).
Seven studies focused on drugs no longer in use, or drugs not used nowadays for obstetric analgesia (Cahal 1960; Cavanagh 1966; Eames 1964; Ransom 1966; Roberts 1957; Sentnor 1966; Walker 1992).
In five studies the same opioid was given to women in both arms of trials and the difference between groups was mode of administration; different modes of administration of parenteral opioids will be considered in a separate Cochrane review (Balki 2007; Isenor 1993; McInnes 2004; Rayburn 1989; Rayburn 1991).
In two studies women in one arm of the trial, as well as receiving an opioid, were also given another add-on drug that the comparison group did not receive. In these studies results are difficult to interpret, as any differences between groups may be due to the add-on drug rather than the opioid (Busacca 1982; Calderon 2006).
In the studies by Calderon 2006, Evron 2005, Li 1995, Nikkola 2000; Shahriari 2007 and Thurlow 2002, different drugs were administered using different methods, and so it is difficult to interpret results as any differences between groups may be due to drug, method or both together.
In one study the effect of the opioid analgesia was not assessed during childbirth, but for second trimester labour following termination of pregnancy (Castro 2004).
Risk of bias in included studies
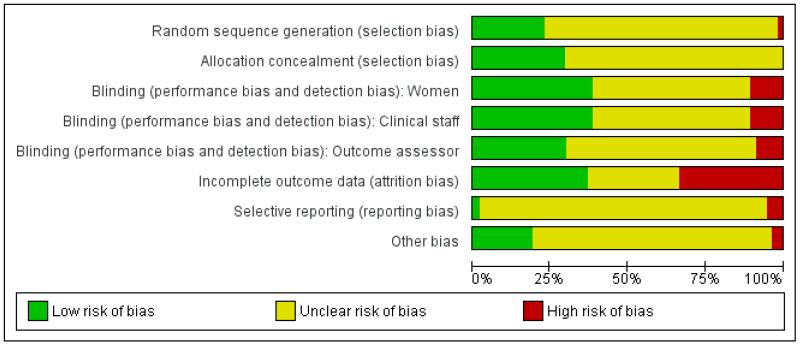
Figure 1. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
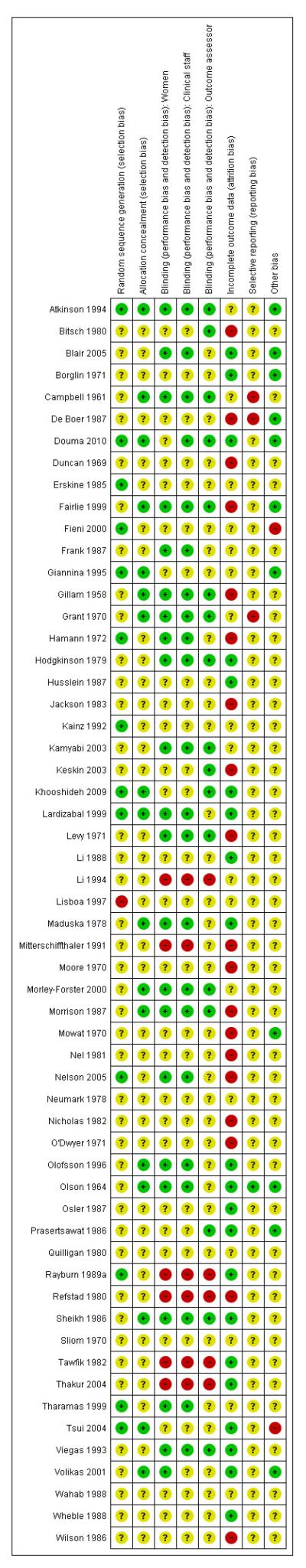
Figure 2. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
Sequence generation
In eight studies, authors stated that a computer-generated random sequence was used (Atkinson 1994; Douma 2010; Fieni 2000; Giannina 1995; Khooshideh 2009; Lardizabal 1999; Nelson 2005; Tsui 2004); in two that an external randomisation service was used (Morley-Forster 2000; Rayburn 1989a); and in four studies that random number tables were consulted (Erskine 1985; Hamann 1972; Kainz 1992; Tharamas 1999). The majority of included studies did not give clear information about how the randomisation sequence was generated.
Allocation concealment
Allocation concealment was not generally described in sufficient detail to allow assessment of risk of bias; it was not always clear at what stage randomisation took place, and whether or not the person carrying out randomisation was aware of group allocation. Four studies described using numbered opaque sealed envelopes to conceal allocation (Giannina 1995; Khooshideh 2009; Tsui 2004; Volikas 2001). Seventeen studies described using identical coded drug boxes (although it may not have been clear who had access to the code or when the code was broken) (Atkinson 1994; Borglin 1971; Campbell 1961; Douma 2010; Fairlie 1999; Gillam 1958; Grant 1970; Lardizabal 1999; Levy 1971; Maduska 1978; Moore 1970; Morley-Forster 2000; Morrison 1987; Olofsson 1996; Olson 1964; Sheikh 1986; Wilson 1986). In the remaining studies it was not clear what steps were taken to conceal allocation at the point of randomisation.
Blinding
Many of the studies were described as double blind; in the majority of these trials women in the control arms were given preparations of similar appearance to those given to women in the experimental arms (either a placebo or an indistinguishable comparison drug). It was not always clear that blinding was effective; for example, some IM drugs may appear similar, but different consistencies may be apparent to experienced staff. It was also not generally clear at what point blinding ended, and whether outcome assessors were blind to group allocation.
In six studies blinding was impractical as women were given different types of treatment (e.g. IM drug versus no treatment; IM drug versus TENS) (Li 1994; Neumark 1978; Rayburn 1989a; Refstad 1980; Tawfik 1982; Thakur 2004), and in a further nine studies methods were not described or were not clear (Bitsch 1980; Erskine 1985; Fieni 2000; Giannina 1995; Husslein 1987; Keskin 2003; Lisboa 1997; Mitterschiffthaler 1991; Wahab 1988).
Incomplete outcome data
Assessing levels of attrition was very difficult in these studies, as denominators were frequently absent from results tables. In addition, even where all women appeared to be accounted for at follow-up, there were frequently missing data for specific outcomes. In some studies loss to follow-up or missing data were greater than 10% (Bitsch 1980; Fairlie 1999; Hamann 1972; Levy 1971; Moore 1970; Mowat 1970; Olson 1964; Wilson 1986), or greater than 20% (De Boer 1987; Frank 1987; Giannina 1995; Gillam 1958; Nicholas 1982; O’Dwyer 1971; Refstad 1980).
In several studies there were missing data on pain outcomes. This may have occurred because drugs were given at a late stage in labour, so that women had already given birth before the first scheduled pain assessment. For example, in Fairlie 1999 17%, and in O’Dwyer 1971 and Refstad 1980 more than one-third of women had given birth within an hour of drug administration.
In some studies women were explicitly excluded from the analysis because of factors that may have related to study medication; in Hamann 1972 13% were excluded after randomisation because they had a long labour or a caesarean section, and in Moore 1970 women were excluded because they had had additional pain relief. Wilson 1986 excluded 10% of the sample because women reported that they had had inadequate pain relief. In the study by Nelson 2005 any woman undergoing artificial rupture of membranes, commencing oxytocin or requesting epidural was excluded after randomisation and were replaced. Further, any women who reached 10 cm cervical dilation within one hour of drug administration were also excluded from the analysis; it was not clear how many women were lost and replaced for these reasons.
Selective reporting
We did not formally assess outcome reporting bias, as we had access only to published study reports and without study protocols it is difficult to assess whether all outcomes have been accounted for. We were not able to explore possible publication bias by using funnel plots as too few studies were included in different comparisons.
Other potential sources of bias
Most of the studies reported that there was no apparent baseline imbalance between groups although this was not always explicit, and where tables describing characteristics of the two groups were provided, they frequently included only a small number of obstetric or demographic variables. In the study by Tsui 2004, there was imbalance between groups in terms of the numbers of women undergoing induction of labour in the two groups (20/25 in the pethidine group and 12/25 in the placebo group), and this may have had an impact on outcomes. In the study by Rayburn 1989a women were only recruited to the study at very limited times (weekdays 8am to 3pm), and while this may not put findings at high risk of bias, it may mean that those recruited were not representative of the population served by the study hospital.
In the Characteristics of included studies and risk of bias tables we have set out more information which will assist in the interpretation of results.
Effects of interventions
In this section where several studies have contributed data to a comparison, we have reported primary and secondary outcomes separately. For some comparisons single studies provided data on a very limited number of outcomes; for these comparisons we have reported outcomes under one heading. We had planned subgroup analysis by parity, by whether or not the labour was induced or augmented, by gestational age (preterm versus term birth) and by whether or not women had continuous support during labour. In this version of the review we were unable to carry out this analysis, as data were not provided by subgroups. In addition, we did not carry out planned sensitivity analysis by study quality as for most outcomes only one or two studies contributed data, and excluding lower-quality studies from the analyses was unlikely to shed any further light on findings.
Intramuscular opioids for pain relief in labour
1. IM pethidine versus placebo
Three studies with 254 women were included in this comparison (Kamyabi 2003; Sliom 1970; Tsui 2004), although for most outcomes only a single study contributed data.
Primary outcomes
One study involving 50 women (Tsui 2004) showed no significant difference in maternal satisfaction 30 minutes after administration of study drug (risk ratio (RR) 7.00, 95% confidence interval (CI) 0.38 to 128); only three of 25 women receiving pethidine and none of the women receiving placebo were ‘satisfied’ or ‘very satisfied’ with analgesia (Analysis 1.1).
One study involving 116 women (Sliom 1970) reported significantly more women in the pethidine group with “fair” or “good” pain relief (RR 1.75, 95% CI 1.24 to 2.47) (Analysis 1.2).
Secondary outcomes
Maternal
Maternal pain relief 30 minutes after study drug administration, defined as a reduction in visual analogue scale (VAS) score of at least 40 mm, was measured in one study with 50 women (Tsui 2004) and was significantly greater for pethidine 100 mg compared with placebo (RR 25, 95% CI 1.56 to 400) though the CI for this estimate is very wide (Analysis 1.3). In this study, although the majority of women in both groups required additional analgesia, this applied to fewer women with pethidine 100 mg compared with placebo (RR 0.71, 95% CI 0.54 to 0.94) (Analysis 1.4).
There was no evidence of differences between groups in the number of women requiring an epidural (RR 0.50, 95% CI 0.14 to 1.78) (Analysis 1.5), in the incidence of nausea and vomiting (RR 1.47, 95% CI 0.65 to 3.31) (Analysis 1.6), assisted vaginal birth (RR 0.86, 95% CI 0.34 to 2.19) (Analysis 1.8), or caesarean section (RR 0.83, 95% CI 0.29 to 2.38) (Analysis 1.9). Significantly more women reported sleepiness with pethidine 100 mg, with half of those receiving pethidine feeling sedated compared with 11% of controls (RR 4.67, 95% CI 2.43 to 8.95) (Analysis 1.7).
In one study, 12/25 women in the placebo group had pethidine at 30 minutes as rescue analgesia confounding interpretation of reported outcomes after 30 minutes (Tsui 2004).
Neonatal
The number of babies with Apgar scores of seven or less at one minute did not differ between the placebo and pethidine groups; for this outcome we used a random-effects model because of high heterogeneity (average RR 1.64, 95% CI 0.52 to 5.18), (heterogeneity: I2 = 61%, Tau2 = 0.46, Chi2 test for heterogeneity P = 0.11) (Analysis 1.10). No babies had Apgar scores less than or equal to seven at five minutes in the one study that reported this outcome (Analysis 1.10). The incidence of newborn resuscitation and admission to neonatal intensive care unit (NICU) was low; no significant differences between groups was detected (Analysis 1.11; Analysis 1.12).
One study reported incidence of fetal respiratory depression, but the study drugs were given late in labour to assess maximum fetal effect. Participants were not included in the analysis if birth was less than 30 minutes or more than four hours after administration of study drugs (Sliom 1970).
We were unable to include any results from one study that met the inclusion criteria, as it was unclear when outcomes were measured how they were defined and how many participants were included in the analysis (Kamyabi 2003). In this study, mean Apgar scores at one minute were reported to be higher (P = 0.008) in the pethidine 75 mg group compared with placebo group (data not shown).
2. IM meptazinol versus IM pethidine
IM meptazinol versus IM pethidine was evaluated in six studies with 1898 women (Morrison 1987; Nel 1981; Nicholas 1982; Osler 1987; Sheikh 1986; Wheble 1988), and in two additional studies where women in both study groups also received add-on drugs (De Boer 1987; Jackson 1983).
Primary outcomes
One study (Morrison 1987) involving 801 women showed no evidence of a difference between meptazinol 100 mg to 150 mg compared with pethidine 100 mg to 150 mg for assessment of analgesic effect measured at three to five days postpartum (RR 1.01, 95% CI 0.91 to 1.12) (Analysis 2.1). In this study, more than half of the women receiving either of these opioids reported that they received no or poor relief despite the fact that women in both groups could also receive an additional dose of study drug, epidural or nitrous oxide as required.
In two studies (Nel 1981; Sheikh 1986) involving 239 women, there was no evidence of a difference between groups in pain intensity one hour after administration of meptazinol 100 mg or pethidine 100 mg; more than two-thirds of women in both groups were rating their pain as severe (four or five on a five-point scale) at one hour (average RR 1.11, 95% CI 0.69 to 1.80 (random-effects; heterogeneity: I2 = 43%, Tau2 = 0.08, Chi2 test for heterogeneity P = 0.18) (Analysis 2.2).
Secondary outcomes
Maternal
Two studies (Osler 1987; Wheble 1988) involving 233 women found no evidence of a difference in requirement for additional analgesia between those who received meptazinol compared with pethidine (RR 1.03, 95% CI 0.88 to 1.20) (Analysis 2.3). This outcome is difficult to interpret as women in the study by Osler 1987 were allowed up to three doses of study drug (meptazinol 100 mg or pethidine 75 mg). Overall, 56 women required a second dose and 15 a third dose, but the number per group was not reported. Whereas in the study by Wheble 1988, women were allowed a second dose of study drug (meptazinol 100 mg or 150 mg or pethidine 100 mg or 150 mg) or epidural or nitrous oxide at the discretion of the caregiver. Additional analgesia relates to a pudendal in the one study (Osler 1987), and a second dose of study drug in the other (Wheble 1988).
The use of epidural analgesia was similar between meptazinol and pethidine (RR 0.96, 95% CI 0.71 to 1.29) in four studies (Nicholas 1982; Osler 1987; Sheikh 1986; Wheble 1988) involving 788 women (Analysis 2.4). Instrumental birth was reported in three studies (Morrison 1987; Osler 1987; Wheble 1988) involving 1266 women, and rates were similar between groups (RR 1.00, 95% CI 0.81 to 1.22) (Analysis 2.7). Overall, there was no evidence of a difference in rates of caesarean section between meptazinol and placebo. However, substantial heterogeneity was detected; therefore, we used a random-effects model (average RR 0.56, 95% CI 0.16, 2.00) (heterogeneity: I2 = 75%, T2 = 0.84, Chi2 test for heterogeneity P = 0.02), (Analysis 2.8).
Three studies each reported nausea, vomiting and sleepiness (Morrison 1987; Nicholas 1982; Sheikh 1986). There was no evidence for a difference in nausea (RR 1.11, 95% CI 0.95 to 1.28); however, significantly more women reported vomiting (RR 1.25, 95% CI 1.06 to 1.47) with meptazinol compared with pethidine. Fewer women in the meptazinol group reported sleepiness (average RR 0.55, 95% CI 0.28 to 1.07), although there was moderate heterogeneity for this outcome (heterogeneity: I2 = 44%, T2 = 0.18, Chi2 test for heterogeneity P = 0.17) and the difference between groups did not reach statistical significance (Analysis 2.6).
Neonatal
Four studies involving 662 women reported number of babies with Apgar scores less than or equal to seven at one minute (Nel 1981; Nicholas 1982; Osler 1987; Wheble 1988), and three studies reported this outcome at five minutes (Nel 1981; Nicholas 1982; Osler 1987). There was no evidence of a difference between groups at one minute (RR 0.76, 95% CI 0.50 to 1.13) or five minutes (RR 0.49, 95% CI 0.05 to 5.37) with three babies with low scores at five minutes reported in one study (Osler 1987) and none in the other two (Nel 1981; Nicholas 1982) (Analysis 2.10). We found no evidence of a difference between meptazinol compared with pethidine for naloxone administration (RR 0.89, 95% CI 0.77 to 1.02), admission to NICU (RR 0.88, 95% CI 0.48 to 1.63) or newborn resuscitation (Analysis 2.11; Analysis 2.12; Analysis 2.13). In one study (Morrison 1987), 40% of the babies were given naloxone, reflecting local practice at the time rather than low Apgar scores; with 41% of the babies having Apgar scores greater than or equal to eight at the time of administration.
Breastfeeding problems were reported by a small number of women in one study (Sheikh 1986); there was no evidence of a difference between groups (RR 0.76, 95% CI 0.17 to 3.30).
Meptazinol versus pethidine with add-on drugs
One study compared IM meptazinol 1.8 mg/kg with IM pethidine 1.8 mg/kg; all women also received promazine 25 mg IM (Jackson 1983). A second study compared IM meptazinol 1.5 mg/kg with IM pethidine 1.5 mg/kg; all women also received metoclopramide 10 mg IM (De Boer 1987). Women could receive a second dose of study drug after three hours in both studies. Both studies were conducted to assess effects of the study drugs on the newborn only.
Primary and secondary outcomes
Maternal outcomes were not measured.
Neonatal
Both studies reported the number of babies with Apgar scores less than or equal to seven at one minute. There was no evidence of a difference between meptazinol compared with pethidine (RR 0.89, 95% CI, 0.47 to 1.67). In the study by De Boer 1987, Apgar at five and 10 minutes were reported as ‘similar’ in both groups and there was no evidence of difference in the number of babies with fetal heart rate changes (decelerations). In the study by Jackson 1983, no babies in either group had Apgar scores less than or equal to seven at 10 minutes. In one study (Jackson 1983), three babies in the meptazinol group and two in the pethidine group required resuscitation.
3. PCA (IM) meptazinol versus PCA (IM) pethidine
One study involving 10 women examined the feasibility of IM meptazinol versus IM pethidine with PCA administration (Li 1988).
Primary and secondary outcomes
All women in both groups were satisfied with the mode of administration (Analysis 3.2).
Pain scores measured one day postpartum were lower with meptazinol compared with pethidine; however, there was no evidence of a significant difference (mean difference (MD) −17.60, 95% CI −49.93 to 14.73) (Analysis 3.1). Epidural rates and nausea and drowsiness scores evaluated one day postpartum were similar between groups (Analysis 3.3; Analysis 3.4; Analysis 3.5).
Neonatal
Naloxone was administered to one baby in each group (Analysis 3.6).
4. IM diamorphine + prochlorperazine versus IM pethidine + prochlorperazine
One study involving 133 women compared IM diamorphine 5 mg to 7.5 mg versus IM pethidine 100 mg to 150 mg. All women also received IM prochlorperazine 12.5 mg at the same time as the study drug (Fairlie 1999).
Primary outcomes
Global assessment of pain relief was evaluated at 24 hours; there was no evidence of a difference between groups in the number of women reporting ‘fair’ or ‘poor’ as opposed to ‘good’ pain relief, with more than half of the women in both groups having inadequate relief (RR 0.88, 95% CI 0.67 to 1.16) (Analysis 4.1).
Secondary outcomes
Maternal
More women reported pain intensity as moderate or severe one hour post administration of study drug with pethidine compared with diamorphine, though there was no evidence of a significant difference between groups, with the majority of women in both groups reporting moderate or severe pain (RR 0.85, 95% CI 0.72 to 1.01) (Analysis 4.2). There was no evidence for a difference between groups in the number of women requiring additional analgesia (RR 1.35, 95% CI 0.53 to 3.4) (Analysis 4.3), an epidural (RR 1.22, 95% CI 0.72 to 2.07) (Analysis 4.4), assisted vaginal birth (RR 0.96, 95% CI 0.46 to 2.02) (Analysis 4.7), or caesarean section (RR 0.52, 95% CI 0.10 to 2.76) (Analysis 4.8).
The number of women vomiting was significantly lower with diamorphine compared with pethidine (RR 0.39, 95% CI 0.17 to 0.86) (Analysis 4.5), but the number of women moderately drowsy or asleep one hour after study drug administration was similar between groups (RR 0.93, 95% CI 0.52 to1.66) (Analysis 4.6).
Neonatal
Significantly fewer babies had Apgar scores less than seven at one minute with diamorphine compared with pethidine (RR 0.41, 95% CI 0.18 to 0.91) (Analysis 4.9). However, there was no evidence of a difference between groups at five minutes, with few babies with an Apgar score less than seven in either group (RR 0.35, 95% CI 0.04 to 3.27) (Analysis 4.10). There were no significant differences between groups for the number of babies needing resuscitation (RR 1.21, 95% CI 0.73 to 2.02) (Analysis 4.11), or admission to NICU (RR 0.58, 95% CI 0.21 to 1.64) (Analysis 4.12).
5. IM tramadol versus IM pethidine
Seven studies involving 569 women compared IM tramadol versus IM pethidine (Bitsch 1980; Fieni 2000; Husslein 1987; Keskin 2003; Khooshideh 2009; Prasertsawat 1986; Viegas 1993). Tramadol and pethidine doses varied between studies and were 50, 75 or 100 mg.
Primary and secondary outcomes
Maternal
Women’s satisfaction with pain relief was not measured in any of the studies.
Pain intensity was defined in disparate ways in the studies; however, significantly more women had poor pain relief with tramadol compared with pethidine (RR 1.56, 95% CI 1.10 to 2.21) (Analysis 5.1).
In three studies which reported requirement for additional analgesia, no evidence of a difference was detected (average RR 1.07, 95% CI 0.60 to 1.91) (Analysis 5.2).
There was no evidence for a difference in incidence of nausea and/or vomiting with tramadol compared with placebo (average RR 0.97, 95% CI 0.34 to 2.76) (Analysis 5.3). There was a substantial level of heterogeneity detected for this outcome (I2 = 72%, T2 = 1.09, Chi2 test for heterogeneity P = 0.003) therefore we used a random-effects model for the analysis. More women in the pethidine group reported sleepiness and the difference between groups reached statistical significance although, again, heterogeneity was high and we used a random-effects model (average RR 0.57, 95% CI 0.33 to 0.97), (heterogeneity I2 = 72%, T2 = 0.24, Chi2 test for heterogeneity P = 0.007) (Analysis 5.4).
Neonatal
Only two studies reported Apgar scores (Khooshideh 2009; Prasertsawat 1986), and reported no babies in either group with Apgar scores less than or equal to seven at one or five minutes, and no babies requiring resuscitation (Analysis 5.7; Analysis 5.8). One study (Keskin 2003) reported the incidence of respiratory distress and admission to NICU which occurred more frequently with tramadol 100 mg compared with pethidine 100 mg, though results were not statistically significant for either outcome (RR 2.26, 95% CI 0.64 to 7.89) (Analysis 5.9; Analysis 5.10).
6. IM tramadol + triflupromazine versus IM pethidine + triflupromazine
One study involving 66 women compared tramadol 500 mg with pethidine 50 mg, and both groups also received triflupromazine 10 mg (Kainz 1992). A third study arm received tramadol 100 mg.
Primary and secondary outcomes
Maternal satisfaction with analgesic effect was not measured. The authors reported that the analgesic effect was equally good in each study arm. Data for effects on pain were not reported (P values for the change within groups were reported; not the between group differences; data not shown).
The incidence of nausea or vomiting was reported and was infrequent, with no evidence of differences between groups (RR 0.82, 95% CI 0.13 to 5.25 and RR 0.40, 95% CI 0.02 to 9.35, respectively) (Analysis 6.1). Sleepiness was more frequently reported by women who received tramadol, though no statistically significant difference between groups was detected (RR 2.86, 95% CI 0.68 to 12.12) (Analysis 6.2).
The authors report that there were no negative effects on the newborn; though no data were presented.
7. IM dihydrocodeine versus IM pethidine
One study involving 106 women compared a single dose of IM dihydrocodeine 50 mg with IM pethidine 100 mg (Sliom 1970). An additional study arm received placebo.
Primary and secondary outcomes
There was no evidence of a difference in pain relief between groups with a substantial proportion of women in each group reporting poor pain relief one hour after administration of study drug (RR 1.09, 95% CI 0.64 to 1.86) (Analysis 7.1).
There was no evidence of a difference between dihydrocodeine and pethidine for nausea and vomiting (RR 0.87, 95% CI 0.40 to 1.88) (Analysis 7.2), or sleepiness (RR 0.67, 95% CI 0.43 to 1.04) (Analysis 7.3).
Significantly fewer babies had Apgar scores less than or equal to seven at one minute with dihydrocodeine compared with pethidine (RR 0.57, 95% CI 0.39 to 0.84) (Analysis 7.4). Apgar score at five minutes was reported as mean scores rather than number of babies in each group: there was no significant difference between groups reported (data not shown).
8. IM pentazocine versus pethidine
Six studies with 877 women are included in this comparison (Borglin 1971; Duncan 1969; Levy 1971; Moore 1970; Mowat 1970; Refstad 1980).
Primary outcomes
Two studies reported on the numbers of women rating pain relief as good or very good at birth (Borglin 1971; Mowat 1970), and there was no statistically significant difference between groups in either study, or when results were pooled (Analysis 8.1).
Four studies reported poor pain relief (Duncan 1969; Levy 1971; Moore 1970; Refstad 1980); more than half of the women in both groups had only partial or poor relief and there was no statistically significant difference between groups (Analysis 8.2).
Secondary outcomes
The use of additional analgesic drugs was reported by two studies (Mowat 1970; Refstad 1980). There was no statistically significant difference between groups in either study (Analysis 8.3).
One or more studies reported nausea, vomiting, sleepiness or assisted vaginal birth; there was no significant evidence of a difference between groups for any of these outcomes (Analysis 8.4; Analysis 8.5; Analysis 8.6).
Two studies reported the incidence of low Apgar scores at one and five minutes (Borglin 1971; Levy 1971) with no statistically significant difference between groups (Analysis 8.7).
9. IM pentazocine + promazine versus pethidine + promazine
One study with 85 women contributed data to this comparison (Refstad 1980).
Primary and secondary outcomes
This study reported on only two of the review’s outcomes: low Apgar score at one and five minutes and naloxone administration. There was no statistically significant difference between groups for either outcome (Analysis 9.1; Analysis 9.2).
10. IM nalbuphine versus pethidine
Four studies with 486 women are included in this comparison (Lardizabal 1999; Lisboa 1997; Mitterschiffthaler 1991; Wilson 1986).
Primary outcomes
One study reported maternal satisfaction with analgesia at 24 hours (Wilson 1986). The majority of women receiving both nalbuphine and pethidine thought that analgesia had been “minimally effective” (63% and 85% respectively), although the difference between groups was statistically significant (RR 0.73, 95% CI 0.55 to 0.96) (Analysis 10.1). One study reported the number of women that were free of pain (Mitterschiffthaler 1991); the difference between groups was not statistically significant, with few women in either group having no pain (Analysis 10.2). Two studies reported pain intensity: one at 30 minutes (Lardizabal 1999) and the other at 60 minutes (Wilson 1986). There was no statistically significant difference between groups in either study (Analysis 10.3; Analysis 10.4).
Secondary outcomes
Maternal
Two studies reported the use of additional analgesia (Lardizabal 1999; Wilson 1986) and there was no statistically significant difference between groups in either study (Analysis 10.5; Analysis 10.6). One study reported nausea and vomiting as separate outcomes (Lardizabal 1999), and another reported nausea and vomiting as a single outcome (Wilson 1986). Statistically significantly fewer women who received nalbuphine reported nausea alone (RR 0.62, 95% CI 0.42 to 0.91, P = 0.02), or vomiting (RR 0.41, 95% CI 0.22 to 0.76) compared with women who received pethidine.
Likewise, fewer women who received nalbuphine reported nausea and vomiting combined (RR 0.41, 95% CI 0.18 to 0.94). There was no evidence of significant differences between groups for maternal sleepiness, assisted or caesarean births in studies reporting these outcomes (Analysis 10.8; Analysis 10.9; Analysis 10.10).
Neonatal
Two studies reported neonatal outcomes (Lardizabal 1999; Wilson 1986). There was no statistically significant difference between groups for low Apgar scores at one, five and 10 minutes, naloxone administration or admission to NICU (Analysis 10.11; Analysis 10.12; Analysis 10.13). One study reported a neonatal neurobehavioural score two to four hours following birth (Wilson 1986); babies of women who received nalbuphine had lower scores than babies born to women in the control group (MD −3.70, 95% CI −6.14 to −1.26).
11. IM phenazocine versus pethidine
One study with 212 women (Grant 1970) compared IM phenazocine versus IM pethidine.
Primary and secondary outcomes
This study reported only two outcomes: epidural uptake and vomiting. There was no statistically significant difference between groups for epidural (Analysis 11.1), but fewer women who received phenazocine vomited (RR 0.39, 95% CI 0.20 to 0.78) compared with those who received pethidine.
12. IM morphine versus pethidine
We included one study with 135 women in this comparison (Prasertsawat 1986).
Primary and secondary outcomes
There was no statistically significant difference between groups in the number of women describing their pain relief as poor (RR 1.22, 95% CI 0.56 to 2.66), additional analgesia (Analysis 12.2), nausea and vomiting (Analysis 12.3), or maternal sleepiness (Analysis 12.4). There was also no statistically significant difference between groups for number of babies born with an Apgar score less than or equal to seven at birth (Analysis 13.1), or requiring resuscitation (Analysis 12.6).
13. IM butorphanol versus pethidine
One study with 80 women compared IM butorphanol with IM pethidine (Maduska 1978).
Primary and secondary outcomes
This study did not report on the review’s primary outcomes. There was no significant evidence of differences between groups for additional analgesia (Analysis 13.1), nausea (Analysis 13.2), or vomiting (Analysis 13.3). Likewise, there was no significant difference between groups for neonatal resuscitation (Analysis 13.4) or naloxone administration (Analysis 13.5).
14. IM tramadol versus no treatment
One study with 60 women compared IM tramadol with no treatment (Li 1994).
Primary and secondary outcomes
This study reported only two outcomes: satisfaction with analgesia and mean blood loss at birth. Only five out of 30 of the women receiving tramadol described it as satisfactory, but the difference between groups was not significant (Analysis 14.1). There was no difference between groups for mean blood loss at birth (Analysis 14.2).
15. IM Avacan® versus IM pentazocine
We included one study with 185 women in this comparison (Hamann 1972).
Primary and secondary outcomes
This study did not report on either of our primary outcomes. There were no statistically significant differences between groups for the uptake of nitrous oxide (Analysis 15.1). More women in the Avacan® group received a pudendal-paracervical block (RR 2.02, 95% CI 1.16 to 3.53). There was no evidence of a difference between groups for the number of women having a caesarean section, or babies born with an Apgar score less than or equal to seven at birth (Analysis 15.3; Analysis 15.4). This study did not report on any other secondary outcomes.
16. IM pentazocine versus IM Pethilorfan®
One trial involving 98 women compared pentazocine with Pethilorfan® (O’Dwyer 1971).
Primary and secondary outcomes
There was no statistically significant difference between study groups in the number of women saying that they did not obtain any relief from medication at one hour (RR 1.22, 95% CI 0.77 to 1.95) (Analysis 16.1).
No statistically or clinically significant differences were reported for any of the secondary outcomes recorded (additional analgesia required, assisted vaginal birth, Apgar score less than eight at one minute, Apgar score less than eight at five minutes) (Analysis 16.2; Analysis 16.3; Analysis 16.4; Analysis 16.5).
Intravenous opioids for pain relief in labour
17. IV fentanyl versus IV pethidine
We included one study with 105 women in this comparison (Rayburn 1989a).
Primary and secondary outcomes
The mean maternal pain score was significantly lower one hour after drug administration for women allocated to the IV fentanyl compared with those in the IV pethidine group; however, women in both groups reported mean pain scores of approximately six on a 10 mm scale (MD −0.20, 95% CI −0.34 to −0.06).
Maternal sedation was significantly lower in women allocated to the IV fentanyl group compared with those in the IV pethidine group (RR 0.05, 95% CI 0.00 to 0.82). There were no statistically significant differences for all other reported outcomes including side effects, interventions in labour and outcomes for babies (Analysis 17.3; Analysis 17.4; Analysis 17.6; Analysis 17.7; Analysis 17.9; Analysis 17.11). The study, however, recruited women only during a limited time period Monday to Friday and allocation was not blinded due to the different half-lives of the treatment options.
18. IV nalbuphine versus IV pethidine
We included one study involving 28 women compared IV nalbuphine with IV pethidine (Giannina 1995).
Primary and secondary outcomes
No outcomes relating to maternal pain during labour were reported.
This study reported estimable data for only two relevant secondary outcomes (caesarean section and low Apgar score at one minute), neither of which showed any significant difference between the two groups (Analysis 18.1; Analysis 18.2).
19. IV phenazocine versus IV pethidine
We included one study including 194 women compared IV phenazocine with IV pethidine (Olson 1964).
Primary and secondary outcomes
There was no statistically significant difference between groups for women’s satisfaction with pain relief (comparing the number of women with “fair” or “poor” pain relief) (RR 0.72, 95% CI 0.48 to 1.10). No other primary outcomes were reported.
Only one identified secondary outcome reported estimable data: nausea with vomiting. There was no statistically significant difference between the two groups for this outcome (Analysis 19.2).
20. IV butorphanol versus IV pethidine
Three studies involving a total of 330 women compared IV butorphanol with IV pethidine (Hodgkinson 1979; Nelson 2005; Quilligan 1980).
Primary outcomes
One study (Quilligan 1980) involving 100 women (findings for these primary outcomes reported for 80 women) included two measures of women’s pain during labour; women’s reported pain relief and pain score. Women’s mean pain relief score was significantly higher for those in the group receiving butorphanol (MD 0.67, 95% CI 0.25 to 1.09). This finding was supported by data regarding reported pain scores one hour after drug administration which were lower for women in the butorphanol group (MD −0.60, 95% CI −1.02 to −0.18). The clinical significance of a difference of this magnitude (i.e. 0.6 on a 10-point scale) is more difficult to determine.
The other two studies comparing IV butorphanol with IV pethidine did not report any outcomes relating to women’s pain during labour.
Secondary outcomes
One study (Hodgkinson 1979) involving 200 women reported a lower incidence of nausea and vomiting associated with butorphanol compared with pethidine (0/100 in the butorphanol group versus 12/100 in the pethidine group; RR 0.04, 95% CI 0.00 to 0.67). Other secondary outcomes reported by one or more of the three studies within this comparison (second dose of analgesia required, epidural analgesia, assisted vaginal birth, caesarean section, Apgar score less than or equal to seven at one and five minutes) showed no statistically significant differences between groups (Analysis 20.3; Analysis 20.4; Analysis 20.6; Analysis 20.7; Analysis 20.8).
21. IV morphine versus IV pethidine
Two trials involving a total of 163 women compared IV morphine with IV pethidine (Campbell 1961; Olofsson 1996).
Primary and secondary outcomes
One study involving 143 women reported women’s satisfaction with pain relief assessed three days postpartum (Campbell 1961). Fewer women allocated to receive IV morphine during labour were satisfied with pain relief than those allocated to receive pethidine (RR 0.87, 95% CI 0.78 to 0.98), although the proportion of women who reported that they were satisfied was high in both groups (60/72 and 66/69).
Campbell 1961 also reported that women allocated to receive IV morphine were significantly more likely to request a second dose of analgesia compared with women allocated to receive IV pethidine (RR 3.41, 95% CI 1.90 to 6.12). This difference may simply reflect a lack of equivalence in the study doses of analgesia given (pethidine initial dose = 100 mg; morphine initial dose = 8 mg) rather than true differences between analgesic effects.
A second study which investigated this comparison (Olofsson 1996) included only 10 women in each trial arm. No statistically significant differences were found for each of the three secondary outcomes reported (nausea, vomiting and caesarean section), although the incidence of nausea was lower in the morphine group (6/10 pethidine versus 1/10 morphine; RR 0.17, 95% CI 0.02 to 1.14) (Analysis 21.3; Analysis 21.4).
22. IV nisentil versus IV pethidine
One study including 395 women compared IV nisentil with IV pethidine (Gillam 1958).
Primary and secondary outcomes
The study did not report any outcomes relating to women’s pain relief.
Women allocated to the nisentil group were less likely to suffer vomiting than those receiving pethidine (RR 0.38, 95% CI 0.22 to 0.66). There was also less risk of nausea in the nisentil group, although this difference was not statistically significant (RR 0.71, 95% CI 0.33 to 1.52).
The incidence of babies requiring resuscitation and/or ventilatory support was two times higher in babies born to women in the nisentil group (14/185) compared to those in the pethidine group (8/210) (RR 1.99, 95% CI 0.85 to 4.63). Although this difference is not statistically significant, and this finding may have occurred by chance, if this is a true reflection of differences between groups then this degree of harmful effect on newborn babies is not clinically acceptable.
23. IV fentanyl versus IV butorphanol
One trial involving 100 women compared IV fentanyl with IV butorphanol (Atkinson 1994).
Primary and secondary outcomes
The study did not report any outcomes relating to women’s pain relief.
Women allocated to receive IV fentanyl were statistically significantly more likely to request additional doses of the study analgesia compared with women allocated to receive IV butorphanol (RR 1.39, 95% CI 1.05 to 1.85). The study author claims the study doses of drug were equivalent (IV fentanyl 50 to 100 mcg every one to two hours; IV butorphanol 1 to 2 mg every one to two hours). Additionally, women in the fentanyl group were twice as likely as those in the butorphanol group to go on to request an epidural (RR 2.00, 95% CI 1.00 to 4.02). Other women’s outcomes reported (drowsiness, caesarean section) showed no statistically significant difference between study groups (Analysis 23.3; Analysis 23.4).
Whilst there were no statistically significant differences observed between groups for any of the neonatal outcomes reported (Apgar score less than seven at five minutes, naloxone administration, need for ventilatory support, neuro-behavioural score at two to four hours and neuro-behavioural score at 24 to 36 hours) babies born to women allocated to the fentanyl group were more likely to need ventilatory support (5/50 versus 0/50; RR 11.00, 95% CI 0.62 to 193.80) and naloxone administration (14/50 versus 8/50; RR 1.75, 95% CI 0.81 to 3.80) (Analysis 23.5; Analysis 23.6; Analysis 23.6; Analysis 23.7; Analysis 23.8; Analysis 23.9).
Intravenous patient controlled opioids for pain relief in labour
24. PCA pentazocine versus PCA pethidine
One trial involving 29 women compared PCA pentazocine with PCA pethidine (Erskine 1985).
Primary and secondary outcomes
Women’s self-reported pain score during labour was found to be lower for those allocated to the pentazocine group compared with women in the pethidine group, although this difference failed to reach statistical significance (SMD −0.76, 95% CI −1.62 to 0.09), a difference of 1.6 cm on a 10 cm pain scale might be considered clinically significant. Similar numbers of women in the two treatment groups rated their pain relief as good one day after the birth (Analysis 24.2).
None of the secondary outcomes studied showed a significant difference between the two study groups (epidural use, sedation, caesarean section, Apgar score less than seven at five minutes, breastfeeding at discharge) (Analysis 24.3; Analysis 24.5; Analysis 24.6; Analysis 24.7; Analysis 24.8), with low numbers of events recorded for a number of these outcomes. Nausea and vomiting was reported more frequently by women allocated to the pethidine group compared with the pentazocine group (5/15 versus 0/14; RR 0.10, 95% CI 0.01 to 1.61) but the difference between groups was not significant.
25. PCA remifentanil versus PCA pethidine
Three trials involving a total of 161 women compared PCA remifentanil with PCA pethidine (Blair 2005; Douma 2010; Volikas 2001).
Primary
No primary outcomes were reported upon in these studies.
Secondary outcomes
Two studies (Volikas 2001; Douma 2010) involving 122 women reported women’s pain score during labour. In both studies pain was assessed using a VAS ranging from 0 (“no pain”) to 10 cm (“worst imaginable pain”). In both studies women were asked to mark the level of pain experienced every hour, starting before analgesia was administered. Results for the Volikas 2001 study were recorded in a graph and so values have been estimated from the graph. There was no evidence of a significant difference in mean pain scores at one hour between the remifentanil and pethidine groups (average MD −8.59, 95% CI −27.61 to 10.44), Analysis 25.1. There was substantial heterogeneity for this outcome and so a random-effects model has been used (heterogeneity I2 = 62%, T2 = 136.73, Chi2 test for heterogeneity P = 0.10) (Analysis 25.1). Two included studies (Blair 2005; Volikas 2001) reported number of women requiring additional analgesia (Entonox®) as an outcome, with most women in both study groups requiring additional analgesia (22/29 versus 24/27; RR 0.86, 95% CI 0.69 to 1.08), Analysis 25.2.
Two studies reported number of women crossing over to epidural as an outcome (Douma 2010; Volikas 2001), with fewer women in the remifentanil group requiring an epidural (RR 0.42, 95% CI 0.20 to 0.89) (Analysis 25.3).
Maternal sleepiness was reported in one study (Douma 2010). This outcome was assessed using an observer sedation score recorded hourly (1, awake; 2, sleepy; 3 eyes closed, but rousable by vocal stimuli; 4, eyes closed, but rousable by physical stimulus; and 5, unrousable). Mean hourly scores at inclusion and then at one, two and three hours after analgesia were reported. There was no evidence of a significant difference in mean sedation scores at one hour between the remifentanil and pethidine groups (MD 0.40, 95% CI 0.14 to 0.66, (Analysis 25.4).
