Abstract
Abundant evidence from both field and lab studies has established that conspecific vocalizations (CVs) are of critical ecological significance for a wide variety of species, including humans, nonhuman primates, rodents, and other mammals and birds. Correspondingly, a number of experiments have demonstrated behavioral processing advantages for CVs, such as in discrimination and memory tasks. Further, a wide range of experiments have described brain regions in many species that appear to be specialized for processing CVs. For example, several neural regions have been described in both mammals and birds wherein greater neural responses are elicited by CVs than by comparison stimuli such as heterospecific vocalizations, nonvocal complex sounds, and artificial stimuli. These observations raise the question of whether these regions reflect domain-specific neural mechanisms dedicated to processing CVs, or alternatively, if these regions reflect domain-general neural mechanisms for representing complex sounds of learned significance. Inasmuch as CVs can be viewed as complex combinations of basic spectrotemporal features, the plausibility of the latter position is supported by a large body of literature describing modulated cortical and subcortical representation of a variety of acoustic features that have been experimentally associated with stimuli of natural behavioral significance (such as food rewards). Herein, we review a relatively small body of existing literature describing the roles of experience, learning, and memory in the emergence of species-typical neural representations of CVs and auditory system plasticity. In both songbirds and mammals, manipulations of auditory experience as well as specific learning paradigms are shown to modulate neural responses evoked by CVs, either in terms of overall firing rate or temporal firing patterns. In some cases, CV-sensitive neural regions gradually acquire representation of non-CV stimuli with which subjects have training and experience. These results parallel literature in humans describing modulation of responses in face-sensitive neural regions through learning and experience. Thus, although many questions remain, the available evidence is consistent with the notion that CVs may acquire distinct neural representation through domain-general mechanisms for representing complex auditory objects that are of learned importance to the animal.
1. Introduction: Ecological and behavioral significance of conspecific vocalizations
Intra-species communication occupies an important niche when considering our most basic need: survival. In many species, there are a wide variety of instances in which communication sounds play a role in warning about predators, signaling reproductive circumstances, relaying information about food sources, and locating genetically-similar neighbors. These communication sounds may also signal the emotional state of the caller as interpreted by the listeners, as well as convey other pieces of information to the listener such as the sex or size of the caller based on frequency and vocal tract length (Fitch, 1997). Thus, for most mammals and birds, conspecific vocalizations (CVs) arguably constitute the most ecologically-significant class of sounds (Simmons et al., 2003; Snowdon et al., 1982).
Given the ecological relevance of CVs, it is perhaps not surprising that a number of experiments with humans and other animals have reported advantages for behavioral processing of CVs over heterospecific vocalizations (HVs) and other sounds. For example, non-human primates (Petersen et al., 1984), as well as several bird species (Dooling et al., 1992; Okanoya and Dooling, 1991) are more readily able to discriminate CVs compared to HVs. Moreover, in a set of studies by Petersen and colleagues (Beecher et al., 1979; Petersen et al., 1978, 1984; Zoloth et al., 1979), Japanese macaques more easily learned to discriminate sets of CVs that were divided by a communicatively-relevant dimension (the position of a frequency-inflection peak), rather than by an arbitrary one (initial pitch). Similarly, European starlings are capable of learning to recognize pitch-shifted conspecific songs, but not pitch-shifted piano melodies (Bregman et al., 2012). A mnemonic advantage for CVs has also been reported in two recent studies of auditory short-term memory in humans (Weiss et al., 2012) and non-human primates (Ng et al., 2009).
In view of the salient ecological and behavioral status of CVs, as well as their distinct representation in the brain (Section 2), several investigators have asked whether CVs are “special” (Belin, 2006; Chartrand et al., 2008; Kuhl, 1986; Moore, 2000). Specifically, this question refers to the possibility of “special” or domain-specific neural mechanisms dedicated to processing CVs. These mechanisms might have been selected for, over evolutionary history, because of the advantage they would grant in interacting with the environment. Alternatively, the “special” or distinctive behavioral status and neural processing of CVs might result from extensive exposure to CVs within the lifespan of the individual organism, including learned associations between CVs and other behaviorally-relevant events. According to this view, domain-general mechanisms for representing meaningful acoustic stimuli would gradually augment representation of CVs based on their learned behavioral significance. Although these questions have only recently received experimental attention, in this review, we present evidence from several species that is consistent with the position that the brain flexibly represents CVs and other meaningful sounds according to experience and learned significance. We begin with a summary of species-typical neural representation of CVs, followed by a brief overview of plasticity in the auditory system, which may enable expanded representation of CVs based on experience and learning.
2. Neural representation of conspecific vocalizations
Consistent with their ecological and behavioral significance, there is abundant evidence for distinctive neural representation of CVs in humans, non-human primates, other mammals, and songbirds. More specifically, brain regions have been identified in a number of species that are selective for CVs in some way, i.e., differential responses are observed for CVs compared to non-CV stimuli, such as HVs, environmental sounds, or artificial sounds. In the survey of literature contained herein, such selectivity is usually assessed in terms of overall neuronal firing rate: some neurons may respond exclusively to CVs, or they may respond to CVs more robustly than other classes of stimuli. Similarly, in neuroimaging studies, greater activation may be observed in response to CVs compared to other classes of sounds. Differential responses to CVs may also be observed in temporal spike patterns, or in the form of asymmetric responses between cerebral hemispheres (see below). Although our understanding of the neural substrates of CVs is far from complete (Romanski and Averbeck, 2009; Wang, 2000), two basic principles have received experimental support in humans and other animals. First, auditory information processing is organized in a hierarchical manner, such that distinctive representation of CVs is more frequently observed in hierarchically-advanced areas. Second, perception of CVs is often associated with asymmetric processing between the cerebral hemispheres.
Many studies in humans and other mammals have shown that auditory processing pathways are organized in a similar fashion to the visual system (Ungerleider and Mishkin, 1982), in that there are at least two major information-processing pathways outside of the primary sensory cortices: one pathway in the dorsal direction specialized for identifying where objects are in space and how to interact with them, and a second pathway in the ventral direction specialized for object identification (Adriani et al., 2003; Kaas and Hackett, 2000; Lomber and Malhotra, 2008; Poremba et al., 2003; Rämä et al., 2004; Rauschecker and Tian, 2000; Romanski et al., 1999b; Wang et al., 1995). A hierarchical organization has been observed along the ventral object-processing pathway in the temporal lobe in humans and non-human primates, such that cells in relatively anterior regions preferentially respond to increasingly complex stimuli, such as CVs over simple, artificial stimuli. Thus, cells in primary sensory cortex tend to respond to specific acoustic features or combinations of acoustic features (Medvedev et al., 2002; Sadagopan and Wang, 2009; Wang, 2007; Wang et al., 2005), such as spectral content and temporal dynamics, whereas hierarchically-advanced areas are more selective for complex auditory objects and sound categories, including CVs (DeWitt and Rauschecker, 2012; Kikuchi et al., 2010; Leaver and Rauschecker, 2010; Miller and Cohen, 2010; Rauschecker et al., 1995; Rauschecker and Scott, 2009). Correspondingly, a number of studies in humans (Belin, 2006; Belin et al., 2000; Belin and Zatorre, 2003; Petkov et al., 2009; Talkington et al., 2012) and monkeys (Kikuchi et al., 2010; Perrodin et al., 2011; Petkov et al., 2008; Poremba et al., 2004) have described regions of the anterior temporal lobe that respond differentially to CVs over control sounds such as HVs, environmental sounds, and simple stimuli. The temporal lobe in turn sends auditory projections to the prefrontal cortex (PFC; Galaburda and Pandya, 1983; Kaas and Hackett, 2000; Morán et. al., 1987; Romanski et al., 1999a), where selectivity for CVs has also been observed (Averbeck and Romanski, 2006; Romanski and Averbeck, 2009; Romanski et al., 2005; Russ et al., 2008a; Wollberg and Sela, 1980). For example, Romanski and Goldman-Rakic (2002) reported that CVs were more effective in driving auditory-responsive neurons in the lateral PFC of monkeys than a wide variety of comparison stimuli including HVs, environmental sounds, and simple stimuli. A neuroimaging study in human subjects similarly found that human speech and nonlinguistic vocalizations elicit greater activation in the lateral PFC than HVs and nonvocal sounds (Fecteau et al., 2005).
Beyond the organization of the two major information processing pathways, hemispheric specialization for processing CVs has been widely reported in humans, and to a lesser degree in non-human primates and other mammals. In humans, left-hemispheric specialization for processing speech and language in humans is well documented (Gazzaniga, 2000; Price et al., 1996). Additional studies have indicated that certain aspects of vocal communication processing in humans are dominated by the left hemisphere, while other functions are dominant in the right hemisphere. Abundant evidence suggests that the left hemisphere is activated preferentially by communication sounds compared to control sounds such as artificial or environmental sounds and HVs (DeWitt and Rauschecker, 2012; Talkington et al., 2012). Additional studies have shown that the left hemisphere may be specialized for processing the fine temporal aspects of speech (Lazard et al., 2012; Zatorre and Belin, 2001). For example, in a study based on detection of brief temporal events, subjects committed fewer errors and responded more rapidly when the stimuli were presented to the left hemisphere (Nicholls et al., 1999). Moreover, electroencephalographic activity recorded during the task was greater at electrodes recorded over the left compared to the right temporal lobe. These results were supported by a subsequent study by Zatorre and Belin (2001), who used positron emission tomography (PET) to examine hemispheric differences in perception of tone sequences that were modulated in terms of temporal or spectral features. Consistent with the results of Nicholls et al. (1999), responses to the temporal features were more lateralized to the left hemisphere. On the other hand, responses to spectral features were more lateralized to the right hemisphere. Additional functions attributed to the right hemisphere include discriminating speaker identity (Belin and Zatorre, 2003; Kriegstein and Giraud, 2004; von Kriegstein et al., 2003), processing spectral information including prosody (Lattner et al., 2005; Lazard et al., 2012; Pell, 2006), and perhaps processing some aspects of the emotional content of vocal communication (Blonder et al., 1991; Kotz et al., 2006).
Studies in non-human primates have also suggested hemispheric specialization for perception of CVs. Heffner and Heffner (1984) first reported that lesions of the left superior temporal gyrus, but not the right, disrupted discrimination of CVs in monkeys. Using PET imaging, Poremba et al. (2004) found significantly greater activation of the left temporal pole when monkeys were exposed to CVs, but not other control stimuli including scrambled CVs, HVs, and environmental stimuli. A follow-up study by Ng (2011) revealed that individual neurons in this area are highly selective, with some responding only to CVs. This outcome is consistent with the findings of Kikuchi et al. (2010), who observed an increasing degree of selectivity for CVs over HVs and other sounds in the most rostral portions of the left superior temporal gyrus. As in the human literature, Petkov et al. (2008) observed sensitivity to caller identity in the right hemisphere of monkeys using fMRI. Several additional studies have provided neural and/or behavioral evidence for lateralized processing of CVs in chimpanzees (Taglialatela et al., 2009), Japanese macaques (Beecher et al., 1979; Petersen et al., 1978, 1984), sea lions (Böye et al., 2005), and mice (Ehret, 1987; Geissler and Ehret, 2004), raising the possibility that hemispheric specialization may be common in mammals.
The organization of the auditory system in songbirds has many characteristics in common with the mammalian system (Bolhuis and Gahr, 2006; Jarvis, 2004). The mesencephalicus lateralis dorsalis (MLd), which is homologous to the mammalian superior colliculus, integrates inputs from several auditory brainstem nuclei, and in turn provides the main input to the thalamo-cortical pathway. The nucleus ovoidalis (Ov), analogous to the ventral portion of the mammalian medial geniculate body, projects to the forebrain structure field L, which is comparable to mammalian primary auditory cortex. Field L is surrounded by two additional auditory regions, the caudal mesopallium (CM) and the caudal medial nidopallium (NCM), which are thought to be similar to mammalian auditory association cortex. Neurons in the MLd as well as each of the auditory forebrain regions have been shown to respond selectively to CVs, particularly the “auditory association” areas CM and NCM. Moreover, hemispheric specialization has also been observed in the NCM, where CVs evoke larger overall responses in the right hemisphere (e.g., Phan and Vicario, 2010). The songbird brain has additional highly specialized circuitry enabling song production, including the sensory/motor nucleus HVC, which exhibits selectivity for the bird’s own song over other conspecific songs (e.g., Lehongre and Del Negro, 2011; Margoliash, 1986). However, because these structures have fewer characteristics in common with the mammalian brain (Miller and Cohen, 2010), we focus herein on the songbird circuitry involved in auditory perception.
In summary, a number of brain regions in humans, non-human primates, and songbirds have been shown to respond selectively to CVs. Regions with the greatest degree of selectivity for CVs in each of these species are found in auditory association areas. In humans, the left hemisphere is preferentially activated by CVs including speech, whereas the right hemisphere may be specialized for processing spectral details in communication sounds, including prosody as well as vocal identity. Hemispheric asymmetries in CV-sensitive areas have similarly been observed in monkeys, songbirds, and several other species.
3. Plasticity of neural representation of acoustic features in the auditory system
Within the last three decades, a large body of research has accumulated showing that plasticity is an inherent property of the auditory system, both in adulthood and early postnatal development (Dahmen and King, 2007; Parks et al., 2004; Suga and Ma, 2003; Syka and Merzenich, 2003; Weinberger, 2004, 2007). Many studies have demonstrated that manipulating the behavioral significance of a particular acoustic feature, through classical or operant conditioning, often leads to a corresponding change in the neural representation of that feature. For example, in rats trained to press a bar for water during the presentation of a 6-kHz tone conditioned stimulus (CS), an increase was observed in the relative proportion of the primary auditory cortex preferentially responsive to 6-kHz tones (Rutkowski and Weinberger, 2005). Further, the degree of expanded representation of the CS tone frequency was related to the level of water deprivation in the rats, underscoring the role of behavioral relevance in reshaping the response properties of the auditory cortex. Responses to a number of additional acoustic features are susceptible to learning-dependent plasticity, including sound intensity (Polley et al., 2006), temporal information (Bao et al., 2004; Fritz et al., 2005a; Kilgard and Merzenich, 1998b), and context-dependent facilitation (Kilgard and Merzenich, 2002). While it should be acknowledged that not all training paradigms have resulted in expanded representation of behaviorally-relevant sound features in the auditory cortex (e.g., Brown et al., 2004, but see also Recanzone et al., 1993), the majority of studies in which a specific acoustic feature was associated with an unconditioned stimulus have reported such changes (see reviews by Suga and Ma, 2003; Weinberger, 2004, 2007). Moreover, these changes in representation have been shown to occur rapidly (e.g., within minutes; Edeline et al., 1993; Fritz et al., 2003, 2005a, 2005b), and can persist for months (Weinberger et al., 1993).
Learning-dependent representational plasticity has been observed in a variety of species in primary and association auditory cortices, as well as subcortical structures (See Figure 1, Table 1 for summaries). The expanded representation of behaviorally-relevant features of the auditory environment may occur in conjunction with, and/or be modulated by other areas such as the amygdala and limbic system (Poremba and Gabriel, 2001; Suga and Ma, 2003; Weinberger, 2004), which may encode the behavioral relevance of the sounds. Indeed, a number of studies have reported that these areas are responsive to CVs, perhaps because of their learned associative value and emotional content (Morris et al., 1999; Parsana et al., 2012; Sander et al., 2007; Wiethoff et al., 2009). Because CVs consist of combinations of acoustic features (Miller and Cohen, 2010) and have a high degree of behavioral significance (Simmons et al., 2003; Snowdon et al., 1982), representational plasticity in the auditory system comprises a plausible means whereby distinctive representation of CVs can emerge.
Figure 1.
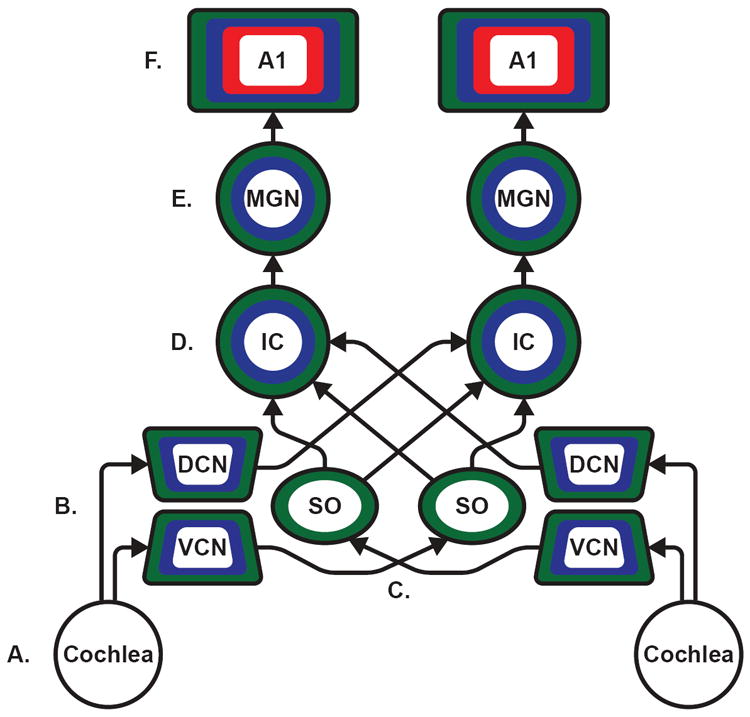
Associative conditioning, short-term and recognition memory, and experience/context-dependent plasticity have been demonstrated throughout the auditory pathway as evidenced by studies using neurophysiological recording techniques from single-unit to electroencephalograms. Illustrated above is the basic mammalian ascending auditory pathway depicting major structures and connections. The color coding depicts different types of neural plasticity: associative conditioning is represented in blue, short-term and recognition memory in red, and experience/context-dependent plasticity in green; see Table 1 for references of examples of each type of finding. A. Auditory information enters through the ear and is translated into neuronal signals via hair cells located in the cochlea. B. Auditory information is then relayed via the auditory portion of the vestibulocochlear nerve into the cochlear nuclei. The first signs of experience-dependent plasticity are observed at this early stage of auditory processing, in the dorsal (DCN) and ventral (VCN) cochlear nuclei. C. The superior olivary complex (SO) receives primary projections from the cochlear nuclei, also implicated in auditory-related experience-dependent plasticity D. Auditory information is then sent to the inferior colliculus (IC) where the signal can be transmitted between the left and right IC. Neurons in the IC have also been suggested to demonstrate plasticity evoked during auditory associative conditioning, see caveat in Table 1 caption. E. The IC projects to the medical geniculate nucleus (MGN). The MGN has been identified in a breadth of research for its role in auditory conditioning, and is thought to be an area which is influenced by, or plays a role in, establishing associative learning involving auditory stimuli. F. Auditory information reaches primary auditory cortex (A1) and associative auditory cortices, which are not differentiated within the figure. Across mammalian species, A1 is implicated in the perception of sound, and all three forms of plasticity have been demonstrated in both primary and associative cortices. Although the prefrontal cortex (PFC) as well as the amygdala and other limbic regions are not shown, they do exhibit plasticity in relation to audition, with the amygdala being heavily implicated in auditory conditioning and the PFC playing a role in auditory short-term and recognition memory. A1: primary auditory cortex; DCN: dorsal cochlear nucleus; IC: inferior colliculus; MGN: medial geniculate nucleus; SO: superior olive; VCN: ventral cochlear nucleus.
Table 1. Neurophysiological studies describing plasticity in the auditory system.
These are example studies listed within each category and are not meant to be an exhaustive listing.
Classical and operant conditioning
Short-term memory, working memory, recognition memory
Active versus passive listening, attention, stimulus presentation history, stimulus-specific adaptation, co-presentation of stimuli
Review article
Please note that the associative conditioning listed for the inferior colliculus and cochlear nucleus may be the result of feedback from higher auditory regions and more studies combining lesions and recording need to be done to verify these results. However, these same type of confirmatory studies with lesions, either permanent or temporary, in upper divisions of the auditory system with recording in the lower structures during acquisition should be done for all regions showing plasticity to determine the origination of plasticity and the sources of feedback or interactions as this may also be occurring in the medial geniculate nucleus (e.g., Poremba and Gabriel, 2001) or auditory cortex as well, perhaps modulated by the type of learning.
4. The effects of experience in shaping neural responses to conspecific vocalizations
4.1. Songbirds
Although there are differences in neural circuitry involved in human speech processing and song learning in songbirds, perception and production of conspecific song has been widely used as a model for understanding the development of human speech perception and vocal learning (Bolhuis and Gahr, 2006; Bolhuis et al., 2010; Doupe and Kuhl, 1999; Jarvis, 2004). Accumulating evidence, particularly within the last decade, strongly suggests that selectivity for conspecific songs over other sounds in the avian brain is heavily influenced by experience (reviewed at length by Woolley, 2012). Cousillas et al. (2004) first reported that, in starlings raised without normal adult song exposure, neurons in field L exhibited abnormally low song selectivity. Specifically, field L neurons in the isolated birds they were broadly responsive to all tested stimuli, whereas precise responses to specific song characteristics were observed in wild-caught birds. Similarly, George et al. (2004) found that the duration of isolation from adult song predicted the degree to which field L neurons in starlings responded broadly to all tested stimuli, instead of selectively to specific song features. Consistent with these deprivation studies, Amin et al. (2007) have observed that overall responsivity and selectivity for songs over artificial stimuli in field L neurons are greater in adult zebra finches than in juveniles. A study by Maul et al. (2010) found that depriving male zebra finches of song from posthatch days 7-29 was sufficient to reduce song selectivity of the auditory forebrain (neural responses did not differ among different songs, syllables, and a pure tone), and that this could be partially, but not completely restored by exposing the birds to 30 s of recorded song playback from posthatch days 30-100. As in field L, neuronal firing rates in NCM are less selective for conspecific song features in starlings that have been deprived of adult song experience than in wild-caught starlings (George et al., 2010). Collectively, these studies show that early auditory experience is crucial for species-typical neural representation of CVs.