There was no significant difference found between groups for any of the other secondary outcomes reported (nausea and vomiting, assisted vaginal birth, caesarean section, Apgar score less than seven at five minutes, naloxone administration, admission to NICU) (Analysis 25.5; Analysis 25.6; Analysis 25.7; Analysis 25.8; Analysis 25.9; Analysis 25.10). Douma 2010 provided mean and standard deviation (SD) values for Apgar scores at five minutes and so these data could not be included in an analysis.
Satisfaction with childbirth experience was reported in one study (Douma 2010). Two hours after delivery women were asked to score their overall satisfaction on a 10-point scale (tool not specified). Women in the remifentanil groups had slightly higher mean satisfaction scores (MD 1.10, 95% CI 0.46 to 1.74) (Analysis 25.11).
Newborn neuro-behavioural scores were reported in one study (Douma 2010). The Neurologic and Adaptive Capacity Score (NACS) was recorded at 15 minutes and two hours after delivery. There was no significant difference found between groups for mean scores at 15 minutes or two hours after delivery (Analysis 25.12; Analysis 25.13).
26. PCA nalbuphine versus PCA pethidine
One trial involving 60 women compared PCA nalbuphine with PCA pethidine (Frank 1987).
Primary and secondary outcomes
Pain score recorded in labour was lower in women allocated to the PCA nalbuphine group compared with women in the PCA pethidine group (SMD −0.51, 95% CI −1.02 to 0.00) (Analysis 26.3). Satisfaction with pain relief recorded one day postnatally was greater for women allocated to receive nalbuphine compared to those allocated to receive pethidine, although this difference was not statistically significant (RR 1.29, 95% CI 0.88 to 1.89) (Analysis 26.1). Similar numbers in the two groups said that they would use the same pain relief method again in a future labour (Analysis 26.2).
No statistically significant differences were found between groups for the three secondary outcomes reported (additional analgesia, nausea and vomiting, Apgar score less than seven at five minutes) (Analysis 26.4; Analysis 26.5; Analysis 26.6).
27. PCA fentanyl versus PCA alfentanil
One study involving 23 women compared PCA fentanyl with PCA alfentanil (Morley-Forster 2000).
Primary and secondary outcomes
Women in the PCA fentanyl group reported lower pain scores on average than those in the alfentanil group, although the observed mean difference of 1.3 cm was not statistically significant (MD −12.80, 95% CI −32.12 to 6.52). In contrast, women allocated to receive fentanyl were less likely to describe their satisfaction with their pain relief as “adequate” or “good” within six hours of giving birth compared with women allocated to receive alfentanil (10/11 versus 7/12; RR 1.56, 95% CI 0.93 to 2.60).
No statistically significant differences were found for any of the other secondary outcomes reported (nausea, caesarean section, naloxone administration) (Analysis 27.3; Analysis 27.4; Analysis 27.5).
28. PCA fentanyl versus PCA pethidine
One trial involving 107 women compared PCA fentanyl with PCA pethidine (Douma 2010)
Primary outcomes
No primary outcomes were reported upon in this study (Douma 2010).
Secondary outcomes
One study (Douma 2010) involving 107 women reported women’s pain score during labour. Pain scores were assessed using a VAS ranging from 0 (“no pain”) to 10 cm (“worst imaginable pain”). Mean pain scores were presented at baseline and at one, two and three hours after analgesia. There was no evidence of a significant difference in mean pain scores at one hour between the fentanyl and pethidine groups (MD −0.65, 95% CI −1.56 to 0.26, Analysis 28.1); however, fewer women in the fentanyl group required epidural (RR 0.44, 95% CI 0.21 to 0.92) (Analysis 28.2). Maternal sleepiness was reported in one study (Douma 2010). This outcome was assessed using an observer sedation score (from 1, awake to 5, unrousable) recorded hourly. There was no evidence of a significant difference in mean sedation scores at one hour between the fentanyl and pethidine groups (MD −0.06, 95% CI −0.25 to 0.13) (Analysis 28.3).
There was no significant difference found between groups for any of the other secondary outcomes reported (nausea and vomiting, assisted vaginal birth, caesarean section) (Analysis 28.4; Analysis 28.5; Analysis 28.6).
Douma 2010 only provided mean and SD values for Apgar scores at five minutes and so these data could not be included in an analysis. NACS were recorded at 15 minutes and two hours after delivery. There was no significant difference found between groups for mean scores at either time point (Analysis 28.7; Analysis 28.8).
Opioids versus TENS for pain relief in labour
29. Opioids versus TENS
Three trials involving 305 women are included in this comparison. One trial compared IV pethidine (50 mg) versus TENS to the lower back (Neumark 1978), another IM pethidine (50 mg) versus TENS to the back (Tawfik 1982) and the third IM tramadol (100 mg) versus TENS to the back (Thakur 2004).
Primary and secondary outcomes
Two studies (Neumark 1978; Tawfik 1982) involving 105 women reported on maternal satisfaction with analgesia measured post delivery. In the study by Neumark 1978 women were asked to rate their satisfaction with analgesia the day after the birth as having “good”, “inadequate” or “no” analgesic effect. In the study by Tawfik 1982 women were asked about the degree of relief they had obtained during the whole period of delivery. This was scored as being “excellent”, “good” or “satisfactory”. We found no evidence of a significant difference in maternal satisfaction with analgesia rated as “good/excellent” between the TENS and opioid groups (RR 1.23, 95% CI 0.79 to 1.92, two studies) (Analysis 29.1).
Three studies (Neumark 1978; Tawfik 1982; Thakur 2004) involving 305 women reported on maternal pain measured in labour. In the study by Neumark 1978 pain was assessed on a six-point pain scale for a 70-minute period (from 1, “no pain” through 6, “unbearable pain”). However, data were reported in graphical form which we were not able to include in the analysis. Tawfik 1982 assessed pain relief 30 minutes after analgesia as being complete, excellent or good versus slight relief, while Thakur 2004 assessed pain on a verbal response scale during labour as complete or moderate relief; versus mild or no relief (the time of measurement was not stated). There was no evidence of a significant difference in maternal pain scores between the opioid and TENS groups (average RR 1.15, 95% CI 0.81 to 1.61, two studies). There was substantial heterogeneity for this outcome and so a random-effects model has been used (heterogeneity I2 = 64%, T2 = 0.04, Chi2 test for heterogeneity P = 0.10) (Analysis 29.2).
Two studies (Tawfik 1982; Thakur 2004) involving 290 women reported on maternal side effects of drowsiness and nausea/vomiting. Women in the opioid group were more likely to report drowsiness (RR 8.96, 95% CI 1.13 to 71.07) (Analysis 29.3) and nausea/vomiting (RR 14.06, 95% CI 1.96 to 100.61) (Analysis 29.4) compared with those in the TENS group, although the 95% CIs were very wide for both of these outcomes.
One study reported on caesarean section and assisted vaginal birth rates (Thakur 2004). There were no caesarean sections reported in either the opioid or TENS groups. There was no evidence of a significant difference in the number of assisted vaginal births between groups (RR 5.00, 95% CI 0.24 to 102.85) (Analysis 29.6).
One study reported on fetal distress (Thakur 2004) and found no evidence of a significant difference between groups (RR 5.00, 95% CI 0.24 to 102.85) (Analysis 29.7).
Two studies reported on Apgar scores (Tawfik 1982; Thakur 2004). However, both studies reported mean scores and these data are very difficult to interpret. None of the studies reported information on the number of babies with Apgar scores less than seven at five minutes (prespecified outcome).
Subgroup analysis
We did not carry out planned subgroup analysis because most meta-analyses included data from only one or two studies and separate breakdown on subgroup categories were rarely provided. We therefore did not think that examining outcomes for subgroups would effect the conclusions of the review or offer any other helpful insights.
DISCUSSION
Summary of main results
We set out to answer the question of whether parenteral opioids provide effective pain relief in labour without causing unpleasant adverse effects or harm to women and babies. We don’t have a simple answer to this question. The review includes 29 different comparisons, where an opioid was compared with placebo, with another opioid, where different modes of administration were used, or with transcutaneous electrical nerve stimulation (TENS). Furthermore, for many comparisons there was a lack of consistency in what outcomes were measured, how they were measured, and when they were recorded. For most comparisons, and many outcomes, only one or two studies contributed data, and there were few opportunities to pool data in meta-analysis. For many comparisons data were not reported for many of our prespecified outcomes.
All of the studies were conducted in hospital settings, on healthy women with uncomplicated pregnancies at 37 to 42 weeks’ gestation. We excluded studies focusing on women with preeclampsia or pre-existing conditions or with a compromised fetus.
Summary of results
Parenteral opioids provided some pain relief during labour.
Up to two-thirds of women who received opioids reported moderate or severe pain following administration of analgesia and/or poor or moderate pain relief.
Opioid drugs were associated with nausea, vomiting and drowsiness, although different types of opioids were associated with different adverse effects.
For most outcomes there was no significant evidence of differences between treatment groups.
There was insufficient evidence to assess the safety of opioids in labour.
Intramuscular administration
For pethidine versus placebo, there was better pain relief with pethidine, with sleepiness as the main adverse effect. There was no evidence of significant differences in adverse effects on the woman or on the neonate.
For meptazinol versus pethidine, there was no evidence of a difference in analgesic effect whether assessed either early or late during labour, although significantly more women had vomiting with meptazinol. There was no evidence of a difference in outcomes for the neonate.
For diamorphine versus pethidine, an antiemetic was given as co-therapy to both groups. There was no evidence of difference in analgesic or adverse effects, with the exception of vomiting which occurred more frequently in women given pethidine. Whilst significantly more babies had Apgar less than seven at one minute with pethidine, by five minutes there was no difference between groups, and no evidence of differences in other neonatal outcomes.
For tramadol versus pethidine, the analgesic effect was better with pethidine than tramadol, and there was no evidence of a difference in adverse effects on mother or baby.
For dihydrocodeine versus pethidine, only one study contributed data and there was no evidence of a difference in analgesic effect or adverse effects. Significantly more babies had Apgar scores less than seven at one minute with pethidine compared with dihydrocodeine, but the difference was not apparent by five minutes, and there was no evidence of other differences in neonatal adverse effects.
Other intramuscular comparisons, most of which were tested in only one study, provided few statistically significant findings. For pentazocine versus pethidine (six studies, one with antiemetic addition to opioid), phenazocine versus pethidine, morphine versus pethidine, butorphanol versus pethidine, and tramadol versus no treatment there was no evidence of a difference in maternal or neonatal outcomes between groups.
For nalbuphine versus pentazocine, one study found a statistically significant difference in maternal satisfaction with analgesia, in favour of nalbuphine. Fewer women who received nalbuphine experienced nausea or vomiting.
Intravenous administration
For most comparisons very few studies contributed data, and for most outcomes there was no evidence of significant differences between groups. Several intravenous opioids (including fentanyl, butorphanol and morphine) appeared to perform better than pethidine in terms of analgesic effect (either satisfaction with analgesia or pain scores). Pethidine was associated with worse side effects: compared with pethidine, sedation was lower with fentanyl (one study), and nausea was less with butorphanol and morphine (one study for each comparison). When fentanyl and butorphanol were compared, butorphanol was associated with fewer requests for further analgesia, a reduced need for neonatal ventilatory support, and fewer babies required naloxone (one study).
Opioids versus transcutaneous electrical nerve stimulation (TENS)
For most outcomes there was no evidence of significant differences between groups (maternal satisfaction with analgesia; maternal pain scores; caesarean section; assisted vaginal birth rates; fetal distress). The only significant finding was that women in the opioid group were more likely to experience drowsiness and nausea and vomiting than women in the TENS group.
Overall completeness and applicability of evidence
This review is one of a series of Cochrane reviews examining pain management in labour; other reviews have examined pharmacological and non-pharmacological methods of pain management in labour including biofeedback (Barragán 2011), aromatherapy (Smith 2011b), relaxation techniques (Smith in progress), acupuncture (Smith 2011a), TENS (Dowswell 2009), epidural analgesia (Anim-Somuah 2005) and a range of other methods of pain management.
Studies included in the review were carried out over a long time period (1958 to 2009) during which there have been major changes in women’s and clinicians’ expectations and views of childbirth and analgesia during labour. Some drugs commonly used in the 1950s and 1960s may no longer be available. The increasing use of epidural analgesia in resource-rich countries means that opioids are now less likely to be the drugs of choice in these settings. Having said this, in many parts of the world epidural analgesia is not available to all women, and throughout the world parenteral opioids are still widely used. It is important for all women to make an informed choice about pain relief options available to them; however, providing clear information on the effectiveness and safety of parenteral opioids is not simple in the light of the findings from this review.
With so many different comparisons and outcomes we are not able to provide clear information on the acceptability, effectiveness and adverse outcomes associated with different opioids. In this review we have not compared the effectiveness of parenteral opioids with other types of analgesia or as a co-therapy. At the same time, in many of the studies we have looked at, women were in fact able to have other analgesia, and this may or may not have been reported. The use of other analgesia and co-interventions may have differed by randomisation group, and may have had an independent or synergistic effect on outcomes for women and babies which we were not able to detect. For example, women’s use of nitrous oxide was not consistently reported; the fact that it was not mentioned in a study does not necessarily mean that it was not used by the women involved. It was also difficult to determine equivalence in terms of dosages of different drugs used, their duration of effect and speed of metabolism. Studies also varied in terms of number of doses available to women, and the stage of labour at which further doses were not allowed in order to avoid detrimental effects on the baby.
There was considerable heterogeneity between studies in the outcomes measured and how they were reported and perceived. In some of the older studies (pre-1970), maternal sedation may have been regarded as a desired effect of opioid drugs, and pain relief was sometimes reported by carers rather than by women themselves. There were varied definitions of similar outcomes such as nausea, vomiting (or both), sleepiness, drowsiness, etc. and even greater variation in the way pain and pain relief were measured, and the time points at which measurements were made.
Despite including 57 studies, there were relatively few statistically significant results. Many of the studies had small samples and most did not have the statistical power (singly or pooled) to detect differences between groups for intended or unintended effects that occur infrequently or rarely. In view of the large number of comparisons and outcomes, it is likely that some of the significant findings we have reported occurred by chance. On the other hand, for some less frequent outcomes (e.g. low Apgar scores or the need for neonatal resuscitation), some findings suggested that there may have been a difference between groups but the studies often had small sample sizes, and differences between groups did not achieve statistical significance. In addition, we are aware that statistical and clinical significance may not be the same thing. For example, it is difficult to know what a 0.6 cm difference in scores on a 10 cm visual analogue scale means in this context.
We were surprised by the number of studies where women’s views of pain relief, or their assessments of pain in labour, were not measured at all. We were also surprised at the paucity of data on breastfeeding outcomes. Even more recent studies did not generally collect data on this important outcome, even though observational studies have suggested that opioids are associated with sedation in babies and suppression of sucking in the minutes and hours after birth. We had also hoped to collect information on the costs associated with using opioid drugs; none of the included studies provided data on the costs incurred by health service providers.
It is known that opioids cross the placental barrier, and short-term effects such as the impact of opioids on fetal heart rate patterns and very early neurological scores have been well documented in observational and randomised studies. It is not clear that these effects have any clinical significance or lasting impact on infant well-being. It has also been suggested that exposure to opioids during labour may predispose children to serious long-term effects; however, much more research is needed to confirm or refute these findings from observational studies (Jacobson 1990; Nyberg 2000). None of the studies included in the review followed up women and babies for more than a few hours or days so we are not able to contribute to these debates.
All of the included studies examined intravenous or intramuscular administration; two excluded studies examined the subcutaneous administration of opioids (Cahal 1960; De Kornfeld 1964); three studies compared opioids with TENS (Neumark 1978; Tawfik 1982; Thakur 2004).
Quality of the evidence
Overall we found the evidence to be of poor quality regarding the analgesic effect of opioids, satisfaction with analgesia, adverse effects and harm to women and babies.
In some studies women were not included in the analysis if they received the study drug within 30 to 60 minutes of giving birth or more than four hours before giving birth. Such exclusions are likely to introduce serious bias; we do not know whether these women had different outcomes from the rest of the sample, and it is possible that outcomes may have differed by randomisation group.
The review’s primary outcomes, maternal satisfaction with analgesia reported during labour and postnatally, were reported in different ways (for example, reports of satisfaction, global assessment of pain relief) and were often poorly reported. It was not always clearly stated to whom women reported their pain levels; indeed in some cases clinicians may have made assessments. These methodological problems may mean there was serious response bias in some studies.
Potential biases in the review process
We are aware that the possibility of introducing bias was present at every stage of the reviewing process. We attempted to minimise bias in a number of ways; two review authors carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
While we attempted to be as inclusive as possible in the search strategy, the literature identified was predominantly written in English and published in North American and European journals. We are also aware that publication bias is a possibility, as the review includes several small studies which reported a number of statistically significant results. Although we did attempt to assess reporting bias, constraints of time meant that this assessment relied on information available in the published trial report and thus, reporting bias was not usually apparent.
We may have introduced some bias by converting three-, four- and five-point categorical scales for the measurement of pain or pain relief into binary outcomes. We attempted to be consistent across studies, but this was not always possible as the wording of categories varied in different studies. We have tried to indicate in the results section, and in forest plots, what event rates in treatment groups signify.
Agreements and disagreements with other studies or reviews
The findings and recommendations of this review are similar to other reviews on this topic (Bricker 2002; NICE 2007) and to an earlier Cochrane review looking at IM opioids (Elbourne 2006). Clinical practice guidelines in the UK recommend that women should be informed of the risks of intravenous and intramuscular opioids and of their limitations; NICE 2007 guidelines suggest that intramuscular and intravenous opioids should be available for women to choose, women should be informed of the alternatives, and should be made aware that parenteral opioids may have side effects (such as nausea and drowsiness) and may interfere with breastfeeding.
AUTHORS’ CONCLUSIONS
Implications for practice
There is little high-quality evidence to inform practice; however, for healthy women with an uncomplicated pregnancy who are giving birth at 37 to 42 weeks we have reached the following conclusions.
Parenteral opioids provide moderate pain relief in labour, but cause sedation, nausea and vomiting in the woman and effects on the newborn are unclear.
There is insufficient evidence from the review to support the choice of one opioid over another.
Implications for research
The question many women would like answered is how opioids compare with other forms of pain relief available for use during labour, in terms of analgesic effectiveness and the risk of adverse effects for both women and babies. Given the paucity of useful information from the current review, it is likely that the evidence underlying this further question is also limited. It is important that this evidence is reviewed, however, so that women can be provided with information that is as complete and accurate as possible, and so that remaining gaps in knowledge can be identified and addressed through further research.
We recommend that a large pragmatic randomised controlled trial (RCT) be undertaken to compare pain relief that includes an opioid with a pain relief regimen not including an opioid, that collects data prospectively on all important prognostic factors such as co-interventions. These include additional analgesia and anti-emetics, labour augmentation by means of artificial rupture of membranes or intravenous infusion of oxytocin, use of electronic fetal monitoring and mode of birth. Outcomes for women and their babies in the short and longer term are also required.
Maternal outcomes that would be important to guide practice are actual pain relief and maternal satisfaction with analgesia, important unintended effects such as nausea, vomiting and sedation. For the neonate, Apgar scores at five and 10 minutes, resuscitation including use of naloxone, neonatal intensive care unit admission, initial effective suckling and establishment of breastfeeding, sedation and irritability.
With respect to measuring the effectiveness of an opioid for labour pain, there are a number of issues. Assessment of pain should be measured in the pause between contractions. In order to minimise response bias, it is important that maternal pain assessment be recorded by the woman herself and not by the woman’s caregiver. Lastly, it is important to assess maternal satisfaction to encompass more than just the effects on pain but include other CNS effects. It would be important to measure satisfaction in the short term (within 24 hours of delivery) and again several days postpartum. In addition, it is known that maintaining control in labour is important to women and this relates to pain and pain control; formal assessment of sense of control in labour would therefore be useful.
Stratification at baseline of two important predictors of outcome should include maternal parity and spontaneous or induced labour onset.
All studies were conducted on women labouring in hospital settings exclusively. Many women labour and give birth in community settings, the proportion of which is likely to increase due to the international initiative to normalise birth, and reduce interventions associated with complications. Therefore, more research in midwifery-led units and at home would inform practitioners using opioids in these settings.
If recruitment of women to RCTs is hampered due to strong maternal preferences for pain relief, then a prospective observational study, across different care settings, which collects data on important predictors and outcomes as described for the RCT would also be informative.
PLAIN LANGUAGE SUMMARY.
The use of opioid intramuscular and intravenous pain relieving drugs in labour
Pain during labour is normal and its management is influenced by an interaction between a woman’s mental and emotional state and the physiological changes that occur during labour. The use of pain-relieving drugs during labour is now part of standard care in many countries throughout the world. In recent years, many women in Western countries have chosen to have epidural analgesia to relieve pain. However, some women prefer not to have an epidural, or in some settings an epidural is not available. In many maternity units intramuscular injections of opioid drugs are widely used for pain relief in labour and options for intravenous infusions may also be available. The opioid drugs used include pethidine (also known as meperidine or demerol), diamorphine, nalbuphine, butorphanol, meptazinol, pentazocine, fentanyl and tramadol, and are relatively inexpensive. It is not clear how effective these drugs are, which opioid is best, and how unpleasant side effects (such as vomiting or sleepiness) or harm to women or their babies can be avoided.
We included 57 randomised controlled trials involving more than 7000 women that compared an opioid with placebo, another opioid or transcutaneous electrical nerve stimulation (TENS). Overall, our findings indicated that opioids provided some pain relief during labour, although substantial proportions of women still reported moderate or severe pain. Opioid drugs were associated with nausea, vomiting and drowsiness, and different types of opioids were associated with different side effects. There was no clear evidence of adverse effects of opioids on the newborn. Maternal satisfaction with opioid analgesia was largely unreported but appeared moderate. We did not have sufficient evidence to assess which opioid drug women were most satisfied with, or which provided the best pain relief with the least side effects for mothers and babies.
In this review the 57 studies reported on 29 different comparisons, and for many outcomes only one study contributed data. We did not examine the effectiveness and safety of intramuscular or intravenous (parenteral) opioids compared with other pharmacological methods of pain relief in labour (such as epidural analgesia) and this review needs to be examined alongside related Cochrane reviews. As parenteral opioid drugs are so widely used it is important that more research is carried out so that women can make informed choices about these forms of pain relief.
ACKNOWLEDGEMENTS
We would like to thank Leanne Jones and the staff in the Cochrane Pregnancy and Childbirth office, GJ Hofmeyr and referees for their helpful feedback on early drafts of the protocol. We also thank the translators of papers assessed for inclusion in the review: Angela Cooke, Graham Dane, Sarah Dodd, John Finch, Catherine Fowler, Youngmee Hahn, Kate Kaminski, Andreas Schwab, Caroline Summers, Bunpode Suwannachat, Andrea Svobodova, Elizabeth Whiteley and Darren J Wright.
This updated review forms part of a series of reviews focusing on pain management in labour that will be included in a Cochrane overview of reviews (Jones 2011b); contributing reviews share a generic protocol (Jones 2011a). We would like to thank Leanne Jones for her valuable help in updating this review so as to improve consistency between this and other pain management reviews.
SOURCES OF SUPPORT
Internal sources
(TD) The University of Liverpool, UK.
External sources
(TD) National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews:CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: (not clear) hospital in Oklahoma, USA. 100 women in early active labour (with regular contractions and cervical dilatation 3-4 cm); at term (at or > 37 weeks’ gestation); no medical or obstetric complications or evidence of fetal distress; requesting a “pain shot” rather than an epidural (all women were offered epidural) |
|
| Interventions | Both groups had continuous electronic fetal monitoring and intrauterine pressure catheters Experimental: IV fentanyl 50-100 mcg every 1-2 hrs to a max of 5 doses Control: IV butorphanol 1-2 mg every 1-2 hrs to a max 5 doses (Doses of drugs were approximately equivalent in both arms of the trial.) |
|
| Outcomes | Maternal uterine activity; adverse effects and side effects (including vomiting and sedation); pain scored using 10-point VAS (0 = no pain, 10 = excruciating pain) scores were recorded by nurses; Apgar scores at 1 and 5 min; infant neurological exam 2-4 and 24-36 hrs after birth | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared identical unlabelled, coded syringes. |
| Blinding (performance bias and detection bias) Women |
Low risk | Identical syringes. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Described as double blind. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Outcome assessors reported as blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | It was not clear at what point women were randomised. 155 women enrolled; 24 decided to have an epidural and were excluded (it was not clear whether or not this was after randomisation); 19 women delivered within one hour of first dose and 12 did not request analgesia and were not included in the analysis. Data available for 100 women; if loss occurred after randomisation this represents a very high level of attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | None apparent. |
| Methods | RCT, 2-arm parallel groups. | |
| Participants | Germany: hospital setting. 45 women, in labour, cephalic presentation. |
|
| Interventions | Experimental: IM tramadol 50 mg (N = 23). Control: IM pethidine 50 mg (N = 22). |
|
| Outcomes | Primary outcome: maternal analgesia. Pain assessed as good, not good relief 5-10 min after injection Secondary outcomes: maternal side effects and fetal heart changes |
|
| Notes | German language paper, translation obtained. Tramadol 100 mg plus antiemetic arm not extracted If additional analgesia required, repeat doses could be administered within < 1 hr Tramadol: could have up to 3 repeat doses, 50 mg. Pethidine: could have up to 3 repeat doses, 25 mg. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Assessor was described as unaware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Women not having a normal birth were excluded from analyses. No information on pain relief was available for 7/45 women |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT, 2-arm parallel groups. | |
| Participants | Setting: Belfast hospital, UK. 40 women (healthy and well) in labour, ASA I or II. Exclusion criteria: women planning to have epidural analgesia, with pre-eclampsia, multiple pregnancy, premature labour, allergy to study medications |
|
| Interventions | Experimental: PCA remifentanil 40 mcg with lock-out of 2 minutes Control: PCA pethidine 15 mg with lock-out of 10 minutes. Nitrous oxide was available to all women and women were free to choose an epidural at any stage |
|
| Outcomes | Maternal sedation score (1-5 fully awake to unrousable); VAS 0-10 for pain and satisfaction with pain relief; nausea; anxiety; Apgar scores at 1 and 5 min; infant neurological adaptive capacity score (2 hrs and 24 hrs after birth) | |
| Notes | VAS scores were reported as median with inter-quartile range. We were not able to enter data into Revman tables but have described findings briefly in the text | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “women were randomly allocated.” |
| Allocation concealment (selection bias) | Unclear risk | Not clear when randomisation occurred or how it was carried out |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as double-blind study. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Double-blind. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | It was reported that for some outcomes assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 40 women were randomised, 1 women was not included in the analysis because of a “protocol violation”. 1 woman that withdrew from the study was included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | No baseline imbalance apparent. |
| Methods | RCT, 2-arm parallel groups. | |
| Participants | Hospital setting. 199 women: in labour, at term gestation, following normal pregnancy No inclusion or exclusion criteria reported. |
|
| Interventions | Experimental: IM pentazocine 20-40 mg (N = 91). Control: IM pethidine 50-100mg(N= 89). |
|
| Outcomes | Primary: analgesic and sedative effects. Pain assessed at time of birth or when second injection administered, as very good, good, moderate or none Secondary: maternal and neonatal side effects. |
|
| Notes | If additional analgesia required opioid repeated once after 3 or > hrs of first injection. Actual dose received by women not reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Ampoules numbered and in random order. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Reported as double blind, but no description of how achieved. Identical volume but appearance not described |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Reported as double blind, but no description of how achieved. Identical volume but appearance not described |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants analysed, but missing data for some outcomes |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | Balanced at baseline for age, parity, blood pressure, pulse, frequency contractions, FHR, augmented labour, intensity of labour, membranes intact or ruptured |
| Methods | RCT, 3-arm parallel group design. | |
| Participants | Setting: hospital in Baltimore, USA. 212 women randomised (141 included in the analyses in this review) Inclusion criteria: women admitted to hospital for planned vaginal birth, at term, requesting analgesia (birth under regional anaesthesia) Exlusions: imminent birth, allergy to any study medication or requiring birth under general anaesthesia |
|
| Interventions | Interventions at 3-4 cm dilatation for primiparous, and 4-5 cm for multiparous women Group 1: pentobarbital IV (initial dose 200 mg) dosage varied Group 2: pethidine IV (initial dose 100 mg), (69 women). Group 3: morphine IV (initial dose 8 mg), (72 women). All 3 groups also received 0.4 mg of scopolamine. If further analgesia was required women were given a half of the initial dose and 0.2 mg of scopolamine. If more than 2 additional doses were required analgesia was at the discretion of the attending doctor In this review we have included groups 2 and 3 only in the analyses; pentobarbital (a barbiturate) is no longer used for pain relief in labour |
|
| Outcomes | Length of labour, amount of analgesia required, obstetric complications and neonatal condition (Apgar score at 1 minute). Maternal perceptions were recorded 3 days after birth (satisfaction and amnesia). A focus of this paper was the perception of staff on whether women were “manageable”. Unmanageable women were those who were “possibly dangerous to others or themselves, perhaps by leaving her bed”. Staff had the option of removing “unmanageable women from the study and prescribing whatever medication was deemed suitable | |
| Notes | All women included delivered under regional anaesthesia. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “in a random manner.” |
| Allocation concealment (selection bias) | Low risk | Coded vials containing study drugs were provided by pharmacy |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as blinded. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | “None of the personnel concerned with the administration of the drugs or the evaluation of the patients’ reaction had access to the master list at any time.” |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | “None of the personnel concerned with the administration of the drugs or the evaluation of the patients’ reaction had access to the master list at any time.” |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | All women appear to be accounted for in the analysis and there were few missing data. The data regarding babies was less clear, denominators were not provided |
| Selective reporting (reporting bias) | High risk | Results were not provided for babies. There was a statement in the text “there were few infant complications in the neonatal period; none of these appeared related to the drugs” |
| Other bias | Unclear risk | Baseline characteristics described as similar. |
| Methods | RCT, 2-arm parallel groups. | |
| Participants | Setting: UK hospital. 46 women (20 primiparous and 14 multiparous women included in the analyses). Uncomplicated pregnancy Exclusions: first stage of labour > 12 hr, second stage > 1 hr, body weight <45 kg, multiple pregnancy, non-vertex presentation, preterm or postmature labour, previous caesarean section, birth weight outside the 5th and 95th centiles for gestational age, congenital fetal abnormality |
|
| Interventions | Experimental: IM meptazinol 1.5 mg/kg body weight plus 10 mg metoclopramide hydrochloride (N = 17) Control: IM pethidine 1.5 mg/kg body weight plus 10 mg metoclopramide hydrochloride (N = 17) |
|
| Outcomes | Neonatal acid-base balance. Maternal pH pre injection, repeated at head crowning, neonatal pH at 10 and 60 min PN | |
| Notes | If additional analgesia required opioid repeated > 3 hrly. Actual dose received by women not reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind but not described. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind but not described. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind but not described. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 12 women excluded from analysis, reasons for all exclusions not explained |
| Selective reporting (reporting bias) | High risk | Reasons why some participant data excluded not explained. 3/12 excluded because problem with pH analyser (meptazinol group) |
| Other bias | Low risk | No baseline imbalances. |
| Methods | RCT, 3-arm parallel groups. | |
| Participants | Setting: The Netherlands, Department of Obstetrics and Gynaecology 180 enrolled, 159 completed the study. Inclusion criteria: healthy ASA physical status I or II term parturients in an active stage of labour, with singleton cephalic presentation, without prior administration of opioid analgesics Exclusion criteria: obesity (BMI ≥ 40 kg m−2), opioid allergy, substance abuse history, and high-risk patients (pre-eclampsia, severe asthma, insulin-dependent diabetes mellitus, hepatic insufficiency, or renal failure) |
|
| Interventions |
|
|
| Outcomes | Outcomes: pain scores (VAS) every hour; sedation score (1 awake, 2 sleepy, 3 eyes closed, 4 eyes closed but rousable, 5 unrousable; overall satisfaction on 10-point scale 2 hours after delivery; side effects - nausea, vomiting, itching; Apgar scores at 1, 5 mins; cord blood gas analysis; NACS scores at 15 min and 2 hr after delivery; oxytocin use; instrumental delivery; CS; spontaneous delivery | |
| Notes | “All women received similar instructions on how to use the PCA device: all parturients were instructed to press the bolus button whenever they needed pain relief.” | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Established using a computer generated random sequence in numbered envelopes.” |
| Allocation concealment (selection bias) | Low risk | “Study medication was prepared and blinded by hospital pharmacy.” |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | “Observants and medical personnel attending to the parturient were unaware of the drug assignment.” |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | “with exception of baseline data, all observations and measurements were made by blinded observers.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 180 enrolled, 159 completed the study: 52 R group; 53 M group; 54 F group; 21 excluded because delivered within 1 hour after randomisation Says “Data analysis was per-protocol”. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes discussed in methods appear to have been reported upon within results. However, the study protocol was not evaluated |
| Other bias | Low risk | Baseline characteristics similar. |
| Methods | RCT, 2-arm parallel groups. | |
| Participants | Setting: UK hospital. 200 women. 66% primips, 34% multips, > 35 weeks’ gestation. Singleton, uncomplicated pregnancy Exclusions: toxaemia, chronic medical disease, isoimmunization, obstetric complication |
|
| Interventions | Experimental: IM pentazocine 48 mg (N = 100). Control: IM pethidine 120 mg (N = 100). Nalorphine hydrobromide + methylphenidate given if opioid administered within 2/24 of second stage diagnosis and, or fetal distress |
|
| Outcomes | Primary outcome: analgesic effects: pain assessed at time of injection and every 30 minutes for 4 hrs. Severe or moderate pain. Pain relief complete, partial or none Secondary outcomes: maternal: vomiting, blood pressure and pulse. Neonatal: Apgar at 1 minute in babies born within 4 hrs of opioid |
|
| Notes | If additional analgesia required opioid repeated after 4 hrs. As inclusion criteria > 35 weeks’ gestation, may include preterm infants | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States ‘double blind’ but does not report how achieved. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States ‘double blind’ but does not report how achieved. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 200 women randomised. Exclusion of women from analyses if inadequacy of records, reached second stage before analgesic assessment, operative birth or another intervention. Exclusion of babies from Apgar analysis if additional analgesia given, GA, antidote given to mother pre-birth or clinical explanation for depressed baby. Denominators for outcomes not clear |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Balanced at baseline for age, parity, height, weight, blood pressure, attendance at antenatal classes and infant weight |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: Cape Town, South Africa. 29 women in established labour, not clear how many primips, mean age 24 years, women were expected to have a vaginal birth and have no antenatal medical or obstetric problems |
|
| Interventions | Experimental: pethidine, IV PCA 10 minute lock out, 0.3 mg per kg Control: pentazocine, IV PCA 10 minute lock out, 0.15 mg per kg |
|
| Outcomes | Pain relief in labour (assessed by midwife); pain relief (measured immediately after labour (10 cm VAS) and 24 hrs postpartum from mother); satisfaction with pain relief; maternal and neonatal serum samples; Apgar score at 1 and 5 min; infant weight; neuro-behavioural examination on 1st and 5th day | |
| Notes | The study also included a non-randomised control group; we have not included this group in the analysis | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | It was reported that women were attended by the same midwife throughout labour who was not informed what medication women received. It is not clear whether this blinding was achieved for all staff |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Outcome assessors of neonatal outcomes were reported to be blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Overall attrition not clear, there was some missing data for some outcomes. Denominators were not provided in all of the results tables |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | No baseline imbalance apparent. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | UK setting: hospital. 161 women randomised, data available for 133 women. 52% primips, 48% multips, cx at least 3 cm dilated, 37 or > weeks’ gestation in spontaneous or induced labour (induction by amniotomy and IV infusion oxytocin) |
|
| Interventions | Experimental: IM diamorphine 7.5 mg (primips), 5 mg (multips) plus 12.5 mg prochlorperazine (N = 65) Control: IM pethidine 150 mg (primips), 100 mg (multips) plus 12.5 mg prochlorperazine (N = 68) |
|
| Outcomes | Primary outcome: maternal pain at 1 hr VAS (0-100), pain intensity (0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain), pain relief (0 = none, 1 = slight, 2 = moderate, 3 = good, 4 = complete) Secondary outcomes: maternal: vomiting, sedation, global analgesia assessment at 24 hr (good or poor). Neonatal: Apgar at 1 and 5 min, resuscitation, naloxone administration, SCBU admission, significant morbidity (seizures, respiratory distress, intraventricular haemorrhage, necrotising enterocolitis) |
|
| Notes | Second dose at maternal request: her choice of drug or epidural. Stratified by maternal parity. Trial stopped early after recruitment of150 women. Planned sample size was 200 women | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block sizes of 6. |
| Allocation concealment (selection bias) | Low risk | Coded drug containers, randomisation code not broken until analysis |
| Blinding (performance bias and detection bias) Women |
Low risk | States double blind, drug containers identical in appearance |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | States double blind, drug containers identical in appearance |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | It was stated that the randomisation code was not broken until the analysis stage |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 28 (17%) excluded as delivered within 1 hr of administration of study drug |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | Balanced at baseline. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Italy: hospital care setting. 40 women. Full-term pregnancy, cx ≥ 4 cm, in spontaneous active labour and requiring analgesia |
|
| Interventions | Experimental: IM tramadol 100 mg (N = 20). Control: IM pethidine 75 mg (N = 20). |
|
| Outcomes | Primary outcome: maternal pain relief and acceptability. Pain assessed hrly up to 5 hrs, VAS 1-3 Secondary outcomes: maternal: observations (pulse, BP, respiratory rate, arterial oxygen saturation). Neonatal: Apgar at 1 and 5 min. Umbilcal cord pH |
|
| Notes | Second dose of study drug allowed after 2 hrs as required. Italian language, translation obtained. Data were presented in a way in which we were not able to incorporate them into data tables in RevMan | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear how many women analysed as only percentages reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | High risk | No baseline characteristics table - unclear re maternal parity Likely response bias as no information on whom women reported to about their pain post injection |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: London hospital, UK. 60 healthy women at term (38-42 weeks) requiring pain relief in labour Women requesting epidural, that had already received opioid analgesia, were receiving treatment for depression or where the fetus was at risk were excluded |
|
| Interventions | Experimental: (30 women) nalbuphine, 3 mg with 3 mg increments to a max of 18 mg per hour; lockout time 10 minutes (total max dose = 42 mg) Control: (30 women) pethidine, 15 mg, 15 mg increments to a max of 90 mg per hr; lockout time 10 minutes (total max dose = 210 mg) Entonox ® was available to women in both groups but was withheld for 30 min for analgesia assessment. Analgesia was stopped in the 2nd stage if there were side effects or if the woman requested an alternative method |
|
| Outcomes | Pain (measured on 5-point scale from 1- no pain to 5 - very severe); pain relief (assessed 1 day after birth; pain relief rated as good or excellent and women saying they would use the same method again); sedation (1 awake, 3 asleep); neuro-behavioural assessment 6 - 10 hrs after birth; FHR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly allocated.” |
| Allocation concealment (selection bias) | Unclear risk | Described as double blind but allocation concealment was not described |
| Blinding (performance bias and detection bias) Women |
Low risk | Very little information. Described as double blind. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Very little information. Described as double blind. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | There was some outcome data for all but one of the women randomised, but there were high levels of missing data for some neonatal outcomes (e.g. neurological infant assessments 40/60 babies available for analysis) |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | There was some baseline imbalance; 6/30 in the nalbuphine group were multiparous compared with 12/30 in the pethidine group. The authors report that they took this into account in the analysis. In this review data have not been adjusted for baseline imbalance |
| Methods | RCT, 2-arm parallel groups. | |
| Participants | New Jersey USA, hospital setting, 1994. 28 women in labour (36 randomised) with uncomplicated pregnancies, singleton, vertex presentation, at term (37 - 41 weeks), 4 cm or less cervical dilatation, at least 3 contractions in 10 minutes, no known maternal or fetal conditions that would affect FHR tracings, fetal reactive, no medications that would affect FHR in the previous 2 weeks Exclusions criteria: meconium staining, pregnancy induced hypertension, fetal tachy- or brady-cardia, arrhythmias or decelerations, chorioamnionitis, FGR, abnormal placenta, maternal fever, fetal chromosomal disorder of structural abnormality |
|
| Interventions | Experimental: IV nalbuphine10 mg. Control: IV pethidine 50 mg. Both groups had continuous fetal monitoring for 1 hour following medication |
|
| Outcomes | FHR (accelerations, high and low variation); Apgar scores < 8 at 1 and 5 min; mode of birth; cord pH <7.15 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Not specified. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 36 women were enrolled. 8 women did not have sufficient FHR tracings and were not included in the analysis (22% attrition) |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | No apparent baseline imbalance. |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: hospital in USA. 500 women admitted to hospital in labour. Little information provided |
|
| Interventions | Experimental: (185 women) alphaprodine (Nisentil), initial dose 40mg IV, subsequent doses IM Control: (210 women) pethidine, initial dose 100 mg IV, subsequent doses IM Both groups received scopolamine. Analgesia was for the first stage of labour, birth was carried out “with rare exception” under “saddle block or pudendal block terminal anesthesia” |
|
| Outcomes | Pain relief (rated just before leaving the room for childbirth); side effects and length of labour | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Low risk | Coded drug containers. |
| Blinding (performance bias and detection bias) Women |
Low risk | Drugs were prepared by pharmacy in coded containers and the codes were not revealed until after birth |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Drugs were prepared by pharmacy in coded containers and the codes were not revealed until after birth |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Drugs were prepared by pharmacy in coded containers and the codes were not revealed until after birth |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 500 women were randomised, 55 women received no analgesia and were excluded, 22 women received more than 1 dose of opioid (not necessarily the same drug) and were excluded, 21 women who were in preterm labour or had a CS were excluded and 1 woman was excluded because she was sensitive to study medication. Data available for 395 women (21% attrition) |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Study medication was for pain relief in the first stage of labour, most women received a pudendal block for birth so outcomes relating to birth may not be attributable to study medication alone |
| Methods | RCT 2-arm parallel group design. | |
| Participants | UK: hospital setting. 212 women in spontaneous or induced labour with cephalic presentation at > 36 weeks’ gestation. Recruited to the trial at 36 week antenatal clinic visit |
|
| Interventions | Experimental: IM phenazocine 3 mg (N = 107). Control: IM pethidine 150 mg (N = 105). |
|
| Outcomes | Primary outcome: maternal analgesia assessed in labour as poor, fair, good, very good. Pain relief also assessed in postnatal questionnaire within 36 hours of birth Secondary outcomes: maternal: amnesia, restlessness, anxiety, vomiting. Neonatal: Apgar at 1 and 5 min |
|
| Notes | Epidural available if further analgesia required. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Code kept by hospital pharmacist and remained unbroken until trial completed |
| Blinding (performance bias and detection bias) Women |
Low risk | States double blind, coded ampoules but no further description given |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | States double blind, coded ampoules but no further description given |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Code kept by hospital pharmacist and remained unbroken until trial completed |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 212 women randomised. Number of women analysed is not reported |
| Selective reporting (reporting bias) | High risk | MW assessed maternal side effects in labour. |
| Other bias | Unclear risk | Although baseline characteristics described as similar - proportion of primips to multips not provided. Balanced for age, parity, height , weight, cx dilatation PN maternal recollection of pain within 36 hr and unclear to whom women reported ratings |
| Methods | RCT. 2-arm parallel group design. | |
| Participants | 185 randomised. Analysis for 160 women in labour. Inclusion criteria: primiparous, no pregnancy complications. Exclusions: women with hypertension or pre-eclampsia. It appeared that women who had any complications during birth (e.g. CS) were excluded after randomisation |
|
| Interventions | Intervention group: Avacan ® 25 mg IM (a spasmolytic). Control group: Fortral ® 20 mg IM (pentazocine) |
|
| Outcomes | Number of requests for analgesia, infant birthweight, Apgar score (at birth) | |
| Notes | Data extraction was done from translation notes. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Described as a double-blind trial but methods were not described |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as double-blind. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Described as double-blind. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 185 women approached, 25 were excluded and results suggest that any women who had CS were excluded from the analysis along with women who had long labours (> 24 hrs) or where no injections were given |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Assessment of risk of bias done using translation notes. |
| Methods | RCT 4-arm parallel group design. | |
| Participants | Setting not clear, USA. 200 women admitted to hospital in the 1st stage of normal labour, mean age 24 years, women received medication if they complained of moderate or severe pain |
|
| Interventions | Experimental: (100 women) (i) IV butorphanol 1 mg (67 women) (ii) IV butorphanol 2 mg (33 women) Control: (100 women) (i) IV pethidine 40 mg (68 women) (ii) IV pethidine 80 mg (32 women) |
|
| Outcomes | Pain intensity (graphs with hourly readings); pain relief (4-point scale); neurobehavioural assessment 1 day after birth (Scanlon scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. Described as “double blind”. |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as double blind but little detail of methods of allocation concealment or blinding |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Described as double blind but little detail of methods of allocation concealment or blinding |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Described as double blind but little detail of methods of allocation concealment or blinding |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss to follow-up apparent. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Very little information on study methods. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Austria: hospital setting. 40 women with no pregnancy complications, in spontaneous and induced labour, cx 3 - 5 cm dilated. 72.5% primips, 27.5% multips |
|
| Interventions | Experimental: IM tramadol 100 mg (N = 20). Control: IM pethidine 100 mg (N = 20). |
|
| Outcomes | Primary: pain relief, assessed 10, 30, 60, 120 min after injection using VAS 0-100, 0 = pain free to 100 strongest pain experienced Secondary: side effects, augmentation and type of birth. |
|
| Notes | Not stated in one dose only. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Blinding not described. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Blinding not described. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All women analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Baseline characteristics stated as similar. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: UK hospital. 100 women in labour at term gestation with uncomplicated pregnancy |
|
| Interventions | Experimental: Meptazinol 1.8 mg/kg body weight (N = 50). Control: pethidine 1.8 mg/kg body weight (N = 50). All participants received promethazine 12.5 mg with first injection |
|
| Outcomes | Primary: newborn effects: Apgar score at 1 and 3 min. | |
| Notes | If additional analgesia required, a repeat injection could be administered 3 hourly 6/50 women from each arm received a second dose at a 3-hourly interval | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind but method not described. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind but method not described. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind but method not described. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 5 babies excluded from analysis due to heart defects and fetal distress |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Balanced for parity, weight and size of baby at baseline. |
| Methods | RCT 3-arm parallel group design. | |
| Participants | Setting: Germany, hospital. 66 women. 38-41 weeks’ gestation, free of complications, in active labour and requiring analgesia, excluded if analgesia received within 4 hours of randomisation Parity: not reported. |
|
| Interventions | Experimental: IM tramadol 100 mg (N = 20); IM tramadol 100 mg + triflupromazine 10 mg (N = 25) Control: IM pethidine 50 mg + triflupromazine 10 mg (N = 21) Unclear if single or multiple doses administered, and if additional analgesia administered |
|
| Outcomes | Maternal outcomes: maternal pain intensity VAS (0-10 cm) 30, 60,120 and 180 minutes, vomiting, drowsiness, blood pressure, heart rate, cardiotocogram | |
| Notes | Tramadol 100 mg only group (A) not included in our analyses. German language, translation obtained | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “zulfallszahlentafel” coincidence number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Stated as double blind but methods not described. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Stated as double blind but methods not described. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Stated as double blind but methods not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 2/66 women excluded due to giving birth within 1 hour of study drug administration |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: hospital in Iran. 88 primiparous women in spontaneous labour, gestation ≥ 37 weeks, and cervix 5 cm dilated Excluded if high-risk pregnancy, narcotic addiction. |
|
| Interventions | Experimental: IM (placebo) normal saline 1.5 ml (N = 44). Control: IM pethidine 75 mg (N = 44). |
|
| Outcomes | Primary: analgesic effect. Pain assessed pre and post injection using Likert Scale VAS: 10 cm line, 0% = minimum effect, 100% = maximum effect Secondary: side effects on uterine contractions (contraction duration and interval recorded 3 times 15-60 min post injection) and neonatal Apgar score at 1 and 5 min |
|
| Notes | Timing of maternal pain assessment not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “divided randomly”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Low risk | Study agents were of identical volume and appearance. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Study agents were of identical volume and appearance. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Study agents were of identical volume and appearance. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Number of participants analysed and planned analysis not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | The number of women allocated to each group is not reported and unclear if there are baseline imbalances in prognostic factors |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Turkey: hospital setting. 59 primiparous women with uncomplicated pregnancy at term gestation, in labour with cervix 3-5 cm dilated and reporting a pain score 4 - 5 according to Wong-Baker Faces Pain Rating Scales with 0 = no pain, 5 = most intense pain Exclusions: maternal medical disorders, history of drug or alcohol abuse |
|
| Interventions | Experimental: IM tramadol 100 mg, single dose (N = 30). Control: IM pethidine 100 mg, single dose (N = 29). |
|
| Outcomes | Primary: analgesic effect assessed 30, 60 and 120 minutes following injection using Wong-Baker Faces Pain Rating Scales with 0 = no pain, 5 = most intense pain Secondary: side effects: nausea, vomiting, drowsiness, fatigue and neonatal effects (Apgar score at 1 and 5 min) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. ‘randomly divided into two groups’. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Outcome assessor unaware of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Losses to follow-up not explained and no intention-to-treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: Iran, hospital. 160 women. Free of complications, spontaneous and induced onset, cx 4 cm dilated, in active labour and requiring analgesia. Women excluded if cx dilated > 5 cm Parity: not reported. |
|
| Interventions | Experimental: IM tramadol 100 mg (N = 80). Control: IM pethidine 50 mg (N = 80). 2nd dose on maternal request after 4 hours but pethidine withheld if cx dilated > 8 cm and tramadol given instead |
|
| Outcomes | Maternal outcomes: maternal pain intensity VAS (0-10 cm) 10, 30 and 1 hourly intervals until birth, maternal satisfaction 24 hours postpartum 5-point scale (excellent, very good, good, fair, poor), drowsiness, nausea, vomiting. Neonatal outcomes: Apgar score at 1 and 5 minutes, naloxone administration, respiratory depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated codes. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Drugs administered by clinician blind to group allocation, but does not state how this was achieved |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Women fed back their maternal pain score to anaesthetist. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Flow chart addresses all data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Baseline characteristics similar. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Argentina: 2 hospitals. 310 women of mixed parity, in labour 37-42 weeks’ gestation with cervix 4-6 cm dilated, cephalic presentation and requiring analgesia Exclusions: maternal medical condition, evidence of fetal distress, previous caesarean section |
|
| Interventions | Experimental: IM nalbuphine 20 mg, single dose (N = 152). Control: IM pethidine 100 mg, single dose (N = 158). |
|
| Outcomes | Primary: neonatal Apgar score < 7 at 1 min. Secondary: maternal pain assessed using VAS pre-injection, and 30 and 120 min afterwards (severe pain 75 or >), nausea, vomiting and type of birth. Neonatal side effects: condition over first 24 hrs, admission to neonatal intensive care nursery |
|
| Notes | Stratified by hospital. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated code. |
| Allocation concealment (selection bias) | Low risk | Coded ampoules, sealed and prepared by independent pharmacist and identical in appearance |
| Blinding (performance bias and detection bias) Women |
Low risk | Identical ampoules. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Identical ampoules. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Few losses to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned if women reported pain to their caregiver. |
| Other bias | Unclear risk | Data analyst unaware of coding. Balanced at baseline. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | USA: hospital setting. 93 primiparous women in labour, uncomplicated pregnancy at 37 or more weeks’ gestation and in pain described as moderate or severe |
|
| Interventions | Experimental: IM pentazocine 60 mg (N = 38). Control: IM pethidine 100 mg (N = 45). |
|
| Outcomes | Primary: pain relief assessed at 1 hr, as good or poor. Secondary: maternal side effects, nausea or vomiting, labour progress. Neonatal Apgar score at 1 and 5 min |
|
| Notes | If additional analgesia was required, a second injection could be administered at the discretion of medic. Not stated if IOL onset included | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Identical vials with code number but no further information given |
| Blinding (performance bias and detection bias) Women |
Low risk | Identical vials with code number. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Identical vials with code number. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | No-one involved with the immediate care of the woman knew the drug identity |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 83/93 women analysed and reasons for missing data not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear how many women randomised to each group and balance at baseline unclear |
| Methods | (Feasibility study) RCT, 2-arm parallel group design. | |
| Participants | 10 primiparous women in labour requesting pain relief, and who had no made any request for alternative analgesia | |
| Interventions | Intervention group: meptazinol (PCA IM) up to 600 mg (75 mg per ml) Comparison group: pethidine (PCA IM) up to 400 mg (50 mg per ml) Doses described as equivalent. Nitrous oxide available to women in both groups |
|
| Outcomes | Pain, drowsiness and nausea on a 100 mm VAS (0 = no pain) during labour and also rated on the day after birth; Apgar score and neonatal weight gain over 3 days | |
| Notes | Feasibility study focusing on PCA IM administration of opioids | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, “randomly allocated”. |
| Allocation concealment (selection bias) | Unclear risk | Described as a double-blind comparison but methods not described |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Described as a double-blind comparison but methods not described |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Described as a double-blind comparison but methods not described |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Described as a double-blind comparison but methods not described |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 10 women randomised and all accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | No baseline imbalance apparent. |
| Methods | RCT. 2-arm parallel groups. | |
| Participants | Setting: Beijing hospital, China. 60 women in early labour (cervical dilatation 2-3 cm) at term, with singleton pregnancy, vertex presentation, with no pregnancy complications |
|
| Interventions | Intervention group: 100 mg IM tramadol. Comparison group: no analgesia. |
|
| Outcomes | Analgesic effect (not clear when measured); satisfactory, some effect or no effect | |
| Notes | Data extraction from translation notes. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were divided “at random” into groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Women |
High risk | Women in the control arm received no treatment. |
| Blinding (performance bias and detection bias) Clinical staff |
High risk | Women in the control arm received no treatment. |
| Blinding (performance bias and detection bias) Outcome assessor |
High risk | Women in the control arm received no treatment. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Denominators not clear. No apparent loss to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | It was not clear whether or not women in the comparison group were given any analgesia or whether they requested any |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Brazil: hospital. 56 women. No information in abstract about participant inclusion criteria or characteristics |
|
| Interventions | Experimental: IM nalbuphine 10 mg. Control: IM pethidine 100 mg. |
|
| Outcomes | Analgesia and side effects. Neonatal: Apgar score. |
|
| Notes | Abstract only: insufficient information about participants. Not reported if > 1 dose given or anti-emetic. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Described as “randomly selected” but not explained how. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Impossible to decipher. |
| Other bias | Unclear risk | Impossible to decipher. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: US hospital. 80 women at term gestation, in spontaneous and induced labour with moderate to severe pain Exclusions: drug abuse history, systemic disease and women who planned to breastfeed their babies |
|
| Interventions | Experimental: IM butorphanol 1 or 2 mg (N = 40). Control: IM pethidine 40 or 80 mg (N = 40). |
|
| Outcomes | Primary: pain intensity assessed 30 and 60 min post injection. Described as 1 = slight relief, 2 = moderate relief, 3 = good relief, 4 = complete relief. Maternal satisfaction of overall drug effect assessed postnatally as 1 = poor, 2 = fair, 3 = very good, 4 = excellent Secondary: neonatal Apgar score at 1 and 5 min, resuscitation. Maternal nausea and vomiting | |
| Notes | If additional analgesia was required, a second dose of original drug could be administered Maternal parity not reported but different drug dosage depending on parity Almost all (77/80) participants were non-Caucasion and all were delivered with local or regional anaesthesia | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Drugs in consecutively numbered, identical vials prepared by independent laboratory |
| Blinding (performance bias and detection bias) Women |
Low risk | States double blind, drugs in identical vials. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | States double blind, drugs in identical vials. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All women analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Balanced at baseline for type of labour, weight, age, type of birth and anaesthetic agent |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: Germany. 40 women. Term pregnancy, cx dilated 2-3 cm, spontaneous labour onset, in active labour and requiring analgesia Parity: not reported. |
|
| Interventions | Experimental: IM nalbuphine 0.1 mg/kg (N = 20). Control: IM pethidine 0.8 mg/kg (N = 20). States dosing was ‘on demand’. Unclear if single or multiple doses administered, and if additional analgesia administered |
|
| Outcomes | Maternal outcomes: maternal pain relief VAS (0-20 cm) 30, 60, 90 and 120 minutes, opinion of pain relief 12 hours postpartum, sedation 4-point scale (awake, tired, sleeping but will wake if spoken to, sleeping but will wake if shaken, asleep not possible to wake up) 30, 60, 90 and 120 minutes, ‘side effects’, blood pressure, heart rate, CTG. Neonatal outcomes: Apgar score at 10 minutes, respiratory depression | |
| Notes | German language - translation obtained. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
High risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
High risk | Not reported. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 4/40 women excluded due to insufficient pain relief. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: UK hospital. 206 mixed parity healthy women, in spontaneous or induced labour, at > 35 weeks’ gestation, cephalic presentation and in pain described as severe, moderate or slight |
|
| Interventions | Experimental: IM pentazocine 40 mg (N = 73). Control: IM pethidine 100mg or 50 mg (N = 133). |
|
| Outcomes | Primary: pain intensity assessed at 30, 60 and 90 min post injection, described as severe, moderate or slight. Asked at 12 - 24 hr postnatal if drug had helped Secondary: neonatal Apgar score at 1 and 5 min, maternal side effects of nausea or vomiting |
|
| Notes | If additional analgesia required, a maximum of 3 further doses of study drug could be administered at 2-3 hrly intervals. Women could also use nitrous oxide and some had a paracervical block > 35 weeks’ gestation therefore preterm babies may be included |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Coded ampoules but no further information given. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind. Coded ampoules but not stated if identical in appearance |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind. Coded ampoules but not stated if identical in appearance |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind. Coded ampoules but not stated if identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 29/206 excluded because delivered or had paracervical block. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: labour ward of a university health centre in Canada 23 women randomised when they requested analgesia, 83% primips, gestational age > 32 weeks, weight < 100 kg or > 50 kg, able to speak English, no history of opioid abuse and normal fetal heart tracing (Women recruited to the study had medical contraindications to epidural although it was no specified what these were.) |
|
| Interventions | Experimental: fentanyl, PCA 10 micro g per ml, initial bolus dose 1 ml, basal infusion rate of 2 ml per hr with PCA bolus 2 ml Control: alfentanil, PCA 100 micro g per ml, initial bolus dose 1 ml, basal infusion rate of 2 ml per hr with PCA bolus 2 ml Doses described as equivalent. Drugs were discontinued in both groups when the attending midwife estimated that birth was likely to take place within 15 min |
|
| Outcomes | Pain (rated on a 100 mm VAS, recorded at baseline and every 30 minutes thereafter); sedation (nurse rated hourly); side effects; satisfaction with pain relief (good, adequate, inadequate); Apgar scores at 5 and 10 minutes; cord blood gases; naloxone dose; neonatal neuro-behavioural score at 4 and 24 hrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation schedule prepared by pharmacy. |
| Allocation concealment (selection bias) | Low risk | Plain, numbered vials prepared by pharmacy. |
| Blinding (performance bias and detection bias) Women |
Low risk | Plain vials prepared by pharmacy. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Plain vials prepared by pharmacy. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Stated that assessment was carried out by staff blind to group assignment |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 25 women were randomised. 2 did not follow the protocol and were not followed up. There was missing data for some variables |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Small sample size and the onset of analgesia varied. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: UK hospital. 1,100 women. 37-42 weeks’ gestation, in active labour and requiring analgesia Parity: 44% primips, 56% multips. |
|
| Interventions | Experimental: IM meptazinol 100 mg ≤ 70 kg, 150 mg > 70 kg (N = 513) Control: IM pethidine 1100 mg ≤ 70 kg, 150 mg > 70 kg (N = 522) Second dose, epidural or inhalation analgesia at maternal request |
|
| Outcomes | Maternal outcomes: maternal pain at 30, 60, 90 and 120 minutes VAS (0-100 mm), nausea, vomiting, sleepiness, use of supplementary analgesia, method of birth, opinion of analgesic effect assessed 3-5 days postpartum (rated excellent, good, poor but just able to cope, no effect and required additional analgesia). Neonatal outcomes: Apgar at 1 and 5 min, resuscitation, naloxone administration, fetal distress, type of feeding | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Coded drug containers prepared at a site remote from the trial |
| Blinding (performance bias and detection bias) Women |
Low risk | States double blind and used coded drug containers. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | States double blind and used coded drug containers. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | States double blind and used coded drug containers. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 65 women excluded due to clerical errors or administration of wrong drug |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Women were balanced at baseline for age, weight, parity and gestation |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: UK hospital. 94 women. > 35 weeks’ gestation, age ≥ 18 years, excluded if diabetic, history of renal or hepatic impairment or taking monoamine oxidase inhibitors, in active labour and requiring analgesia Parity: ≤ 3. |
|
| Interventions | Experimental: IM pentazocine 60 ≤ mg (N = 46). Control: IM pethidine 15 ≤ 0 mg (N = 48). Up to 3 injections > 3 hours apart at maternal request. |
|
| Outcomes | Maternal outcomes: satisfied with analgesia, nausea, vomiting, sleepiness, use of additional analgesia (study drug), method of birth. Neonatal outcomes: Apgar at 1 and 5 min | |
| Notes | Data for some outcomes available after first dose. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind but how achieved not reported. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind but how achieved not reported. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind but how achieved not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Exclusions from most analyses. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | Balanced at baseline for age, parity, induced labour onset. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: South Africa - hospital. 75 women. Healthy with no clinically detectable abnormality, in active labour, spontaneous and induced, and requiring analgesia. Excluded if history of hypersensitivity to any drug, previous caesarean, preterm labour, cardiac, pulmonary or renal disease and significant hypertension Parity: mixed. |
|
| Interventions | Experimental: IM meptazinol 100 mg (N = 37). Control: IM pethidine 100 mg (N = 38). No concomitant analgesia given, metoclopramide 10 mg as required for nausea |
|
| Outcomes | Maternal outcomes: pain at 1 hr 5-point VAS scale, drug-related side effects. Neonatal outcomes: Apgar at 1 and 5 min, paediatrician assessment at 24 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind but does not describe how blinding achieved |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind but does not describe how blinding achieved |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind but does not describe how blinding achieved |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Number of women randomised not reported only number analysed, not same numbers analysed for all outcomes |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Women requiring caesarean or epidural were excluded from further study, unclear if this is pre- or post-randomisation |
| Methods | RCT, 3-arm parallel group design. | |
| Participants | Setting: hospital in North Carolina USA. 45 healthy women with singleton pregnancies requesting analgesia Women with allergies to the study medication, those that had already had medication and those taking opioids for chronic conditions were excluded, along with those with any signs of fetal distress |
|
| Interventions | Experimental: (15 women) IV butorphanol, 1 mg bolus. Control: (15 women) IV pethidine, 50 mg bolus. (A second control group received IV pethidine 25 mg plus 0.5 mg butorphanol; this group has not been included in the analyses in this review.) |
|
| Outcomes | Pain (measured on a 0 - 10 numerical rating scale); sedation and nausea, Apgar scores at 1 and 5 minutes | |
| Notes | Results for pain outcomes were reported on bar charts and are difficult to interpret. We have not included these results in the analyses in this review | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated balanced block design”. Block size not stated |
| Allocation concealment (selection bias) | Unclear risk | Study described as double-blind but not details on allocation concealment provided |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as double blind. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | The “drug was prepared by an anaesthesiologist not involved with the treatment of the patient or obtaining study measures” |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | It was not clear how many women were randomised. Any women undergoing ARM, commencing oxytocin or requesting epidural were excluded after randomisation and were replaced “their randomization was re-entered for another patient”. Women who reached 10 cm dilation within 1 hr of drug administration were also excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | Randomised trial (methods unclear). | |
| Participants | 30 women. Inclusion criteria: “co-operative patients” with no drug dependency. Various ages and social groups Exclusion criteria: unclear. |
|
| Interventions | 5 study groups:
|
|
| Outcomes | Pain intensity (grades 1 - 6 - no pain, light, bearable, heavy, very heavy, unbearable) over 70-minute period. Satisfaction with analgesia 1 day after the birth “Reaction of the subjects the day after the birth to analgesia - rated as “good”, “inadequate analgesia” or “none” - table 2. Progress in labour | |
| Notes | Paper in German. Translation notes used for data extraction. We were unable to use the data from this paper in the review. We had intended comparing outcomes for women receiving IV pethidine versus no treatment. The only outcome reported in the paper was the amount of relief obtained from the analgesia and no outcomes were reported for the control group (no treatment). 5 women received pethidine and 5 women no treatment. It was reported that 2/5 women receiving pethidine had “good relief”, 3 had insufficient or no relief. All women in the control group were reported as having an increase in pain Results - categories for pain relief (good, insufficient, none) do not correspond with pain scale - 6 perceptions reported in the translation |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described - “randomly divided”. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | 1 group received no treatment. TENS groups - 1 without current and 1 where it was applied to wrong positions were blinded to the TENS intervention. Pethidine group presumably were not blinded |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Not clear. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not clear. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | 1 woman was lost to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | Small study and results were difficult to interpret. |
| Other bias | Unclear risk | Translation, so difficult to evaluate other bias. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: UK hospital. 450 women. Healthy women with no obstetric complications, full-term pregnancy, in active labour and requiring analgesia. Excluded if history of hypersensitivity to any drug, previous caesarean, preterm labour, cardiac, pulmonary or renal disease and significant hypertension Parity: not reported. |
|
| Interventions | Experimental: IM meptazinol (N = 186 analysed). Control: IM pethidine (N = 172 analysed). Both given according to body weight. 75 mg if 38-50 kg, 100 mg if 51-69 kg or 150 mg if 70-85 mg. Each patient received up to 2 injections of study drug, and if analgesia still inadequate epidural given |
|
| Outcomes | Maternal outcomes: maternal assessment of pain relief at 15, 30, 45, 60, 90 and 120 min (rated none, poor, satisfactory, good, very good or complete), type of birth, epidural, sleepiness, nausea and vomiting. Neonatal outcomes: Apgar at 1 and 5 min, apnoea, resuscitation, and lethargy, muscle tone, irritability success of feeding within first 24hour period | |
| Notes | Does not report number randomised to each group. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind but does not describe methods used. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind but does not describe methods used. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind but does not describe methods used. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 79.5% follow-up but no intention-to-treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: UK hospital. 100 women. Age > 18 years, > 35 weeks’ gestation, uncomplicated singleton, vaginal birth expected, in active labour and requiring analgesia Parity: 9% primips, 76% multips, 15% grand multips. |
|
| Interventions | Experimental: IM pentazocine 30 mg (N = 48 analysed). Control: IM Pethilorfan ®100 mg (N = 50 analysed). Second injection possible after 2 hr, each patient could receive up to 4 injections of study drug, and nitrous oxide or trilene to supplement analgesia if required |
|
| Outcomes | Maternal outcomes: maternal assessment of pain relief (numbers obtaining or not obtaining pain relief), type of birth, additional analgesia required (study drug). Neonatal outcomes: Apgar at 1 and 5 min, naloxone administration | |
| Notes | Does not state actual number randomised to each group. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind but does not describe how this was achieved |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind but does not describe how this was achieved |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind but does not describe how this was achieved |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 31/98 excluded from primary outcome as delivered within 1 hour of administration of study drug, and 16 babies excluded from Apgar assessment as study drug administered more than 4 hours before birth |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Balanced at baseline for age, parity, contractions and vital signs |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Stockholm Sweden, hospital setting. 20 healthy nulliparous women in active labour after spontaneous rupture of the membranes, cephalic presentation. No exclusion criteria specified |
|
| Interventions | Experimental: 0.05 mg/kg IV morphine up to 3 doses (max 0.15 mg/kg body weight) Control: 0.5 mg/kg IV pethidine up to 3 doses (max 1.5 mg/kg body weight) Both groups had continuous FHR monitoring. |
|
| Outcomes | Sedation rates; CS, nausea and vomiting. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “assigned at random.” |
| Allocation concealment (selection bias) | Low risk | Coded ampoules provided by pharmacy. |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as double blind; pharmacy provided identical coded ampoules |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Described as double blind; pharmacy provided identical coded ampoules |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No apparent loss to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Small sample and no clear information that groups were comparable at baseline. Range of cervical dilations at recruitment between 4 and 9 cm |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: Washington, USA. 194 women in established labour. Analgesia was given at approximately 4-5 cm cervical dilatation |
|
| Interventions | Experimental: IV phenazocine 1 mg. Control: IV pethidine 50 mg. Both groups received promethazine 50 mg, and for both groups “birth was accomplished under pudendal nerve block anaesthesia with terminal self-administered trichloroethylene” |
|
| Outcomes | Pain relief (recorded by women on the first postpartum day); nausea and vomiting; adverse effects; progress in labour; Apgar scores at 1 and 5 mins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Drugs were prepared by pharmacy in identical coded vials and the code was not broken by the pharmacist until the study had been completed |
| Blinding (performance bias and detection bias) Women |
Low risk | Drugs in identical vials. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Pharmacy prepared identical coded drugs. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Missing data for some outcomes (approximately 5% for maternal postpartum outcomes, and 10% for nurse recorded evaluations in labour) |
| Selective reporting (reporting bias) | Low risk | None apparent. |
| Other bias | Low risk | None apparent. |
| Methods | RCT 2-arm parallel group design. | |
| Participants | Setting: Denmark - hospital. 199 women. Spontaneous or induced labour onset, in active labour and requiring analgesia Parity: 78% nullips, 22% multips. |
|
| Interventions | Experimental: IM meptazinol 100 mg (N = 100). Control: IM pethidine 750 mg (N = 99). Each patient could receive up to 3 injections of study drug with an interval of not less than 2 hours between doses | |
| Outcomes | Maternal outcomes: maternal assessment of pain relief 5, 15, 30, 60, 90, 120 min (rated complete, good, satisfactory, unsatisfactory), type of birth, additional analgesia required, epidural, adverse effects. Neonatal outcomes: Apgar at 1 and 5 min, neonatal distress, admission to SCBU | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Described as double blind but no methods described. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Described as double blind but no methods described. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Described as double blind but no methods described. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All women analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | No baseline imbalance in age, weight, height or number of previous deliveries |
| Methods | RCT 3-arm parallel group design. | |
| Participants | Setting: Thailand - hospital. 135 women. 37 to 42 weeks’ gestation, cx ≥ 3 cm, inactive labour and requiring analgesia Parity: not reported. |
|
| Interventions | Experimental: IM tramadol 100 mg (N = 45); IM morphine 100 mg (N = 45). Control: IM pethidine 100 mg (N = 45). Second injection possible after 1 hr of half original study dose, each participant could receive maximum of 2 doses | |
| Outcomes | Maternal outcomes: pain severity/relief 30 min, 1, 2, 3, and 4 hrs (rated good, satisfactory, no response), drowsiness, nausea, vomiting. Neonatal outcomes: Apgar at 1 and 5 min, neonatal resuscitation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States blind but does not describe the method. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States blind but does not describe the method. |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Medical students unaware of group allocation assessed outcome |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | Age and maternal weight balanced at baseline. |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting not clear (hospital in USA). 100 women in good health in active labour, with no addiction to or tolerance to drugs and complaining of moderate to severe pain. Women who “planned to nurse” were excluded |
|
| Interventions | Experimental: (50 women) IV butorphanol 1-2 mg (44 women had an initial dose of 1 mg and 6 an initial dose of 2 mg, after one hr or more a 2nd dose was given if requested) Control: (50 women) IV pethidine 40-80 mg (45 women had an initial dose of 40 mg and 5 an initial dose of 80 mg, a 2nd dose was given after 1hr or more if requested) | |
| Outcomes | Pain (5-point scale 0 - no pain, 4 - very severe pain); pain relief (5-point scale 0 - none, 4 - complete relief); FHR; Apgar scores at 1 and 5 minutes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Described as double blind study but no details provided. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Outcome data were available for all women randomised. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | No baseline imbalance was apparent although 8 women in the butorphanol group were induced compared with 1 woman in the pethidine group |
| Methods | RCT. 2-arm parallel groups. | |
| Participants | Setting: Nebraska university hospital, USA. 105 women in early active labour (3-4 cm cervical dilation); at or beyond 37 weeks’ gestation with no medical or obstetric complications, with no signs of fetal distress and requesting narcotic analgesia rather than an epidural. (Intervention group: 55% nulliparous, 71% non-white race, mean age 23 years; control group: 48% nulliparous, 70% non-white race, mean age 23 years.) |
|
| Interventions | Experimental: (49 women) IV fentanyl 50-100 mcg per hr. Control: (56 women) IV pethidine 25-50 mg per hr. |
|
| Outcomes | Pain (measured on 10-point VAS recorded by labour ward nurses); nausea and vomiting; sedation; itching; fetal heart rate changes | |
| Notes | Women were recruited only between 8 am and 3 pm on weekdays. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacy randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Women |
High risk | Decribed as not blinded. |
| Blinding (performance bias and detection bias) Clinical staff |
High risk | Staff not blind to group allocation. |
| Blinding (performance bias and detection bias) Outcome assessor |
High risk | Staff not blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All women randomised seem to be included in the results. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Women were recruited only on weekdays between 8am and 3pm so may not represent the population attending the study hospital |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: Norway - hospital. 85 women. Healthy women at term, expected to have a normal birth in active labour and requiring analgesia Parity: not reported. |
|
| Interventions | Experimental: IM pentazocine 45 mg (N = 43). Control: IM pethidine 100 mg (N = 42). Half dose repeated after 1 hr if required and further full dose after 3 hr if labour prolonged. All women received promazine 25 mg IM before 1st injection, nitrous oxide or pudendal block or both allowed at end of 2nd stage |
|
| Outcomes | Maternal outcomes: pain relief at 1 hr (0 = no pain, 1 = slight pain, 2 = moderate pain, 3 = severe pain), type of birth, additional analgesia required. Neonatal outcomes: Apgar at 1 and 5 min, naloxone administration, fetal heart rate changes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
High risk | No blinding. |
| Blinding (performance bias and detection bias) Clinical staff |
High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessor |
High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 25/85 women excluded from analysis as delivered within 1 hour of 1 st dose of study drug |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Nitrous oxide or pudendal block permitted during second stage |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: UK - hospital. 205 women. Healthy women 38-41 weeks’ gestation, uncomplicated pregnancy, spontaneous or induced labour onset, in active labour and requiring analgesia. Excluded if epidural or forceps birth likely Parity: mixed. |
|
| Interventions | Experimental: IM meptazinol 100 mg (N = 98). Control: IM pethidine 100 mg (N = 99). Additional doses of test drug allowed at intervals no less than 2 hrs if required to a maximum of 3 doses. All women could receive nitrous oxide if required and prochlorperazine 12.5 mg IM for nausea and vomiting. Epidural at midwife discretion |
|
| Outcomes | Maternal outcomes: pain intensity 30 min and then hourly intervals until birth (rated none, mild, moderate, severe), pain relief (rated none, slight, moderate, strong or complete), type of birth, additional analgesia required, nausea and vomiting. Neonatal outcomes: Apgar at 1 and 5 min, resuscitation. Within 72 hrs postpartum feeding problems, irritability and muscle tone | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Coded ampoules kept at a site remote from trial. |
| Blinding (performance bias and detection bias) Women |
Low risk | Described as double blind, used coded ampoules and states that identity of drug unknown |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Described as double blind, used coded ampoules and states that identity of drug unknown |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | Blind outcome assessor for all bar 15% of women. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 8 women excluded from analysis as delivered within 30 minutes of administration |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Balanced at baseline for age and weight, but imbalance in parity. 43/98 multip meptazinol group versus 34/99 in pethidine group |
| Methods | RCT, 3-arm parallel group design. | |
| Participants | Setting: SA - hospital. 196 women. Healthy women at term, uncomplicated labour, in active labour expected to deliver in next 4 hours and requiring analgesia. Excluded if likely to deliver within 30 min and had received analgesia within previous 6 hours Parity: mixed. |
|
| Interventions | Experimental: IM dihydrocodeine 50 mg (N = 80). Control: IM pethidine 100 mg (N = 58), placebo (saline) (N = 58) Single dose of study drug. |
|
| Outcomes | Maternal outcomes: pain relief at 1 hour (rated good, fair, poor), sedation (rated drowsy, alert but calm, restless), nausea, vomiting. Neonatal outcomes: Modified Apgar at 1 and 5 min (minus colour) | |
| Notes | Women excluded after randomisation if delivered more than 4 hours after injection of study drug | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind. Not reported how blinding was achieved. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind. Not reported how blinding was achieved. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind. Not reported how blinding was achieved. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Number of women randomised not reported, authors only report the number of women analysed |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unequal number of women in each treatment group due to post-randomisation exclusions. Exclusions included women who delivered < 30 minutes or > 4 hours after administration of study agents |