A particularly interesting study by Woolley et al. (2010) investigated neural responses to both zebra finch and Bengalese finch songs in zebra finches that had been cross-fostered by Bengalese finches. The neural responses in the cross-fostered birds were compared to normally-reared zebra finches and normally-reared Bengalese finches. In both the midbrain (MLd) and forebrain (field L), neural responses of the normally-reared zebra finches had a greater capacity to encode information about both the zebra finch and Bengalese finch songs than the neural responses of the Bengalese finches (based on firing rate, change in firing rate, rate of change in firing rate, and response reliability), perhaps resulting from differences in the songs produced by each species. In the cross-fostered zebra finches, the information-coding capacity of neural responses to both zebra finch and Bengalese finch songs did not differ significantly from the neural responses of the Bengalese finches, whereas both groups differed significantly from the normally-reared zebra finches. In other words, the information coding capacity of neurons in MLd and field L of zebra finches cross-fostered by Bengalese finches was more similar to the that of the Bengalese finches than that of the zebra finches. Curiously, in both zebra finch groups, field L neurons did not exhibit significant selectivity for either zebra finch or Bengalese finch songs in terms of overall firing rate, whereas field L neurons in the Bengalese finches were selective for conspecific songs. Thus, while the cross-rearing manipulation in this study did not significantly affect selectivity among the tested song types, the species-typical information coding capacity of neurons in this study was strongly influenced by early auditory experience.
Phan and Vicario (2010) recently investigated the role of auditory experience in shaping hemispheric specialization for CV processing in zebra finches. The auditory environment of the developing finches was manipulated in two ways: a first set of birds was divided into two groups that either received song exposure (“tutored”) or were deprived of normal song during development (“untutored”); a second set of birds was surgically devocalized, rendering them incapable of producing audible vocalizations, and then similarly divided into tutored and untutored groups. In the intact adults, the response magnitude of NCM neurons to both conspecific songs and calls was greater in the right hemisphere than the left, regardless of whether they had been exposed to song. Similarly, devocalized birds that had experienced conspecific song exhibited a right-hemispheric preference for songs and vocalizations, although the extent of specialization was less than in the intact birds. Critically, however, the devocalized birds that did not receive exposure to conspecific song did not exhibit a hemispheric preference for songs or vocalization as was observed in the other groups. These results strongly suggest experience with CVs as a key factor in the emergence of species-typical neural representations of CVs. They further demonstrate the ability of the animals’ own vocalizations to shape these representations.
In summary, sensitivity of neuronal responses to CVs in the songbird auditory forebrain (field L and NCM), as well as the midbrain (MLd), are heavily reliant on exposure to song and vocalizations. There are several differences between the neural substrates of birdsong and the representation of CVs in humans and other mammals that call for caution when drawing inferences about representation of CVs in the mammalian brain from the birdsong literature, such as sex differences (Hauber et al., 2007; Maul et al., 2010; Phan and Vicario, 2010), hormonal influences (Del Negro et al., 2005; Fusani and Gahr, 2006; Tremere and Pinaud, 2011), and differences between songs and other communication vocalizations. However, it is clear in these bird species that significant brain resources are devoted to perception of these sounds, and that experience with the CVs including self-vocalizations plays a significant role in elevating these sounds above others.
4.2. Humans and other mammals
Relatively few studies have directly investigated the role of experience in shaping responses to CVs in humans and other mammals. However, the existing studies suggest that, like in songbirds, experience is a key factor in the emergence of species-typical neural representations of CVs. One such study by Cheung et al. (2005) investigated the neural representation of CVs in marmosets that had undergone a vocal tract modification surgery that permanently lowered the frequency content of the twitter call (e.g., the minimum frequency of the altered call was approximately one octave lower than the natural call). After 5–15 months of experience with the altered self-vocalizations, the response properties of neurons in the auditory cortex were assessed for both altered and natural CVs. Compared to controls, neurons in the vocally-modified subjects exhibited reduced temporal precision and overall response magnitude in response to both the altered and natural calls. The authors suggested that the responses to the altered calls might have been diminished rather than augmented because of their reduced communicative efficacy. The receptive-field properties of neurons defined by simple tone bursts as well as the tonotopic organization of the auditory cortex did not differ between controls and vocally-modified subjects, indicating that the observed plasticity was specific to the change in experience with CVs.
Another notable study by Liu and Schreiner (2007) provided evidence for the role of experience in shaping neural representation of CVs in the auditory cortex in the context of the ultrasonic communication system between mouse pups and mothers. A variety of pup calls were presented to both mothers who exhibited behavioral preference for pup calls over neutral sounds, and to pup-naïve females who did not exhibit behavioral preference for the calls. While the mean firing rate evoked by the pup calls did not differ between the mothers and naïve females, the peak evoked firing rate was greater, and of shorter latency in the mothers. Further analyses revealed that the information contained in the evoked responses for detection and discrimination of pup calls, but not for unnatural control sounds, was significantly improved in mothers compared to naïve females. Thus, although traditional measures of selectivity for CVs were not assessed in this study, the communicative significance of the pup calls was evident in the detection and discrimination abilities of auditory cortical neurons in the experienced mothers.
Nourski et al. (2012, 2013) have recently presented data from human neurosurgical patients highlighting the plasticity of regions of the brain that typically respond to speech. In normal-hearing patients undergoing invasive monitoring for refractory epilepsy, neurophysiological responses were recorded in response to naturally-voiced syllables and syllables that had been spectrally degraded to replicate the signal that would reach the brain after being processed by a cochlear implant. For the overwhelming majority of sites in the posterolateral portion of the superior temporal gyrus (PLST), the amplitude and spatial extent of the responses was greater in response to the natural syllables than the spectrally-degraded stimuli (Nourski et al., 2012). Nourski and colleagues (2013) also reported a rare case study of a patient undergoing invasive monitoring for epilepsy that had also been a cochlear implant user for approximately 20 years. In this patient, speech sounds (which had been spectrally degraded by the cochlear implant) evoked activity in the PLST that was remarkably similar to the natural-speech evoked responses in the normal-hearing patients. The observation that extensive experience with spectrally-degraded speech leads to neural representation in the PLST that closely resembles natural speech suggests that the “speech sensitivity” of this area relies on experience with stimuli of communicative significance, even if the sounds are relatively artificial.
Additional indirect evidence that experience contributes to CV-specific responses comes from studies comparing neuronal responses elicited by natural CVs compared to time-reversed CVs, which contain similar spectral content and acoustic complexity. Preferential responding to natural over time-reversed CVs has been observed in several studies, including in the auditory cortex of marmosets (Wang et al., 1995), the posterior ectosylvian gyrus and ventral part of the auditory cortex in cats (Gourévitch and Eggermont, 2007), the FM-FM area of bats (Esser et al., 1997), and in the forebrain of songbirds (Doupe and Konishi, 1991; Margoliash, 1983). It should be noted, however, that other studies have failed to observe any selectivity for natural over time-reversed CVs, including in the auditory cortex of squirrel monkeys (Glass and Wollberg, 1983a, 1983b), and in the auditory cortex and thalamus of rats and guinea pigs (Huetz et al., 2009; Philibert et al., 2005), while others have reported ambiguous results (e.g., greater onset responses to natural CVs in the cat auditory cortex, but greater sustained responses to time-reversed CVs; Gehr et al., 2000). Bearing these caveats in mind, the idea that selectivity for natural CVs is attributable to their acquired behavioral significance is supported by a study by Wang and Kadia (2001) comparing selectivity for natural over time-reversed marmoset twitter calls in the auditory cortex of marmosets and cats. As in previous studies, the auditory cortex in marmosets exhibited greater firing rates for the natural twitters. In cats, however, for which neither the natural nor the time-reversed marmoset calls have any behavioral relevance, the auditory cortex did not respond differentially between the two call types. Although these findings do not necessarily rule out potential species-specific or innate mechanisms of CV representation, a recent computational study has found that selectivity for natural over time-reversed CVs can plausibly arise from experience-related short-term plasticity alone (Lee and Buonomano, 2012).
In addition to the neurophysiological studies cited above, a number of developmental studies have provided evidence at the behavioral level that species-typical responses to CVs are influenced by experience (Seyfarth and Cheney, 1997). For instance, Fischer et al. (2000) observed behavioral responses elicited by CVs in a field study of developing baboons. The baboon vocalizations ranged from tonal, harmonically-rich calls, which were typically produced when the caller became distanced from another individual or group (“contact barks”), to noisy, harsh calls, which were typically produced in response to predators (“alarm barks”). In adult baboons, noisy alarm barks reliably provoked strong behavioral responses, whereas the tonal contact barks elicited only a weak response or no response at all. In the infant baboons, adult-like responses to the two call types developed with age: at two and a half months, neither call type elicited a behavioral response; at four months, a similar behavioral response was elicited by either call type; at six months, only the noisy alarm bark elicited a strong behavioral response.
Additional studies have provided behavioral evidence that hemispheric specialization for processing vocal communication emerges through experience. Using a head-orienting task, Böye et al. (2005) found that adult sea lions exhibited a strong right-ear (left hemisphere) preference for CVs, but not HVs. This orienting asymmetry was not observed in infant sea lions, which the authors attributed to a lack of adequate experience with CVs. These observations corroborate an earlier report by Hauser and Andersson (1994) that adult but not infant rhesus monkeys preferentially orient to the right when CVs but not HVs are presented. Similarly, Lemasson et al. (2010) have recently reported that Japanese macaques exhibit a right-orientation bias for vocalizations of familiar but not unfamiliar primates. Although there are limitations to the orienting asymmetry paradigm (Teufel et al., 2010), these studies are consistent with the neurophysiological demonstration by Phan and Vicario (2010) of experience-dependent development of hemispheric specialization in songbirds.
5. The effects of learning and memory in shaping neural responses to conspecific vocalizations
Experience and learning are closely-related concepts. Whereas the term “experience” can encompass the entire range of events that an organism is exposed to, herein we use the terms “learning and memory” to refer to the more specific instances of experience in which organisms identify contingent relationships among events (i.e., associative learning). Evidence in birds indicates that species-typical behavioral responses to CVs develop more rapidly when the CVs are part of a specific, contingent relationship than when they are merely experienced by the birds. For example, bobwhite quail chicks require at least 240 minutes of passive experience with the maternal bobwhite call in order to acquire a preference for that call (Lickliter and Hellewell, 1992). However, when playback of the maternal call is made contingent upon the chicks’ own vocalizations, a single 5-minute session is sufficient to induce a preference for the call (Harshaw and Lickliter, 2007). The roles of experience and learning can sometimes be difficult to distinguish. As noted above, in marmosets that had surgical modifications of the vocal tract, diminished neurophysiological responses were observed following 5–15 months of experience with the altered calls (Cheung et al., 2005). However, the vocal tract modification altered not only the spectral content of the calls, but also reduced their communicative efficacy. Thus, as suggested by the authors, the lack of reward for successful communicative interchanges with conspecifics might have been a key factor in producing the suppressed responses. In this section, we review several studies in which neural responses in vocalization-sensitive brain regions are shown to be influenced specifically by training.