| Methods | RCT: methods not clear. | |
| Participants | Setting: Egypt. 90 primiparous women with normal presentation and position and expected to deliver normally |
|
| Interventions | Intervention: pethidine 50 mg IM 4-5 hourly. Comparison: TENS applied to back. The position arranged to suit the mother and moved to lower abdomen if preferred Both groups were given 10 mg diazepam IM. Both groups had artificial rupture of membranes at 5 cm and oxytocin augmentation |
|
| Outcomes | Pain intensity (scored as being: severe = 3; moderate = 2; mild = 1) - only measured before intervention; pain relief scored (complete = 4, excellent = 3, good = 2, slight (satisfactory) = 1) at 30 mins, 5 cm and at full cervical dilatation; patient’s opinion on the technique - satisfaction (during whole period of delivery), scored as (excellent = 3, good = 2, satisfactory = 1); Apgar score; side effects (drowsiness, nausea, vomiting) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly divided between 2 groups.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Women |
High risk | Not reported - but not feasible with nature of interventions |
| Blinding (performance bias and detection bias) Clinical staff |
High risk | Not reported - but not feasible with nature of interventions |
| Blinding (performance bias and detection bias) Outcome assessor |
High risk | Not reported - but not feasible with nature of interventions |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No apparent loss to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described within the methods are reported upon within the results. However, the study protocol was not evaluated |
| Other bias | Unclear risk | Unbalanced groups; 35 in the intervention group and 55 in the comparison group |
| Methods | RCT. | |
| Participants | Setting: Indore, India. 300 women in established labour attending for care in a hospital in India. The participants were described as being predominantly from low socio-economic groups and from urban areas Inclusion criteria: term pregnancy (37-42 weeks), vertex presentation, cervical dilatation 3 cm or more with contractions Exclusion criteria: previous uterine scar, malpresentation, multiple pregnancy, cephalo-pelvic disproportion, antepartum haemorrhage, pre-eclampsia or other medical disorders |
|
| Interventions | Interventions group: TENS to back. Comparison group 1: 100 mg intramuscular tramadol. Comparison group 2: no intervention. |
|
| Outcomes | Maternal pain score measured on a verbal response scale during labour “degree of analgesia” (degree of pain relief: no relief, mild relief, moderate relief, complete relief - dichotomised as a percentage); mean time for onset and duration of analgesia; duration of stages of labour; mode of delivery (normal, forceps, CS); mean Apgar score of neonates; side effects for mothers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “randomly allocated” but groups were of identical size with identical numbers of primiparous and multiparous women in each group |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation not described. |
| Blinding (performance bias and detection bias) Women |
High risk | No blinding reported - but not possible due to nature of intervention |
| Blinding (performance bias and detection bias) Clinical staff |
High risk | No blinding reported. |
| Blinding (performance bias and detection bias) Outcome assessor |
High risk | No blinding reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Apparently there was no loss to follow-up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described within the methods are reported upon within the results. However, the study protocol was not evaluated |
| Other bias | Unclear risk | Groups were unusually similar and it was not clear that there had been stratification to achieve such balanced groups |
| Methods | RCT. 2-arm parallel groups. | |
| Participants | 200 nulliparous women in labour. Inclusion criteria: at term (37-42 weeks) spontaneous labour, in active labour, vertex presentation Exclusions: age > 16 or < 35, weight > 50 or < 75 kg, infant birthweight estimated > 2500 or < 4000 g, medical or surgical complication or unable to understand VAS |
|
| Interventions | Intervention group: IM buprenorphine 300 mcg. Comparison group: IM pethidine 75 mg. |
|
| Outcomes | Analgesic effect at 1, 2, 3, 4 hrs, side effects (nausea, drowsiness, use of antidote) | |
| Notes | Data extraction from translation notes. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Women |
Low risk | Treatment described as blinded. |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | Treatment described as blinded. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Denominators in tables not clear. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: Hong Kong - hospital. 50 women. Healthy women in early active labour and requiring analgesia. Uncomplicated singleton term pregnancy, cephalic presentation. Spontaneous and induced labour onset. Excluded if epidural already requested Parity: 3:2 nullip:multip ratio. |
|
| Interventions | Experimental: IM pethidine 100 mg (N = 25). Control: placebo (saline) (N = 25). Single dose of study drug. Rescue analgesia allowed after 30 min nitrous-oxide or epidural for women in pethidine group and pethidine for women in placebo group |
|
| Outcomes | Maternal outcomes: pain intensity at 15 and 30 min VAS (0-100), maternal assessment of sedation at 15 and 30 min VAS (0-100), type of birth, additional analgesia required, vomiting, maternal satisfaction at 30 min 5-point scale (1 = totally dissatisfied to 5 = very satisfied). Neonatal outcomes: Apgar at 1 and 5 min, resuscitation and admission to SCBU | |
| Notes | Study terminated after 50 women pethidine. Stratified by parity recruited as interim analysis demonstrated benefit for | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque sealed envelopes. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind and women blind to contents of syringe. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind and staff blind to contents of syringe. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | States double blind and assessor blind to contents of syringe |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All women accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | High risk | 20/25 women in pethidine group versus 12/25 women in placebo group had labour induced which may affect maternal and neonatal outcomes |
| Methods | RCT, 3-arm parallel group design. | |
| Participants | Setting: Singapore - hospital. 90 women. Women aged 18 to 35 years in active labour and requiring analgesia, cx 3-5 cm, uncomplicated term pregnancy with uncomplicated birth expected, spontaneous or induced labour onset. Excluded if preterm labour Parity: 100% nullips. |
|
| Interventions | Experimental: IM tramadol 50 mg (N = 30), tramadol 100 mg (N = 30) Control: IM pethidine 75 mg (N = 30). Single dose of study drug. |
|
| Outcomes | Maternal outcomes: pain relief at 10, 20, 30, 45 and 1 hour 4-point scale (0 = none, 1 = insufficient, 2 = sufficient, 3 = complete pain relief), type of birth, drowsiness, nausea, vomiting. Neonatal outcomes: Apgar at 1 and 5 min, resuscitation and admission to SCBU | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Low risk | States double blind, identical syringes prepared separately from clinical observer |
| Blinding (performance bias and detection bias) Clinical staff |
Low risk | States double blind, identical syringes prepared separately from clinical observer |
| Blinding (performance bias and detection bias) Outcome assessor |
Low risk | States double blind, identical syringes prepared separately from clinical observer |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: hospital in Surrey, UK. 17 healthy women 36-40 weeks’ gestation requesting pethidine for pain relief in labour, ASA I or II. Women with a contraindication to pethidine or remifentanil or requesting epidural were excluded |
|
| Interventions | Experimental: IV PCA remifentanil, 0.5 mcg bolus per kg (based on antenatal booking weight) with 2 min lock-out, no hourly max Control: IV PCA pethidine, 10 mg bolus, 5 min lock-out, 100 mg hourly max All women were given 10 mg metoclopramide IV over 8 hrs. |
|
| Outcomes | Maternal: pain on 10 cm VAS recorded hourly; nausea recorded on a 10 cm VAS; itching; BP pulse and resps Neonate: 1 and 5 min Apgar scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described “randomly allocated”. |
| Allocation concealment (selection bias) | Low risk | “by selecting the next in a series of sealed envelopes prepared by pharmacy.” |
| Blinding (performance bias and detection bias) Women |
Low risk | Women were described as blind. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | “One investigator selected the envelope and prepared the PCA pump. the pump was covered so that the other investigator, the observer, was unable to see which drug the woman was receiving.” |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss to follow-up apparent although for some outcomes it was not clear what the denominators were |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | None apparent. |
| Methods | RCT. 4-arm parallel groups. | |
| Participants | Setting: hospital in Cairo, Egypt. 80 multiparous women at term (39-41 weeks), 19-27 years (parity 2-6), in the first stage of labour following uncomplicated pregnancies, spontaneous labour Women with respiratory or cardiac disease were excluded. |
|
| Interventions | Group 1: IM nalbuphine 0.13 mg/kg. Group 2: IM butorphanol 0.16 mg/kg. Group 3: IM pentazocine 0.4 mg/kg. Group 4: IM placebo. |
|
| Outcomes | Pain relief 0 = complete relief, 3 = no relief. Apgar score at 1 and 5 min. Maternal and fetal blood gases | |
| Notes | Data were reported as means and have not been included in data tables. We describe findings briefly in the text | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described “randomly divided”. |
| Allocation concealment (selection bias) | Unclear risk | Not described “four equal groups”. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Not clear. Placebo controlled, but not clear if women or staff were aware of group assignment |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Unclear risk | Not clear when randomisation took place and denominators in tables not clear |
| Other bias | Unclear risk | The equal division into groups suggests that there may not have been true random allocation |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: UK - hospital. 47 women. Women in active labour and requiring analgesia, 37-42 weeks’ gestation, singleton pregnancies with no known disorders, spontaneous or induced labour onset Parity: mixed. |
|
| Interventions | Experimental: IM meptazinol (N = 17). Control: IM pethidine (N = 17). Study dose dependent on woman’s weight: 100 mg if weight < 70 kg, 150 mg if weight ≥ 70 kg. Additional analgesia at discretion of caregiver, either 2nd dose of study drug, epidural or nitrous oxide, metoclorpromide as required for nausea and vomiting |
|
| Outcomes | Maternal outcomes: type of birth, additional analgesia, epidural. Neonatal outcomes: Apgar at 1 and 5 min, fetal heart rate changes | |
| Notes | Open non-randomised control arm. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | Described as double blind but methods not described. |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | Described as double blind but methods not described. |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Described as double blind but methods not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All patients analysed in an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Balanced at baseline for height, weight, age, socioeconomic group, gestation, cervical dilation, parity and smoking |
| Methods | RCT, 2-arm parallel group design. | |
| Participants | Setting: UK - hospital. 80 women. Healthy women in active labour and requiring analgesia, ≥ 38 weeks’ gestation, uncomplicated pregnancy Parity: 4 or less. |
|
| Interventions | Experimental: IM nalbuphine 20 mg (N = 37). Control: IM pethidine 100 mg (N = 35). Additional doses of test drug allowed at intervals no less than 2 hrs if required to a maximum of 3 doses. Epidural if analgesia inadequate at discretion of caregiver and subsequently removed from trial | |
| Outcomes | Maternal outcomes: pain intensity at peak of contraction at 30, 60 and 90 min (rated very severe, severe, moderate, slight) and with VAS (0-100), type of birth, sleepiness, nausea and vomiting. Neonatal outcomes: Apgar at 1 and 5 min, naloxone administration, Scanlon score (neuro-behavioural score) at 2-4 and 24 hrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Women |
Unclear risk | States double blind and study drugs were dispensed in coded ampoules |
| Blinding (performance bias and detection bias) Clinical staff |
Unclear risk | States double blind and study drugs were dispensed in coded ampoules |
| Blinding (performance bias and detection bias) Outcome assessor |
Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | 8/80 excluded from analyses due to inadequate pain relief. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Does not report actual number randomised per group. Broadly comparable at baseline with respect to physical and obstetric characteristics |
ARM: artificial rupture of the membranes
ASA: American Society of Anesthesiologists Classification
BMI: body mass index
BP: blood pressure
CS: caesarean section
CTG: cardiotocograph
cx: cervix
FGR: fetal growth restriction
FHR: fetal heart rate
GA: gestational age
IM: intramuscular
IOL: induction of labour
ITT: intention to treat
IV: intravenous
min: minutes
multips: multiparous women
MW: midwife
nullips: nulliparous women
PCA: patient controlled analgesia
PN: postnatal
primips: primiparous women
RCT: randomised controlled trial
resps: respirations
SC: subcutaneous
SCBU: special care baby unit
TENS: transcutaneous electrical nerve stimulation
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Aiken 1971 | This study compares the use of diazepam versus a placebo. Both groups had pethidine |
| Balcioglu 2007 | In this study group allocation was by order of hospital admission (alternate allocation). Not an RCT |
| Balki 2007 | In this study both groups received the same drug (remifentanil) by PCA. The focus of the study was on variation in the bolus size versus variation in the background infusion rate. Studies that examine variation in mode of administration will be considered in a separate related Cochrane review |
| Ballas 1976 | There was no evidence that this study was an RCT. There were 3 study groups and all 3 received pethidine (1 after 1-hour delay). The aim of the study was to monitor uterine activity over 60 minutes |
| Bare 1962 | This study examined the effects of hydroxine hydrochloride, an antihistamine. None of the study groups received an opioid analgesic drug |
| Bredow 1992 | This study was not an RCT. Alternate allocation to groups. |
| Brelje 1966 | This was a quasi-randomised study with group allocation by month of birth. The aim of the study was to look at hydroxine as an adjunct to pethidine. both study groups had pethidine |
| Busacca 1982 | In this study 1 group received pethidine with promethazine and 1 received no treatment. As the opioid group received a combination of drugs any differences between groups may have been due to the effect of the add on drug |
| Cahal 1960 | This study had 3 groups: SC pethidine, SC benzethidine and SC flurethidine. We are not aware that, apart from pethidine, these drugs are used any longer for pain relief in labour |
| Calderon 2006 | In this study 1 group received IV remifentanil and 1 group received IM pethidine with haloperidol. With 1 group receiving an add-on drug it would not be possible to compare the effects of the 2 opioids |
| Callaghan 1966 | In this study pethidine was compared with the use of a sedative. It was not clear that this was an RCT |
| Camann 1992 | This study compared IV sufentanil with epidural analgesia. Epidural analgesia in labour is covered in a related Cochrane review |
| Castro 2004 | This study was for pain relief during second trimester labour for termination of pregnancy and so not for pain relief for labour of childbirth |
| Cavanagh 1966 | This study had 4 groups: pethidine IM, amileridine IM, pethidine + perphenazine IM and amileridine + perphenazine IM. We are not aware that amileridine is used any longer in obstetric practice |
| Chang 1976 | It was not clear that participants in this trial were all in labour. The aim of the study was to examine fetal acid balance, with maternal and fetal blood sampling 30 and 60 minutes after administering the drugs. No other outcomes were recorded |
| Cincadze 1978 | Brief conference abstract. It was not clear that this was an RCT. We attempted to trace the authors for more information without success |
| Cullhed 1961 | This was not an RCT. Groups were divided into groups according to date of hospital attendance |
| Dan 1991 | In this study 1 group received IV nalbuphine and the other pethidine with promethazine, as the pethidine group had an add-on drug it is not possible to compare the 2 opioids |
| De Kornfeld 1964 | This study was excluded for methodological reasons; there was extremely high attrition for some outcomes (> 50%). SC pethidine and placebo were compared in this study; however, it appeared that the drugs were administered very late in labour. Of 224 women included in the analysis, it appeared that more than half had given birth within an hour of drug administration. There were data on pain relief for only approximately 103 women at 1 hour. Results were very difficult to interpret |
| De Lamerens 1964 | All study groups received pethidine. The aim of the study was to examine the effects of tranquillisers as adjuncts to analgesics |
| Eames 1964 | This study had 2 groups: pethidine 100 mg IM and oxymorphone 1.5 mg IM. Oxymorphone is no longer used for pain relief in labour |
| El-Kerdawy 2010 | This study compared opioids with epidural analgesia. Epidural analgesia in labour is covered in a related Cochrane review |
| Eliot 1975 | There was no evidence that there was random allocation in this study. There were 2 study groups and both received pethidine, the aim of the study was to compare drugs administered as an adjunct to the opioid analgesia (diazepam vs promazine) |
| Evron 2005 | In this study 2 different drugs using different modes of administration were compared. IV pethidine (with dummy PCA) was compared with PCA remifentanil (with dummy background IV infusion). With both the drug and method being different in each arm of the trial results from this study are very difficult to interpret |
| Evron 2007 | PCA IV pethidine was compared with epidural analgesia. |
| Evron 2008 | In this study with 4 different treatment arms, 1 group received IV remifentanil, the remaining 3 received epidural analgesia. Epidurals are covered in a separate Cochrane review |
| Gambling 1998 | This study compared IV pethidine versus a combined spinal epidural |
| Ginosar 2003 | Study examining IV versus epidural fentanyl. |
| Goodlin 1988 | Entry in trials register. It is not clear that this study was completed. We attempted to contact the author and searched for any published results relating to this trial without success |
| Grandjean 1979 | Study examining IV versus epidural analgesia. |
| Greer 1988 | The study evaluated the effects of the interventions on platelet function in the newborn |
| Hodgkinson 1978 | In this study both randomised groups received pethidine. 1 group also received naloxone. A third, non-randomised “matched” group received no narcotic drugs |
| Isenor 1993 | In this study both groups received the same drug (pethidine). The focus of the study was on variation in route of administration; IM was compared with PCA (IV) pethidine. Studies that examine variation in mode of administration will be considered in a separate related Cochrane review |
| Kalaskar 2007 | No results were reported in this brief abstract. We attempted to contact the author without success |
| Kaltreider 1967 | Only women in preterm labour were recruited to this study. This study was excluded for methodological reasons: there was no information about the number of women randomised and women who received any additional non-study medications were excluded post randomisation. Under these circumstances interpreting the findings of this study are very difficult |
| Krins 1969 | Study participants were not women in labour |
| Li 1995 | In this study 2 opioid drugs were compared (tramadol and dihydroetorphine hydrochloride). However, the drugs were administered by different routes (sublingual versus oral) and results are therefore very difficult to interpret |
| MacVicar 1960 | Not an RCT; consecutive allocation to groups. Study examining the sedative effects of drugs and their effects on memory |
| Malkasian 1967 | In this study both groups received pethidine. The focus of the trial was on the use of promethazine versus hydroxyzine as add-on drugs |
| McDonald 1964 | This study included 5 study arms and focused specifically on neonatal serum bilirubin, an outcome not relevant to this review |
| McGrath 1992 | A study examining epidural versus IV analgesia. |
| McInnes 2004 | In this study both groups received the same drug (diamorphine) either by PCA or IM. Studies that examine variation in mode of administration will be considered in a separate related Cochrane review |
| McQuitty 1967 | This study focused on promethazine, promazine and propiomazine ad adjuncts to pethidine. All study groups received pethidine |
| Moore 1974 | It was not clear that this was a randomised trial. Women were paired and then allocated in sequence to 4 study arms |
| Morgan 2004 | This was a pilot study reported as an abstract only and there was too little information on methods and results to assess risk of bias and results did not include outcomes relevant to this review |
| Morris 1994 | Study focusing on IV versus epidural fentanyl. |
| Nafisi 2006 | Study comparing IV pethidine versus epidural. |
| Nikkola 2000 | In this study women in the 2 arms of the trials were given different drugs with different routes of administration. PCA IV fentanyl was compared with paracervical blockade; 10 ml 0.25% bupivacaine injected into 4 locations in the cervix |
| Overton 1992 | This study comparing sublingual diamorphine with IM pethidine was reported in a brief abstract; no denominators for study groups were provided. We attempted to contact the study author for more information without success |
| Pandole 2003 | In this study women received either IM tramadol or IM pethidine. It was not clear that this was an RCT |
| Polley 2000 | This study compared IV vs epidural fentanyl (epidural analgesia is the subject of separate Cochrane reviews) |
| Posner 1960 | In this study both groups received pethidine; the focus of the study was on a narcotic antagonist (levallorphan) as an adjunct to pethidine |
| Powe 1962 | All 3 groups in this study received pethidine. The aim of the study was to examine the effects of promethazine and propiomazine as adjuncts to pethidine |
| Rabie 2006 | This study compared the use of IV PCA remifentanil versus epidural |
| Ransom 1966 | This study had 2 groups: pethidine 125 mg IM and oxymorphone 1.25 mg IM |
| Rayburn 1989 | In this study both groups received the same drug (pethidine) by PCA versus nurse administered (IV). Studies that examine variation in mode of administration will be considered in a separate related Cochrane review |
| Rayburn 1991 | In this study both groups received the same drug (fentanyl) 1 group by PCA and 1 nurse administered (IV). Studies that examine variation in mode of administration will be considered in a separate related Cochrane review |
| Roberts 1957 | In this study a mood enhancing drug (methylpentonol) was compared with an analgesic (pethidine). The outcome was not pain relief but fetal expiratory volume. There was no comparison of analgesic drugs in labour. We are not aware that methylpentonol is any longer used during childbirth |
| Roberts 1960 | In this study both groups received the same IM opioid analgesia (alphaprodine). The study examined the effects of a narcotic antagonist (levallorphan) as an adjunct to the opioid |
| Robinson 1980 | This study compared different ways of administering pethidine (IM vs IV); the IM group received an antiemetic the IV group didn’t. 386 women were randomised but there appears to have been serious attrition with complete data for only approximately a third of women randomised. Attrition was mainly due to protocol deviations. With these methodological problems findings from this study are very difficult to interpret |
| Ron 1984 | Study examining the value of promethazine as an adjunct to pethidine. The study did include a placebo group but the only result reported was maternal blood pressure ten minutes after injection of the drug/ placebo |
| Rowley 1963 | This was a quasi-randomised study. The outcomes collected in this study were neonate bilirubin levels |
| Savage 1955 | Quasi-randomised study with alternate allocation. |
| Sentnor 1966 | This study had 4 groups: pethidine 50 mg, 75 mg or 100 mg IM, oxymorphone 0.75 mg, 1.125 mg or 1. 5 mg, pethidine + noroxymorphone IM and oxymorphone + noroxymorphone IM. Oxymorphone is no longer used in clinical practice |
| Shahriari 2007 | In this study IV remifentanil was compared with IM pethidine. As both the drug and the route were different we excluded this study as results are difficult to interpret |
| Singh 2001 | Not an RCT |
| Solek-Pastuszka 2009 | This study compared opioids with epidural analgesia. Epidural analgesia in labour is covered in a related Cochrane review |
| Soontrapa 2002 | This was a quasi-randomised study and allocation could be anticipated |
| Sosa 2004 | This study focused on women with dystocia and the use of pethidine to promote progress in labour. Women requiring pain relief were excluded |
| Spellacy 1966 | All study groups received pethidine; the aim of the study was to look at the effects of adjuncts |
| Suvonnakote 1986 | In this study comparing IM pethidine and IM tramadol the report states that the sample was randomly selected, but there was no indication that there was random allocation to groups |
| Taskin 1993 | In this study the focus was on the rate of cervical dilatation rather than pain relief. The study was reported in a brief abstract; we attempted to contact the authors for more information without success |
| Thurlow 2002 | In this study 2 different drugs with different modes of administration were compared. IM pethidine (with an antiemetic) was compared with PCA remifentanil. In view of the different modes of administration we decided to exclude this study as results are very difficult to interpret |
| Tomlin 1965 | It was not clear that the women included in this study were in labour; women were recruited in the third trimester admitted to hospital following complications or “awaiting caesarean section or the birth of multiple pregnancies” |
| Tournaire 1980 | This study, otherwise eligible for the review, focused on the effect of pethidine on the frequency and intensity of uterine contractions and the rate of cervical dilatation; no other outcomes were reported |
| Treisser 1981 | This study did not focus on pain relief in labour; rather, it examined the effects of different drugs on progress in labour for women with dystocia (oxytocin, chlorpromazine, ritodine and pethidine were compared) |
| Tripti 2006 | Quasi-randomised study with alternate allocation. |
| Vavrinkova 2005 | There was no evidence that this was an RCT. |
| Volmanen 2005 | This study compares IV remifentanil with inhaled 50% nitrous oxide in a cross-over trial. Results were not reported separately for the first stage of this trial |
| Volmanen 2008 | This study compared IV remifentanil versus epidural analgesia |
| Von Vorherr 1963 | This study focused on speeding up progress in labour. In this group study groups received oxytocin as well as analgesics and women in the control arm received an higher dose of oxytocin |
| Walker 1992 | In this study pethidine was compared with a NSAID ketorolac. Ketorolac is not used nowadays in obstetric analgesia |
| Wan 1965 | Both study groups received pethidine; the aim of the study was to look at the effects of a sedative as an adjuvant therapy |
| Wiener 1979 | In this study epidural analgesia was compared with IM pethidine. It was not clear that this was an RCT |
| Williams 1962 | Both groups in this study received pethidine. The aim of the study was to examine the effects of a narcotic antagonist (levallorphan) as an adjunct to pethidine |
| Wong 2005 | This study is reported in a series of papers and conference abstracts. The study examined the use of an intrathecal opioid as part of a combined spinal epidural compared to a systemic opioid. Epidural analgesia is covered in a separate related Cochrane review |
IM: intramuscular
IV: intravenous
NSAID: non-steroidal anti-inflammatory drug
PCA: patient controlled analgesia
RCT: randomised controlled trial
SC: subcutaneous
Characteristics of ongoing studies [ordered by study ID]
DATA AND ANALYSES
Comparison 1. Pethidine 100 mg IM versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal satisfaction at 30 minutes (number of women satisfied or very satisfied) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| 2 Maternal pain relief good or fair (1 hour) | 1 | 116 | Risk Ratio (M-H, Fixed, 95% CI) | 1.75 [1.24, 2.47] |
| 3 Pain relief at 30 minutes (reduction in VAS of at least 40 mm) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 25.0 [1.56, 400.54] |
| 4 Additional analgesia | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.54, 0.94] |
| 5 Epidural | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.14, 1.78] |
| 6 Nausea and vomiting | 2 | 166 | Risk Ratio (M-H, Fixed, 95% CI) | 1.47 [0.65, 3.31] |
| 7 Maternal sleepiness | 2 | 166 | Risk Ratio (M-H, Fixed, 95% CI) | 4.67 [2.43, 8.95] |
| 8 Assisted vaginal delivery | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.34, 2.19] |
| 9 Caesarean section | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.29, 2.38] |
| 10 Low Apgar score (≤ 7) at 1 and 5 minutes | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 10.1 Low scores at 1 minute | 2 | 166 | Risk Ratio (M-H, Random, 95% CI) | 1.64 [0.52, 5.18] |
| 10.2 Low scores at 5 minutes | 1 | 50 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Neonatal resuscitation | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 1.67 [0.45, 6.24] |
| 12 Admission to NICU | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
Comparison 2. Meptazinol versus pethidine.
| Outcome or subgroup title | No. of No. of studies participants | Statistical method | Effect size | |
|---|---|---|---|---|
| 1 Maternal pain relief poor or none (3-5 PN) | 1 | 801 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.91, 1.12] |
| 2 Pain intensity 4 or 5 on 5-point scale (1 hour) | 2 | 239 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.69, 1.80] |
| 3 Additional analgesia required | 2 | 233 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 4 Epidural | 4 | 788 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.71, 1.29] |
| 5 Nausea and vomiting | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 3 | 1590 | Risk Ratio (M-H, Fixed, 95% CI) | 1.11 [0.95, 1.28] |
| 5.2 Vomiting | 3 | 1589 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [1.06, 1.47] |
| 6 Maternal sleepiness | 3 | 1590 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.28, 1.07] |
| 7 Assisted vaginal delivery | 3 | 1266 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.81, 1.22] |
| 8 Caesarean section | 3 | 1266 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.16, 2.00] |
| 9 Fetal heart rate changes (decelerations) | 1 | 34 | Risk Ratio (M-H, Fixed, 95% CI) | 1.23 [0.92, 1.64] |
| 10 Low Apgar score (≤ 7) at 1 and 5 minutes | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Low scores at 1 minute | 4 | 662 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.50, 1.13] |
| 10.2 Low scores at 5 minutes | 3 | 616 | Risk Ratio (M-H, Fixed, 95% CI) | 0.50 [0.05, 5.37] |
| 11 Naloxone administration | 1 | 998 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 11.1 <36 weeks‐ gestation | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.49, 1.89] |
| 11.2 ≥ 36 weeks‐ gestation | 1 | 975 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 12 Neonatal resuscitation | 2 | 1356 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.95, 1.05 |
| 12.1 < 36 weeks‐ gestation | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.69, 1.16] |
| 12.2 ≥ 36 weeks‐ gestation | 2 | 1333 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 13 Admission to NICU | 1 | 199 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.48, 1.63;] |
| 14 Breastfeeding problems | 1 | 197 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.17, 3.30] |
| 15 Apgar less than or equal to 7 at 1 minute | 2 | 129 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.47, 1.67] |
| 16 Neonatal resuscitation | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 1.5 [0.26, 8.60] |
Comparison 3. PCA (IM) meptazinol versus PCA (IM) pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (measured 1 day after delivery) | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | −17.60 [−49.93, 14. 73] |
| 2 Satisfied with mode of administration (PCA IM) | 1 | 10 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| 3 Epidural | 1 | 10 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.15, 59.89] |
| 4 Nausea score in labour (rated 1 day after delivery) | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | −8.0 [−48.70, 32.70] |
| 5 Drowsiness score in labour (rated 1 day after delivery) | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [−28.19, 39.39] |
| 6 Naloxone administered | 1 | 10 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.08, 11.93] |
Comparison 4. Diamorphine + prochloprerazine versus pethidine + prochloprerazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global assessment of pain relief at 24 hours | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| 2 Pain intensity at 1 hour (moderate or severe) | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 3 Additonal analgesia required | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 1.35 [0.53, 3.40] |
| 4 Epidural | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.72, 2.07] |
| 5 Vomiting | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.17, 0.86] |
| 6 Maternal sleepiness | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.52, 1.66] |
| 7 Assisted vaginal delivery | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.46, 2.02] |
| 8 Caesarean section | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.52 [0.10, 2.76] |
| 9 Apgar < 7 at 1 minute | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.41 [0.18, 0.91] |
| 10 Apgar < 7 at 5 minutes | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.35 [0.04, 3.27] |
| 11 Neonatal resuscitation | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 1.21 [0.73, 2.02] |
| 12 Admission to NICU | 1 | 133 | Risk Ratio (M-H, Fixed, 95% CI) | 0.58 [0.21, 1.64] |
Comparison 5. Tramadol versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity: women with poor pain relief | 4 | 243 | Risk Ratio (M-H, Fixed, 95% CI) | 1.56 [1.10, 2.21] |
| 2 Additional analgesia required | 3 | 295 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.60, 1.91] |
| 3 Nausea and vomiting | 6 | 454 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.34, 2.76] |
| 4 Maternal sleepiness | 5 | 409 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.33, 0.97] |
| 5 Assisted vaginal delivery | 3 | 260 | Risk Ratio (M-H, Fixed, 95% CI) | 0.56 [0.12, 2.56] |
| 6 Caesarean section | 3 | 260 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 7 Low Apgar scores (≤ 7) at 1 and 5 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Low scores at 1 minute | 2 | 250 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Low scores at 5 minutes | 1 | 160 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Neonatal resuscitation | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neonatal respiratory distress | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| 10 Admission to NICU | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
Comparison 6. Tramadol + triflupromazine versus pethidine + triflupromazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea and vomiting | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nausea | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.13, 5.25] |
| 1.2 Vomiting | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.02, 9.35] |
| 2 Maternal sleepiness | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 2.86 [0.68, 12.12] |
Comparison 7. Dihydrocodeine 50 mg IM versus pethidine 100 mg IM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal pain relief poor (1 hour) | 1 | 138 | Risk Ratio (M-H, Fixed, 95% CI) | 1.09 [0.64, 1.86] |
| 2 Nausea and vomiting | 1 | 138 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.40, 1.88] |
| 3 Maternal sleepiness | 1 | 138 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.43, 1.04] |
| 4 Apgar ≤ 7 at 1 minute | 1 | 138 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
Comparison 8. Pentazocine IM versus pethidine IM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief (good or very good) at delivery | 2 | 253 | Risk Ratio (M-H, Fixed, 95% CI) | 1.08 [0.92, 1.27] |
| 2 Pain relief poor (partial, none or worse) | 4 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 No add-on drugs | 3 | 365 | Risk Ratio (M-H, Random, 95% CI) | 1.23 [0.74, 2.05] |
| 2.2 With promazine | 1 | 85 | Risk Ratio (M-H, Random, 95% CI) | 1.53 [0.66, 3.58] |
| 3 Additional analgesia required | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pentazocine | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.50, 1.65] |
| 3.2 Pentazocine + promazine | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 1.67 [0.73, 3.84] |
| 4 Nausea and vomiting | 3 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 3 | 391 | Risk Ratio (M-H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 4.2 Vomiting | 1 | 73 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.27, 3.14] |
| 5 Assisted vaginal delivery | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 No add-on drugs | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 5.22 [0.63, 42.97] |
| 5.2 With promazine | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.23, 2.71] |
| 6 Maternal sleepiness | 3 | 391 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 7 Low Apgar score (≤ 7) at 1 and 5 minutes | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 7.1 Low score at 1 minute | 2 | 242 | Risk Ratio (M-H, Random, 95% CI) | 1.39 [0.06, 32.97] |
| 7.2 Low score at 5 minutes | 1 | 62 | Risk Ratio (M-H, Random, 95% CI) | 0.23 [0.01, 4.34] |
Comparison 9. Pentazocine + promazine versus pethidine + promazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low Apgar score (≤ 7) at 1 and 5 minutes (with promazine) | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low score at 1 minute | 1 | 66 | Risk Ratio (M-H, Fixed, 95% CI) | 1.13 [0.07, 17.30] |
| 1.2 Low score at 5 minutes | 1 | 66 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.02, 8.88] |
| 2 Naloxone administration (neonatal) | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| 2.1 With promazine | 1 | 85 | Risk Ratio (M-H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
Comparison 10. Nalbuphine versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal satisfaction with analgesia at 24 hours; numbers dissatisfied | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 0.73 [0.55, 0.96] |
| 2 Pain free | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 6.0 [0.79, 45.42] |
| 3 Pain intensity at 30 minutes: women with severe pain | 1 | 295 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| 4 VAS at 60 minutes (at peak of contraction) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | −8.0 [−18.55, 2.55] |
| 5 Additional analgesia required | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 1.26 [0.49, 3.27] |
| 6 Epidural | 1 | 307 | Risk Ratio (M-H, Fixed, 95% CI) | 1.65 [0.55,4.94] |
| 7 Nausea and vomiting | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Nausea | 1 | 301 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.42, 0.91] |
| 7.2 Vomiting | 1 | 301 | Risk Ratio (M-H, Fixed, 95% CI) | 0.41 [0.22, 0.76] |
| 7.3 Nausea and vomiting | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 0.41 [0.18, 0.94] |
| 8 Maternal sleepiness | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 3.78 [0.86, 16.60] |
| 9 Assisted vaginal delivery | 2 | 382 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.25, 3.85] |
| 10 Caesarean section | 1 | 310 | Risk Ratio (M-H, Fixed, 95% CI) | 0.45 [0.12, 1.69] |
| 11 Low Apgar score (≤ 7) at 1 and 5 minutes | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 11.1 Low score at 1 minute | 2 | 382 | Risk Ratio (M-H, Random, 95% CI) | 1.18 [0.72, 1.95] |
| 11.2 Low score at 5 minutes | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 0.47 [0.04, 4.99] |
| 12 Naloxone administration (neonatal) | 1 | 72 | Risk Ratio (M-H, Fixed, 95% CI) | 6.63 [0.35, 123.93] |
| 13 Admission to NICU | 1 | 299 | Risk Ratio (M-H, Fixed, 95% CI) | 1.07 [0.61, 1.89] |
| 14 Neonatal neurobehavioural (Scanlon) 2-4 hours PN | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | −3.70 [−6.14, −1.26] |
Comparison 11. Phenazocine versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Epidural | 1 | 212 | Risk Ratio (M-H, Fixed, 95% CI) | 1.31 [0.58, 2.97] |
| 2 Vomiting | 1 | 212 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.20, 0.78] |
Comparison 12. Morphine versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief described as poor | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.56, 2.66] |
| 2 Additional analgesia required | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 1.14 [0.45, 2.89] |
| 3 Nausea and vomiting | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.21, 4.69] |
| 4 Maternal sleepiness | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 0.6 [0.29, 1.23] |
| 5 Apgar < 7 at 1 minute | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal resuscitation | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Butorphanol versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional analgesia required | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| 2 Nausea | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.2 [0.01, 4.04] |
| 3 Vomiting | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 4 Neonatal resuscitation | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.01,7.95] |
| 5 Naloxone administration (neonatal) | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.01,7.95] |
Comparison 14. IM tramadol versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anagesic effect described as satisfactory (not clear when measured) | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| 2 Mean blood loss at delivery (ml) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 25.70 [−9.83, 61.23] |
Comparison 15. IM Avacan ® versus IM pentazocine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Further analgesia required (nitrous oxide) | 1 | 160 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.53, 1.63] |
| 2 Further analgesia required (pudendal-paracervical block) | 1 | 160 | Risk Ratio (M-H, Fixed, 95% CI) | 2.02 [1.16, 3.53] |
| 3 Caesarean section | 1 | 184 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.21, 1.84] |
| 4 Low Apgar score (< 7) ”at birth” | 1 | 160 | Risk Ratio (M-H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
Comparison 16. IM pentazocine versus IM pethilorfan.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief (women NOT obtaining pain relief) at 1 hour | 1 | 69 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.77, 1.95] |
| 2 Additional analgesia required | 1 | 98 | Risk Ratio (M-H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| 3 Assisted vaginal delivery | 1 | 98 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.07, 16.19] |
| 4 Apgar < 8 at 1 minute | 1 | 82 | Risk Ratio (M-H, Fixed, 95% CI) | 5.71 [0.72, 45.39] |
| 5 Apgar < 8 at 5 minutes | 1 | 82 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. IV fentanyl versus IV pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (1 hour after drug administration) | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | −0.20 [−0.34, −0.06] |
| 2 Mean doses of analgesia | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| 3 Nausea and/or vomiting | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 0.51 [0.17, 1.55] |
| 4 Anti-emetic required | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 0.09 [0.01, 1.52] |
| 5 Maternal sedation | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 0.05 [0.00, 0.82] |
| 6 Caesarean section | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 1.14 [0.24, 5.40] |
| 7 Apgar score < 7 at 1 and 5 minutes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Low score at 1 minute | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.23, 1.77] |
| 7.2 Low score at 5 minutes | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.02, 9.12] |
| 8 Naloxone administered | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| 9 Babies requiring resuscitation/ventilatory support | 1 | 105 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.46, 2.32] |
| 10 Neurobehavioural score (1 - 2 hours after delivery) | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.15, 2.45] |
| 11 Neurobehavioural score (2 hours - 24 hours) | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [−0.42, 2.22] |
Comparison 18. IV nalbuphine versus IV pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.26, 95.61] |
| 2 Apgar score < 7 at 1 and 5 minutes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low score at 1 minute | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.13, 67.91] |
| 2.2 Low score at 5 minutes | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. IV phenazocine versus IV pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction with pain relief (women with fair or poor relief) | 1 | 194 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.48, 1.10] |
| 2 Nausea with vomiting | 1 | 194 | Risk Ratio (M-H, Fixed, 95% CI) | 0.4 [0.08, 2.01] |
| 3 Perinatal death | 1 | 194 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Apgar score < 7 at 1 minute | 1 | 194 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 20. IV butorphanol versus IV pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief score | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.25, 1.09] |
| 2 Pain score (1 hour after drug administration) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−1.02, −0.18] |
| 3 Further analgesia (2nd dose) required | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.63, 1.45] |
| 4 Epidural | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.30, 3.35] |
| 5 Nausea and/or vomiting | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 6 Assisted vaginal delivery | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 1.3 [0.60, 2.83] |
| 7 Caesarean section | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.8 [0.22, 2.89] |
| 8 Apgar score < 7 at 1 and 5 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Low score at 1 minute | 2 | 230 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.15, 1.61] |
| 8.2 Low score at 5 minutes | 2 | 230 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.06, 13.77] |
Comparison 21. IV morphine versus IV pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women satisfied with analgesia (assessed 3 days postpartum) | 1 | 141 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.78, 0.98] |
| 2 Further dose of study analgesia required | 1 | 143 | Risk Ratio (M-H, Fixed, 95% CI) | 3.41 [1.90, 6.12] |
| 3 Nausea and vomiting | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 0.17 [0.02, 1.14] |
| 3.2 Vomiting | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 4 Caesarean section | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 22. IV nisentil versus IV pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea and vomiting | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nausea | 1 | 395 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 1.2 Vomiting | 1 | 395 | Risk Ratio (M-H, Fixed, 95% CI) | 0.38 [0.22, 0.66] |
| 2 Babies requiring resuscitation/ventilatory support | 1 | 395 | Risk Ratio (M-H, Fixed, 95% CI) | 1.99 [0.85,4.63] |
Comparison 23. IV fentanyl versus IV butorphanol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Additional analgesia required (women requesting two or more doses) | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 1.39 [1.05, 1.85] |
| 2 Epidural | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 2.0 [1.00, 4.02] |
| 3 Matenal drowsiness (required tactile rousing) | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.64, 14.16] |
| 4 Caesarean section | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 0.8 [0.23, 2.81] |
| 5 Apgar score < 7 at 5 minutes | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 1.2 [0.39, 3.68] |
| 6 Babies requiring ventilatory support | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 11.0 [0.62, 193.80] |
| 7 Naloxone required | 1 | 100 | Risk Ratio (M-H, Fixed, 95% CI) | 1.75 [0.81, 3.80] |
| 8 Neurobehavioural score at 2-4 hours | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [−1.61, 1.61] |
| 9 Neurobehavioural score at 24-36 hours | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | −0.50 [−1.62, 0.62] |
Comparison 24. PCA pentazocine versus PCA pethidine.
| No. of Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score in labour | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.76 [−1.62, 0.09] |
| 2 Pain relief rated as good one day after birth | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.51, 1.32] |
| 3 Epidural | 1 | 28 | Risk Ratio (M-H, Fixed, 95% CI) | 1.5 [0.29, 7.65] |
| 4 Nausea and vomiting | 1 | 29 | Risk Ratio (M-H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| 5 Sedation | 1 | 29 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.01, 4.09] |
| 6 Caesarean section | 1 | 29 | Risk Ratio (M-H, Fixed, 95% CI) | 0.36 [0.02, 8.07] |
| 7 Apgar score < 7 at 5 minutes | 1 | 29 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Breastfeeding at discharge | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.83, 1.17] |
Comparison 25. PCA remifentanil versus PCA pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score in labour | 2 | 122 | Mean Difference (IV, Random, 95% CI) | −8.59 [−27.61, 10. 44] |
| 2 Women receiving other analgesia (Entonox) | 2 | 56 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 3 Epidural | 2 | 122 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| 4 Maternal sleepiness during labour | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| 5 Nausea and vomiting | 2 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.61, 1.49] |
| 6 Assisted vaginal birth | 2 | 97 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.46, 2.00] |
| 7 Caesarean section | 2 | 97 | Risk Ratio (M-H, Fixed, 95% CI) | 1.81 [0.60, 5.46] |
| 8 Apgar score < 7 at 5 minutes | 1 | 17 | Risk Ratio (M-H, Fixed, 95% CI) | 0.13 [0.01, 2.16] |
| 9 Naloxone administered | 2 | 56 | Risk Ratio (M-H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| 10 Admission to NICU | 1 | 17 | Risk Ratio (M-H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| 11 Satisfaction with childbirth experience | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.46, 1.74] |
| 12 Neurobehavioural score (15 minutes post delivery) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−0.93, 1.33] |
| 13 Neurobehavioural score (2 hours post delivery) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [−0.66, 1.86] |
Comparison 26. PCA nalbuphine versus PCA pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief in labour measured in the postnatal period (rated good or excellent) | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.29 [0.88, 1.89] |
| 2 Would use the same pain relief again | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 3 Pain score in labour | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.51 [−1.02, 0.00] |
| 4 Women receiving other analgesia (Entonox) | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 0.83 [0.46, 1.48] |
| 5 Nausea and vomiting | 1 | 59 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.30, 1.54] |
| 6 Apgar score < 7 at 5 minutes | 1 | 41 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 [0.02, 9.76] |
Comparison 27. PCA fentanyl versus PCA alfentanil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief described as adequate (recorded after delivery) | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 1.56 [0.93, 2.60] |
| 2 Pain score at 4-6 cm cervical dilatation | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | −12.80 [−32.12, 6. 52] |
| 3 Nausea | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 2.73 [0.66, 11.30] |
| 4 Caesarean section | 1 | 23 | Risk Ratio (M-H, Fixed, 95% CI) | 1.64 [0.33, 8.03] |
| 5 Naloxone required | 1 | 24 | Risk Ratio (M-H, Fixed, 95% CI) | 2.36 [0.33, 10.33] |
Comparison 28. PCA fentanyl versus PCA pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score in labour | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | −0.65 [−1.56, 0.26] |
| 2 Epidural | 1 | 107 | Risk Ratio (M-H, Fixed, 95% CI) | 0.44 [0.21, 0.92] |
| 3 Maternal sleepiness during labour | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | −0.06 [−0.25, 0.13] |
| 4 Nausea and vomiting | 1 | 102 | Risk Ratio (M-H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 5 Assisted vaginal birth | 1 | 81 | Risk Ratio (M-H, Fixed, 95% CI) | 0.57 [0.22, 1.49] |
| 6 Caesarean section | 1 | 81 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.03, 2.34] |
| 7 Neurobehavioural score (15 minutes post delivery) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | −0.90 [−2.31, 0.51] |
| 8 Neurobehavioural score (2 hours | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | −0.5 [−1.95, 0.95] |
Comparison 29. Opioids versus TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal satisfaction with analgesia measured post delivery (rated as good) | 2 | 104 | Risk Ratio (M-H, Fixed, 95% CI) | 1.23 [0.79, 1.92] |
| 2 Maternal pain score measured during labour | 2 | 290 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.81, 1.61] |
| 3 Drowsiness | 2 | 290 | Risk Ratio (M-H, Fixed, 95% CI) | 8.96 [1.13, 71.07] |
| 4 Nausea and vomiting | 2 | 290 | Risk Ratio (M-H, Fixed, 95% CI) | 14.06 [1.96, 100.61] |
| 5 Caesarean section | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Assisted vaginal birth | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
| 7 Fetal distress | 1 | 200 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
Analysis 1.1. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 1 Maternal satisfaction at 30 minutes (number of women satisfied or very satisfied).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 1 Maternal satisfaction at 30 minutes (number of women satisfied or very satisfied)
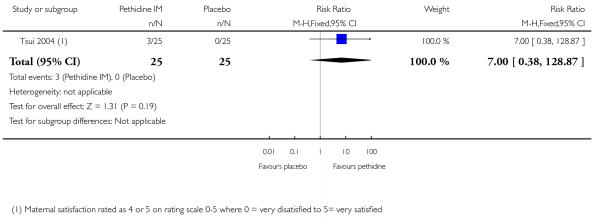 |
Analysis 1.2. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 2 Maternal pain relief good or fair (1 hour).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 2 Maternal pain relief good or fair (1 hour)
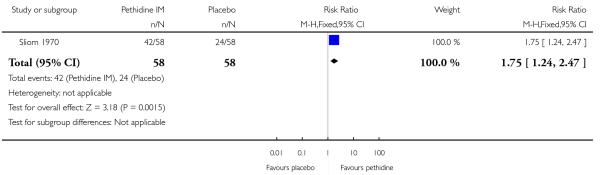 |
Analysis 1.3. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 3 Pain relief at 30 minutes (reduction in VAS of at least 40 mm).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 3 Pain relief at 30 minutes (reduction in VAS of at least 40 mm)
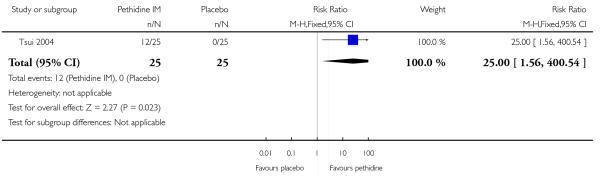 |
Analysis 1.4. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 4 Additional analgesia.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 4 Additional analgesia
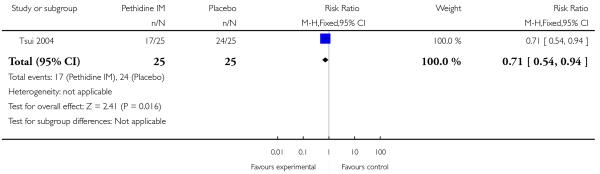 |
Analysis 1.5. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 5 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 5 Epidural
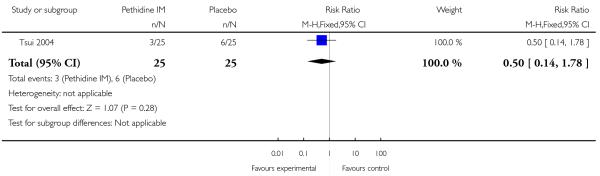 |
Analysis 1.6. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 6 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 6 Nausea and vomiting
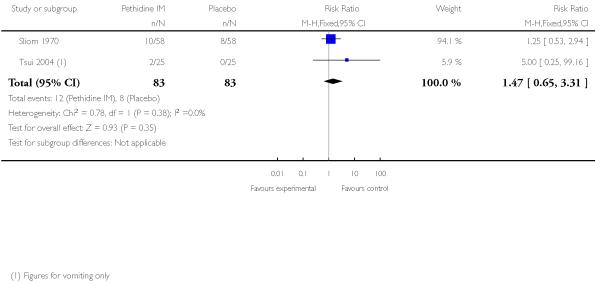 |
Analysis 1.7. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 7 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 7 Maternal sleepiness
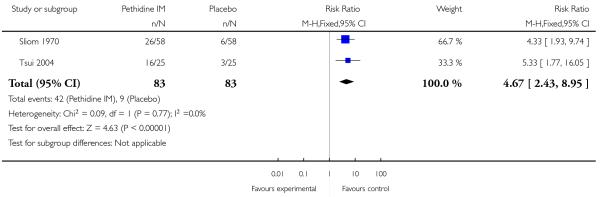 |
Analysis 1.8. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 8 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 8 Assisted vaginal delivery
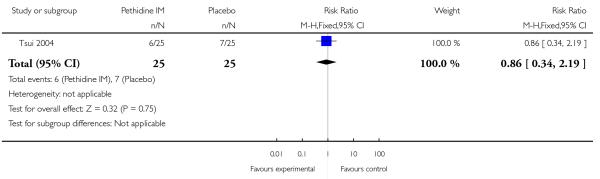 |
Analysis 1.9. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 9 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 9 Caesarean section
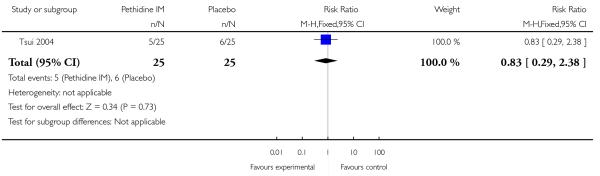 |
Analysis 1.10. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 10 Low Apgar score (≤ 7) at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 10 Low Apgar score (≤7) at 1 and 5 minutes
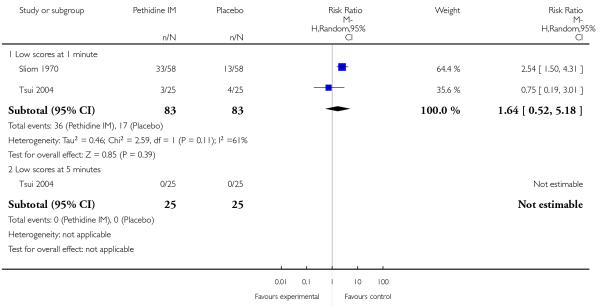 |
Analysis 1.11. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 11 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 11 Neonatal resuscitation
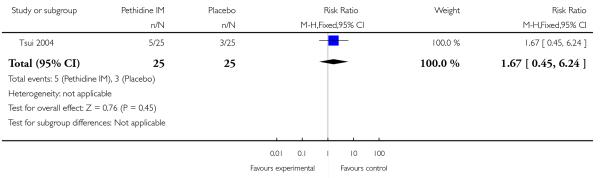 |
Analysis 1.12. Comparison 1 Pethidine 100 mg IM versus placebo, Outcome 12 Admission to NICU.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 1 Pethidine 100 mg IM versus placebo
Outcome: 12 Admission to NICU
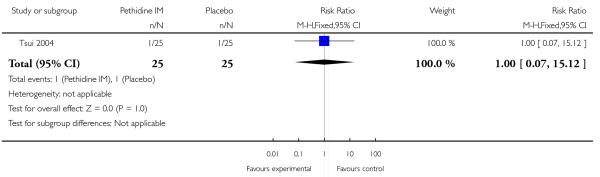 |
Analysis 2.1. Comparison 2 Meptazinol versus pethidine, Outcome 1 Maternal pain relief poor or none (3-5 PN).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 1 Maternal pain relief poor or none (3-5 PN)
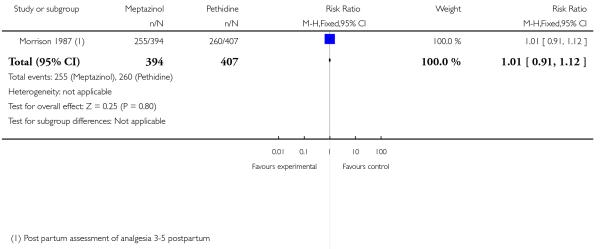 |
Analysis 2.2. Comparison 2 Meptazinol versus pethidine, Outcome 2 Pain intensity 4 or 5 on 5-point scale (1 hour).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 2 Pain intensity 4 or 5 on 5-point scale (1 hour)
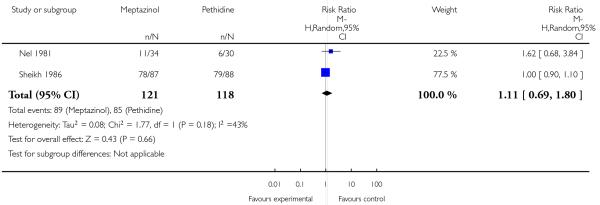 |
Analysis 2.3. Comparison 2 Meptazinol versus pethidine, Outcome 3 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 3 Additional analgesia required
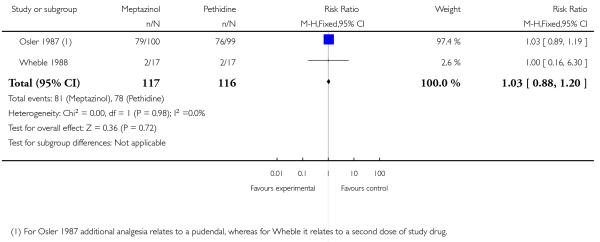 |
Analysis 2.4. Comparison 2 Meptazinol versus pethidine, Outcome 4 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 4 Epidural
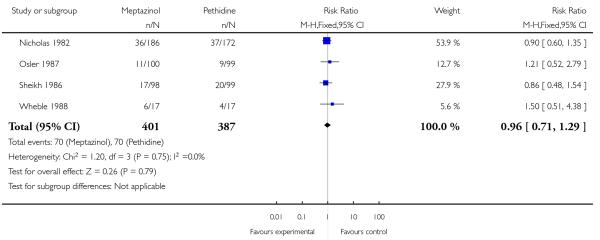 |
Analysis 2.5. Comparison 2 Meptazinol versus pethidine, Outcome 5 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 5 Nausea and vomiting
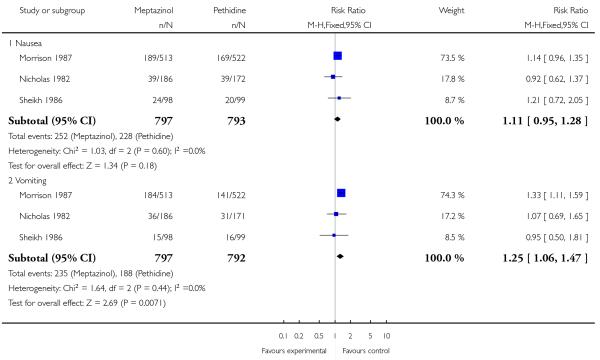 |
Analysis 2.6. Comparison 2 Meptazinol versus pethidine, Outcome 6 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 6 Maternal sleepiness
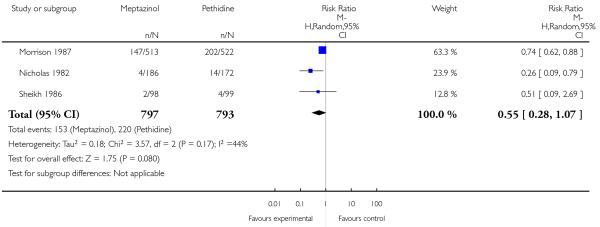 |
Analysis 2.7. Comparison 2 Meptazinol versus pethidine, Outcome 7 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 7 Assisted vaginal delivery
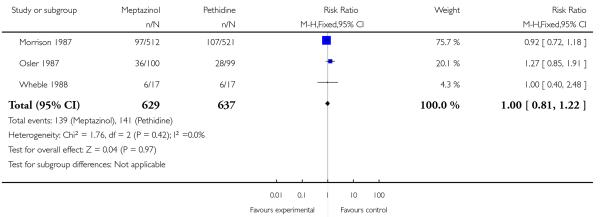 |
Analysis 2.8. Comparison 2 Meptazinol versus pethidine, Outcome 8 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 8 Caesarean section
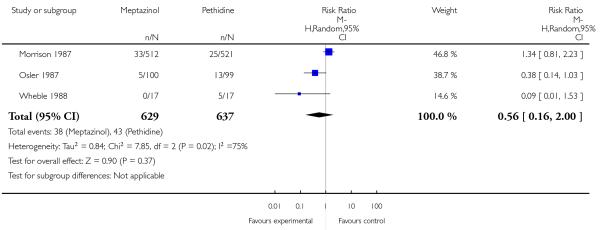 |
Analysis 2.9. Comparison 2 Meptazinol versus pethidine, Outcome 9 Fetal heart rate changes (decelerations).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 9 Fetal heart rate changes (decelerations)
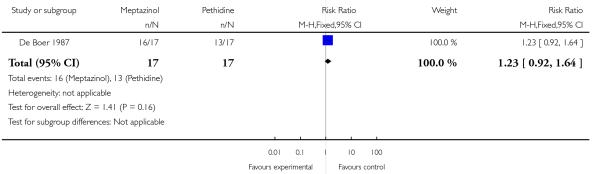 |
Analysis 2.10. Comparison 2 Meptazinol versus pethidine, Outcome 10 Low Apgar score (≤ 7) at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 10 Low Apgar score (≤ 7) at 1 and 5 minutes
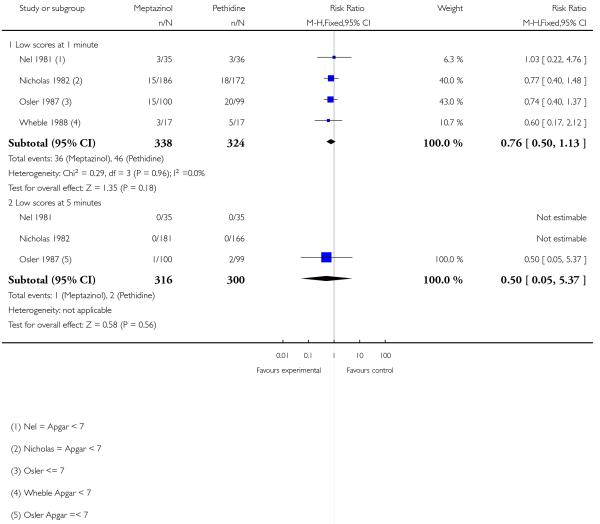 |
Analysis 2.11. Comparison 2 Meptazinol versus pethidine, Outcome 11 Naloxone administration.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 11 Naloxone administration
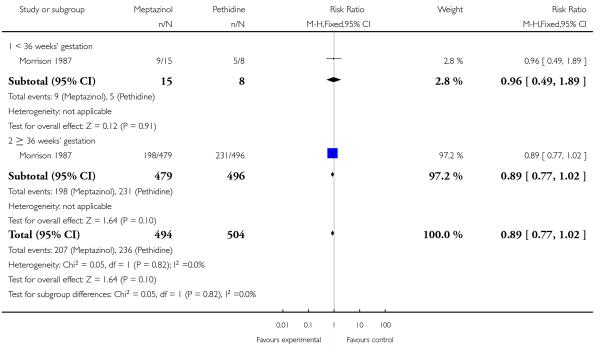 |
Analysis 2.12. Comparison 2 Meptazinol versus pethidine, Outcome 12 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 12 Neonatal resuscitation
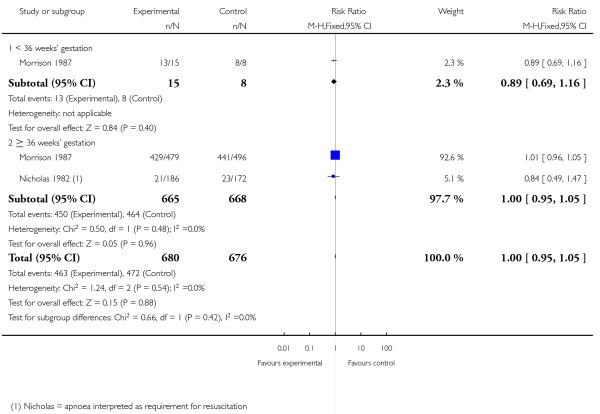 |
Analysis 2.13. Comparison 2 Meptazinol versus pethidine, Outcome 13 Admission to NICU.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 13 Admission to NICU
 |
Analysis 2.14. Comparison 2 Meptazinol versus pethidine, Outcome 14 Breastfeeding problems.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 14 Breastfeeding problems
 |
Analysis 2.15. Comparison 2 Meptazinol versus pethidine, Outcome 15 Apgar less than or equal to 7 at 1 minute.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 15 Apgar less than or equal to 7 at 1 minute
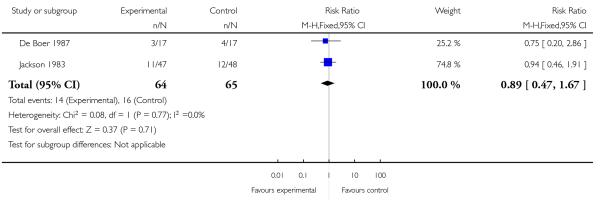 |
Analysis 2.16. Comparison 2 Meptazinol versus pethidine, Outcome 16 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 2 Meptazinol versus pethidine
Outcome: 16 Neonatal resuscitation
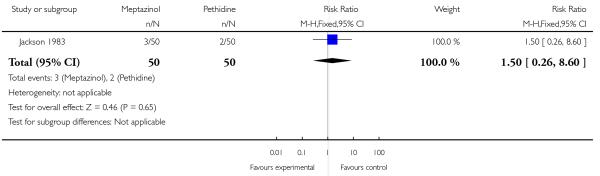 |
Analysis 3.1. Comparison 3 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 1 Pain score (measured 1 day after delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 3 PCA (IM) meptazinol versus PCA (IM) pethidine
Outcome: 1 Pain score (measured 1 day after delivery)
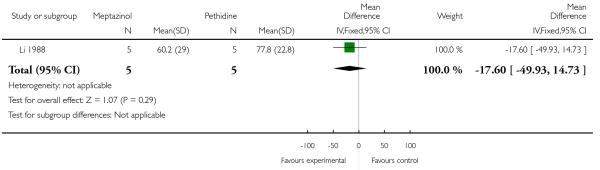 |
Analysis 3.2. Comparison 3 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 2 Satisfied with mode of administration (PCA IM).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 3 PCA (IM) meptazinol versus PCA (IM) pethidine
Outcome: 2 Satisfied with mode of administration (PCA IM)
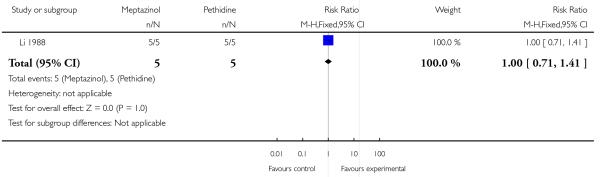 |
Analysis 3.3. Comparison 3 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 3 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 3 PCA (IM) meptazinol versus PCA (IM) pethidine
Outcome: 3 Epidural
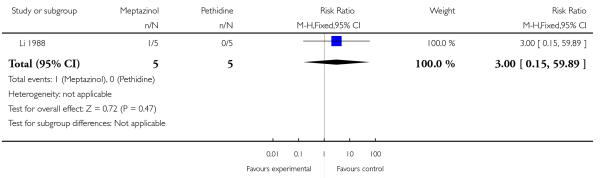 |
Analysis 3.4. Comparison 3 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 4 Nausea score in labour (rated 1 day after delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 3 PCA (IM) meptazinol versus PCA (IM) pethidine
Outcome: 4 Nausea score in labour (rated 1 day after delivery)
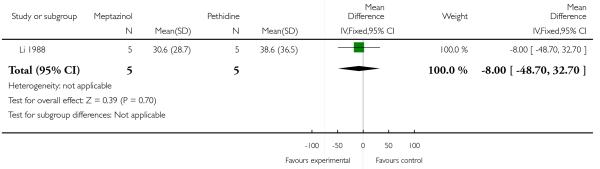 |
Analysis 3.5. Comparison 3 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 5 Drowsiness score in labour (rated 1 day after delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 3 PCA (IM) meptazinol versus PCA (IM) pethidine
Outcome: 5 Drowsiness score in labour (rated 1 day after delivery)
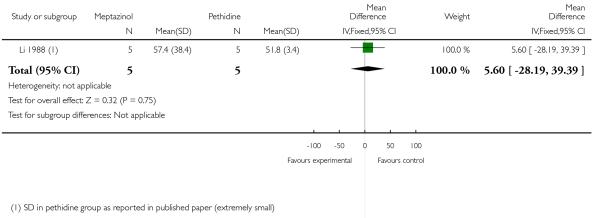 |
Analysis 3.6. Comparison 3 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 6 Naloxone administered.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 3 PCA (IM) meptazinol versus PCA (IM) pethidine
Outcome: 6 Naloxone administered
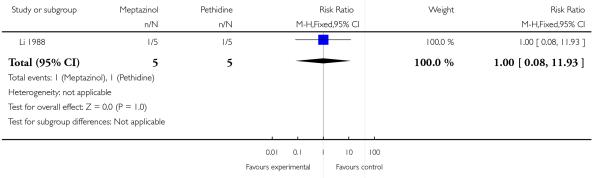 |
Analysis 4.1. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 1 Global assessment of pain relief at 24 hours.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 1 Global assessment of pain relief at 24 hours
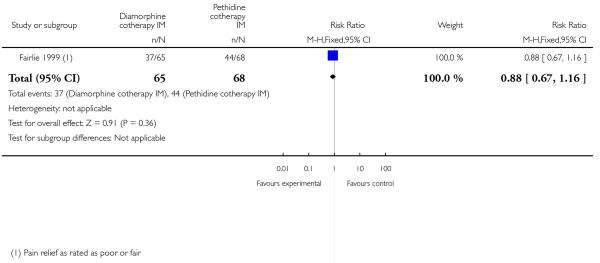 |
Analysis 4.2. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine, Outcome 2 Pain intensity at 1 hour (moderate or severe).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 2 Pain intensity at 1 hour (moderate or severe)
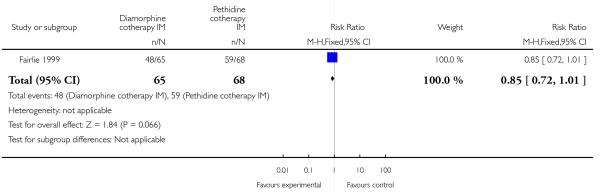 |
Analysis 4.3. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine, Outcome 3 Additonal analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 3 Additonal analgesia required
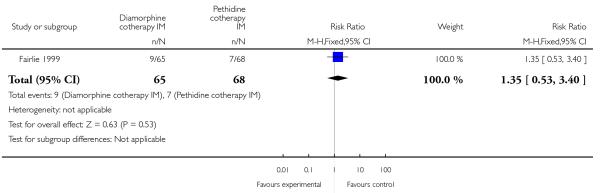 |
Analysis 4.4. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 4 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 4 Epidural
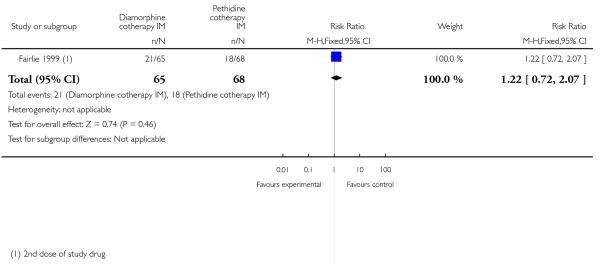 |
Analysis 4.5. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 5 Vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 5 Vomiting
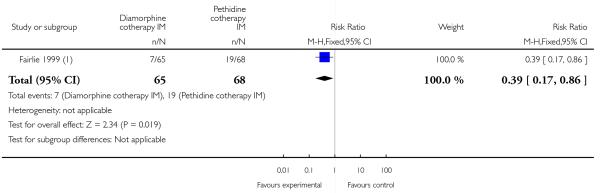 |
Analysis 4.6. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 6 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 6 Maternal sleepiness
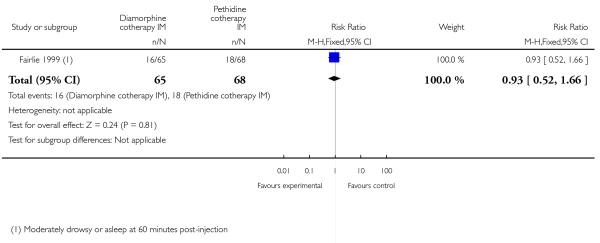 |
Analysis 4.7. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 7 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 7 Assisted vaginal delivery
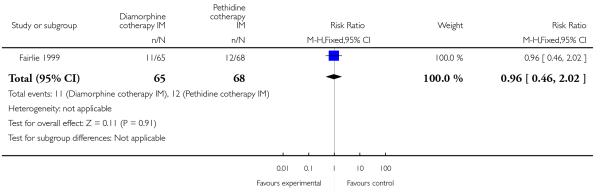 |
Analysis 4.8. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 8 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 8 Caesarean section
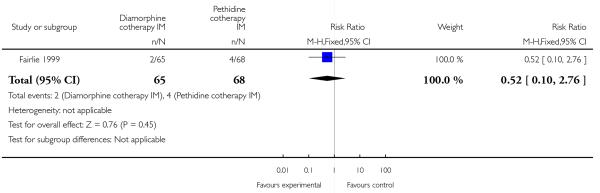 |
Analysis 4.9. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine, Outcome 9 Apgar > 7 at 1 minute.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 9 Apgar > 7 at 1 minute
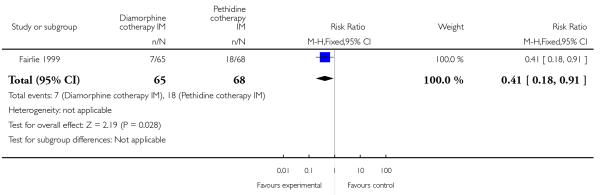 |
Analysis 4.10. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 10 Apgar < 7 at 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 10 Apgar < 7 at 5 minutes
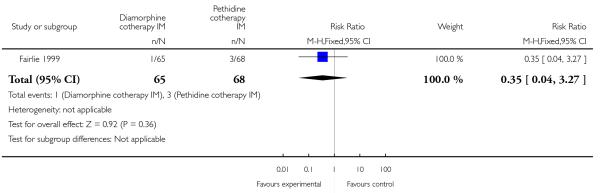 |
Analysis 4.11. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 11 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 11 Neonatal resuscitation
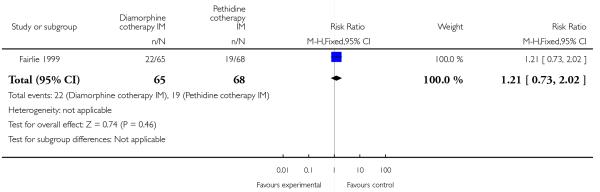 |
Analysis 4.12. Comparison 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine,Outcome 12 Admission to NICU.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 4 Diamorphine + prochloprerazine versus pethidine + prochloprerazine
Outcome: 12 Admission to NICU
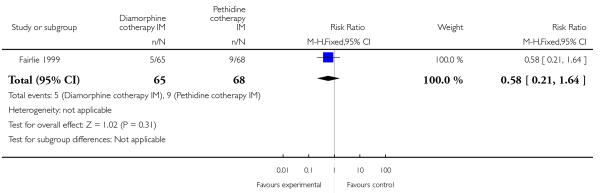 |
Analysis 5.1. Comparison 5 Tramadol versus pethidine, Outcome 1 Pain intensity: women with poor pain relief.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 1 Pain intensity: women with poor pain relief
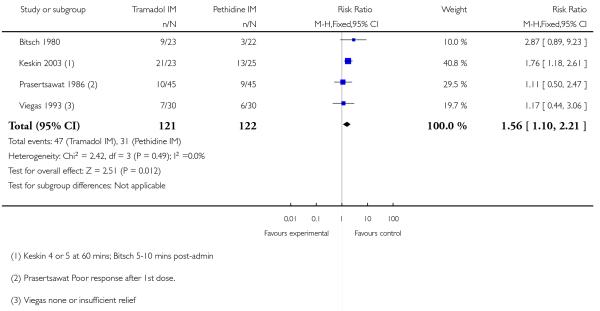 |
Analysis 5.2. Comparison 5 Tramadol versus pethidine, Outcome 2 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 2 Additional analgesia required
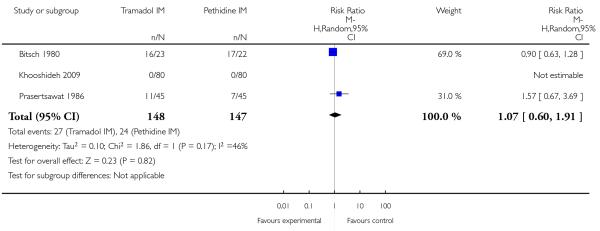 |
Analysis 5.3. Comparison 5 Tramadol versus pethidine, Outcome 3 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 3 Nausea and vomiting
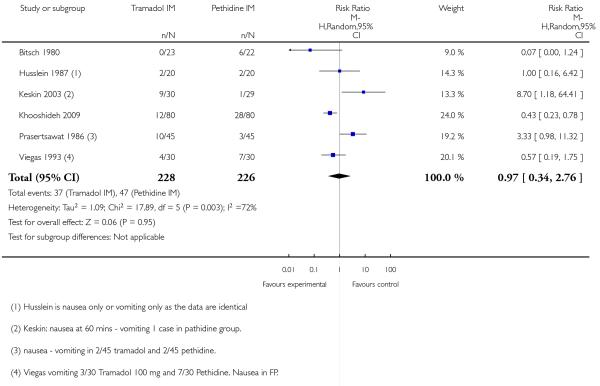 |
Analysis 5.4. Comparison 5 Tramadol versus pethidine, Outcome 4 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 4 Maternal sleepiness
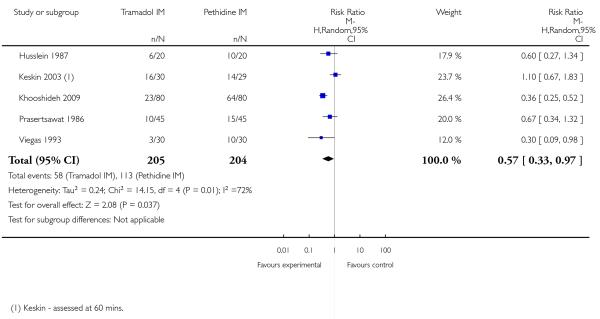 |
Analysis 5.5. Comparison 5 Tramadol versus pethidine, Outcome 5 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 5 Assisted vaginal delivery
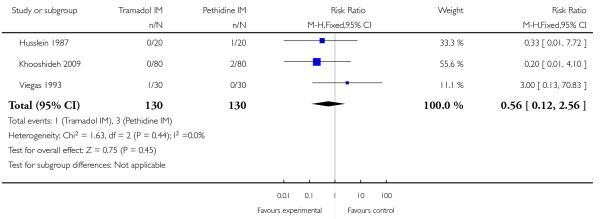 |
Analysis 5.6. Comparison 5 Tramadol versus pethidine, Outcome 6 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 6 Caesarean section
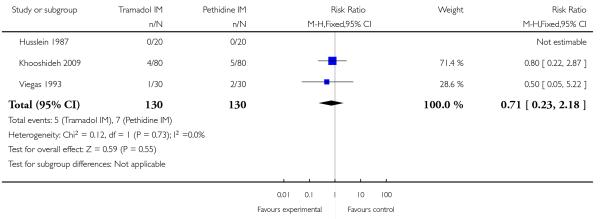 |
Analysis 5.7. Comparison 5 Tramadol versus pethidine, Outcome 7 Low Apgar scores (≤ 7) at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 7 Low Apgar scores (≤ 7) at 1 and 5 minutes
 |
Analysis 5.8. Comparison 5 Tramadol versus pethidine, Outcome 8 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 8 Neonatal resuscitation
 |
Analysis 5.9. Comparison 5 Tramadol versus pethidine, Outcome 9 Neonatal respiratory distress.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 9 Neonatal respiratory distress
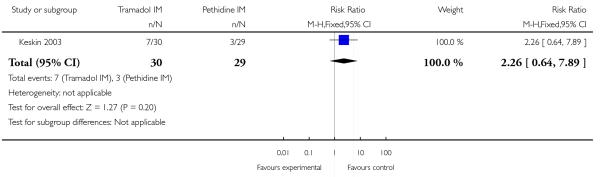 |
Analysis 5.10. Comparison 5 Tramadol versus pethidine, Outcome 10 Admission to NICU.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 5 Tramadol versus pethidine
Outcome: 10 Admission to NICU
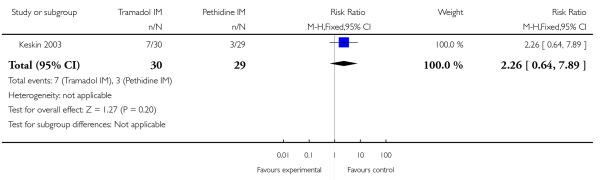 |
Analysis 6.1. Comparison 6 Tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 1 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 6 Tramadol + triflupromazine versus pethidine + triflupromazine
Outcome: 1 Nausea and vomiting
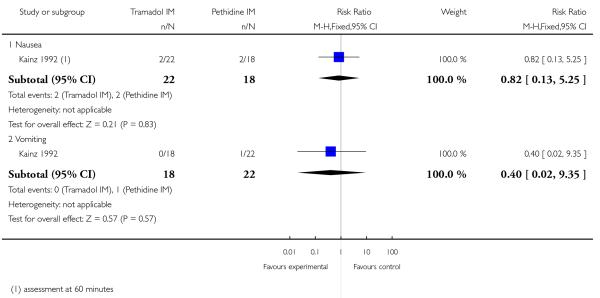 |
Analysis 6.2. Comparison 6 Tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 2 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 6 Tramadol + triflupromazine versus pethidine + triflupromazine
Outcome: 1 Nausea and vomiting
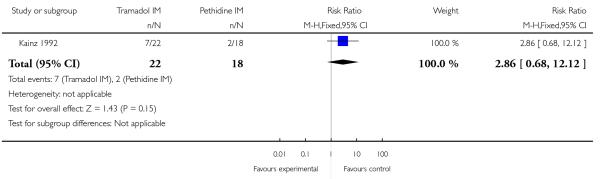 |
Analysis 7.1. Comparison 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM, Outcome 1 Maternal pain relief poor (1 hour).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM
Outcome: 1 Maternal pain relief poor (1 hour)
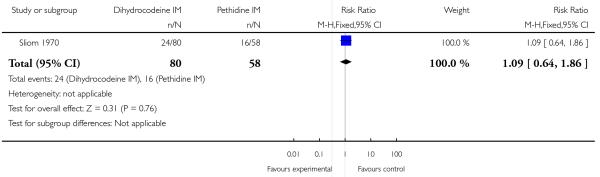 |
Analysis 7.2. Comparison 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM, Outcome 2 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM
Outcome: 2 Nausea and vomiting
 |
Analysis 7.3. Comparison 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM, Outcome 3 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM
Outcome: 3 Maternal sleepiness
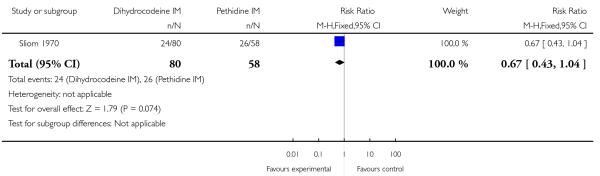 |
Analysis 7.4. Comparison 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM, Outcome 4 Apgar ≤ 7 at 1 minute.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 7 Dihydrocodeine 50 mg IM versus pethidine 100 mg IM
Outcome: 4 Apgar ≤ 7 at 1 minute
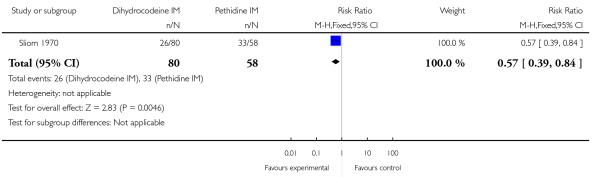 |
Analysis 8.1. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 1 Pain relief (good or very good) at delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 1 Pain relief (good or very good) at delivery
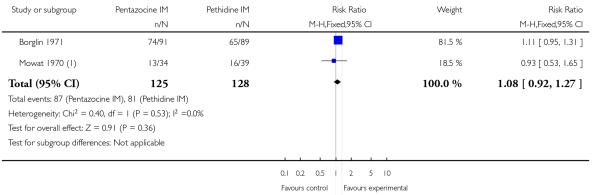 |
Analysis 8.2. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 2 Pain relief poor (partial, none or worse).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 2 Pain relief poor (partial, none or worse)
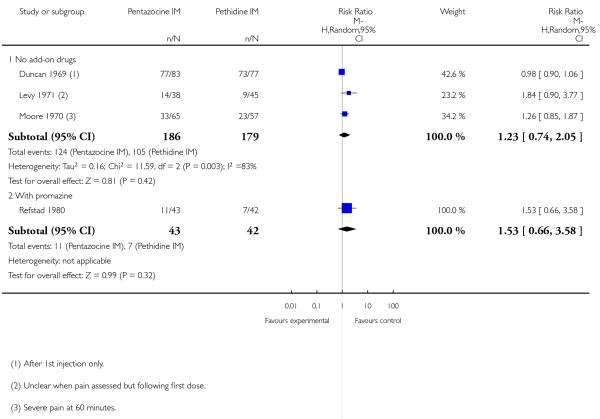 |
Analysis 8.3. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 3 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 3 Additional analgesia required
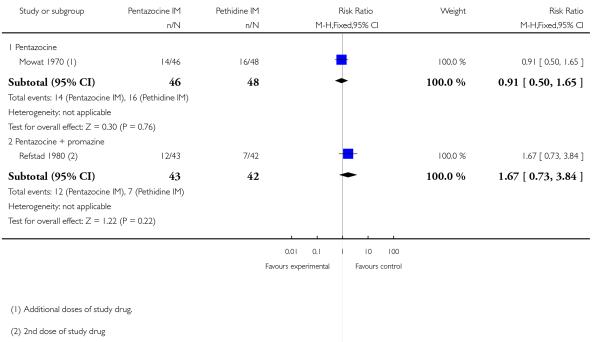 |
Analysis 8.4. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 4 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 4 Nausea and vomiting
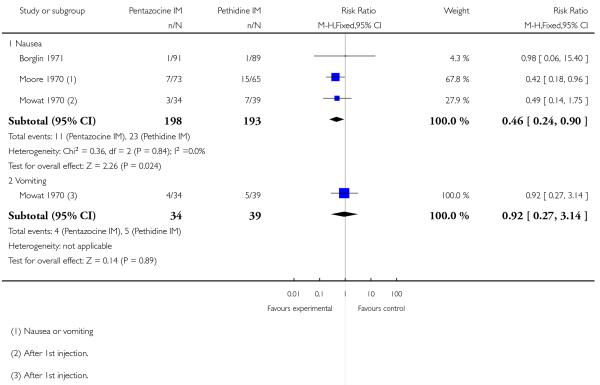 |
Analysis 8.5. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 5 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 5 Assisted vaginal delivery
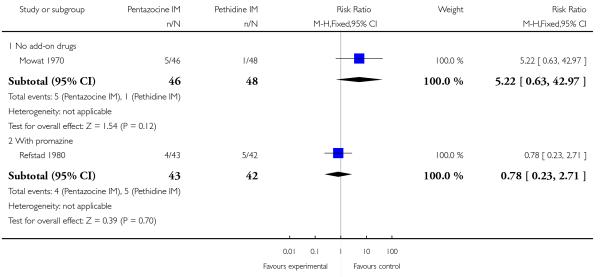 |
Analysis 8.6. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 6 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 6 Maternal sleepiness
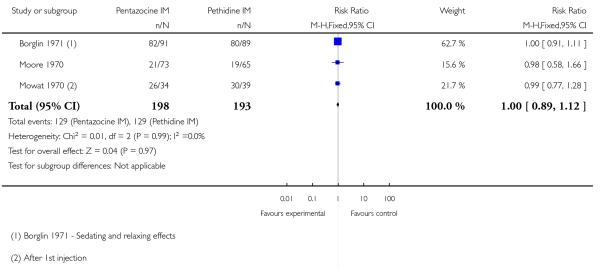 |
Analysis 8.7. Comparison 8 Pentazocine IM versus pethidine IM, Outcome 7 Low Apgar score (≤ 7) at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 8 Pentazocine IM versus pethidine IM
Outcome: 7 Low Apgar score (≤ 7) at 1 and 5 minutes
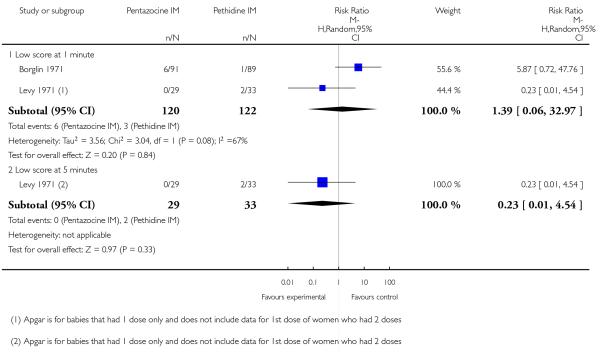 |
Analysis 9.1. Comparison 9 Pentazocine + promazine versus pethidine + promazine, Outcome 1 Low Apgar score (≤ 7) at 1 and 5 minutes (with promazine).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 9 Pentazocine + promazine versus pethidine + promazine
Outcome: 1 Low Apgar score (≤ 7) at 1 and 5 minutes (with promazine)
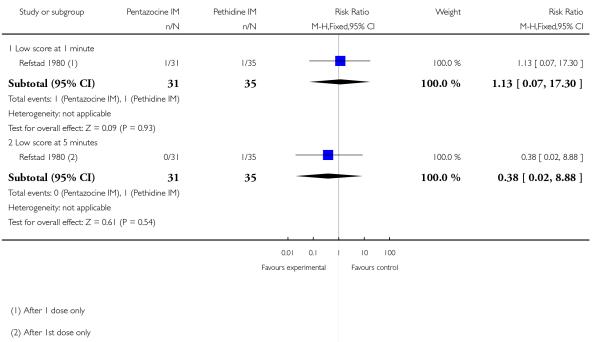 |
Analysis 9.2. Comparison 9 Pentazocine + promazine versus pethidine + promazine, Outcome 2 Naloxone administration (neonatal).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 9 Pentazocine + promazine versus pethidine + promazine
Outcome: 2 Naloxone administration (neonatal)
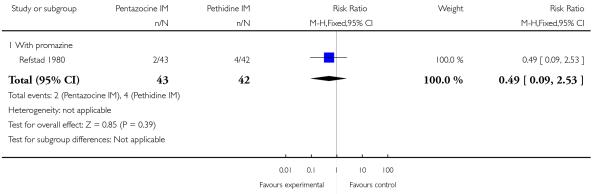 |
Analysis 10.1. Comparison 10 Nalbuphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia at 24 hours; numbers dissatisfied.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 1 Maternal satisfaction with analgesia at 24 hours; numbers dissatisfied
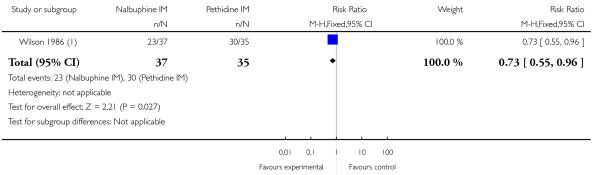 |
Analysis 10.2. Comparison 10 Nalbuphine versus pethidine, Outcome 2 Pain free.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 2 Pain free
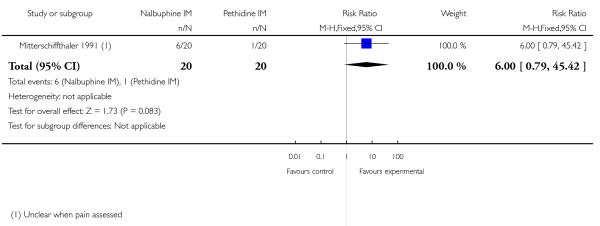 |
Analysis 10.3. Comparison 10 Nalbuphine versus pethidine, Outcome 3 Pain intensity at 30 minutes: women with severe pain.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 3 Pain intensity at 30 minutes: women with severe pain
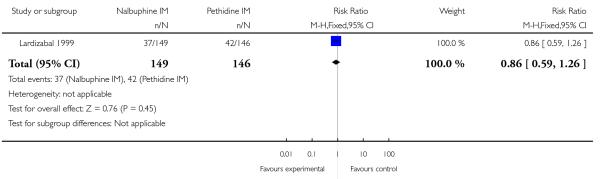 |
Analysis 10.4. Comparison 10 Nalbuphine versus pethidine, Outcome 4 VAS at 60 minutes (at peak of contraction).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 4 VAS at 60 minutes (at peak of contraction)
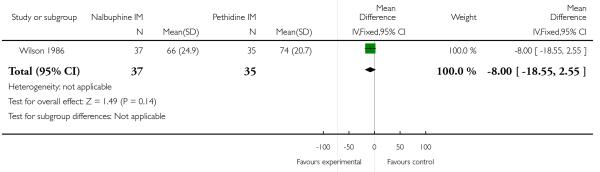 |
Analysis 10.5. Comparison 10 Nalbuphine versus pethidine, Outcome 5 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 5 Additional analgesia required
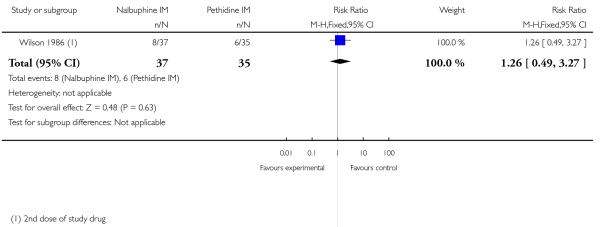 |
Analysis 10.6. Comparison 10 Nalbuphine versus pethidine, Outcome 6 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 6 Epidural
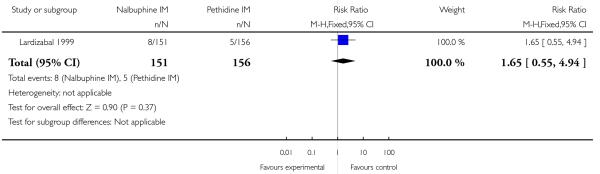 |
Analysis 10.7. Comparison 10 Nalbuphine versus pethidine, Outcome 7 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 7 Nausea and vomiting
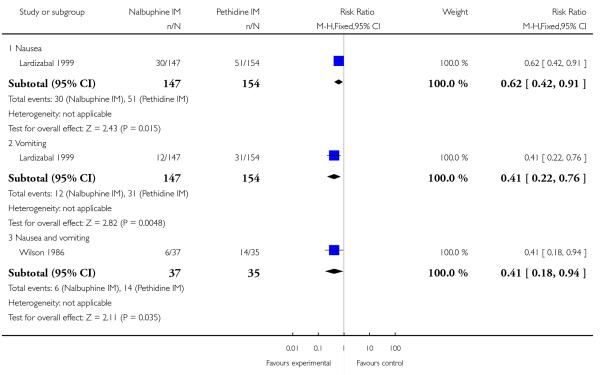 |
Analysis 10.8. Comparison 10 Nalbuphine versus pethidine, Outcome 8 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 8 Maternal sleepiness
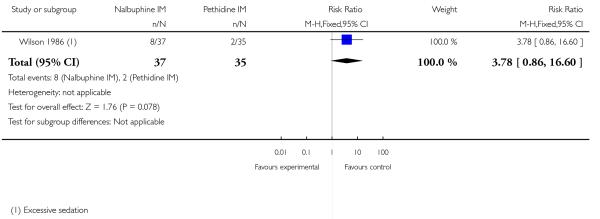 |
Analysis 10.9. Comparison 10 Nalbuphine versus pethidine, Outcome 9 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 9 Assisted vaginal delivery
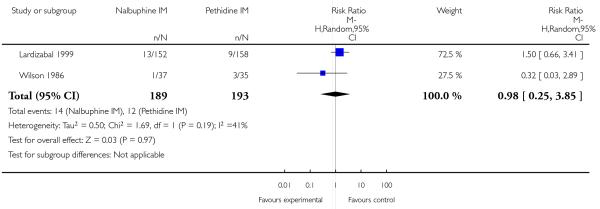 |
Analysis 10.10. Comparison 10 Nalbuphine versus pethidine, Outcome 10 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 10 Caesarean section
 |
Analysis 10.11. Comparison 10 Nalbuphine versus pethidine, Outcome 11 Low Apgar score (≤ 7) at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 11 Low Apgar score (≤ 7) at 1 and 5 minutes
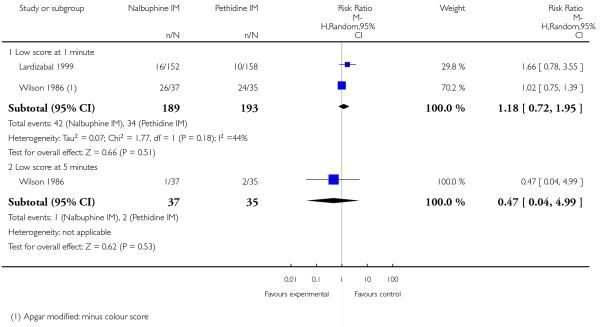 |
Analysis 10.12. Comparison 10 Nalbuphine versus pethidine, Outcome 12 Naloxone administration (neonatal).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 12 Naloxone administration (neonatal)
 |
Analysis 10.13. Comparison 10 Nalbuphine versus pethidine, Outcome 13 Admission to NICU.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 13 Admission to NICU
 |
Analysis 10.14. Comparison 10 Nalbuphine versus pethidine, Outcome 14 Neonatal neurobehavioural (Scanlon) 2-4 hours PN.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 10 Nalbuphine versus pethidine
Outcome: 14 Neonatal neurobehavioural (Scanlon) 2-4 hours PN
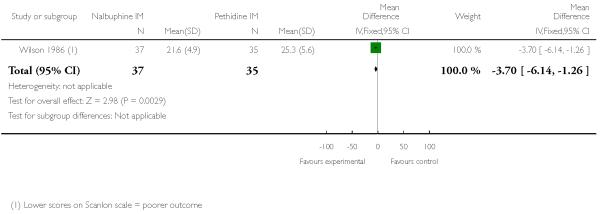 |
Analysis 11.1. Comparison 11 Phenazocine versus pethidine, Outcome 1 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 11 Phenazocine versus pethidine
Outcome: 1 Epidural
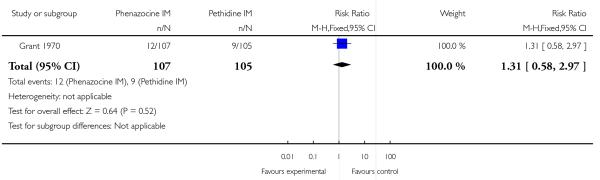 |
Analysis 11.2. Comparison 11 Phenazocine versus pethidine, Outcome 2 Vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 11 Phenazocine versus pethidine
Outcome: 2 Vomiting
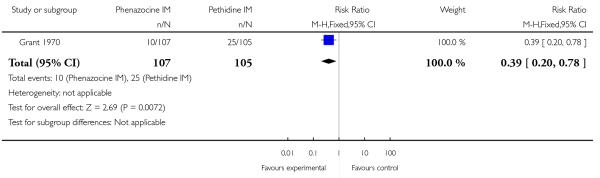 |
Analysis 12.1. Comparison 12 Morphine versus pethidine, Outcome 1 Pain relief described as poor.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 12 Morphine versus pethidine
Outcome: 1 Pain relief described as poor
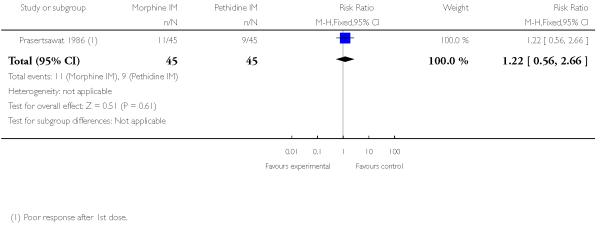 |
Analysis 12.2. Comparison 12 Morphine versus pethidine, Outcome 2 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 12 Morphine versus pethidine
Outcome: 2 Additional analgesia required
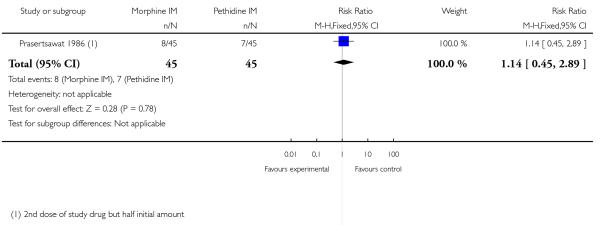 |
Analysis 12.3. Comparison 12 Morphine versus pethidine, Outcome 3 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 12 Morphine versus pethidine
Outcome: 3 Nausea and vomiting
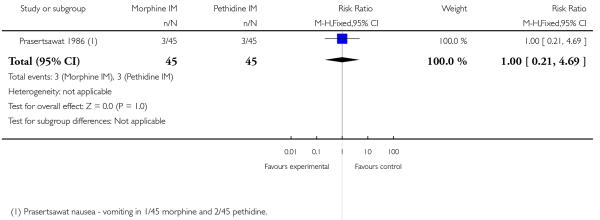 |
Analysis 12.4. Comparison 12 Morphine versus pethidine, Outcome 4 Maternal sleepiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 12 Morphine versus pethidine
Outcome: 4 Maternal sleepiness
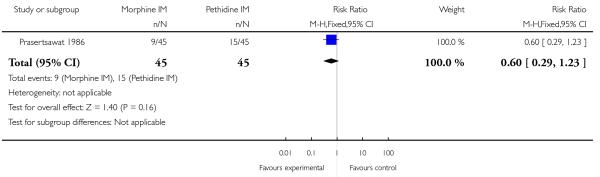 |
Analysis 12.5. Comparison 12 Morphine versus pethidine, Outcome 5 Apgar < 7 at 1 minute.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 12 Morphine versus pethidine
Outcome: 5 Apgar < 7 at 1 minute
 |
Analysis 12.6. Comparison 12 Morphine versus pethidine, Outcome 6 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 12 Morphine versus pethidine
Outcome: 6 Neonatal resuscitation
 |
Analysis 13.1. Comparison 13 Butorphanol versus pethidine, Outcome 1 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 13 Butorphanol versus pethidine
Outcome: 1 Additional analgesia required
 |
Analysis 13.2. Comparison 13 Butorphanol versus pethidine, Outcome 2 Nausea.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 13 Butorphanol versus pethidine
Outcome: 2 Nausea
 |
Analysis 13.3. Comparison 13 Butorphanol versus pethidine, Outcome 3 Vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 13 Butorphanol versus pethidine
Outcome: 3 Vomiting
 |
Analysis 13.4. Comparison 13 Butorphanol versus pethidine, Outcome 4 Neonatal resuscitation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 13 Butorphanol versus pethidine
Outcome: 4 Neonatal resuscitation
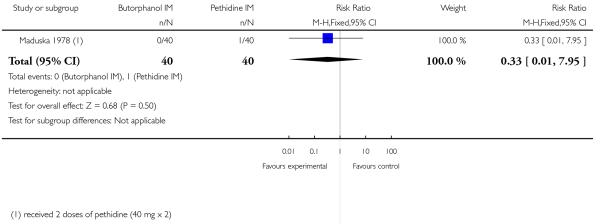 |
Analysis 13.5. Comparison 13 Butorphanol versus pethidine, Outcome 5 Naloxone administration (neonatal).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 13 Butorphanol versus pethidine
Outcome: 5 Naloxone administration (neonatal)
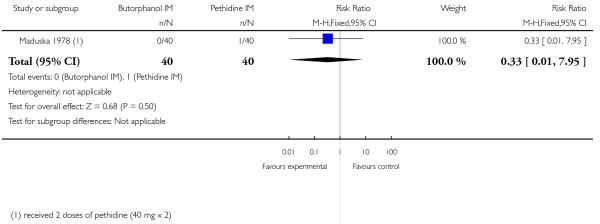 |