5.1. Songbirds
Gentner and Margoliash (2003) trained adult starlings to discriminate between two sets of conspecific songs using either a two-choice or go/no-go task. In the two-choice task, birds were rewarded for pecking the key corresponding to the correct song set, whereas in the go/no-go task they were only rewarded for pecking a key in response to the correct song set (S+). After reaching asymptotic performance of approximately 90% correct responses, neurophysiological responses in area CM were measured in responses to the learned songs and an additional set of novel songs. In birds trained under both the two-choice and go/no-go paradigms, the overall evoked response magnitude was greater for the learned song sets than the novel songs. For birds trained in the two-choice task, the response magnitude elicited by songs from each set (“peck left” and “peck right”) were approximately equal. However, for birds trained in the go/no-go paradigm, the songs that had been rewarded (S+) elicited significantly greater responses than songs from the unrewarded set (S-), though the unrewarded songs elicited greater responses than the novel, unlearned songs. Moreover, the responses elicited by the rewarded songs in birds trained in the go/no-go task were greater than the responses elicited by either song set in the birds trained on the two-choice alternative task, even though both sets had been rewarded. A similar study by Thompson and Gentner (2010) found that area NCM exhibited similar selectivity for learned over unlearned songs, with the exception that the selectivity for learned songs was manifest in the form of relatively suppressed firing rates. These outcomes support the idea that selectivity for CVs, at least in areas CM and NCM in the songbird brain, is closely tied to the behavioral relevance of CVs.
Like songbirds, parrots learn their vocalizations from a tutor, and the auditory association areas CM and NCM that are typically differentially responsive to CVs are homologous in songbirds and parrots. Eda-Fujiwara et al. (2012) took advantage of the ability of budgerigars, a parrot species, to mimic human vocalizations in addition to learning their own CVs. In their experiment, an experimental group of budgerigars were trained to discriminate two Japanese words that were vocalized by another budgerigar. Following training, neuronal activation in response to the learned words was measured by expression of the immediate early gene ZENK in areas CM and NCM. Neuronal activation in both areas was significantly greater in trained birds than untrained control birds that were exposed to the same stimuli. Neuronal activation in untrained birds exposed to the budgerigar-spoken words did not differ from a third group of birds exposed to silence. There were no differences in neural activation in the hippocampus among the three groups, suggesting that the training-induced changes were specific to areas of the brain that are typically involved in representing CVs. Finally, the degree of neuronal activation in area CM and in the dorsal portion of NCM was positively correlated with behavioral accuracy during discrimination training. The observation that, through training, these areas became robustly responsive to auditory stimuli that would not normally be encountered by budgerigars in the wild provides further support for the idea that selectivity for CVs may be shaped by their learned behavioral significance.
5.2. Humans and other mammals
Using event-related potentials, Kraus and colleagues have shown that a number of forms of short-term and long-term auditory training influence cortical and subcortical responses evoked by speech features (Chandrasekaran and Kraus, 2010; Kraus and Banai, 2007). For example, individuals with music training have enhanced representation of native speech syllables (Musacchia et al., 2007), vocally-expressed emotion (Strait et al., 2009), and linguistic pitch contours, which is correlated with the extent of musical training (Wong et al., 2007). Similarly, short-term auditory training programs in children have been shown to improve the representation of speech syllables and pitch contours (Russo et al., 2005; Song et al., 2008). While these studies demonstrate training-induced malleability of neural representations of speech, only more recent studies using neuroimaging techniques have been able to identify specific brain regions that are susceptible to these influences.
Using fMRI, Leech et al. (2009) recently reported that training human subjects to recognize and categorize complex artificial sounds led to differential activation of a speech-sensitive cortical area, the left posterior superior temporal sulcus (pSTS). The task consisted of a video game in which subjects had to learn the relationship between a visually-presented alien and its corresponding sound category. After approximately five hours of experience with the game, passive exposure to the training sounds resulted in significantly decreased deactivation of the pSTS relative to pretraining exposure to the sounds. Further, the difference between pretraining and posttraining activation in this area was significantly correlated with behavioral accuracy in categorizing the sounds. The finding that artificial, spectrotemporally-complex sounds produce changes in pSTS activation following training suggest that responses to speech in this area may be related to expertise with speech sounds. This conclusion was further supported by a subsequent study showing that this area was strongly activated bilaterally by both speech and music in individuals with musical expertise (violinists), but only by speech in a control group of experts (actors) without extensive musical training (Dick et al., 2011). A comparable pattern of results was observed in the left planum temporale and an anterior portion of the left superior temporal gyrus. Taken together, these studies provide evidence that the “speech selectivity” of these regions may reflect expertise with spectrotemporally-complex stimuli rather than an innate, domain-specific bias for speech processing.
As reviewed above, cells in the auditory cortex of marmosets frequently exhibit increased overall firing rates for natural over time-reversed marmoset calls, whereas cells in the cat auditory cortex fail to make this distinction (Wang and Kadia, 2001). Schnupp et al. (2006) tested whether learned behavioral significance was sufficient to induce selectivity for natural marmoset calls in the auditory cortex of adult ferrets by training them to discriminate marmoset twitter calls (S+) from other natural sounds (S-) in a go/no-go paradigm. Whereas no significant difference in the overall firing rate elicited by natural versus reversed calls was observed in control animals, there was a small but significant decrease in firing rate elicited by the natural calls compared to the reversed calls in the trained subjects. However, the degree of this effect was so small that the authors suggested that it was of little physiological significance. On the other hand, the information contained in the temporal spike patterns increased substantially following training. Thus, the learned significance of the HVs was evident in temporal pattern codes, but only weakly in the overall firing rate. The latter observation indicates that the auditory cortex does not inevitably exhibit greater selectivity, as evidenced by increased firing rate, for complex sounds of acquired behavioral significance. It should be noted, however, that the adult ferrets used in this study presumably had well-established neural representations of their own CVs, which could have interfered with expansion of the representation of the natural marmoset call as defined by overall firing rate. However, additional studies surrounding this topic are needed to make this determination.
Collectively, the studies reviewed in Sections 4 and 5 have uniformly shown that neural representations of CVs are susceptible to the influences of experience, learning, and memory. These findings are consistent across a number of species, including songbirds, humans, and other mammals. In some of these studies, neural regions that typically represent CVs came to respond to non-CV sounds according to their acquired behavioral significance, including HVs and artificial, complex sounds. These observations are consistent with the notion that CVs acquire distinctive neural representation through domain-general mechanisms for representing complex auditory objects that are of importance to the animal.
6. Comparison of vocalizations and faces
The visual and auditory systems share much in common in terms of sensory processing strategies (Nelken and Calford, 2011) and experience-dependent plasticity (Rauschecker, 1999). As mentioned above, both sensory systems appear to be organized into dorsal and ventral processing streams that are specialized for processing non-spatial and spatial stimulus attributes, i.e., the “what” and “where” pathways, respectively (Kaas and Hackett, 1999; Poremba et al., 2003; Rauschecker and Tian, 2000; Ungerleider and Mishkin, 1982). This includes the observation that cells in hierarchically-advanced regions of the ventral processing stream exhibit selectivity for more complex objects, such that vocalization- and face-sensitive regions lie anterior to primary sensory areas in the temporal lobe (Fujita et al., 1992; Gross and Sergent, 1992; Kanwisher et al., 1997; Perrett et al., 1988; Petkov et al., 2008; Poremba et al., 2004). These regions in turn project to the prefrontal cortex, where integration of CVs and faces has been observed (Sugihara et al., 2006; Romanski, 2012; Romanski and Averbeck, 2009).
In humans, the fusiform gyrus of the right hemisphere, or fusiform face area (FFA), is preferentially activated by faces over a number of control stimuli, such as houses and scrambled faces (Kanwisher et al., 1997). Initial evidence that the FFA might be activated by visual stimuli with which subjects have extensive experience came from a study by Gauthier et al. (1999), in which subjects were trained to become experts at categorizing a novel class of visual objects (greebles). Following training, increased activation was observed during categorization of upright versus inverted greebles. Further, passive viewing of greebles produced greater activation of the FFA in the trained experts than in novices. A subsequent study revealed that these results generalized to expertise with birds and cars (Gauthier et al., 2000). In bird experts, passive viewing of birds compared to other familiar objects produced significant activation of the FFA, whereas cars failed to elicit similar activation. In car experts, passive viewing of cars compared with other objects, but not birds, elicited significant FFA activity. A more recent study has similarly reported that FFA activity in radiologists detecting abnormalities in chest radiographs is positively correlated with expertise (Harley et al., 2009). Although there is debate as to whether face selectivity in the FFA depends entirely on experience (Bukach et al., 2006; Kanwisher, 2000; McKone et al., 2007; Tarr and Gauthier, 2000), at minimum, these studies indicate that experience with behaviorally-relevant, non-face stimuli leads to activation in the FFA.
These findings corroborate earlier behavioral evidence that face-specific processing may be driven by experience. Similar to the advantages for discriminating and remembering CVs, there are advantages for processing natural faces at the behavioral level. For example, recognition memory for faces is disrupted to a greater degree by inverted presentation than other classes of images. Diamond and Carey (1986) showed that, in dog experts, memory for inverted images of dogs is similarly disrupted relative to natural images, suggesting that this bias results from extensive experience. Similarly, recent evidence suggests that infants’ preference for faces and face-discrimination abilities, initially assumed to be innate, may arise from domain-general perceptual biases and experience (Turati, 2004).
In summary, both behavioral and neural studies have suggested experience and learning play a central role in the distinctive processing of faces. These outcomes provide a visual parallel to the studies in the auditory system reviewed above, indicating that preferential behavioral and neural processing of CVs is closely tied to experience with CVs and their learned behavioral significance. Taken together, these findings support the position that representation of meaningful stimuli is acquired through experience and learning, rather than by domain-specific mechanisms that have evolved to invariantly represent stimuli of ecological significance.
7. Additional factors influencing the neural representation of conspecific vocalizations: arousal, attention, and short-term memory
The majority of studies describing neural regions that are differentially responsive to CVs have employed passive-listening paradigms in which sounds are exposed to subjects with little or no behavioral interaction. It should be noted, however, that neural responses evoked by a given sound may vary depending on the behavioral state of the animal, or the context in which the sounds occur. For instance, it is well known that anesthesia has a major impact on the sound-responsiveness of auditory brain regions, including the primary auditory cortex (Cheung et al., 2001; Gaese and Ostwald, 2001; Howard et al., 2000). Similarly, responses elicited by sounds including CVs in primary and secondary auditory cortices often differ during wakefulness and sleep (Edeline et al., 2001; Issa and Wang, 2008, 2011). Moreover, whether or not a subject is attending to a sound or performing well during a behavioral task may modulate the evoked response characteristics (Mesgarani and Chang, 2012; Niwa et al., 2012; Otazu et al., 2009; Poremba and Bigelow, 2013; Ryan et al., 1984); however, even the influence of attention will need to be separated from the effects of experience with sounds (Baumann et al., 2008). The influence of attention on neural encoding of CVs may be a strong modulator of the neural processing discussed herein.
Historically, studies of the neural circuitry underlying short-term memory have focused on visual stimuli. Typically, single- or multi-unit recordings are conducted while the subject performs a delay task in which a sample stimulus is presented, followed by retention interval, after which one or more test stimuli are presented. These studies have revealed a prominent role for the prefrontal cortex in short-term memory, where changes in firing rate are often observed during the retention interval (Shafi et al., 2007; Fuster and Alexander, 1971). Further, test stimuli that “match” the sample stimulus are often associated with enhanced responses (e.g., Miller et al., 1996). Recordings in the temporal lobe, on the other hand, show that matching test stimuli are more commonly associated with suppressed responses (Miller et al., 1993; Nakamura and Kubota, 1995). Although neurophysiological investigations of auditory short-term memory are relatively sparse, the existing evidence suggests that many of the same memory-related phenomena reported in the visual literature are also observed during auditory short-term memory (Poremba and Bigelow, 2013). For example, Plakke et al. (2013) reported that matching sounds elicited elevated firing rates in prefrontal neurons during an auditory short-term memory task. Using the same paradigm, Ng (2011) found that neurons in the dorsal temporal pole exhibited suppressed firing rates when matching sounds were presented. Neurophysiological as well as neuropsychological studies also suggest a role for the auditory cortex in short-term memory (Gottlieb et al., 1989; Kuśmierek et al., 2007; Sakurai, 1990, 1994), suggesting that memory-related modulation of sound-evoked activity may be widespread in auditory cortical pathways.
In summary, neural responses to sounds including CVs are not “fixed” across behavioral states; rather, they are strongly modulated by arousal and attention, and also depend on stimulus presentation history and the behavioral context in which sounds are presented. Although our current understanding of the neural substrates of these phenomena is incomplete, these influences should be taken into consideration when investigating the neural representation of CVs.
8. Relationship to human language development
Any information about the mechanisms underlying human language development may be beneficial to animal work utilizing vocalizations, as some relevant components of these processes may be similar across species. The debate over language development has spanned decades but has heated up in the past 25 years even as the timeline of language learning has become well mapped. The relative contributions of experience versus ‘innateness’ are still debated, but support for a more experience-based explanation of language acquisition has taken precedence (Spencer et al., 2009; Werker and Vouloumanos, 2001). Saffran and colleagues originally showed that experience might play a greater role in language acquisition than previously thought, suggesting that infants separate and segment speech using statistical learning methods as they determine which syllables are commonly found together and which syllables are infrequently grouped (Saffran et al., 1996). This general set of hypotheses regarding the learning of language has now been expanded by other recent findings (McMurray, 2007; Samuleson et al., 2011). Language development’s reliance on speech exposure (Huttenlocher et al., 1991) is also supported by studies of feral children who do not develop language without early speech experience; this is similar to the studies of songbird learning manipulations discussed in section 4.1.
Word-learning is an essential stepping stone to understanding speech, as well as for garnering meaning from one’s environment. The processes by which children learn and navigate their environments early in life can also be the same processes that account for the development of vocabulary (Saffran et al., 1996). Studying shape bias is a popular method of studying word learning in children, and research in this area suggests that attention, which modulates and pervades many types of learning both in early childhood and adulthood, is an essential factor for children to develop into effective word learners (Saffran et al., 1996; Samuelson et al., 2011). The shape bias refers to a child’s ability to hear a novel name for a novel object, and then to generalize that name to other novel objects, which are similar to the original object presented. This research suggests that what children are really learning is the association between the object and the context that adults will use to name that object. By forming that association and by paying attention to novel objects, children can focus on the relevant features of the object that need to be recognized and categorized in the future (Smith and Samuelson, 2006; Spencer et al., 2009).
The process of forming associations is critical in early word learning and also the environment of the child, including social interactions, may be important. In conversation, words often function to direct a listener to a specific region of space with what we say often reflected in where we look. Spatial constancy of the objects presented to the children also affects how well new words are learned (Samuelson et al., 2011; Smith et al., 2003). Social interaction, often between parent and child, assists not only the learning of new words, but also the perception and production of speech (Cousillas et al., 2008). Language learning in children is fostered by a social environment in which interaction promotes attention on the part of the child, and learning is grounded in general processes such as association dependent upon experience. Social interaction is critical for language learning not only in children, but also in other species such as songbirds. Social deprivation has devastating effects on the acquisition of language in humans and even, in the case of songbirds, leading to perceptual abnormalities within the auditory pathway (Cousillas et al., 2008; Kuhl, 2004). In general, these additional processes such as social interactions and attention can modulate the quality and quantity of experience, learning, and memory that can occur for CVs.
9. Summary and future research directions
We have been discussing whether CVs are “special” and whether they deserve special status when studying auditory processing. There is ample evidence that CVs hold special significance in terms of behavioral meaning (Section 1) and neural representation (Section 2). However, the available evidence reviewed herein is consistent with the notion that this special status may be acquired through experience, learning, and memory, rather than through innate, domain-specific mechanisms for processing CVs. This tentative conclusion is not unexpected in light of a large body of research describing the auditory system as inherently plastic (Figure 1; Table 1) and capable of augmenting the neural representation of behaviorally-relevant acoustic features. Moreover, these observations are consistent with studies in the visual system suggesting face-selective neural regions and face-specific behavioral processing rely at least in part on experience. Together, these findings are difficult to reconcile with the idea that domain-specific neural modules have evolved to exclusively represent ecologically-salient stimuli such as CVs and faces, regardless of experience or developmental history.
These advances notwithstanding, there are still a great many questions surrounding the emergence of species-typical behavioral processing and neural representation of CVs. Many of these questions surround early auditory development, including the possibility of an early “sensitive period” for acquiring species-typical behavioral and neural responses to CVs (Braun et al., 2003; Kuhl, 2010; Nakahara et al., 2004; Phan et al., 2006; Wang, 2004; Ziabreva et al., 2003). In this regard, it is worth noting that self-vocalizations alone have been shown to be sufficient to induce at least some forms of behavioral and neural sensitivity to CVs in several bird species (Gottlieb, 1991; Phan and Vicario, 2010). Moreover, several studies have documented prenatal sensitivity to auditory stimulation (Harshaw and Lickliter, 2011; Lickliter and Stoumbos, 1992), including the observation that human infants’ behavioral and neural responses to speech sounds are influenced by prenatal maternal speech (Byers-Heinlein et al., 2010; DeCasper and Fifer, 1980; DeCasper and Spence, 1986; May et al., 2011; Minagawa-Kawai et al., 2011; see also Seebach et al., 1994). Thus, developmental studies should be mindful of these influences when seeking to identify the variables underlying the emergence of species-typical representation of CVs.
In adults, additional studies of auditory expertise could be useful for addressing the specificity of regions that are typically selective for CVs. For example, as pointed out by Chartrand et al. (2008), studies of auditory perception in musicians and bird experts could provide parallels to studies in the visual system showing that objects with which subjects have expertise activate face-selective areas (Section 6). In the same way, extensive training with complex artificial stimuli could induce activity in regions that typically represent CVs. As reviewed in Section 5, there is already some evidence that this is the case. Another potentially interesting avenue of research in animals would be to compare the behavioral and neural responses elicited by CVs and other behaviorally-relevant vocalizations with which subjects have extensive experience, such as those of predators. Additional opportunities for future research include direct comparisons of the effects of auditory exposure versus associative learning, and investigations of potential differences in the degree to which experience, learning, and memory affect representation of CVs among different brain regions, as well as among different species.
If experience and learning play important roles in making communication sounds “special”, then their influence and modulating effects need to be taken into consideration when using them as stimuli in experiments and in their comparison to other non-communication sounds. Comparisons between stimuli are particularly important in imaging and neurophysiological studies in helping to determine which components of the auditory system may be contributing during sound processing. The amount of experience with the sounds, associations that these sounds may have gained, developmental trajectory of the experience, social pairings, etc., should be considered when drawing conclusions, or the assumptions even tested when possible. If communication signals are primarily learned about or driven by experience, then are all presentations of communication stimuli equal across experiments? Although this possibility exists in human experiments too, the number of subjects can be increased to help control for differences in their experience levels. In animal studies, one possibility is that for some experiments, housing parameters may play a role particularly if some animals are housed in pairs or individually. However, a study of lab-housed animals suggests that communication processing may be normal in these animals as cross-habituation was observed when the monkey calls were functionally equivalent, but acoustically distinct (Gifford et al, 2003). Associations between the sounds and meanings may not be as strong, although CVs are most likely the most frequently heard auditory stimulus. However, these possibilities should be considered and tested when possible. On the plus side, there are several studies showing that different types of sounds in non-human primates elicit different behavioral and neural responses (Poremba et al., 2004; Ng et al., 2009), but future controlled testing of sounds with a similar frequency range to vocalizations and consideration of the amount of experience with the sounds will need further consideration. In addition, we must study whether the neural processes underlying associative conditioning also underlie learning of CVs and the relationship between conditioning, experience, and memory encoding.
Another caveat to studying CVs in a variety of situations is to be careful in our testing of why these sounds are occurring. The study of Blumberg and colleagues provides cautionary evidence (Blumberg and Alberts, 1990). Although it was assumed that when rat pups became separated from their dams they made specialized cries that brought the dam to them. They discovered that under hypothermic conditions these cries were instead a byproduct of temperature regulation, which induces increased breathing. Yes, the rat dams are alerted by the calls and retrieve the rat pups, but the meaning and mechanism behind the pup “call” may be different than what human researchers had assumed. Hypotheses derived from other studies suggest that the rat pups may eventually learn to make this isolation call when separated and further study needs to ascertain when its production changes from an unconditioned response to a conditioned one with the stimulus being the colder temperatures away from the dam (Hofer and Shair, 1993). At some point as we continue investigating CVs in animals, we must be concerned with what meaning is meant to be conveyed by the subject making the CVs, what may be interpreted by the listener, and how that may change or develop over time.