Analysis 14.1. Comparison 14 IM tramadol versus no treatment, Outcome 1 Anagesic effect described as satisfactory (not clear when measured).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 14 IM tramadol versus no treatment
Outcome: 1 Anagesic effect described as satisfactory (not clear when measured)
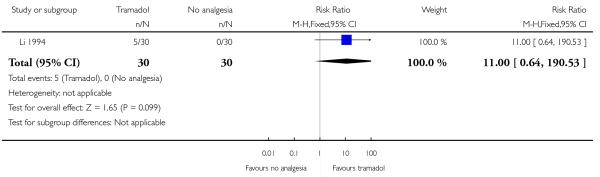 |
Analysis 14.2. Comparison 14 IM tramadol versus no treatment, Outcome 2 Mean blood loss at delivery (ml).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 14 IM tramadol versus no treatment
Outcome: 2 Mean blood loss at delivery (ml)
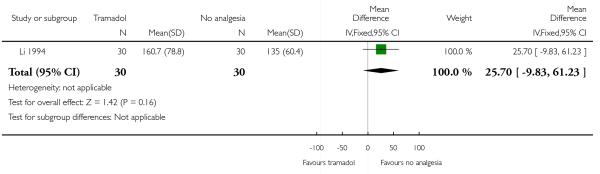 |
Analysis 15.1. Comparison 15 IM Avacan ® versus IM pentazocine, Outcome 1 Further analgesia required (nitrous oxide).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 15 IM Avacan versus IM pentazocine
Outcome: 1 Further analgesia required (nitrous oxide)
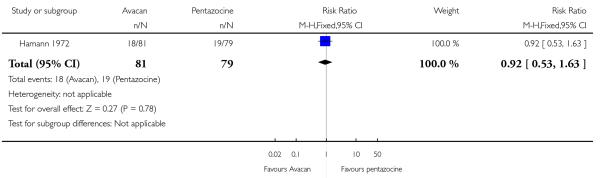 |
Analysis 15.2. Comparison 15 IM Avacan ® versus IM pentazocine, Outcome 2 Further analgesia required (pudendal-paracervical block).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 15 IM Avacan versus IM pentazocine
Outcome: 2 Further analgesia required (pudendal-paracervical block)
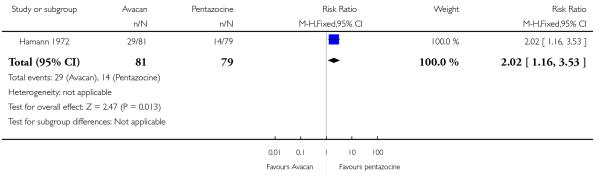 |
Analysis 15.3. Comparison 15 IM Avacan ® versus IM pentazocine, Outcome 3 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 15 IM Avacan versus IM pentazocine
Outcome: 3 Caesarean section
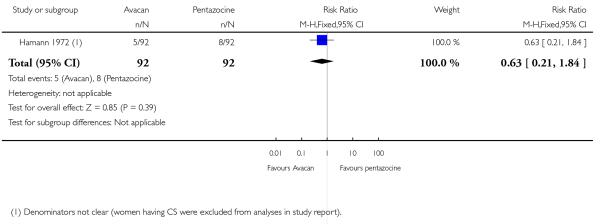 |
Analysis 15.4. Comparison 15 IM Avacan ® versus IM pentazocine, Outcome 4 Low Apgar score (< 7) “at birth”.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 15 IM Avacan versus IM pentazocine
Outcome: 4 Low Apgar score (< 7) “at birth”
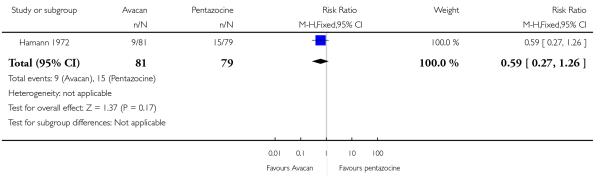 |
Analysis 16.1. Comparison 16 IM pentazocine versus IM pethilorfan, Outcome 1 Pain relief (women NOT obtaining pain relief) at 1 hour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 16 IM pentazocine versus IM pethilorfan
Outcome: 1 Pain relief (women NOT obtaining pain relief) at 1 hour
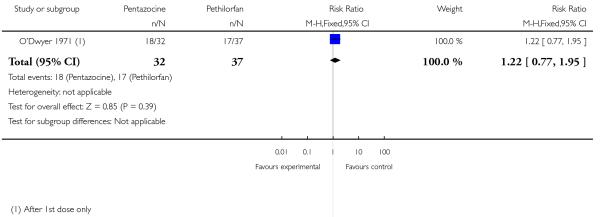 |
Analysis 16.2. Comparison 16 IM pentazocine versus IM pethilorfan, Outcome 2 Additional analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 16 IM pentazocine versus IM pethilorfan
Outcome: 2 Additional analgesia required
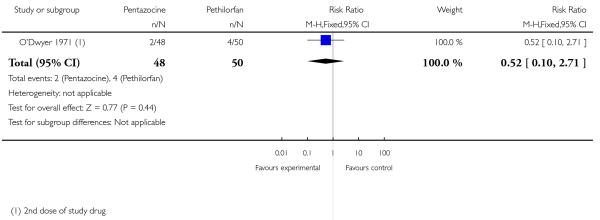 |
Analysis 16.3. Comparison 16 IM pentazocine versus IM pethilorfan, Outcome 3 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 16 IM pentazocine versus IM pethilorfan
Outcome: 3 Assisted vaginal delivery
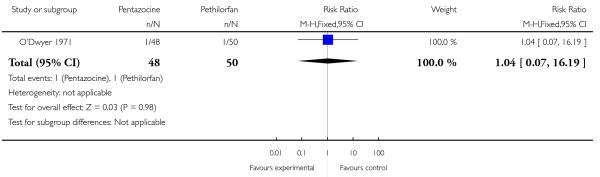 |
Analysis 16.4. Comparison 16 IM pentazocine versus IM pethilorfan, Outcome 4 Apgar < 8 at 1 minute.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 16 IM pentazocine versus IM pethilorfan
Outcome: 4 Apgar < 8 at 1 minute
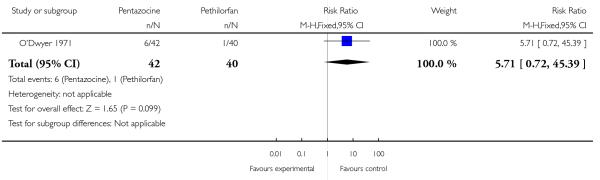 |
Analysis 16.5. Comparison 16 IM pentazocine versus IM pethilorfan, Outcome 5 Apgar < 8 at 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 16 IM pentazocine versus IM pethilorfan
Outcome: 5 Apgar < 8 at 5 minutes
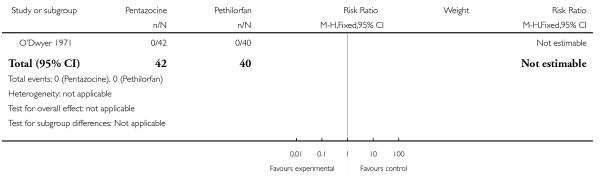 |
Analysis 17.1. Comparison 17 IV fentanyl versus IV pethidine, Outcome 1 Pain score (1 hour after drug administration).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 1 Pain score (1 hour after drug administration)
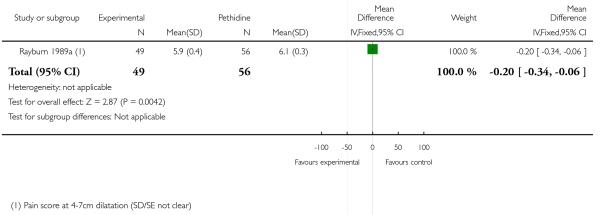 |
Analysis 17.2. Comparison 17 IV fentanyl versus IV pethidine, Outcome 2 Mean doses of analgesia.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 2 Mean doses of analgesia
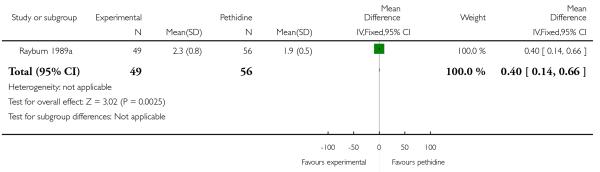 |
Analysis 17.3. Comparison 17 IV fentanyl versus IV pethidine, Outcome 3 Nausea and/or vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 3 Nausea and/or vomiting
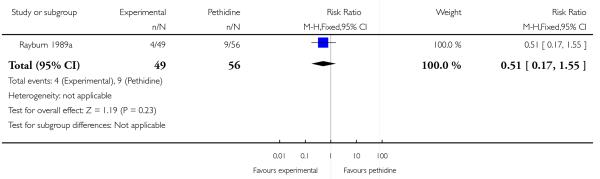 |
Analysis 17.4. Comparison 17 IV fentanyl versus IV pethidine, Outcome 4 Anti-emetic required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 4 Anti-emetic required
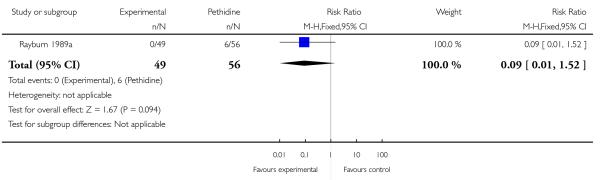 |
Analysis 17.5. Comparison 17 IV fentanyl versus IV pethidine, Outcome 5 Maternal sedation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 5 Maternal sedation
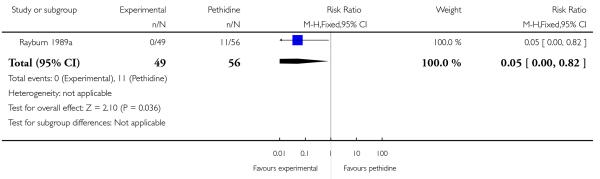 |
Analysis 17.6. Comparison 17 IV fentanyl versus IV pethidine, Outcome 6 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 6 Caesarean section
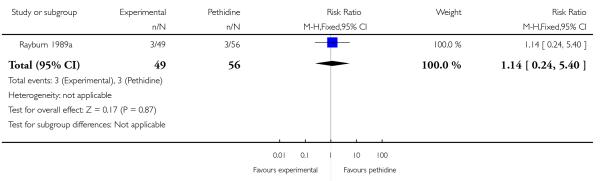 |
Analysis 17.7. Comparison 17 IV fentanyl versus IV pethidine, Outcome 7 Apgar score < 7 at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 7 Apgar score < 7 at 1 and 5 minutes
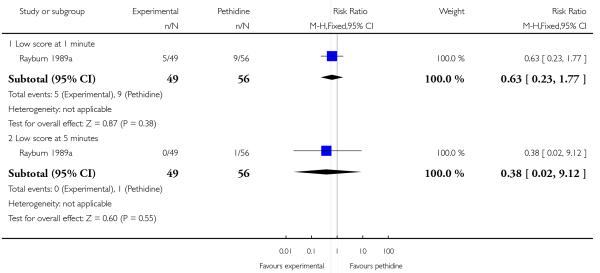 |
Analysis 17.8. Comparison 17 IV fentanyl versus IV pethidine, Outcome 8 Naloxone administered.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 8 Naloxone administered
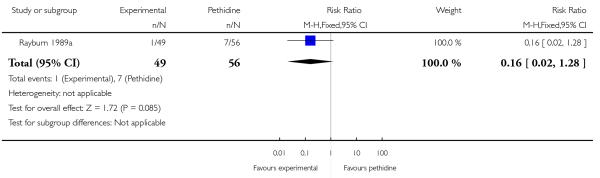 |
Analysis 17.9. Comparison 17 IV fentanyl versus IV pethidine, Outcome 9 Babies requiring resuscitation/ventilatory support.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 9 Babies requiring resuscitation/ ventilatory support
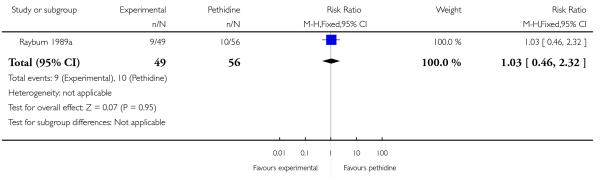 |
Analysis 17.10. Comparison 17 IV fentanyl versus IV pethidine, Outcome 10 Neurobehavioural score (1 - 2 hours after delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 10 Neurobehavioural score (1 - 2 hours after delivery)
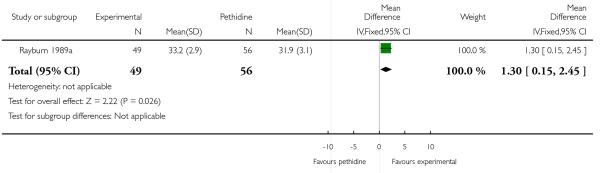 |
Analysis 17.11. Comparison 17 IV fentanyl versus IV pethidine, Outcome 11 Neurobehavioural score (2 hours - 24 hours).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 17 IV fentanyl versus IV pethidine
Outcome: 11 Neurobehavioural score (2 hours - 24 hours)
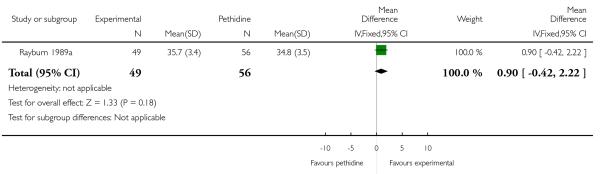 |
Analysis 18.1. Comparison 18 IV nalbuphine versus IV pethidine, Outcome 1 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 18 IV nalbuphine versus IV pethidine
Outcome: 1 Caesarean section
 |
Analysis 18.2. Comparison 18 IV nalbuphine versus IV pethidine, Outcome 2 Apgar score < 7 at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 18 IV nalbuphine versus IV pethidine
Outcome: 2 Apgar score < 7 at 1 and 5 minutes
 |
Analysis 19.1. Comparison 19 IV phenazocine versus IV pethidine, Outcome 1 Satisfaction with pain relief (women with fair or poor relief).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 19 IV phenazocine versus IV pethidine
Outcome: 1 Satisfaction with pain relief (women with fair or poor relief)
 |
Analysis 19.2. Comparison 19 IV phenazocine versus IV pethidine, Outcome 2 Nausea with vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 19 IV phenazocine versus IV pethidine
Outcome: 2 Nausea with vomiting
 |
Analysis 19.3. Comparison 19 IV phenazocine versus IV pethidine, Outcome 3 Perinatal death.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 19 IV phenazocine versus IV pethidine
Outcome: 3 Perinatal death
 |
Analysis 19.4. Comparison 19 IV phenazocine versus IV pethidine, Outcome 4 Apgar score < 7 at 1 minute.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 19 IV phenazocine versus IV pethidine
Outcome: 4 Apgar score < 7 at 1 minute
 |
Analysis 20.1. Comparison 20 IV butorphanol versus IV pethidine, Outcome 1 Pain relief score.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 1 Pain relief score
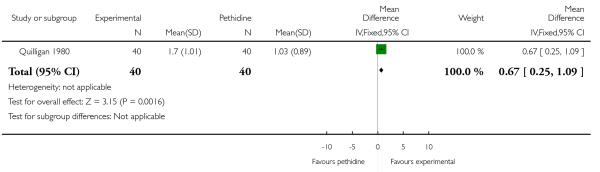 |
Analysis 20.2. Comparison 20 IV butorphanol versus IV pethidine, Outcome 2 Pain score (1 hour after drug administration).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 2 Pain score (1 hour after drug administration)
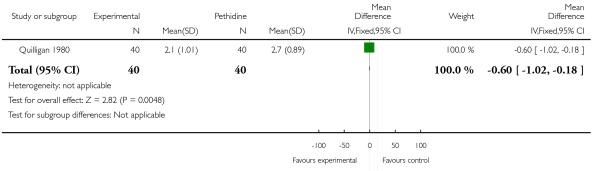 |
Analysis 20.3. Comparison 20 IV butorphanol versus IV pethidine, Outcome 3 Further analgesia (2nd dose) required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 3 Further analgesia (2nd dose) required
 |
Analysis 20.4. Comparison 20 IV butorphanol versus IV pethidine, Outcome 4 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 4 Epidural
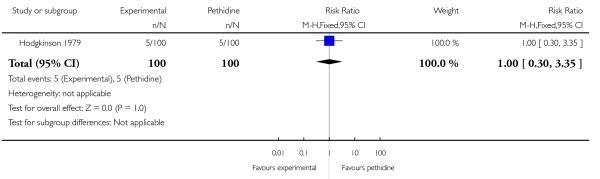 |
Analysis 20.5. Comparison 20 IV butorphanol versus IV pethidine, Outcome 5 Nausea and/or vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 5 Nausea and/or vomiting
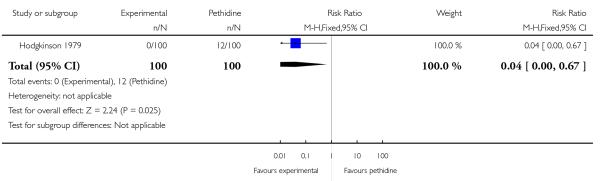 |
Analysis 20.6. Comparison 20 IV butorphanol versus IV pethidine, Outcome 6 Assisted vaginal delivery.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 6 Assisted vaginal delivery
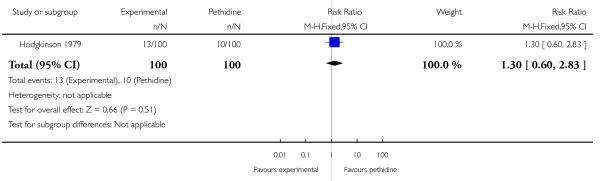 |
Analysis 20.7. Comparison 20 IV butorphanol versus IV pethidine, Outcome 7 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 7 Caesarean section
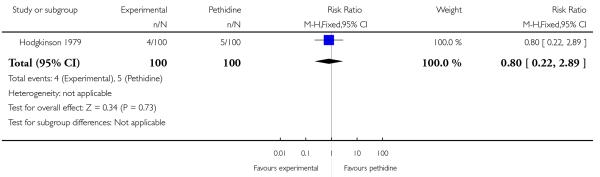 |
Analysis 20.8. Comparison 20 IV butorphanol versus IV pethidine, Outcome 8 Apgar score < 7 at 1 and 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 20 IV butorphanol versus IV pethidine
Outcome: 8 Apgar score < 7 at 1 and 5 minutes
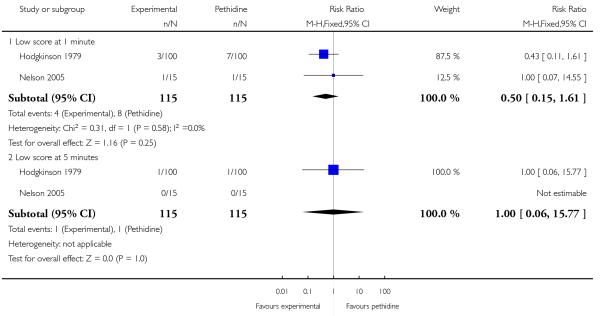 |
Analysis 21.1. Comparison 21 IV morphine versus IV pethidine, Outcome 1Women satisfied with analgesia (assessed 3 days postpartum).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 21 IV morphine versus IV pethidine
Outcome: 1 Women satisfied with analgesia (assessed 3 days postpartum)
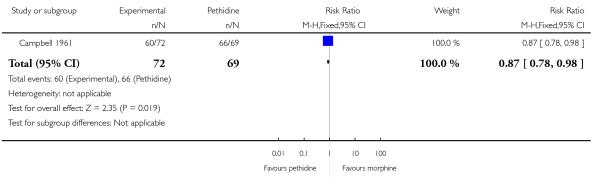 |
Analysis 21.2. Comparison 21 IV morphine versus IV pethidine, Outcome 2 Further dose of study analgesia required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 21 IV morphine versus IV pethidine
Outcome: 2 Further dose of study analgesia required
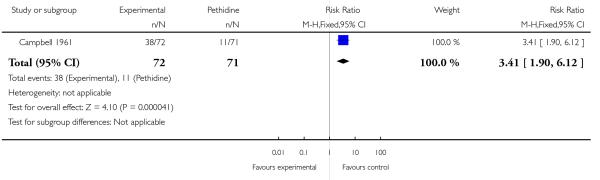 |
Analysis 21.3. Comparison 21 IV morphine versus IV pethidine, Outcome 3 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 21 IV morphine versus IV pethidine
Outcome: 3 Nausea and vomiting
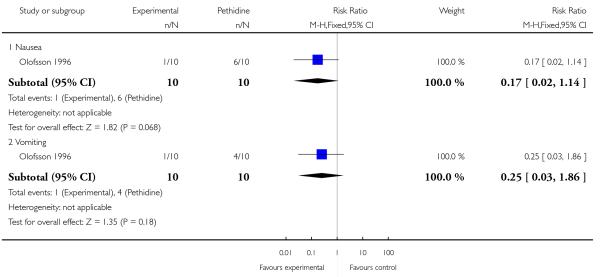 |
Analysis 21.4. Comparison 21 IV morphine versus IV pethidine, Outcome 4 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 21 IV morphine versus IV pethidine
Outcome: 4 Caesarean section
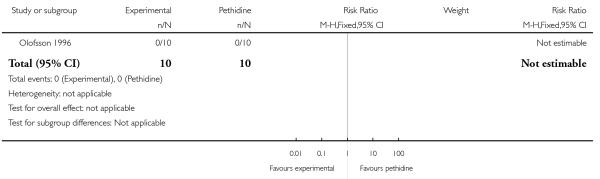 |
Analysis 22.1. Comparison 22 IV nisentil versus IV pethidine, Outcome 1 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 22 IV nisentil versus IV pethidine
Outcome: 1 Nausea and vomiting
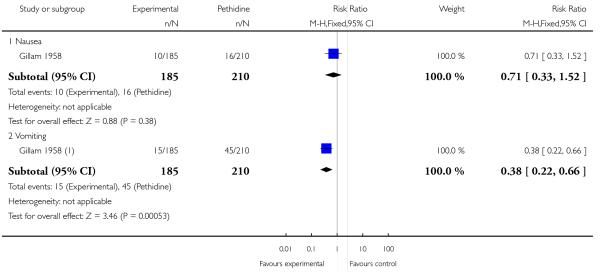 |
Analysis 22.2. Comparison 22 IV nisentil versus IV pethidine, Outcome 2 Babies requiring resuscitation/ventilatory support.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 22 IV nisentil versus IV pethidine
Outcome: 2 Babies requiring resuscitation/ventilatory support
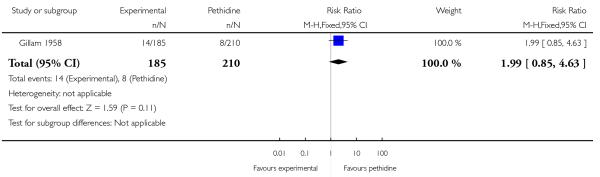 |
Analysis 23.1. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 1 Additional analgesia required (women requesting two or more doses).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 1 Additional analgesia required (women requesting two or more doses)
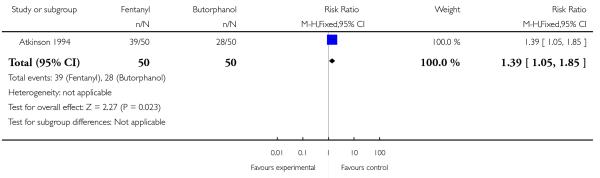 |
Analysis 23.2. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 2 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 2 Epidural
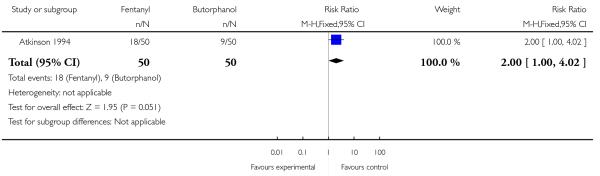 |
Analysis 23.3. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 3 Matenal drowsiness (required tactile rousing).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 3 Matenal drowsiness (required tactile rousing)
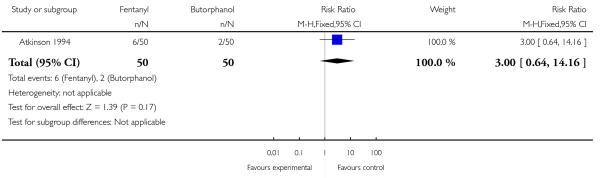 |
Analysis 23.4. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 4 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 4 Caesarean section
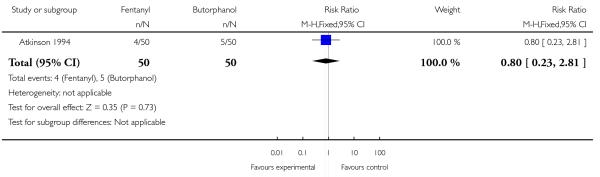 |
Analysis 23.5. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 5 Apgar score < 7 at 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 5 Apgar score < 7 at 5 minutes
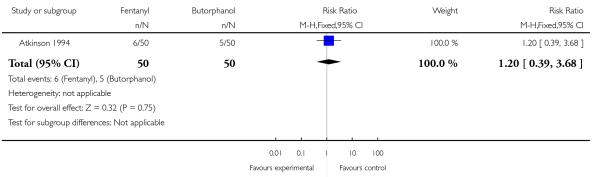 |
Analysis 23.6. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 6 Babies requiring ventilatory support.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 6 Babies requiring ventilatory support
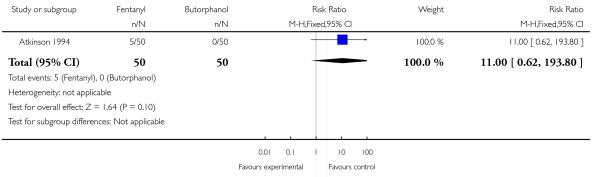 |
Analysis 23.7. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 7 Naloxone required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 7 Naloxone required
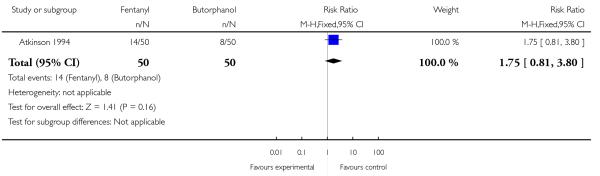 |
Analysis 23.8. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 8 Neurobehavioural score at 2-4 hours.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 8 Neurobehavioural score at 2-4 hours
 |
Analysis 23.9. Comparison 23 IV fentanyl versus IV butorphanol, Outcome 9 Neurobehavioural score at 24-36 hours.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 23 IV fentanyl versus IV butorphanol
Outcome: 9 Neurobehavioural score at 24-36 hours
 |
Analysis 24.1. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 1 Pain score in labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 1 Pain score in labour
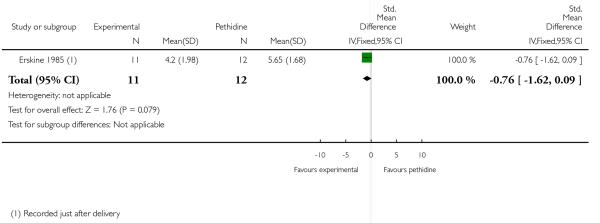 |
Analysis 24.2. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 2 Pain relief rated as good one day after birth.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 2 Pain relief rated as good one day after birth
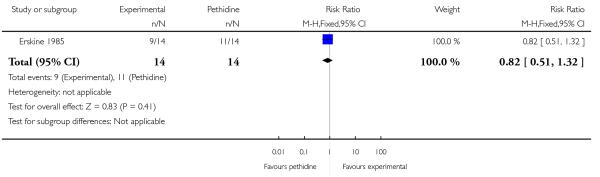 |
Analysis 24.3. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 3 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 3 Epidural
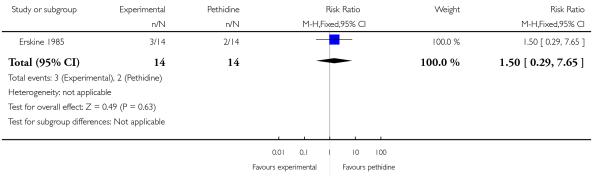 |
Analysis 24.4. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 4 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 4 Nausea and vomiting
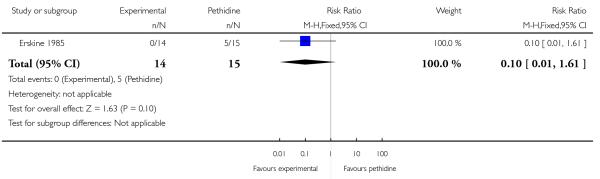 |
Analysis 24.5. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 5 Sedation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 5 Sedation
 |
Analysis 24.6. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 6 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 6 Caesarean section
 |
Analysis 24.7. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 7 Apgar score < 7 at 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 7 Apgar score < 7 at 5 minutes
 |
Analysis 24.8. Comparison 24 PCA pentazocine versus PCA pethidine, Outcome 8 Breastfeeding at discharge.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 24 PCA pentazocine versus PCA pethidine
Outcome: 8 Breastfeeding at discharge
 |
Analysis 25.1. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 1 Pain score in labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 1 Pain score in labour
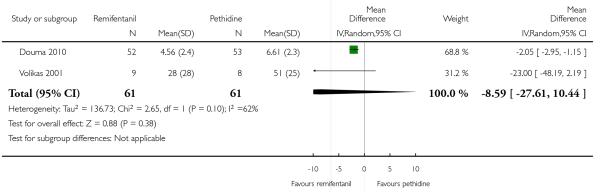 |
Analysis 25.2. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 2Women receiving other analgesia (Entonox).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 2 Women receiving other analgesia (Entonox)
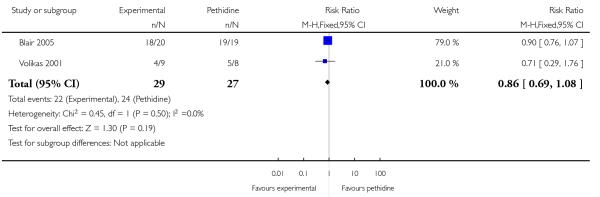 |
Analysis 25.3. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 3 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 3 Epidural
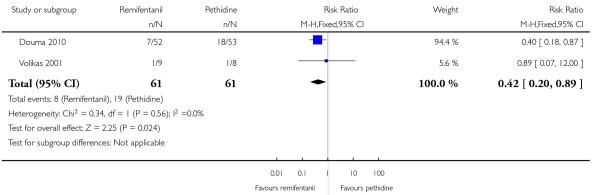 |
Analysis 25.4. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 4 Maternal sleepiness during labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 4 Maternal sleepiness during labour
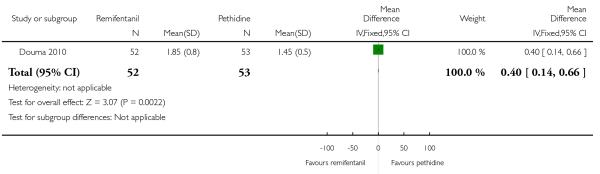 |
Analysis 25.5. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 5 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 5 Nausea and vomiting
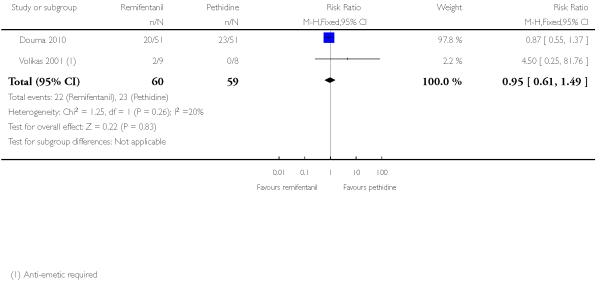 |
Analysis 25.6. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 6 Assisted vaginal birth.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 6 Assisted vaginal birth
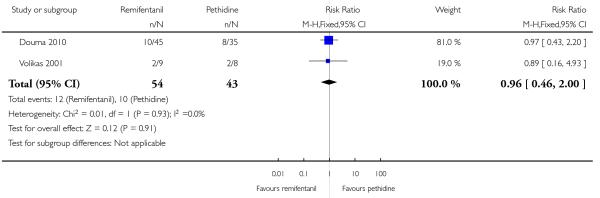 |
Analysis 25.7. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 7 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 7 Caesarean section
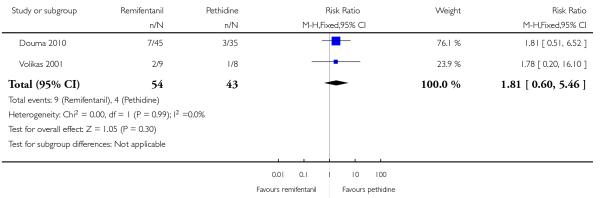 |
Analysis 25.8. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 8 Apgar score < 7 at 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 8 Apgar score < 7 at 5 minutes
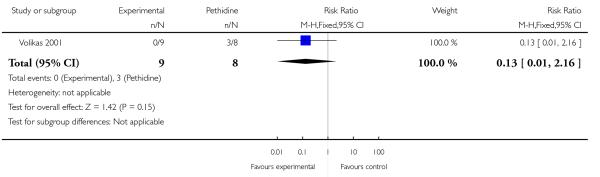 |
Analysis 25.9. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 9 Naloxone administered.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 9 Naloxone administered
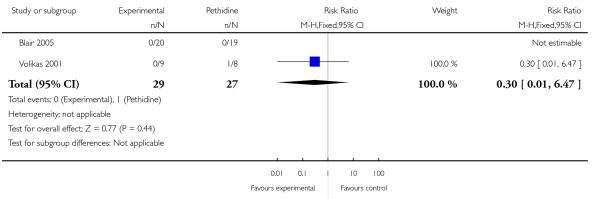 |
Analysis 25.10. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 10 Admission to NICU.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 10 Admission to NICU
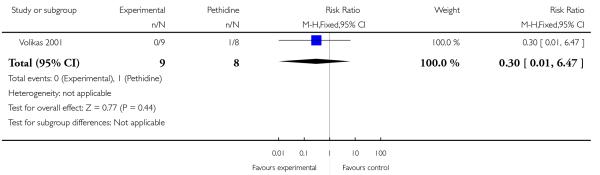 |
Analysis 25.11. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 11 Satisfaction with childbirth experience.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 11 Satisfaction with childbirth experience
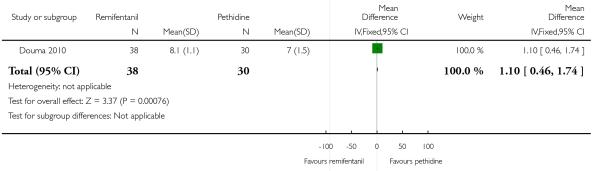 |
Analysis 25.12. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 12 Neurobehavioural score (15 minutes post delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 12 Neurobehavioural score (15 minutes post delivery)
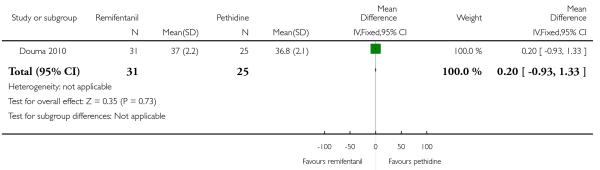 |
Analysis 25.13. Comparison 25 PCA remifentanil versus PCA pethidine, Outcome 13 Neurobehavioural score (2 hours post delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 25 PCA remifentanil versus PCA pethidine
Outcome: 13 Neurobehavioural score (2 hours post delivery)
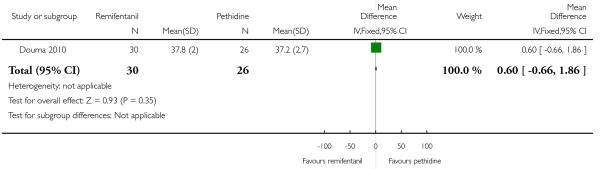 |
Analysis 26.1. Comparison 26 PCA nalbuphine versus PCA pethidine, Outcome 1 Pain relief in labour measured in the postnatal period (rated good or excellent).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 26 PCA nalbuphine versus PCA pethidine
Outcome: 1 Pain relief in labour measured in the postnatal period (rated good or excellent)
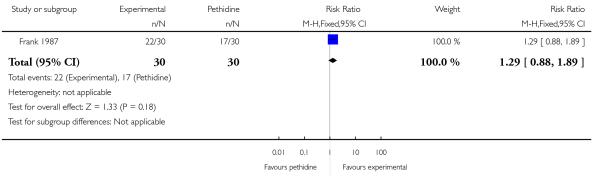 |
Analysis 26.2. Comparison 26 PCA nalbuphine versus PCA pethidine, Outcome 2Would use the same pain relief again.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 26 PCA nalbuphine versus PCA pethidine
Outcome: 2 Would use the same pain relief again
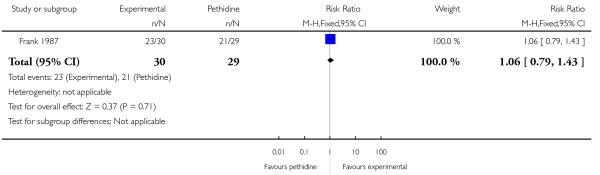 |
Analysis 26.3. Comparison 26 PCA nalbuphine versus PCA pethidine, Outcome 3 Pain score in labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 26 PCA nalbuphine versus PCA pethidine
Outcome: 3 Pain score in labour
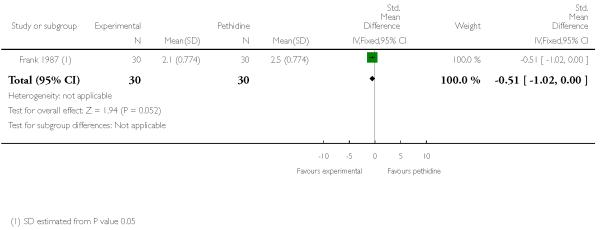 |
Analysis 26.4. Comparison 26 PCA nalbuphine versus PCA pethidine, Outcome 4Women receiving other analgesia (Entonox).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 26 PCA nalbuphine versus PCA pethidine
Outcome: 4 Women receiving other analgesia (Entonox)
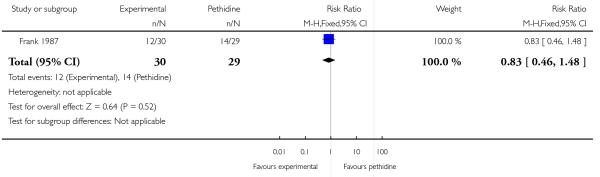 |
Analysis 26.5. Comparison 26 PCA nalbuphine versus PCA pethidine, Outcome 5 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 26 PCA nalbuphine versus PCA pethidine
Outcome: 5 Nausea and vomiting
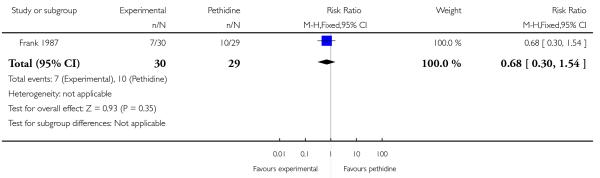 |
Analysis 26.6. Comparison 26 PCA nalbuphine versus PCA pethidine, Outcome 6 Apgar score < 7 at 5 minutes.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 26 PCA nalbuphine versus PCA pethidine
Outcome: 6 Apgar score < 7 at 5 minutes
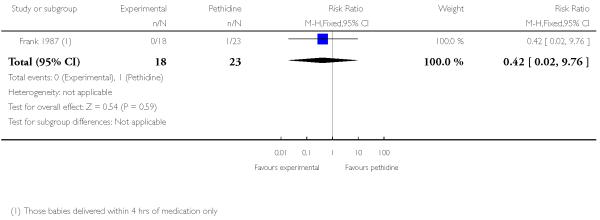 |
Analysis 27.1. Comparison 27 PCA fentanyl versus PCA alfentanil, Outcome 1 Pain relief described as adequate (recorded after delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 27 PCA fentanyl versus PCA alfentanil
Outcome: 1 Pain relief described as adequate (recorded after delivery)
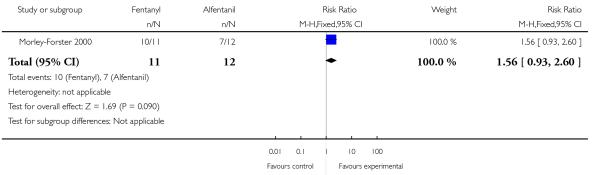 |
Analysis 27.2. Comparison 27 PCA fentanyl versus PCA alfentanil, Outcome 2 Pain score at 4-6 cm cervical dilatation.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 27 PCA fentanyl versus PCA alfentanil
Outcome: 2 Pain score at 4-6 cm cervical dilatation
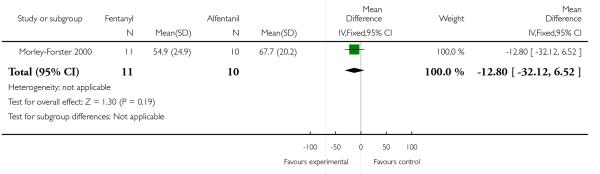 |
Analysis 27.3. Comparison 27 PCA fentanyl versus PCA alfentanil, Outcome 3 Nausea.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 27 PCA fentanyl versus PCA alfentanil
Outcome: 3 Nausea
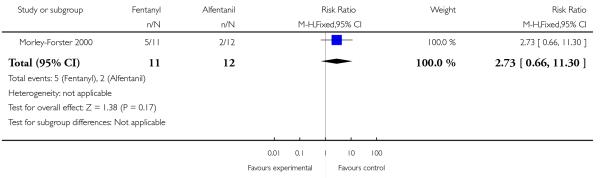 |
Analysis 27.4. Comparison 27 PCA fentanyl versus PCA alfentanil, Outcome 4 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 27 PCA fentanyl versus PCA alfentanil
Outcome: 4 Caesarean section
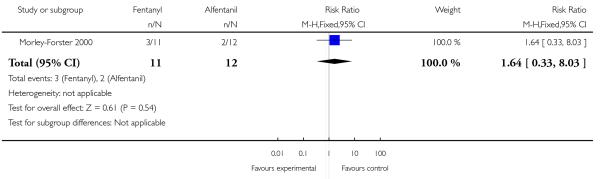 |
Analysis 27.5. Comparison 27 PCA fentanyl versus PCA alfentanil, Outcome 5 Naloxone required.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 27 PCA fentanyl versus PCA alfentanil
Outcome: 5 Naloxone required
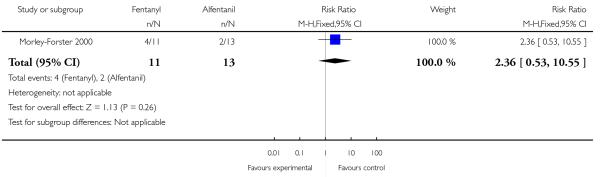 |
Analysis 28.1. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 1 Pain score in labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 1 Pain score in labour
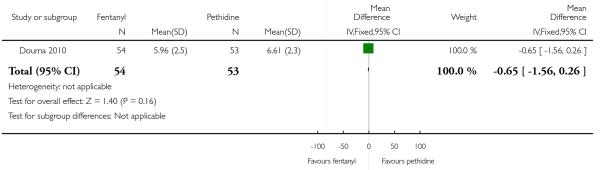 |
Analysis 28.2. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 2 Epidural.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 2 Epidural
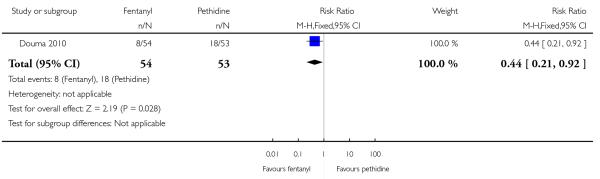 |
Analysis 28.3. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 3 Maternal sleepiness during labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 3 Maternal sleepiness during labour
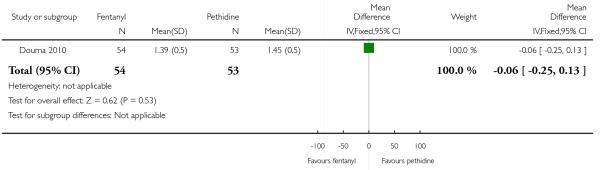 |
Analysis 28.4. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 4 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 4 Nausea and vomiting
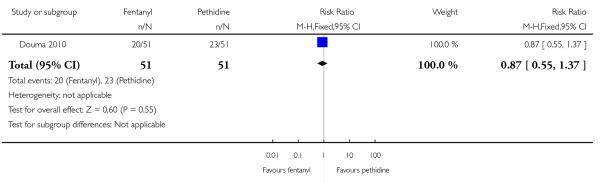 |
Analysis 28.5. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 5 Assisted vaginal birth.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 5 Assisted vaginal birth
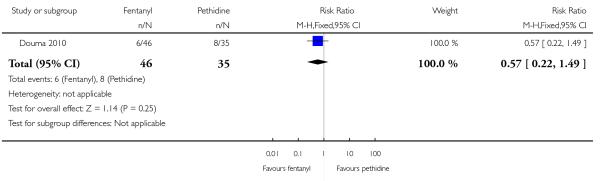 |
Analysis 28.6. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 6 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 6 Caesarean section
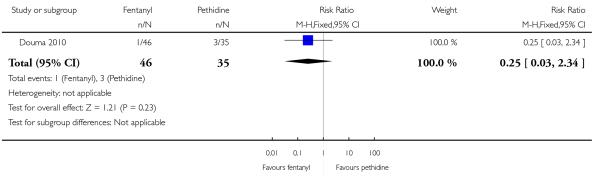 |
Analysis 28.7. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 7 Neurobehavioural score (15 minutes post delivery).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 28 PCA fentanyl versus PCA pethidine
Outcome: 7 Neurobehavioural score (15 minutes post delivery)
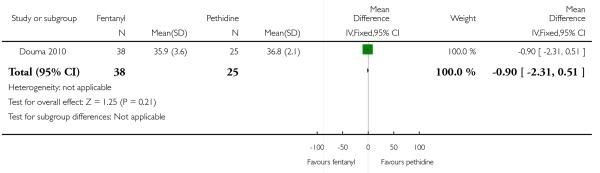 |
Analysis 28.8. Comparison 28 PCA fentanyl versus PCA pethidine, Outcome 8 Neurobehavioural score (2 hours post delivery).
Review: Parenteral opioids for maternal pain management in labour
Outcome: 8 Neurobehavioural score (2 hours post delivery)
Outcome: 7 Neurobehavioural score (15 minutes post delivery)
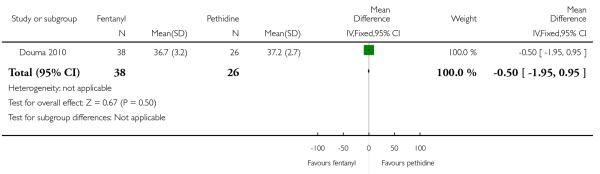 |
Analysis 29.1. Comparison 29 Opioids versus TENS, Outcome 1 Maternal satisfaction with analgesia measured post delivery (rated as good).
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 1 Maternal satisfaction with analgesia measured post delivery (rated as good)
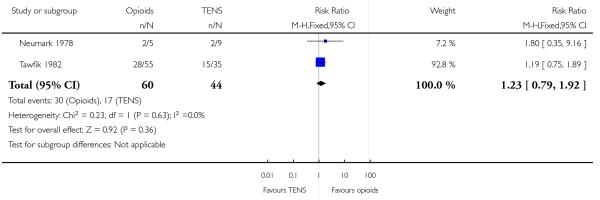 |
Analysis 29.2. Comparison 29 Opioids versus TENS, Outcome 2 Maternal pain score measured during labour.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 2 Maternal pain score measured during labour
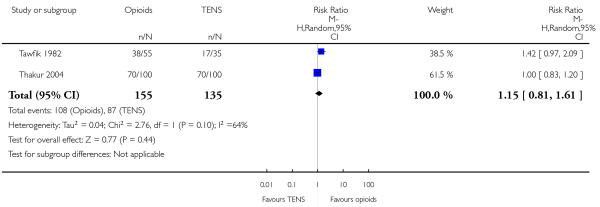 |
Analysis 29.3. Comparison 29 Opioids versus TENS, Outcome 3 Drowsiness.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 3 Drowsiness
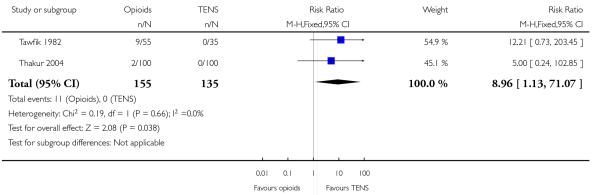 |
Analysis 29.4. Comparison 29 Opioids versus TENS, Outcome 4 Nausea and vomiting.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 4 Nausea and vomiting
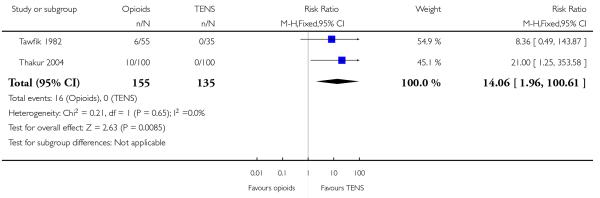 |
Analysis 29.5. Comparison 29 Opioids versus TENS, Outcome 5 Caesarean section.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 5 Caesarean section
 |
Analysis 29.6. Comparison 29 Opioids versus TENS, Outcome 6 Assisted vaginal birth.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 6 Assisted vaginal birth
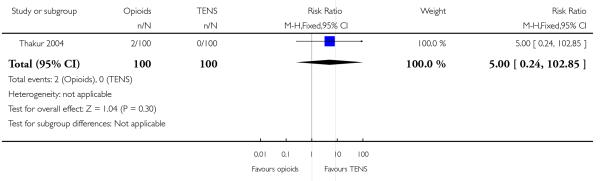 |
Analysis 29.7. Comparison 29 Opioids versus TENS, Outcome 7 Fetal distress.
Review: Parenteral opioids for maternal pain management in labour
Comparison: 29 Opioids versus TENS
Outcome: 7 Fetal distress
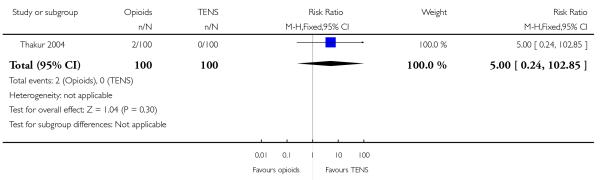 |
WHAT’S NEW
Last assessed as up-to-date: 6 July 2011.
| Date | Event | Description |
|---|---|---|
| 21 June 2011 | New search has been performed | Search updated. We have included data from three new studies (Douma 2010; Tawfik 1982; Thakur 2004). These changes have not altered the conclusions of the review New outcome added - see Differences between protocol and review. |
HISTORY
Protocol first published: Issue 4, 2008
Review first published: Issue 9, 2010
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The Background section of the review has been updated and amended since publication of the protocol. Two new review authors that joined the team at the review stage (L Smith and E Burns) considered that it was important to make these amendments.
The focus of some of the reports we identified using the search strategy was on the route of administration, rather than on the effectiveness of opioids compared with placebo or other opioids. That is, in several trials, women in both arms received the same opioid and the same dose but the drug was given by a different route (e.g. intravenous (staff administered) versus patient-controlled analgesia, or intramuscular versus intravenous). Although in the original protocol we had specified that we would examine different routes, in retrospect we thought that including such comparisons would add several more potentially large sections to the review (each report requiring a different comparison) and would throw little light on the main review questions: whether opioids are effective for pain relief in labour without causing unpleasant side effects or harm to mothers and babies. Studies focusing on route of administration will be examined in the future in a separate, related Cochrane review.
This review is one of a series of reviews to be included in an overview of reviews examining methods of pain management in labour (in development). It has been updated to follow the generic protocol developed in 2011 for reviews contributing to the overview (Jones 2011a), as a result of which we have added a new comparison (opioids versus TENS).
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
- Atkinson 1994 {published data only} .Atkinson BD, Truitt LJ, Rayburn WF, Turnbull GL, Christensen HD, Wlodaver A. Double-blind comparison of intravenous butorphanol (Stadol) and fentanyl (Sublimaze) for analgesia during labor. American Journal of Obstetrics and Gynecology. 1994;171:993–8. doi: 10.1016/0002-9378(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Bitsch 1980 {published data only} .Bitsch M, Emmrich J, Hary J, Lippach G, Rindt W. Obstetrical analgesia with tramadol [Geburtshilfliche Analgesie mit Tramadol] Fortschritte der Medizin. 1980;98(16):632–4. [PubMed] [Google Scholar]
- Blair 2005 {published data only} .Blair JM, Dobson GT, Hill DA, Fee JPH. Patient-controlled analgesia for labor: a comparison of remifentanil and pethidine [abstract] Anesthesiology. 2001;95 doi: 10.1111/j.1365-2044.2004.03975.x. Abstract no: A1063. [DOI] [PubMed] [Google Scholar]
- *; Blair JM, Dobson GT, Hill DA, McCracken GR, Fee JPH. Patient controlled analgesia for labour: a comparison of remifentanil with pethidine. Anaesthesia. 2005;60:22–7. doi: 10.1111/j.1365-2044.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- Borglin 1971 {published data only} .Borglin NE, Klottrup P. Pethidine and pentazocine. A double blind investigation in obstetric material. Lakartidningen. 1971;68:41–4. [PubMed] [Google Scholar]
- Campbell 1961 {published data only} .Campbell C, Phillips OC, Frazier TM. Analgesia during labor: a comparison of pentobarbital, meperidine and morphine. Obstetrics & Gynecology. 1961;17:714–8. [PubMed] [Google Scholar]