One advantage of the view that the neural representation of CVs is not consigned to an innate, modular “black box” is that the underlying mechanisms can be better delineated and understood. More direct comparisons to the study of human language become possible as we systematically determine which mechanisms exist in animals and which may be lacking, under-developed, or missing completely. Understanding where and how auditory objects are encoded and communicative signals are processed in animals may delineate the precursor neural framework for the evolution of language; it may also reveal species’ differences in communication systems and the lack of a sophisticated language system in animals. Ultimately, defining the neural circuits and cellular interactions necessary for encoding CVs and auditory objects as well as for auditory associative learning, recognition, and short-term memory, will lead to a better understanding of the etiology underlying auditory aphasias like word deafness, potentially leading to treatments for learning disabilities (Goll et al., 2010), and shedding light on communicative disorders.
Learning, memory, and experience instantiate communication sounds as unique representations
Conspecific vocalizations acquire neural representations through domain-general mechanisms
Exposure to sounds during development is crucial for later communication processing
Conspecific vocalization processing may be modulated by associate conditioning
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani M, Maeder P, Meuli R, Thiran AB, Frischknecht R, Villemure JG, Mayer J, Annoni JM, Bogousslavsky J, Fornari E, Thiran JP, Clarke S. Sound recognition and localization in man: specialized cortical networks and effects of acute circumscribed lesions. Exp Brain Res. 2003;153:591–604. doi: 10.1007/s00221-003-1616-0. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992;257:1412–1415. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]
- Amin N, Doupe A, Theunissen FE. Development of selectivity for natural sounds in the songbird auditory forebrain. J Neurophysiol. 2007;97:3517–3531. doi: 10.1152/jn.01066.2006. [DOI] [PubMed] [Google Scholar]
- Artchakov D, Tikhonravov D, Ma Y, Neuvonen T, Linnankoski I, Carlson S. Distracters impair and create working memory-related neuronal activity in the prefrontal cortex. Cereb Cortex. 2009;19:2680–2689. doi: 10.1093/cercor/bhp037. [DOI] [PubMed] [Google Scholar]
- Artchakov D, Tikhonravov D, Vuontela V, Linnankoski I, Korvenoja A, Carlson S. Processing of auditory and visual location information in the monkey prefrontal cortex. Exp Brain Res. 2007;180:469–479. doi: 10.1007/s00221-007-0873-8. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Romanski LM. Probabilistic encoding of vocalizations in macaque ventral lateral prefrontal cortex. J Neurosci. 2006;26:11023–11033. doi: 10.1523/JNEUROSCI.3466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Bäuerle P, von der Behrens W, Kössl M, Gaese BH. Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system. J Neurosci. 2011;31:9708–9722. doi: 10.1523/JNEUROSCI.5814-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Meyer M, Jancke L. Enhancement of auditory-evoked potentials in musicians reflects and influence of expertise but not selective attention. J Cogn Neurosci. 2008;12:2238–2249. doi: 10.1162/jocn.2008.20157. [DOI] [PubMed] [Google Scholar]
- Beecher MD, Petersen MR, Zoloth SR, Moody DB, Stebbins WC. Perception of conspecific vocalizations by Japanese macaques. Evidence for selective attention and neural lateralization. Brain Behav Evol. 1979;16:443–460. doi: 10.1159/000121881. [DOI] [PubMed] [Google Scholar]
- Belin P. Voice processing in human and non-human primates. Philos Trans R Soc Lond B Biol Sci. 2006;361:2091–2107. doi: 10.1098/rstb.2006.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ. Adaptation to speaker’s voice in right anterior temporal lobe. Neuroreport. 2003;14:2105–2109. doi: 10.1097/00001756-200311140-00019. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blair HT, Tinkelman A, Moita MA, LeDoux JE. Associative plasticity in neurons of the lateral amygdala during auditory fear conditioning. Ann NY Acad Sci. 2003;985:485–487. doi: 10.1111/j.1749-6632.2003.tb07106.x. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Bowers D, Heilman KM. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–1127. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: an acoustic by-product of laryngeal braking? Behav Neurosci. 1990;104:808–817. doi: 10.1037//0735-7044.104.5.808. [DOI] [PubMed] [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7:1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010;11:747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Böye M, Güntürkün O, Vauclair J. Right ear advantage for conspecific calls in adults and subadults, but not infants, California sea lions (Zalophus californianus): hemispheric specialization for communication? Eur J Neurosci. 2005;21:1727–1732. doi: 10.1111/j.1460-9568.2005.04005.x. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Experience-dependent plasticity in the inferior colliculus: a site for visual calibration of the neural representation of auditory space in the barn owl. J Neurosci. 1993;13:4589–4608. doi: 10.1523/JNEUROSCI.13-11-04589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Kremz P, Wetzel W, Wagner T, Poeggel G. Influence of parental deprivation on the behavioral development in Octodon degus: modulation by maternal vocalizations. Dev Psychobiol. 2003;42:237–445. doi: 10.1002/dev.10096. [DOI] [PubMed] [Google Scholar]
- Bregman MR, Patel AD, Gentner TQ. Stimulus-dependent flexibility in non-human auditory pitch processing. Cognition. 2012;122:51–60. doi: 10.1016/j.cognition.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 2005;25:6797–6806. doi: 10.1523/JNEUROSCI.1571-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Irvine DR, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–965. doi: 10.1093/cercor/bhh056. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Humphrey GL. Response plasticity in cochlear nucleus of decerebrate cats during acoustic habituation procedures. J Neurophysiol. 1972;35:864–878. doi: 10.1152/jn.1972.35.6.864. [DOI] [PubMed] [Google Scholar]
- Bukach CM, Gauthier I, Tarr MJ. Beyond faces and modularity: the power of an expertise framework. Trends Cogn Sci. 2006;10:159–166. doi: 10.1016/j.tics.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Byers-Heinlein K, Burns TC, Werker JF. The roots of bilingualism in newborns. Psychol Sci. 2010;21:343–348. doi: 10.1177/0956797609360758. [DOI] [PubMed] [Google Scholar]
- Calford MB. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3:2350–2364. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology. 2010;47:236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand JP, Peretz I, Belin P. Auditory recognition expertise and domain specificity. Brain Res. 2008;1220:191–198. doi: 10.1016/j.brainres.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Nagarajan SS, Bedenbaugh PH, Schreiner CE, Wang X, Wong A. Auditory cortical neuron response differences under isoflurane versus pentobarbital anesthesia. Hear Res. 2001;156:115–127. doi: 10.1016/s0378-5955(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Nagarajan SS, Schreiner CE, Bedenbaugh PH, Wong A. Plasticity in primary auditory cortex of monkeys with altered vocal production. J Neurosci. 2005;25:2490–2503. doi: 10.1523/JNEUROSCI.5289-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behav Neurosci. 1991;105:416–430. doi: 10.1037//0735-7044.105.3.416. [DOI] [PubMed] [Google Scholar]
- Cousillas H, George I, Henry L, Richard JP, Hausberger M. Linking social and vocal brains: could social segregation prevent a proper development of a central auditory area in a female songbird? PLoS One. 2008;3:e2194. doi: 10.1371/journal.pone.0002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousillas H, Richard JP, Mathelier M, Henry L, George I, Hausberger M. Experience-dependent neuronal specialization and functional organization in the central auditory area of a songbird. Eur J Neurosci. 2004;19:3343–3352. doi: 10.1111/j.0953-816X.2004.03376.x. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol. 2007;17:456–464. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proc Natl Acad Sci USA. 2003;109:2144–2149. doi: 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behav Dev. 1986;9:133–150. [Google Scholar]
- Del Negro C, Lehongre K, Edeline JM. Selectivity of canary HVC neurons for the bird’s own song: modulation by photoperiodic conditions. J Neurosci. 2005;25:4952–4963. doi: 10.1523/JNEUROSCI.4847-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA. 2012;109:E505–514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Physiological plasticity of single neurons in auditory cortex of the cat during acquisition of the pupillary conditioned response: II. Secondary field (AII) Behav Neurosci. 1984;98:189–210. [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–360. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: an effect of expertise. J Exp Psychol Gen. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Dick F, Lee HL, Nusbaum H, Price CJ. Auditory-motor expertise alters “speech selectivity” in professional musicians and actors. Cereb Cortex. 2011;21:938–948. doi: 10.1093/cercor/bhq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Brown SD, Klump GM, Okanoya K. Auditory perception of conspecific and heterospecific vocalizations in birds: evidence for special processes. J Comp Psychol. 1992;106:20–28. doi: 10.1037/0735-7036.106.1.20. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci USA. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Eda-Fujiwara H, Imagawa T, Matsushita M, Matsuda Y, Takeuchi HA, Satoh R, Watanabe A, Zandbergen MA, Manabe K, Kawashima T, Bolhuis JJ. Localized brain activation related to the strength of auditory learning in a parrot. PLoS One. 2012;7:e38803. doi: 10.1371/journal.pone.0038803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM. Frequency-specific plasticity of single unit discharges in the rat medial geniculate body. Brain Res. 1990;529:109–119. doi: 10.1016/0006-8993(90)90817-u. [DOI] [PubMed] [Google Scholar]
- Edeline JM. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: a critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Prog Neurobiol. 1999;57:165–224. doi: 10.1016/s0301-0082(98)00042-2. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Dutrieux G, Manunta Y, Hennevin E. Diversity of receptive field changes in auditory cortex during natural sleep. Eur J Neurosci. 2001;14:1865–1880. doi: 10.1046/j.0953-816x.2001.01821.x. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Neuenschwander-el Massioui N, Dutrieux G. Frequency-specific cellular changes in the auditory system during acquisition and reversal of discriminative conditioning. Psychobiol. 1990;18:382–393. [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Subcortical adaptive filtering in the auditory system: associative receptive field plasticity in the dorsal medial geniculate body. Behav Neurosci. 1991a;105:154–175. doi: 10.1037//0735-7044.105.1.154. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Thalamic short-term plasticity in the auditory system: associative returning of receptive fields in the ventral medial geniculate body. Behav Neurosci. 1991b;105:618–639. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325:249–251. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- Esser KH, Condon CJ, Suga N, Kanwal JS. Syntax processing by auditory cortical neurons in the FM-FM area of the mustached bat Pteronotus parnellii. Proc Natl Acad Sci USA. 1997;94:14019–14024. doi: 10.1073/pnas.94.25.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser KH, Eiermann A. Tonotopic organization and parcellation of auditory cortex in the FM-bat Carollia perspicillata. Eur J Neurosci. 1999;11:3669–3682. doi: 10.1046/j.1460-9568.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Armony JL, Joanette Y, Belin P. Sensitivity to voice in human prefrontal cortex. J Neurophysiol. 2005;94:2251–2254. doi: 10.1152/jn.00329.2005. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Adam TJ. Excitatory and inhibitory response adaptation in the superior olive complex affects binaural acoustic processing. Hear Res. 1997a;103:1–18. doi: 10.1016/s0378-5955(96)00158-x. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Adam TJ. Short-term adaptation of excitation and inhibition shapes binaural processing. Acta Otolaryngol. 1997b;117:187–191. doi: 10.3109/00016489709117766. [DOI] [PubMed] [Google Scholar]