- De Boer 1987 {published data only} .De Boer F, Shortland D, Simpson RL, Clifford WA, Catley DM. A comparison of the effects of maternally administered meptazinol and pethidine on neonatal acid-base status. British Journal of Obstetrics and Gynaecology. 1987;94:256–61. doi: 10.1111/j.1471-0528.1987.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Douma 2010 {published data only} .Douma MR. [accessed 15 February 2007];Obstetric analgesia: a comparison of patient controlled pethidine, remifentanil and fentanyl in labour. doi: 10.1093/bja/aep359. http://www.controlled-trials.com/ISRCTN12122492. [DOI] [PubMed]
- Douma MR, Verwey RA, Kam-Endtz CE, Van der Linden PD, Stienstra R. Obstetric analgesia: a comparison of patient-controlled meperidine, remifentanil, and fentanyl in labour. British Journal of Anaesthesia. 2010;104(2):209–15. doi: 10.1093/bja/aep359. [DOI] [PubMed] [Google Scholar]
- Duncan 1969 {published data only} .Duncan SLB, Ginsburg J, Morris NF. Comparison of pentazocine and pethidine in normal labor. American Journal of Obstetrics and Gynecology. 1969;105:197–202. doi: 10.1016/0002-9378(69)90057-x. [DOI] [PubMed] [Google Scholar]
- Erskine 1985 {published data only} .Erskine WAR, Dick A, Morrell DF, Vital M, Van Den Heever J. Self-administered intravenous analgesia during labour. A comparison between pentazocine and pethidine. South African Medical Journal. 1985;67:764–7. [PubMed] [Google Scholar]
- Fairlie 1999 {published data only} .Fairlie FM, Marshall L, Walker JJ. Pethidine compared with diamorphine for pain relief in labour. American Journal of Obstetrics and Gynecology. 1992;166:394. [Google Scholar]
- *; Fairlie FM, Marshall L, Walker JJ, Elbourne D. Intramuscular opioids for maternal pain relief in labour: a randomised controlled trial comparing pethidine with diamorphine. British Journal of Obstetrics and Gynaecology. 1999;106:1181–7. doi: 10.1111/j.1471-0528.1999.tb08145.x. [DOI] [PubMed] [Google Scholar]
- Fieni 2000 {published data only} .Fieni S, Angeri F, Kaihura CT, Ricci L, Bedocchi L, Galanti B, et al. Evaluation of the peripartum effects of 2 analgesics: meperidine and tramadol, used in labor. Acta Bio-Medica de l Ateneo Parmense. 2000;71(Suppl 1):397–400. [PubMed] [Google Scholar]
- Frank 1987 {published data only} .Frank M, McAteer EJ, Cattermole R, Loughnan B, Stafford LB, Hitchcock AM. Nalbuphine for obstetric analgesia: a comparison of nalbuphine with pethidine for pain relief in labour when administered by patient-controlled analgesia (PCA) Anaesthesia. 1987;42:697–703. doi: 10.1111/j.1365-2044.1987.tb05313.x. [DOI] [PubMed] [Google Scholar]
- Giannina 1995 {published data only} .*; Giannina G, Guzman ER, Lai YL, Lake MF, Cernadas M, Vintzileos AM. Comparison of the effects of meperidine and nalbuphine on intrapartum fetal heart rate tracings. Obstetrics & Gynecology. 1995;86:441–5. doi: 10.1016/0029-7844(95)00164-M. [DOI] [PubMed] [Google Scholar]
- Gillam 1958 {published data only} .Gillam JS, Hunter GW, Darner CB, Thompson GR. Meperidine hydrochloride and alphaprodine hydrochloride as obstetric analgesic agents. A double blind study. American Journal of Obstetrics and Gynecology. 1958;75:1105–10. doi: 10.1016/0002-9378(58)90833-0. [DOI] [PubMed] [Google Scholar]
- Grant 1970 {published data only} .Grant A, Holt EM, Noble AD. A comparison between pethidine and phenazocine (Narphen) for relief of pain in labour. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1970;77:824–9. doi: 10.1111/j.1471-0528.1970.tb04408.x. [DOI] [PubMed] [Google Scholar]
- Hamann 1972 {published data only} .Hamann GO. Avacan vs fortral. A controlled double blind investigation on parturient patients. Ugeskrift for Laeger. 1972;134:2261–5. [PubMed] [Google Scholar]
- Hodgkinson 1979 {published data only} .Hodgkinson R, Huff RW, Hayashi RH, Husain FJ. Double-blind comparison of maternal analgesia and neonatal neurobehaviour following intravenous butorphanol and meperidine. Journal of International Medical Research. 1979;7(3):224–30. doi: 10.1177/030006057900700310. [DOI] [PubMed] [Google Scholar]
- Husslein 1987 {published data only} .Husslein P, Kubista E, Egarter C. Obstetrical analgesia with tramadol - results of a prospective randomized comparison with pethidine. Zeitschrift fur Geburtshilfe und Perinatologie. 1987;191:234–7. [PubMed] [Google Scholar]
- Jackson 1983 {published data only} .Jackson MB, Robson PJ. Preliminary clinical and pharmacokinetic experiences in the newborn when meptazinol is compared with pethidine as an obstetric analgesic. Postgraduate Medical Journal. 1983;59(Suppl 1):47–51. [PubMed] [Google Scholar]
- Kainz 1992 {published data only} .Kainz C, Joura E, Obwegeser R, Plockinger B, Gruber W. Efficacy and tolerance of tramadol with or without antiemetic compared to pethidine in obstetric analgesia. Zeitschrift fur Geburtshilfe und Perinatologie. 1992;196:78–82. [PubMed] [Google Scholar]
- Kamyabi 2003 {published data only} .*; Kamyabi Z, Naderi T, Ramazani A. A randomized, placebo-controlled trial of the effects of pethidine on labor pain, uterine contractions and infant Apgar score. Annals of Saudi Medicine. 2003;23(5):318–20. doi: 10.5144/0256-4947.2003.318. [DOI] [PubMed] [Google Scholar]
- Kamyabi Z, Zamiri Z, Ramazani A. A randomized double-blind survey of the effects of pethidine on the relief of labour pains, length of labour, uterine contractions and infant’s Apgar score. Journal of Obstetrics and Gynaecology Research. 2002;28(1):47. [Google Scholar]
- Keskin 2003 {published data only} .Keskin HL, Aktepe Keskin E, Avsar AF, Tabuk M, Caglar GS. Pethidine versus tramadol for pain relief during labor. International Journal of Gynecology & Obstetrics. 2003;82(1):11–6. doi: 10.1016/s0020-7292(03)00047-x. [DOI] [PubMed] [Google Scholar]
- Khooshideh 2009 {published data only} .Khooshideh M, Shahriari A. A comparison of tramadol and pethidine analgesia on the duration of labour: a randomised clinical trial. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2009;49(1):59–63. doi: 10.1111/j.1479-828X.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Lardizabal 1999 {published data only} .Lardizabal JL, Belizan JM, Carroli G, Gonzalez L, Campodonico L, Aguillaume CJ. A randomized trial of nalbuphine versus meperidine for analgesia during labor. References en Gynecologie Obstetrique. 1999;6:245–8. [Google Scholar]
- Levy 1971 {published data only} .Levy DL. Obstetric analgesia. Pentazocine and meperidine in normal primiparous labor. Obstetrics & Gynecology. 1971;38:907–11. [PubMed] [Google Scholar]
- Li 1988 {published data only} .Li DFH, Rees GAD, Rosen M. Feasibility of self-administration analgesia by the intramuscular route in labour. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1988;27:99–104. doi: 10.1016/0028-2243(88)90002-0. [DOI] [PubMed] [Google Scholar]
- Li 1994 {published data only} .Li YF, Li E, Tie LP, Weng LJ. Tramadol in labor analgesia. Beijing Medical Journal. 1994;16:265–8. [Google Scholar]
- Lisboa 1997 {published data only} .Lisboa APT, Cunha RD, Silva JCP, Mamede JAV, Ennes DK. Random clinical study, comparing nalbufine (IM) and meperidine (IM) during labour - 1996. [abstract] Acta Obstetricia et Gynecologica Scandinavica. 1997;76(167):1. [Google Scholar]
- Maduska 1978 {published data only} .Maduska AL, Hajghassemali M. A double-blind comparison of butorphanol and meperidine in labour: maternal pain relief and effect on the newborn. Canadian Anaesthetists Society Journal. 1978;25:398–404. doi: 10.1007/BF03006569. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler 1991 {published data only} .Mitterschiffthaler G, Huter O. Pethidin or nalbuphin for analgesia in labour? Geburtshilfe und Frauenheilkunde. 1991;51:362–5. doi: 10.1055/s-2007-1026158. [DOI] [PubMed] [Google Scholar]
- Moore 1970 {published data only} .Moore J, Carson RM, Hunter RJ. A comparison of the effects of pentazocine and pethidine administered during labour. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1970;77:830–6. doi: 10.1111/j.1471-0528.1970.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Morley-Forster 2000 {published data only} .Morley-Forster PK, Reid DW, Vandeberghe H. A comparison of patient-controlled analgesia fentanyl and alfentanil for labour analgesia. Canadian Journal of Anaesthesia. 2000;47(2):113–9. doi: 10.1007/BF03018845. [DOI] [PubMed] [Google Scholar]
- Morrison 1987 {published data only} .Morrison CE, Dutton D, Howie H, Gilmour H. Pethidine compared with meptazinol during labour. Anaesthesia. 1987;42:7–14. doi: 10.1111/j.1365-2044.1987.tb02937.x. [DOI] [PubMed] [Google Scholar]
- Mowat 1970 {published data only} .Mowat J, Garrey MM. Comparison of pentazocine and pethidine in labour. BMJ. 1970;2:757–9. doi: 10.1136/bmj.2.5712.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel 1981 {published data only} .Nel CP, Bloch B, Rush JM. A comparison of meptazinol and pethidine for pain relief during the first stage of labour. South African Medical Journal. 1981;59:908–10. [PubMed] [Google Scholar]
- Nelson 2005 {published data only} .Nelson KE, Eisenach JC. A comparison of butorphanol and meperidine for labor analgesia [abstract] Anesthesiology. 2004;101(Suppl) Abstract no: A1221. [Google Scholar]
- *; Nelson KE, Eisenach JC. Intravenous butorphanol, meperidine, and their combination relieve pain and distress in women in labour. Anesthesiology. 2005;105:1008–13. doi: 10.1097/00000542-200505000-00021. [DOI] [PubMed] [Google Scholar]
- Neumark 1978 {published data only} .Neumark J, Pauser G, Scherzer W. Pain relief in childbirth; an analysis of the analgesic effects of transcutaneous nerve stimulation (TNS), pethidine and placebos. Praktische Anaesthesie, Wiederbelebung und Intensivtherapie. 1978;13:13–20. [PubMed] [Google Scholar]
- Nicholas 1982 {published data only} .Nicholas ADG, Robson PJ. Double-blind comparison of meptazinol and pethidine in labour. British Journal of Obstetrics and Gynaecology. 1982;89:318–22. doi: 10.1111/j.1471-0528.1982.tb04704.x. [DOI] [PubMed] [Google Scholar]
- O’Dwyer 1971 {published data only} .O’Dwyer E. A comparison of the analgesics pentazocine and pethilorfan in the relief of pain during labour. Journal of the Irish Medical Association. 1971;64(408):173–6. [PubMed] [Google Scholar]
- Olofsson 1996 {published data only} .Olofsson Ch, Ekblom A, Ekman-Ordeberg G, Hjelm A, Irestedt L. Lack of analgesic effect of systemically administered morphine or pethidine on labour pain. British Journal of Obstetrics and Gynaecology. 1996;103:968–72. doi: 10.1111/j.1471-0528.1996.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Olson 1964 {published data only} .Olson RO, Riva HL. Evaluation of phenazocine with meperidine as an analgesic agent during labor, by the double blind method. American Journal of Obstetrics and Gynecology. 1964;88:601–11. doi: 10.1016/0002-9378(64)90887-7. [DOI] [PubMed] [Google Scholar]
- Osler 1987 {published data only} .Osler M. A double-blind study comparing meptazinol and pethidine for pain relief in labour. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1987;26:15–8. doi: 10.1016/0028-2243(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Prasertsawat 1986 {published data only} .Prasertsawat OP, Herabutya Y, Chaturachinda K. Obstetric analgesia: comparison between tramadol, morphine, and pethidine. Current Therapeutic Research, Clinical and Experimental. 1986;40(6):1022–8. [Google Scholar]
- Quilligan 1980 {published data only} .Quilligan EJ, Keegan KA, Donahue MJ. Double-blind comparison of intravenously injected butorphanol and meperidine in parturients. International Journal of Gynecology & Obstetrics. 1980;18:363–7. doi: 10.1002/j.1879-3479.1980.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Rayburn 1989a {published data only} .Rayburn WF, Smith CV, Parriott JE, Woods RE. Randomized comparison of meperidine and fentanyl during labor. Obstetrics & Gynecology. 1989;74:604–6. [PubMed] [Google Scholar]
- Refstad 1980 {published data only} .Refstad SO, Lindbaek E. Ventilatory depression of the newborn of women receiving pethidine or pentazocine. A double-blind comparative trial. British Journal of Anaesthesia. 1980;52:265–71. doi: 10.1093/bja/52.3.265. [DOI] [PubMed] [Google Scholar]
- Sheikh 1986 {published data only} .Sheikh A, Tunstall ME. Comparative study of meptazinol and pethidine for the relief of pain in labour. British Journal of Obstetrics and Gynaecology. 1986;93:264–9. doi: 10.1111/j.1471-0528.1986.tb07905.x. [DOI] [PubMed] [Google Scholar]
- Sliom 1970 {published data only} .Sliom CM. Analgesia during labour: a comparison between dihydrocodeine and pethidine. South African Medical Journal. 1970;44(11):317–9. [PubMed] [Google Scholar]
- Tawfik 1982 {published data only} .Tawfik O, Badraoui MHH, El-Ridi FS. The value of transcutaneous nerve stimulation (TNS) during labour in Egyptian mothers. Schmerz. 1982;2:98–105. [Google Scholar]
- Thakur 2004 {published data only} .Thakur R, Rekha P. Comparative study of transcutaneous electrical nerve stimulation (TENS) and tramadol hydrochloride for pain relief in labor. Journal of Obstetrics and Gynecology of India. 2004;54(4):346–50. [Google Scholar]
- Tharamas 1999 {published data only} .Tharamas W. Buprenorphine versus meperidine for analgesia in labor in nullipara at Chon Buri Hospital. Chon Buri Hospital Journal. 1999;24(2):25–34. [Google Scholar]
- Tsui 2004 {published data only} .Tsui MHY, Warwick D, Kee N, Ng FF, Lau TK. A double blinded placebo-controlled study of intramuscular pethidine for pain relief in the first stage of labour. British Journal of Obstetrics and Gynaecology. 2004;111:648–55. doi: 10.1111/j.1471-0528.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- Viegas 1993 {published data only} .Viegas OAC, Khaw B, Ratnam SS. Tramadol in labour pain in primiparous patients: a prospective comparative clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1993;49:131–5. doi: 10.1016/0028-2243(93)90260-j. [DOI] [PubMed] [Google Scholar]
- Volikas 2001 {published data only} .Volikas I, Male D. A comparison of pethidine and remifentanil patient-controlled analgesia in labour. International Journal of Obstetric Anesthesia. 2001;10:86–90. doi: 10.1054/ijoa.2000.0831. [DOI] [PubMed] [Google Scholar]
- Wahab 1988 {published data only} .Wahab SA, Askalani AH, Amar RA, Ramadan ME, Neweigy SB, Saleh AA. Effect of some recent analgesics on labor pain and maternal and fetal blood gases and pH. International Journal of Gynecology & Obstetrics. 1988;26:75–80. doi: 10.1016/0020-7292(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Wheble 1988 {published data only} .Wheble AM, Dawes GS, Gillmer MDG, Sykes GS. A double blind quantitative study of the effects of meptazinol and pethidine on the fetal heart rate in labour. Journal of Obstetrics and Gynaecology. 1988;8:248–52. [Google Scholar]
- Wilson 1986 {published data only} .*; Wilson CM, McClean E, Moore J, Dundee JW. A double-blind comparison of intramuscular pethidine and nalbuphine in labour. Anaesthesia. 1986;41:1207–13. doi: 10.1111/j.1365-2044.1986.tb13005.x. [DOI] [PubMed] [Google Scholar]
- Wilson CM, Moore J, McClean E, Dundee JW. Maternal analgesia and neonatal neuro-behaviour following nalbuphine and pethidine (abstract) British Journal of Clinical Pharmacology. 1986;21:613P. [Google Scholar]
References to studies excluded from this review
- Aiken 1971 {published data only} .Aiken RA, Cope E. The value of promazine and diazepam as adjuncts to pethidine in labour; Proceedings of 3rd International Congress on Psychosomatic Medicine in Obstetrics and Gynaecology; 1971; London, UK. 1971.pp. 241–3. [Google Scholar]
- Balcioglu 2007 {published data only} .Balcioglu O, Akin S, Demir S, Aribogan A. Patient-controlled intravenous analgesia with remifentanil in nulliparous subjects in labor. Expert Opinion on Pharmacotherapy. 2007;8(18):3089–96. doi: 10.1517/14656566.8.18.3089. [DOI] [PubMed] [Google Scholar]
- Balki 2007 {published data only} .Balki M, Kasodekar S, Dhumne S, Bernstein P, Carvalho J. Patient-controlled analgesia with background remifentanil infusion for labor pain [abstract] Anesthesiology. 2006;104(Suppl 1):13. [Google Scholar]
- *; Balki M, Kasodekar S, Dhumne S, Bernstein P, Carvalho JC. Remifentanil patient-controlled analgesia for labour:optimizing drug delivery regimens. Canadian Journal of Anaesthesia. 2007;54(8):626–33. [PubMed] [Google Scholar]
- Ballas 1976 {published data only} .Ballas S, Toaff ME, Toaff R. Effects of intravenous meperidine and meperidine with promethazine on uterine activity and fetal heart rate during labor. Israel Journal of Medical Sciences. 1976;12:1141–7. [PubMed] [Google Scholar]
- Bare 1962 {published data only} .Bare WW. Double-blind evaluation of hydroxyzine hydrochloride for labour and delivery. American Journal of Obstetrics and Gynecology. 1962;83:18–21. [Google Scholar]
- Bredow 1992 {published data only} .Bredow V. Use of tramadol versus pethidine versus denaverine suppositories in labor--a contribution to noninvasive therapy of labor pain [Die Anwendung von Tramadol-versus Pethidin-versus Denaverin-suppositorien unter der Geburt—-ein Beitrag zur nichtinvasiven Geburtsschmerztherapie] Zentralblatt fur Gynakologie. 1992;114(11):551–4. [PubMed] [Google Scholar]
- Brelje 1966 {published data only} .Brelje MC, Garcia-Bunuel R. Meperidine-hydroxyzine in obstetric analgesia. Obstetrics & Gynecology. 1966;27:350–4. [PubMed] [Google Scholar]
- Busacca 1982 {published data only} .Busacca M, Gementi P, Gambini E, Lenti C, Meschi F, Vignali M. Neonatal effects of the administration of meperidine and promethazine to the mother in labor. Double blind study. Journal of Perinatal Medicine. 1982;10:48–53. doi: 10.1515/jpme.1982.10.1.48. [DOI] [PubMed] [Google Scholar]
- Cahal 1960 {published data only} .Cahal DA, Dare JG, Keith D. A sequential trial of analgesics in labour. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1961;63:88–93. doi: 10.1111/j.1471-0528.1961.tb02689.x. [DOI] [PubMed] [Google Scholar]
- Calderon 2006 {published data only} .Calderon E, Martinez E, Roman MD, Pernia A, Garcia-Hernandez R, Torres LM. Intravenous remifentanil delivered through an elastomeric device versus intramuscular meperidine comparative study for obstetric analgesia [Remifentalino intravenoso mediante infusor elastomerico frente a meperidina intramuscular. Estudio comparativo en analgesia obstetrica] Revista de la Sociedad Espanola del Dolor. 2006;13(7):462–7. [Google Scholar]
- Callaghan 1966 {published data only} .Callaghan PE, Zelenik JS. Methotrimeprazine for obstetric analgesia. American Journal of Obstetrics and Gynecology. 1966;95:636–9. doi: 10.1016/s0002-9378(16)34738-x. [DOI] [PubMed] [Google Scholar]
- Camann 1992 {published data only} .Camann WR, Denney RA, Holby ED, Datta S. A comparison of intrathecal, epidural and intravenous sufentanil for labor analgesia. Anesthesiology. 1992;77:884–7. doi: 10.1097/00000542-199211000-00008. [DOI] [PubMed] [Google Scholar]
- Castro 2004 {published data only} .Castro C, Tharmaratnam U, Brockhurst N, Tureanu L, Tam K, Windrim R. Patient-controlled analgesia with fentanyl provides effective analgesia for second trimester labour: a randomized controlled study. Canadian Journal of Anaesthesia. 2004;50(10):1039–46. doi: 10.1007/BF03018370. [DOI] [PubMed] [Google Scholar]
- Cavanagh 1966 {published data only} .Cavanagh D, Le Cart C, Cassady JC, Kiem IM. A comparison of anileridine and meperidine as obstetric analgesia. A double-blind study of 471 patients. American Journal of Obstetrics and Gynecology. 1966;96:213–20. doi: 10.1016/0002-9378(66)90317-6. [DOI] [PubMed] [Google Scholar]
- Chang 1976 {published data only} .Chang A, Wood C, Humphrey M, Gilbert M, Wagstaff C. The effects of narcotics on fetal acid base status. British Journal of Obstetrics and Gynaecology. 1976;83:56–61. doi: 10.1111/j.1471-0528.1976.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Cincadze 1978 {published data only} .Cincadze, Bagdany S, Kintraia PJ, Mamtavrisvili Intensive fetal monitoring within obstetrical analgesia; Proceedings of 6th European Congress of Perinatal Medicine; 1978; Vienna, Austria. 1978; Aug-Sep. Abstract no: 52. [Google Scholar]
- Cullhed 1961 {published data only} .Cullhed S, Lofstrom B. Obstetric analgesia with pethidine and scopolamine. Lancet. 1961;1:75–7. doi: 10.1016/s0140-6736(61)92119-5. [DOI] [PubMed] [Google Scholar]
- Dan 1991 {published data only} .Dan U, Barkai G, Rabinovici J, Echin A, Modan M, Mashiah S. A prospective double blind comparison between intravenous nalbuphine and pethidine administered during labor; Proceedings of 11th European Congress of Perinatal Medicine; 1988; Rome, Italy. 1988.Apr 10-13, [Google Scholar]
- *; Dan U, Rabinovici Y, Barkai G, Modan M, Etchin A, Mashiach S. Intravenous pethidine and nalbuphine during labor: a prospective double-blind comparative study. Gynecologic and Obstetric Investigation. 1991;32:39–43. doi: 10.1159/000292990. [DOI] [PubMed] [Google Scholar]
- De Kornfeld 1964 {published data only} .De Kornfeld TJ, Pearson JW, Lasagna L. Methotrimeprazine in the treatment of labor pain. New England Journal of Medicine. 1964;270:391–4. doi: 10.1056/NEJM196402202700803. [DOI] [PubMed] [Google Scholar]
- De Lamerens 1964 {published data only} .De Lamerens S, Tuttle AH, Aballi AJ. Neonatal bilirubin levels after use of phenothiazine derivatives for obstetrical analgesia. Journal of Pediatrics. 1964;65:925–8. doi: 10.1016/s0022-3476(64)80016-0. [DOI] [PubMed] [Google Scholar]
- Eames 1964 {published data only} .Eames GM, Pool KRS. Clinical trial of oxymorphone in labour. BMJ. 1964;2:353–5. doi: 10.1136/bmj.2.5405.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kerdawy 2010 {published data only} .El-Kerdawy H, Farouk A. Labor analgesia in preeclampsia: remifentanil patient controlled intravenous analgesia versus epidural analgesia. Middle East Journal of Anesthesiology. 2010;20(4):539–45. [PubMed] [Google Scholar]
- Eliot 1975 {published data only} .Eliot BW, Hill JG, Cole AP, Hailey DM. Continuous pethidine/diazepam infusion during labour and its effects on the newborn. British Journal of Obstetrics and Gynaecology. 1975;82:126–31. doi: 10.1111/j.1471-0528.1975.tb02209.x. [DOI] [PubMed] [Google Scholar]
- Evron 2005 {published data only} .Evron S, Glezerman M, Sadan O, Boaz M. Remifentanil patient controlled analgesia for labor pain. Anesthesiology. 2002;96(Suppl) Abstract no: A1032. [Google Scholar]
- *; Evron S, Glezerman M, Sadan O, Boaz M, Ezri T. Remifentanil: a novel systematic analgesic for labor pain. Anesthesia & Analgesia. 2005;100:233–8. doi: 10.1213/01.ANE.0000143351.64538.BC. [DOI] [PubMed] [Google Scholar]
- Evron S, Sadan O, Ezri T, Boaz M, Glezerman M. Remifentanil: a new systemic analgesic for labor pain and an alternative to dolestine [abstract] American Journal of Obstetrics and Gynecology. 2001;185(6 Suppl):S210. [Google Scholar]
- Evron 2007 {published data only} .Evron S, Parameswaran R, Zipori D, Ezri T, Sadan O, Koren R. Activin beta A in term placenta and its correlation with placental inflammation in parturients having epidural or systemic meperidine analgesia: a randomized study. Journal of Clinical Anesthesia. 2007;19(3):168–74. doi: 10.1016/j.jclinane.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Evron 2008 {published data only} .Evron S, Ezri T, Protianov M, Muzikant G, Sadan O, Herman A, et al. The effects of remifentanil or acetaminophen with epidural ropivacaine on body temperature during labor. Journal of Anesthesia. 2008;22(2):105–11. doi: 10.1007/s00540-007-0589-8. [DOI] [PubMed] [Google Scholar]
- Gambling 1998 {published data only} .Gambling DR, Sharma SK, Ramin SM, Lucas MJ, Leveno KJ, Wiley J, et al. A randomized study of combined spinal-epidural analgesia versus intravenous meperidine during labor: impact on cesarean delivery rate. Anesthesiology. 1998;89:1336–44. doi: 10.1097/00000542-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Ginosar 2003 {published data only} .Ginosar Y, Columb MO, Cohen SE, Mirikatani E, Tingle MS, Ratner EF, et al. The site of action of epidural fentanyl infusions in the presence of local anesthetics: a minimum local analgesic concentration infusion study in nulliparous labor. Anesthesia & Analgesia. 2003;97:1439–45. doi: 10.1213/01.ANE.0000081792.84877.A2. [DOI] [PubMed] [Google Scholar]
- Goodlin 1988 {published data only} .Goodlin RC. Prevention of in utero meconium aspiration by narcotic administration. 1988. Personal communication. [Google Scholar]
- Grandjean 1979 {published data only} .Grandjean H, De Mouzon J, Cabot JA, Desprats R, Pontonnier G. Peridural analgesia and by phenoperidine in normal labor. Therapeutic trial with a control series. Archives Francaises de Pediatrie. 1979;36(9 Suppl):LXXV–LXXXI. [PubMed] [Google Scholar]
- Greer 1988 {published data only} .Greer IA, Johnston J, Tulloch I, Walker JJ. Effect of maternal ketorolac administration on platelet function in the newborn. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1988;29:257–60. doi: 10.1016/0028-2243(88)90065-2. [DOI] [PubMed] [Google Scholar]
- Hodgkinson 1978 {published data only} .Hodgkinson R, Bhatt M, Grewal G, Marx GF. Neonatal neurobehavior in the first 48 hours of life: effect of the administration of meperidine with and without naloxone in the mother. Pediatrics. 1978;62:294–8. [PubMed] [Google Scholar]
- Isenor 1993 {published data only} .Isenor L, Penny-MacGillivray T. Intravenous meperidine infusion for obstetric analgesia. Journal of Obstetric, Gynecologic and Neonatal Nursing. 1993;22(4):349–56. doi: 10.1111/j.1552-6909.1993.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Kalaskar 2007 {published data only} .Kalaskar A, Nayak AH. Comparative evaluation of analgesic efficacy and safety of intramuscular tramadol with pentazocine for labour analgesia: a prospective study; 31st British International Congress of Obstetrics and Gynaecology; 2007; London, UK. 2007.Jul 4-6, p. 112. [Google Scholar]
- Kaltreider 1967 {published data only} .Kaltreider DF. Premature labor and meperidine analgesia. American Journal of Obstetrics and Gynecology. 1967;99:989–93. doi: 10.1016/0002-9378(67)90256-6. [DOI] [PubMed] [Google Scholar]
- Krins 1969 {published data only} .Krins AJ, Mitchell WR, Wood C. Effect of morphine upon maternal capillary blood oxygen and carbon dioxide tension. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1969;76:359–61. doi: 10.1111/j.1471-0528.1969.tb05846.x. [DOI] [PubMed] [Google Scholar]
- Li 1995 {published data only} .Li E, Weng L. Influence of dihydroetorphine hydrochloride and tramadol on labor pain and umbilical blood gas. Chung-Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynaecology] 1995;30(6):345–8. [PubMed] [Google Scholar]
- MacVicar 1960 {published data only} .MacVicar J, Murray MH. Clinical evaluation of promazine as an adjunct to predelivery sedation. British Medical Journal. 1960;1:595–8. doi: 10.1136/bmj.1.5173.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkasian 1967 {published data only} .Malkasian GD, Smith RA, Decker DG. Comparison of hydroxyzine-meperidine and promethazine-meperidine for analgesia during labor. Obstetrics & Gynecology. 1967;30:568–75. [PubMed] [Google Scholar]
- McDonald 1964 {published data only} .McDonald R, Shaw M, Craig C. Effect of phenothiazines and analgesics given during labour on neonatal serum bilirubin. British Medical Journal. 1964;1:677. doi: 10.1136/bmj.1.5384.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath 1992 {published data only} .McGrath J, Chestnut D, Debruyn C. The effect of epidural bupivacaine vs intravenous nalbuphine on fetal heart rate during labor. Anesthesiology. 1992;77:A983. [Google Scholar]