- Fischer J, Cheney DL, Seyfarth RM. Development of infant baboons’ responses to graded bark variants. Proc Biol Sci. 2000;267:2317–2321. doi: 10.1098/rspb.2000.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Fritz JB, David SV, Radtke-Schuller S, Yin P, Shamma SA. Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nat Neurosci. 2010;13:1011–1019. doi: 10.1038/nn.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Active listening: task-dependent plasticity of spectrotemporal receptive fields in primary auditory cortex. Hear Res. 2005a;206:159–76. doi: 10.1016/j.heares.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J Neurosci. 2005b;25:7623–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Fujita I, Tanaka K, Ito M, Cheng K. Columns for visual features of objects in monkey inferotemporal cortex. Nature. 1992;360:343–346. doi: 10.1038/360343a0. [DOI] [PubMed] [Google Scholar]
- Fusani L, Gahr M. Hormonal influence on song structure and organization: the role of estrogen. Neuroscience. 2006;138:939–946. doi: 10.1016/j.neuroscience.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Saltwick SE, Miller JD. Conditioning and reversal of short-latency multiple-unit responses in the rabbit medial geniculate nucleus. Science. 1975;189:1108–1109. doi: 10.1126/science.1162365. [DOI] [PubMed] [Google Scholar]
- Gaese BH, Ostwald J. Anesthesia changes frequency tuning of neurons in the rat primary auditory cortex. J Neurophysiol. 2001;86:1062–1066. doi: 10.1152/jn.2001.86.2.1062. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 1983;221:169–184. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Gehr DD, Komiya H, Eggermont JJ. Neuronal responses in cat primary auditory cortex to natural and altered species-specific calls. Hear Res. 2000;150:27–42. doi: 10.1016/s0378-5955(00)00170-2. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Auditory perception vs. recognition: representation of complex communication sounds in the mouse auditory cortical fields. Eur J Neurosci. 2004;19:1027–1040. doi: 10.1111/j.1460-9568.2004.03205.x. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I, Alcaix S, Henry L, Richard JP, Cousillas H, Hausberger M. Neural correlates of experience induced deficits in learned vocal communication. PLoS ONE. 2010;5:e14347. doi: 10.1371/journal.pone.0014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I, Cousillas H, Vernier B, Richard JP, Henry L, Mathelier M, Lengagne T, Hausberger M. Sound processing in the auditory-cortex homologue of songbirds: functional organization and developmental issues. J Physiol Paris. 2004;98:385–394. doi: 10.1016/j.jphysparis.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Gifford GW, Hauser MD, Cohen YE. Discrimination of functionally referential calls by laboratory-housed rhesus macaques: Implications for neuroethological studies. Brain Behav Evol. 2003;61:213–224. doi: 10.1159/000070704. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Glass I, Wollberg Z. Auditory cortex responses to sequences of normal and reversed squirrel monkey vocalizations. Brain Behav Evol. 1983a;22:13–21. doi: 10.1159/000121503. [DOI] [PubMed] [Google Scholar]
- Glass I, Wollberg Z. Responses of cells in the auditory cortex of awake squirrel monkeys to normal and reversed species-specific vocalizations. Hear Res. 1983b;9:27–33. doi: 10.1016/0378-5955(83)90131-4. [DOI] [PubMed] [Google Scholar]
- Goll JC, Crutch SJ, Warren JD. Central auditory disorders: toward a neuropsychology of auditory objects. Curr Opin Neurol. 2010;23:617–627. doi: 10.1097/WCO.0b013e32834027f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Lima F, Scheich H. Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose. II. Auditory cortex plasticity. Behav Brain Res. 1986;20:281–293. doi: 10.1016/0166-4328(86)90228-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Results. Dev Psychol. 1991;27:35–39. [Google Scholar]
- Gottlieb Y, Vaadia E, Abeles M. Single unit activity in the auditory cortex of a monkey performing a short term memory task. Exp Brain Res. 1989;74:139–148. doi: 10.1007/BF00248287. [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Eggermont JJ. Spatial representation of neural responses to natural and altered conspecific vocalizations in cat auditory cortex. J Neurophysiol. 2007;97:144–158. doi: 10.1152/jn.00807.2006. [DOI] [PubMed] [Google Scholar]
- Gross CG, Sergent J. Face recognition. Curr Opin Neurobiol. 1992;2:156–161. doi: 10.1016/0959-4388(92)90004-5. [DOI] [PubMed] [Google Scholar]
- Harley E, Pope W, Villablanca J, Mumford J, Suh R, Mazziotta J, Enzmann D, Engel S. Engagement of fusiform cortex and disengagement of lateral occipital cortex in the acquisition of radiological expertise. Cereb Cortex. 2009;19:2746–2754. doi: 10.1093/cercor/bhp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshaw C, Lickliter R. Interactive and vicarious acquisition of auditory preferences in Northern bobwhite (Colinus virginianus) chicks. J Comp Psychol. 2007;121:320–331. doi: 10.1037/0735-7036.121.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshaw C, Lickliter R. Biased embryos: prenatal experience alters the postnatal malleability of auditory preferences in bobwhite quail. Dev Psychobiol. 2011;53:291–302. doi: 10.1002/dev.20521. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Woolley SMN, Theunissen FE. Experience-dependence of neural responses to social vs. isolate conspecific songs in the forebrain of female zebra finches. J Ornithol. 2007;148:S231–S239. [Google Scholar]
- Hauser MD, Andersson K. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: field experiments. Proc Natl Acad Sci USA. 1994;91:3946–3948. doi: 10.1073/pnas.91.9.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science. 1984;226:75–76. doi: 10.1126/science.6474192. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair HN. Ultrasonic vocalization, laryngeal braking, and thermogenesis in rat pups: a reappraisal. Behav Neurosci. 1993;107:354–362. doi: 10.1037//0735-7044.107.2.354. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline JM. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J Neurosci. 2009;29:334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher J, Wendy H, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: relation to language input and gender. Dev Psychol. 1991;27:236–248. [Google Scholar]
- Issa EB, Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci. 2008;28:14467–14480. doi: 10.1523/JNEUROSCI.3086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, Wang X. Altered neural responses to sounds in primate primary auditory cortex during slow-wave sleep. J Neurosci. 2011;31:2965–2973. doi: 10.1523/JNEUROSCI.4920-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann NY Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Gao E, Suga N. Effects of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophysiol. 2001;86:211–225. doi: 10.1152/jn.2001.86.1.211. [DOI] [PubMed] [Google Scholar]
- Joseph JP, Barone P. Prefrontal unit activity during a delayed oculomotor task in the monkey. Exp Brain Res. 1987;67:460–468. doi: 10.1007/BF00247279. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2:1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nat Neurosci. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Logothetis NK. Visual modulation of neurons in auditory cortex. Cereb Cortex. 2008;18:1560–1574. doi: 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Horwitz B, Mishkin M. Hierarchical auditory processing directed rostrally along the monkey’s supratemporal plane. J Neurosci. 2010;30:13021–13030. doi: 10.1523/JNEUROSCI.2267-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Yorioka Y, Sawaguchi T. Parallel visuospatial and audiospatial working memory processes in the monkey dorsolateral prefrontal cortex. Nat Neurosci. 2000;3:1075–1076. doi: 10.1038/80581. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzes M, Buchwald J. Progressive alterations in cochlear nucleus, inferior colliculus, and medial geniculate responses during acoustic habituation. Exp Neurol. 1969;25:85–105. doi: 10.1016/0014-4886(69)90073-9. [DOI] [PubMed] [Google Scholar]
- Komura Y, Tamura R, Uwano T, Nishijo H, Kaga K, Ono T. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–549. doi: 10.1038/35087595. [DOI] [PubMed] [Google Scholar]
- Komura Y, Tamura R, Uwano T, Nishijo H, Ono T. Auditory thalamus integrates visual inputs into behavioral gains. Nat Neurosci. 2005;8:1203–1209. doi: 10.1038/nn1528. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Meyer M, Paulmann S. Lateralization of emotional prosody in the brain: an overview and synopsis on the impact of study design. Prog Brain Res. 2006;156:285–294. doi: 10.1016/S0079-6123(06)56015-7. [DOI] [PubMed] [Google Scholar]
- Kraus N, Banai K. Auditory-processing malleability: focus on language and music. Curr Dir Psychol Sci. 2007;16:105–110. [Google Scholar]
- Kraus N, Disterhoft JF. Response plasticity of single neurons in rabbit auditory association cortex during tone-signalled learning. Brain Res. 1982;246:205–215. doi: 10.1016/0006-8993(82)91168-4. [DOI] [PubMed] [Google Scholar]
- Kriegstein KV, Giraud AL. Distinct functional substrates along the right superior temporal sulcus for the processing of voices. Neuroimage. 2004;22:948–955. doi: 10.1016/j.neuroimage.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Theoretical contributions of tests on animals to the special-mechanisms debate in speech. Exp Biol. 1986;45:233–265. [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nat Rev Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśmierek P, Malinowska M, Kowalska DM. Different effects of lesions to auditory core and belt cortex on auditory recognition in dogs. Exp Brain Res. 2007;180:491–508. doi: 10.1007/s00221-007-0868-5. [DOI] [PubMed] [Google Scholar]
- Lattner S, Meyer ME, Friederici AD. Voice perception: Sex, pitch, and the right hemisphere. Hum Brain Mapp. 2005;24:11–20. doi: 10.1002/hbm.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazard DS, Collette JL, Perrot X. Speech processing: from peripheral to hemispheric asymmetry of the auditory system. Laryngoscope. 2012;122:167–173. doi: 10.1002/lary.22370. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Rauschecker JP. Cortical representation of natural complex sounds: effects of acoustic features and auditory object category. J Neurosci. 2010;30:7604–7612. doi: 10.1523/JNEUROSCI.0296-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TP, Buonomano DV. Unsupervised formation of vocalization-sensitive neurons: a cortical model based on short-term and homeostatic plasticity. Neural Comput. 2012;24:2579–2603. doi: 10.1162/NECO_a_00345. [DOI] [PubMed] [Google Scholar]