- McInnes 2004 {published data only} .Hillan E. [accessed 12 June 2002];Diamorphine for pain relief in labour: a randomised controlled trial comparing intramuscular injection and patient controlled analgesia. 2002 doi: 10.1111/j.1471-0528.2004.00131.x. http://controlled-trials.com. [DOI] [PubMed]
- Hillan EM. Diamorphine for pain relief in labour: a randomized controlled trial comparing intramuscular injection and patient-controlled analgesia; 16th International Nursing Research Congress; 2005; Hawaii. 2005; Jul 14-16, [DOI] [PubMed] [Google Scholar]
- *; McInnes RJ, Hillan E, Clark D, Gilmour H. Diamorphine for pain relief in labour: a randomised controlled trial comparing intramuscular injection and patient-controlled analgesia. BJOG: an International Journal of Obstetrics & Gynaecology. 2004;111(10):1081–9. doi: 10.1111/j.1471-0528.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- McQuitty 1967 {published data only} .McQuitty FM. Relief of pain in labour A controlled double-blind trial comparing pethidine and various phenothiazine derivatives. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1967;74:925–8. doi: 10.1111/j.1471-0528.1967.tb15580.x. [DOI] [PubMed] [Google Scholar]
- Moore 1974 {published data only} .Moore J, Ball HG. A sequential study of intravenous analgesic treatment during labour. British Journal of Anaesthesia. 1974;46:365–72. doi: 10.1093/bja/46.5.365. [DOI] [PubMed] [Google Scholar]
- Morgan 2004 {published data only} .Morgan PJ, Palmer SK, Kung R, Halpern SH, Yee JA. Effect of intramuscular opioids on subsequent epidural analgesia [abstract] Canadian Journal of Anesthesia. 2004;51(Suppl 1):A58. [Google Scholar]
- Morris 1994 {published data only} .Morris GF, Gore-Hickman W, Lang SA, Yip RW. Can parturients distinguish between intravenous and epidural fentanyl? Canadian Journal of Anaesthesia. 1994;41:667–72. doi: 10.1007/BF03015618. [DOI] [PubMed] [Google Scholar]
- Nafisi 2006 {published data only} .Nafisi S. Effects of epidural lidocaine analgesia on labor and delivery: a randomized, prospective, controlled trial. BMC Anesthesiology. 2006;6:15. doi: 10.1186/1471-2253-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkola 2000 {published data only} .Nikkola EM, Jahnukainen TJ, Eklad UU, Kero PO, Salonen MAO. Neonatal monitoring after maternal fentanyl analgesia in labor. Journal of Clinical Monitoring & Computing. 2000;16:597–608. doi: 10.1023/a:1012268617009. [DOI] [PubMed] [Google Scholar]
- Overton 1992 {published data only} .Overton C, Haddad N, Williams J. Trial to study the effectiveness and suitability of sublingual diamorphine hydrochloride as an alternative in labour and to compare it with the presently available methods; Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992; Manchester, UK. 1992.Jul 7-10, p. 431. [Google Scholar]
- Pandole 2003 {published data only} .Pandole A, Akolekar R, Sardeshpande N, Kore S, Ambiye VR. A comparative study between tramadol and pethidine as a form of labour analgesia. Bombay Hospital Journal. 2003;45:4. [Google Scholar]
- Polley 2000 {published data only} .Polley LS, Columb MO, Naughton NN, Wagner DS, Dorantes DM, Van de Ven JMC. Effect of intravenous versus epidural fentanyl on the minimum local analgesic concentration of epidural bupivacaine in labor. Anesthesiology. 2000;93(1):122–8. doi: 10.1097/00000542-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Posner 1960 {published data only} .Posner AC. Combined pethidine and antagonists in obstetrics. British Medical Journal. 1960;1:124–5. [Google Scholar]
- Powe 1962 {published data only} .Powe CE, Kiem IM, Fromhagen C, Cavanagh D. Propiomazine hydrochloride in obstetrical analgesia. JAMA. 1962;181:290–4. doi: 10.1001/jama.1962.03050300010002. [DOI] [PubMed] [Google Scholar]
- Rabie 2006 {published data only} .Rabie ME, Negmi HH, Moustafa AM, Al Oufi H. Remifentanil by patient controlled analgesia compared with epidural analgesia for pain relief in labour [abstract] Regional Anesthesia and Pain Management. 2006;31(5 Suppl 1):52. [Google Scholar]
- Ransom 1966 {published data only} .Ransom S. Oxymorphone as an obstetric analgesic - a clinical trial. Anaesthesia. 1966;21:464–71. doi: 10.1111/j.1365-2044.1966.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Rayburn 1989 {published data only} .Rayburn W, Leuschen MP, Earl R, Woods M, Lorkovic M, Gaston-Johansson F. Intravenous meperidine during labor: a randomized comparison between nursing- and patient-controlled administration. Obstetrics & Gynecology. 1989;74:702–6. [PubMed] [Google Scholar]
- Rayburn 1991 {published data only} .Rayburn WF, Smith CV, Leuschen MP, Hoffman KA. Patient-controlled analgesia using fentanyl during labor; Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990; Houston, Texas, USA. 1990.Jan 23-27, p. 51. [Google Scholar]
- *; Rayburn WF, Smith CV, Leuschen MP, Hoffman KA, Flores CS. Comparison of patient-controlled and nurse-administered analgesia using intravenous fentanyl during labor. Anesthesiology Review. 1991;18(1):31–6. [PubMed] [Google Scholar]
- Roberts 1957 {published data only} .Roberts H, Kane KM, Percival N, Snow P, Please NW. Effects of some analgesic drugs used in childbirth with special reference to variation in respiratory minute volume of the newborn. Lancet. 1957;1:128–32. [PubMed] [Google Scholar]
- Roberts 1960 {published data only} .Roberts H, Kuck MAC. Use of alphaprodine and levallorphan during labour. Canadian Medical Association Journal. 1960;83:1088–93. [PMC free article] [PubMed] [Google Scholar]
- Robinson 1980 {published data only} .Evans JM, David H, Rosen M, Revill SI, Robinson J, McCarthy J, et al. Patient activated intravenous narcotic. Obstetric Anaesthetists Assn Report. Anaesthesia. 1976;31:847. [Google Scholar]
- *; Robinson JO, Rosen M, Evans JM, Revill SI, David H, Rees GAD. Self-administered intravenous and intramuscular pethidine - a controlled trial in labour. Anaesthesia. 1980;35:763–70. doi: 10.1111/j.1365-2044.1980.tb03916.x. [DOI] [PubMed] [Google Scholar]
- Ron 1984 {published data only} .Ron M, Menashe M, Hochner-Celnikier D, Palti Z. Maternal blood pressure response to the intravenous administration of pethidine-promethazine during labor. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1984;18:25–8. doi: 10.1016/0028-2243(84)90029-7. [DOI] [PubMed] [Google Scholar]
- Rowley 1963 {published data only} .Rowley WF, Tannrikulu O, Grossman A, Hsia DY. A controlled study on effect of promethazine hydrochloride and meperidine hydrochloride upon serum bilirubin levels in the newborn infant. Journal of Pediatrics. 1963;62:934–5. doi: 10.1016/s0022-3476(63)80111-0. [DOI] [PubMed] [Google Scholar]
- Savage 1955 {published data only} .Savage D. Chlorpromazine (Largactil) as an analgesic in labour. British Journal of Anaesthesia. 1955;27:346–53. doi: 10.1093/bja/27.7.346. [DOI] [PubMed] [Google Scholar]
- Sentnor 1966 {published data only} .*; Sentnor MH, Posner NA, Kohl SG, Pomerance W. Oxymorphone re-evaluated. The addition of a respiratory antagonist. American Journal of Obstetrics and Gynecology. 1966;96:430–6. [PubMed] [Google Scholar]
- Sentnor MH, Solomons E, Kohl SG. An evaluation of oxymorphone in labor. American Journal of Obstetrics and Gynecology. 1962;84:956–61. doi: 10.1016/0002-9378(62)90074-1. [DOI] [PubMed] [Google Scholar]
- Shahriari 2007 {published data only} .Shahriari A, Khooshideh M. A randomized controlled trial of intravenous remifentanil compared with intramuscular meperidine for pain relief in labor. Journal of Medical Sciences. 2007;7(4):635–9. [Google Scholar]
- Singh 2001 {published data only} .Singh S, Mathur V, Srivastava U, Pandey DN, Gupta N. Comparative evaluation of efficacy of tramadol with pentazocine for labour analgesia and their effects on foetal outcome. Journal of Obstetrics and Gynecology of India. 2001;51(2):55–7. [Google Scholar]
- Solek-Pastuszka 2009 {published data only} .Solek-Pastuszka J, Kepinski S, Makowski A, Celewicz Z, Zukowski M, Safranow K, et al. Patient-controlled continuous epidural analgesia vs intravenous remifentanil infusion for labour anaesthesia [Porownanie jakosci znieczulenia u rodzacych otrzymujacych remifentanil metoda analgezji dozylnej sterowanej przez pacjenta lub ciaglego znieczulenia zewnatrzoponowego] Anestezjologia Intensywna Terapia. 2009;41(2):84–8. [PubMed] [Google Scholar]
- Soontrapa 2002 {published data only} .Sookpanya S, Suntrapa S, Komwilaisak R. Effectiveness of intravenous pethidine for pain relief in the first stage of labour. Thai Journal of Obstetrics and Gynaecology. 1999;11(4):272. [Google Scholar]
- Soontrapa S, Somboonporn W, Komwilaisak R, Sookpanya S, Soontrapa Sukree, Somboonporn Woraluk, et al. Effectiveness of intravenous meperidine for pain relief in the first stage of labour. Journal of the Medical Association of Thailand. 2002;85(11):1169–75. [PubMed] [Google Scholar]
- Sosa 2004 {published data only} .*; Sosa CG, Balaguer E, Alonso JG, Panizza R, Laborde A, Berrondo C. Meperidine for dystocia during the first stage of labor: a randomized controlled trial. American Journal of Obstetrics and Gynecology. 2004;191:1212–8. doi: 10.1016/j.ajog.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Sosa CG, Buekens P, Hughes JM, Balaguer E, Sotero G, Panizza R, et al. Effect of pethidine administered during the first stage of labor on the acid-base status at birth. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2006;129(2):135–9. doi: 10.1016/j.ejogrb.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Spellacy 1966 {published data only} .Spellacy WN, Shattuck CA, Loffer FD. A double-blind study of the comparative effects of meperidine with secobarbital, hydroxyzine, or a placebo on labor and delivery. Obstetrics & Gynecology. 1966;27:290–3. [PubMed] [Google Scholar]
- Suvonnakote 1986 {published data only} .Suvonnakote T, Thitadilok W, Atisook R. Pain relief during labour. Journal of the Medical Association of Thailand. 1986;69(11):575–9. [PubMed] [Google Scholar]
- Taskin 1993 {published data only} .Taskin O, Saade G, Belfort M, Moise K. The effect of narcotics and spasmolytics on cervical dilatation in labor: a randomized placebo-controlled study. American Journal of Obstetrics and Gynecology. 1993;168:362. [Google Scholar]
- Thurlow 2002 {published data only} .Thurlow JA, Laxton CH, Dick A, Waterhouse P. A comparison of patient controlled analgesia (PCA) using remifentanil with intramuscular pethidine for pain relief in labour [abstract] International Journal of Obstetric Anesthesia. 2000;9:200. [Google Scholar]
- *; Thurlow JA, Laxton CH, Dick A, Waterhouse P, Sherman L, Goodman NW. Remifentanil by patient-controlled analgesia compared with intramuscular meperidine for pain relief in labour. British Journal of Anaesthesia. 2002;88(3):374–8. doi: 10.1093/bja/88.3.374. [DOI] [PubMed] [Google Scholar]
- Tomlin 1965 {published data only} .Tomlin PJ. Pethidine compared with pethidine plus levallorphan and with a placebo. British Journal of Anaesthesia. 1965;37:23–8. doi: 10.1093/bja/37.1.23. [DOI] [PubMed] [Google Scholar]
- Tournaire 1980 {published data only} .Tournaire M, Catinat-Ozil D, Breart G, Scherrer P, Baron JM, Leroy B. The influence of pethidine on uterine activity and dilatation of the cervix in spontaneous labour. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 1980;9:261–6. [PubMed] [Google Scholar]
- Treisser 1981 {published data only} .Treisser A, Breart G, Blum F, Jouhet P, Pigne A, Barrat J. Dystocia at the onset of labour. An evaluation of the different treatments available. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction. 1981;10:91–8. [PubMed] [Google Scholar]
- Tripti 2006 {published data only} .Tripti N, Jyotsna A. Pain relief in labor tramadol versus pentazocine. Journal of Obstetrics and Gynecology of India. 2006;56(5):406–9. [Google Scholar]
- Vavrinkova 2005 {published data only} .Vavrinkova B, Oborna L, Binder T, Horak J. Nalbuphine in obstetrical analgesia [Nalbuphine v porodnicke analgezii] Ceska Gynekologie. 2005;70(3):180–3. [PubMed] [Google Scholar]
- Volmanen 2005 {published data only} .*; Volmanen P, Akural E, Raudakoski T, Ohtonen P, Alahuhta S. Comparison of remifentanil and nitrous oxide in labour analgesia. Acta Anaesthesiologica Scandinavica. 2005;49:453–8. doi: 10.1111/j.1399-6576.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- Volmanen P, Akural E, Raudaskoski, Alahuhta S. Comparison of maternal haemodynamic effects and respiratory indices during remifentanil and nitrous oxide labour analgesia. International Journal of Obstetric Anesthesia. 2004;13(3):S19. [Google Scholar]
- Volmanen 2008 {published data only} .Volmanen P, Sarvela J, Akural EI, Raudaskoski T, Korttila K, Alahuhta S. Intravenous remifentanil vs. epidurallevobupivacaine with fentanyl for pain relief in early labour: a randomised, controlled, double-blinded study. Acta Anaesthesiologica Scandinavica. 2008;52(2):249–55. doi: 10.1111/j.1399-6576.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- Von Vorherr 1963 {published data only} .Von Vorherr H. Is there a pharmacological acceleration of childbirth? [Gibt es eine medikamentose Geburtsbeschleunigung?] Deutsche Medizinische Wochenschrift. 1963;88:1426–30. doi: 10.1055/s-0028-1112246. [DOI] [PubMed] [Google Scholar]
- Walker 1992 {published data only} .Walker JJ, Johnstone J, Lloyd J, Rocha CL. The transfer of ketorolac tromethamine from maternal to foetal blood. European Journal of Clinical Pharmacology. 1988;34:509–11. doi: 10.1007/BF01046711. [DOI] [PubMed] [Google Scholar]
- Walker JJ, Johnston J. A randomised study of non-steroidal anti-inflammatory drug ketolorac against pethidine in labour; Proceedings of 11th European Congress of Perinatal Medicine; 1988; Rome, Italy. 1988.Apr 10-13, p. 163. [Google Scholar]
- *; Walker JJ, Johnston J, Fairlie FM, Lloyd J, Bullingham R. A comparative study of intramuscular ketorolac and pethidine in labour pain. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1992;46:87–94. doi: 10.1016/0028-2243(92)90251-s. [DOI] [PubMed] [Google Scholar]
- Wan 1965 {published data only} .Wan LS, Emich JP. Use of propiomazine hydrochloride during labor - a double-blind study on 236 patients. Obstetrics & Gynecology. 1965;25:68–71. [PubMed] [Google Scholar]
- Wiener 1979 {published data only} .Wiener PC, Hogg MI, Rosen M. Neonatal respiration, feeding and neurobehavioural state: effects of intrapartum bupivacaine, pethidine and pethidine reversed by naloxone. Anaesthesia. 1979;34:996–1004. doi: 10.1111/j.1365-2044.1979.tb06247.x. [DOI] [PubMed] [Google Scholar]
- Williams 1962 {published data only} .Williams G, Cope I. An evaluation of a combination of pethidine and levallorphan (‘Pethilorfan’) in labour. Medical Journal of Australia. 1962;49:499–503. [PubMed] [Google Scholar]
- Wong 2005 {published data only} .Scavone BM, Sullivan JT, Peaceman AM, Strauss-Hoder TP, Wong CA. Fetal heart rate and uterine contraction pattern abnormalities after combined spinal/epidural vs systemic labor analgesia [abstract] Anesthesiology. 2002;96(Suppl) Abstract no: A1049. [Google Scholar]
- Sullivan JT, Scavone BM, McCarthy RJ, Wong CA. Does type of labor analgesia alter the pattern of oxytocin use? [abstract] Anesthesiology. 2002;96(Suppl 1) Abstract no: P48. [Google Scholar]
- Sullivan JT, Scavone BM, McCarthy RJ, Wong CA. Neuraxial labor analgesia is associated with an altered pattern of oxytocin use [abstract] Anesthesiology. 2002;96(Suppl) Abstract no: A1039. [Google Scholar]
- Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstetrics & Gynecology. 2009;113(5):1066–74. doi: 10.1097/AOG.0b013e3181a1a9a8. [DOI] [PubMed] [Google Scholar]
- *; Wong CA, Scavone BM, Peaceman AM, McCarthy RJ, Sullivan JT, Diaz NT, et al. The risk of cesarean delivery with neuraxial analgesia given early versus late in labor. [See comment] New England Journal of Medicine. 2005;352(7):655–65. doi: 10.1056/NEJMoa042573. [DOI] [PubMed] [Google Scholar]
- Wong CA, Scavone BM, Sullivan JT, Ebarvia MJ, McCarthy RJ. The risk of cesarean delivery with early neuraxial analgesia in nulliparous induction of labor. Anesthesiology. 2007;107 Abstract no: A1204. [Google Scholar]
- Wong CA, Scavone BM, Sullivan JT, Strauss-Hoder TP, McCarthy RJ. Randomized trial of neuraxial vs systemic analgesia for latent phase labor: effect on incidence of cesarean delivery [abstract] Anesthesiology. 2002;96(Suppl) Abstract no: A1047. [Google Scholar]
- Wong CA, Sullivan JT, McCarthy RJ, Scavone BM, Patel R, Ebarvia MJ. Randomized trial of neuraxial vs. systemic analgesia for labor induction: effect on incidence of cesarean delivery [abstract] Anesthesiology. 2007;106(Suppl 1):21. [Google Scholar]
Additional references
- Anim-Somuah 2005 .Anim-Somuah M, Smyth R, Howell C. Epidural versus non-epidural or no analgesia in labour. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD000331.pub2. DOI: 10.1002/14651858.CD000331.pub2. [DOI] [PubMed] [Google Scholar]
- Barragán 2011 .Barragán Loayza IM, Solà I, Juandó Prats C. Biofeedback for pain management during labour. Cochrane Database of Systematic Reviews. 2011;(6) doi: 10.1002/14651858.CD006168.pub2. DOI: 10.1002/14651858.CD006168.pub2. [DOI] [PubMed] [Google Scholar]
- Bricker 2002 .Bricker L, Lavender T. Parenteral opioids for labor pain relief: a systematic review. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl):S94–S109. doi: 10.1067/mob.2002.121549. [DOI] [PubMed] [Google Scholar]
- Callister 1995 .Callister LC. Cultural meanings of childbirth. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1995;24(4):327–31. doi: 10.1111/j.1552-6909.1995.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain 1993 .Chamberlain G, Wraight A, Steer P. Pain ands its relief in childbirth. The results of a National Survey conducted by the National Birthday Trust. Churchill Livingstone; Edinburgh: 1993. [Google Scholar]
- Cluett 2009 .Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD000111.pub4. DOI: 10.1002/14651858.CD000111.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- Derry 2011 .Derry S, Straube S, Moore RA, Hancock H, Collins SL. Intracutaneous or subcutaneous sterile water injection for relieving pain in labour. Cochrane Database of Systematic Reviews. 2011;(5) doi: 10.1002/14651858.CD009107.pub2. DOI: 10.1002/14651858.CD009107. [DOI] [PubMed] [Google Scholar]
- Dowswell 2009 .Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD007214.pub2. DOI: 10.1002/14651858.CD007214.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2006 .Elbourne D, Wiseman RA. Types of intra-muscular opioids for maternal pain relief in labour. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD001237.pub2. DOI: 10.1002/14651858.CD001237.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley 1999 .Findley I, Chamberlain G. ABC of labour care: relief of pain. BMJ. 1999;318:927–30. doi: 10.1136/bmj.318.7188.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesi 1998a .Genesi L, Niescierowicz R. Neuroendocrinology and birth 1: stress. British Journal of Midwifery. 1998;6(10):659–64. [Google Scholar]
- Genesi 1998b .Genesi L, Niescierowicz R. Neuroendocrinology and birth 2: the role of oxytocin. British Journal of Midwifery. 1998;6(12):791–6. [Google Scholar]
- Green 2003 .Green JM, Baston HA. Feeling in control during labour: concepts, correlates, and consequences. Birth. 2003;30:235–47. doi: 10.1046/j.1523-536x.2003.00253.x. [DOI] [PubMed] [Google Scholar]
- Hatem 2008 .Hatem M, Sandall J, Devane D, Soltani H, Gates S. Midwife-led versus other models of care for childbearing women. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD004667.pub2. DOI: 10.1002/14651858.CD004667.pub2. [DOI] [PubMed] [Google Scholar]
- Hawkins 1999 .Hawkins JL, Beaty BR, Gibbs CP. Update on obstetric anesthesia practices in the U.S. Anesthesiology. 1999;91:A1060. [Google Scholar]
- Hawkins 2003 .Hawkins J. Obstetric analgesia and anaesthesia. In: Scott JR, Gibbs RS, Karlan BY, Haney AF, editors. Darnforth’s Obstetrics and Gynaecology. 9th Edition Lippincott, Williams and Wilkins; London: 2003. [Google Scholar]
- Healthcare Commission 2007 .Commission for Healthcare Audit . Women’s experiences of maternity care in the NHS in England. Key findings from a survey of NHS Trusts. Commission for Healthcare Audit and Inspection; 2007. [Google Scholar]
- Higgins 2011 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Hodnett 2002 .Hodnett ED. Pain and women’s satisfaction with the experience of childbirth: a systematic review. American Journal of Obstetrics and Gynecology. 2002;186(5 Suppl Nature):S160–S172. doi: 10.1067/mob.2002.121141. [DOI] [PubMed] [Google Scholar]
- Hodnett 2007 .Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during labour. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD003766.pub2. DOI: 10.1002/14651858.CD003766.pub2. [DOI] [PubMed] [Google Scholar]
- Hogg 1977 .Hogg MI, Wiener PC, Rosen M, Mapleson WW. Urinary excretion and metabolism of pethidine and norpethidine in the newborn. British Journal of Anaesthesia. 1977;49(9):891–9. doi: 10.1093/bja/49.9.891. [DOI] [PubMed] [Google Scholar]
- Hunter 2007 .Hunter S, Hofmeyr GJ, Kulier R. Hands and knees position in late pregnancy or labour for fetal malposition (lateral or posterior) Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD001063.pub3. DOI: 10.1002/14651858.CD001063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson 1990 .Jacobson B, Nyberg K, Grönbladh L, Eklund G, Bygdeman M, Ryberg U. Opiate addiction in adult offspring through possible imprinting after obstetric treatment. BMJ. 1990;301:1067–70. doi: 10.1136/bmj.301.6760.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones 2011a .Jones L, Dou L, Dowswell T, Alfirevic Z, Neilson JP. Pain management for women in labour: generic protocol. Cochrane Database of Systematic Reviews. 2011;(6) doi: 10.1002/14651858.CD009234.pub2. DOI: 10.1002/14651858.CD009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones 2011b .Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: an overview of systematic reviews. Cochrane Database of Systematic Reviews. 2011;(7) doi: 10.1002/14651858.CD009234.pub2. DOI: 10.1002/14651858.CD009234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence 2009 .Lawrence A, Lewis L, Hofmeyr GJ, Dowswell T, Styles C. Maternal positions and mobility during first stage labour. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD003934.pub2. DOI: 10.1002/14651858.CD003934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leap 2004 .Leap N, Anderson T. The role of pain in normal birth and the empowerment of women. Chapter 2. In: Downe S, editor. Normal childbirth: evidence and debate. Churchill Livingstone; 2004. [Google Scholar]
- Lee 2004 .Lee K, Ho KM. Obstetric regional analgesia services in New Zealand: a national survey. New Zealand Medical Journal. 2004;117(1206):U1177. [PubMed] [Google Scholar]
- Lowe 1993 .Lowe NK. Maternal confidence for labor: development of the Childbirth Self-Efficacy Inventory. Research in Nursing and Health. 1993;16(2):141–9. doi: 10.1002/nur.4770160209. [DOI] [PubMed] [Google Scholar]
- Lowe 1996 .Lowe NK. The pain and discomfort of labor and birth. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1996;25(1):82–92. doi: 10.1111/j.1552-6909.1996.tb02517.x. [DOI] [PubMed] [Google Scholar]
- MHRA 2007 .The Medicines and Healthcare products Regulatory Agency (MHRA) [accessed 5 May 2010];Midwives: Prescribing. 2007 http://www.mhra.gov.uk/Howweregulate/Medicines/Availabilityprescribingsellingandsupplyingofmedicines/ExemptionsfromMedicinesActrestrictions/Midwives/index.htm.
- NICE 2007 .National Institute for Health and Clinical Excellence (NICE) NICE clinical guideline 55. Intrapartum care: care of healthy women and their babies during childbirth. NICE; London: 2007. [Google Scholar]
- Nissen 1995 .Nissen E, Lilja G, Matthiesen AS, Ransjo-Arvidsson AB, Uvnas-Moberg K, Widstrom AM. Effects of maternal pethidine on infants’ developing breast feeding behaviour. Acta Paediatrica. 1995;84(2):140–5. doi: 10.1111/j.1651-2227.1995.tb13596.x. [DOI] [PubMed] [Google Scholar]
- Novikova 2011 .Novikova N, Cluver C. Local anaesthetic nerve block for pain management in labour. Cochrane Database of Systematic Reviews. 2011;(7) doi: 10.1002/14651858.CD009200.pub2. DOI: 10.1002/14651858.CD009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg 2000 .Nyberg K, Buka SL, Lipsitt LP. Perinatal medication as a potential risk factor for adult drug abuse in a North American cohort. Epidemiology. 2000;11:715–6. doi: 10.1097/00001648-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Othman 2011 .Othman M, Jones L, Neilson JP. Non-opioid drugs for pain management in labour. Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD009223.pub2. in press. [DOI] [PubMed] [Google Scholar]
- Ransjo-Arvidson 2001 .Ransjo-Arvidson AB, Matthiesen AS, Lilja G, Nissen E, Widstrom AM, Uvnas-Moberg K. Maternal analgesia during labor disturbs newborn behavior: effects on breastfeeding, temperature, and crying. Birth. 2001;28(1):5–12. doi: 10.1046/j.1523-536x.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Redshaw 2007 .Redshaw M, Rowe R, Hockley C, Brocklehurst P. Recorded delivery: a national survey of women’s experience of maternity care 2006. National Perintal Epidemiology Unit, University of Oxford; Oxford: 2007. [Google Scholar]
- RevMan 2011 .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Reynolds 2000 .Reynolds F, editor. Regional Analgesia in Obstetrics: a Millennium Update. Springer; 2000. [Google Scholar]
- Righard 1990 .Righard L, Alade MO. Effect of delivery room routines on success of first breast-feed. Lancet. 1990;336:1105–7. doi: 10.1016/0140-6736(90)92579-7. [DOI] [PubMed] [Google Scholar]
- Saravanakumar 2007 .Saravanakumar K, Garstang JS, Hasan K. Intravenous patient-controlled analgesia for labour: a survey of UK practice. International Journal of Obstetric Anesthesia. 2007;16:221–5. doi: 10.1016/j.ijoa.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sekhavat 2009 .Sekhavat L, Behdad S. The effects of meperidine analgesia during labor on fetal heart rate. International Journal of Biomedical Science. 2009;5(1):59–62. [PMC free article] [PubMed] [Google Scholar]
- Simmons 2007 .Simmons SW, Cyna AM, Dennis AT, Hughes D. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD003401.pub2. DOI: 10.1002/14651858.CD003401.pub2. [DOI] [PubMed] [Google Scholar]
- Smith 2011a .Smith CA, Collins CT, Crowther CA, Levett KM. Acupuncture or acupressure for pain management in labour. Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.CD009232. in press. [DOI] [PubMed] [Google Scholar]
- Smith 2011b .Smith CA, Collins CT, Crowther CA. Aromatherapy for pain management in labour. Cochrane Database of Systematic Reviews. 2011;(7) doi: 10.1002/14651858.CD009215. DOI: 10.1002/14651858.CD009215. [DOI] [PubMed] [Google Scholar]
- Solt 2002 .Solt I, Ganadry S, Weiner Z. The effect of meperidine and promethazine on fetal heart rate indices during the active phase of labor. Israel Medical Association Journal. 2002;4(3):178–80. [PubMed] [Google Scholar]
- Trout 2004 .Trout KK. The neuromatrix theory of pain: implications for selected nonpharmacologic methods of pain relief for labor. Journal of Midwifery and Womens Health. 2004;49(6):482–8. doi: 10.1016/j.jmwh.2004.07.009. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study