- Leech R, Holt LL, Devlin JT, Dick F. Expertise with artificial nonspeech sounds recruits speech-sensitive cortical regions. J Neurosci. 2009;29:5234–5239. doi: 10.1523/JNEUROSCI.5758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehongre K, Del Negro C. Representation of the bird’s own song in the canary HVC: contribution of broadly tuned neurons. Neuroscience. 2011;173:93–109. doi: 10.1016/j.neuroscience.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Lemasson A, Koda H, Kato A, Oyakawa C, Blois-Heulin C, Masataka N. Influence of sound specificity and familiarity on Japanese macaques’ (Macaca fuscata) auditory laterality. Behav Brain Res. 2010;208:286–289. doi: 10.1016/j.bbr.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency-specific receptive field plasticity in the medial geniculate body induced by pavlovian fear conditioning is expressed in the anesthetized brain. Behav Neurosci. 1992;106:484–497. doi: 10.1037//0735-7044.106.3.484. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Hellewell TB. Contextual determinants of auditory learning in bobwhite quail embryos and hatchlings. Dev Psychobiol. 1992;25:17–31. doi: 10.1002/dev.420250103. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Stoumbos J. Modification of prenatal auditory experience alters postnatal auditory preferences of bobwhite quail chicks. Q J Exp Psychol B. 1992;44:199–214. doi: 10.1080/02724999208250612. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983;3:1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci. 1986;6:1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul KK, Voss HU, Parra LC, Salgado-Commissariat D, Ballon D, Tchernichovski O, Helekar SA. The development of stimulus-specific auditory responses requires song exposure in male but not female zebra finches. Dev Neurobiol. 2010;70:28–40. doi: 10.1002/dneu.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front Psychol. 2011;2:222. doi: 10.3389/fpsyg.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Bracewell RM, Barash S, Andersen RA. Spatially tuned auditory responses in area LIP of macaques performing delayed memory saccades to acoustic targets. J Neurophysiol. 1996;75:1233–1241. doi: 10.1152/jn.1996.75.3.1233. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Green EJ, Winters RW, Nolen TG, Schneiderman N, McCabe PM. Changes of synaptic efficacy in the medial geniculate nucleus as a result of auditory classical conditioning. J Neurosci. 1996;16:1273–1283. doi: 10.1523/JNEUROSCI.16-03-01273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Simultaneous single unit recording in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit’s classically conditioned heart rate. Brain Res. 1995;682:157–166. doi: 10.1016/0006-8993(95)00331-j. [DOI] [PubMed] [Google Scholar]
- McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends Cogn Sci. 2007;11:8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- McMurray B. Defusing the childhood vocabulary explosion. Science. 2007;317:631. doi: 10.1126/science.1144073. [DOI] [PubMed] [Google Scholar]
- Medvedev AV, Chiao F, Kanwal JS. Modeling complex tone perception: grouping harmonics with combination-sensitive neurons. Biol Cybern. 2002;86:497–505. doi: 10.1007/s00422-002-0316-3. [DOI] [PubMed] [Google Scholar]
- Mesgarani N, Chang EF. Selective cortical representation of attended speaker in multi-talker speech perception. Nature. 2012;485:233–236. doi: 10.1038/nature11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Cohen YE. Vocalizations as auditory objects: behavior and neurophysiology. In: Platt M, Ghazanfar A, editors. Primate Neuroethology. Oxford University Press; 2010. pp. 237–255. [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, van der Lely H, Ramus F, Sato Y, Mazuka R, Dupoux E. Optical brain imaging reveals general auditory and language-specific processing in early infant development. Cereb Cortex. 2011;21:254–261. doi: 10.1093/cercor/bhq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR. Auditory neuroscience: is speech special? Curr Biol. 2000;10:R362–364. doi: 10.1016/s0960-9822(00)00479-6. [DOI] [PubMed] [Google Scholar]
- Morán MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Morris JS, Scott SK, Dolan RJ. Saying it with feeling: neural responses to emotional vocalizations. Neuropsychologia. 1999;37:1155–1163. doi: 10.1016/s0028-3932(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Musacchia G, Sams M, Skoe E, Kraus N. Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proc Natl Acad Sci USA. 2007;104:15894–15898. doi: 10.1073/pnas.0701498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Zhang LI, Merzenich MM. Specialization of primary auditory cortex processing by sound exposure in the “critical period”. Proc Natl Acad Sci USA. 2004;101:7170–7174. doi: 10.1073/pnas.0401196101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. J Neurophysiol. 1995;74:162–178. doi: 10.1152/jn.1995.74.1.162. [DOI] [PubMed] [Google Scholar]
- Nelken I, Calford MB. Processing strategies in auditory cortex: comparison with other sensory modalities. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. Springer; New York: 2011. pp. 643–656. [Google Scholar]
- Ng CW. (Doctoral dissertation) Retrieved from ProQuest Dissertations and Theses. 2011. Behavioral and neural correlates of auditory encoding and memory functions in rhesus macaques. 879629871. [Google Scholar]
- Ng CW, Plakke B, Poremba A. Primate auditory recognition memory performance varies with sound type. Hear Res. 2009;256:64–74. doi: 10.1016/j.heares.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls ME, Schier M, Stough CK, Box A. Psychophysical and electrophysiologic support for a left hemisphere temporal processing advantage. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:11–16. [PubMed] [Google Scholar]
- Niwa M, Johnson JS, O’Connor KN, Sutter ML. Active engagement improves primary auditory cortical neurons’ ability to discriminate temporal modulation. J Neurosci. 2012;32:9323–9334. doi: 10.1523/JNEUROSCI.5832-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourski K, Brugge J, Steinschneider M, Oya H, Kawasaki H, Reale R, Howard M. Auditory cortical responses to spectrally degraded speech: an intracranial electrophysiology study. 18th Annual Meeting of the Organization for Human Brain Mapping; Beijing, China. 2012. [Google Scholar]
- Nourski KV, Etler CP, Brugge JF, Oya H, Kawasaki H, Reale RA, Abbas PJ, Brown CJ, Howard MA., 3rd Direct Recordings from the Auditory Cortex in a Cochlear Implant User. J Assoc Res Otolaryngol. 2013 doi: 10.1007/s10162-013-0382-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KN, Allison TL, Rosenfield ME, Moore JW. Neural activity in the medial geniculate nucleus during auditory trace conditioning. Exp Brain Res. 1997;113:534–556. doi: 10.1007/pl00005605. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Differential frequency conditioning enhances spectral contrast sensitivity of units in auditory cortex (field Al) of the alert Mongolian gerbil. Eur J Neurosci. 1996;8:1001–1017. doi: 10.1111/j.1460-9568.1996.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Perception of distance calls by budgerigars (Melopsittacus undulatus) and zebra finches (Poephila guttata): assessing species-specific advantages. J Comp Psychol. 1991;105:60–72. doi: 10.1037/0735-7036.105.1.60. [DOI] [PubMed] [Google Scholar]
- Oleson TD, Ashe JH, Weinberger NM. Modification of auditory and somatosensory system activity during pupillary conditioning in the paralyzed cat. J Neurophysiol. 1975;38:1114–1139. doi: 10.1152/jn.1975.38.5.1114. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishino H, Fukuda M, Sasaki K, Nishijo H. Single neuron activity in dorsolateral prefrontal cortex of monkey during operant behavior sustained by food reward. Brain Res. 1984;311:323–332. doi: 10.1016/0006-8993(84)90095-7. [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Brand A, Koch U, Ikebuchi M, Grothe B. Dynamic changes in level influence spatial coding in the lateral superior olive. Hear Res. 2008;238:58–67. doi: 10.1016/j.heares.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN, Rubel EW, Fay RR. Plasticity of the Auditory System. Springer; New York: 2004. [Google Scholar]
- Parsana AJ, Li N, Brown TH. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav Brain Res. 2012;226:77–86. doi: 10.1016/j.bbr.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell MD. Cerebral mechanisms for understanding emotional prosody in speech. Brain Lang. 2006;96:221–234. doi: 10.1016/j.bandl.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Mistlin AJ, Chitty AJ, Smith PA, Potter DD, Broennimann R, Harries M. Specialized face processing and hemispheric asymmetry in man and monkey: evidence from single unit and reaction time studies. Behav Brain Res. 1988;29:245–258. doi: 10.1016/0166-4328(88)90029-0. [DOI] [PubMed] [Google Scholar]
- Perrodin C, Kayser C, Logothetis NK, Petkov CI. Voice cells in the primate temporal lobe. Curr Biol. 2011;21:1408–1415. doi: 10.1016/j.cub.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MR, Beecher MD, Zoloth SR, Green S, Marler PR, Moody DB, Stebbins WC. Neural lateralization of vocalizations by Japanese macaques: communicative significance is more important than acoustic structure. Behav Neurosci. 1984;98:779–790. doi: 10.1037//0735-7044.98.5.779. [DOI] [PubMed] [Google Scholar]
- Petersen MR, Beecher MD, Zoloth SR, Moody DB, Stebbins WC. Neural lateralization of species-specific vocalizations by Japanese macaques (Macaca fuscata) Science. 1978;202:324–327. doi: 10.1126/science.99817. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Kayser C, Steudel T, Whittingstall K, Augath M, Logothetis NK. A voice region in the monkey brain. Nat Neurosci. 2008;11:367–374. doi: 10.1038/nn2043. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Logothetis NK, Obleser J. Where are the human speech and voice regions, and do other animals have anything like them? Neuroscientist. 2009;15:419–429. doi: 10.1177/1073858408326430. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proc Natl Acad Sci USA. 2010;107:2301–2306. doi: 10.1073/pnas.0900091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert B, Laudanski J, Edeline JM. Auditory thalamus responses to guinea-pig vocalizations: a comparison between rat and guinea-pig. Hear Res. 2005;209:97–103. doi: 10.1016/j.heares.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ, Rigdon GC. Single-unit and slow potential responses from rat frontal cortex during associative conditioning. Exp Neurol. 1983;82:118–130. doi: 10.1016/0014-4886(83)90247-9. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ, Rigdon GC. Conditioning-related single unit activity in the frontal cortex of urethane anesthetized rats. Int J Neurosci. 1985;25:263–271. doi: 10.3109/00207458508985379. [DOI] [PubMed] [Google Scholar]
- Plakke B. (Doctoral dissertation) Retrieved from ProQuest Dissertations and Theses. 2010. Auditory working memory: contributions of lateral prefrontal cortex and acetylcholine in non-human primates. 880271144. [Google Scholar]
- Plakke B, Ng CW, Poremba A. Neural correlates of auditory recognition memory in primate lateral prefrontal cortex. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.04.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Bigelow J. Neurophysiology of attention and memory processing. In: Cohen YE, Popper AN, Fay RR, editors. Neural Correlates of Auditory Cognition. Springer; New York: 2013. pp. 215–250. [Google Scholar]
- Poremba A, Gabriel M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. J Neurosci. 2001;21:270–278. doi: 10.1523/JNEUROSCI.21-01-00270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- Poremba A, Saunders RC, Crane AM, Cook M, Sokoloff L, Mishkin M. Functional mapping of the primate auditory system. Science. 2003;299:568–572. doi: 10.1126/science.1078900. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rämä P, Poremba A, Sala JB, Yee L, Malloy M, Mishkin M, Courtney SM. Dissociable functional cortical topographies for working memory maintenance of voice identity and location. Cereb Cortex. 2004;14:768–780. doi: 10.1093/cercor/bhh037. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner C, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM. Integration of faces and vocalizations in ventral prefrontal cortex: implications for the evolution of audiovisual speech. Proc Natl Acad Sci USA. 2012;109:10717–10724. doi: 10.1073/pnas.1204335109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB. The primate cortical auditory system and neural representation of conspecific vocalizations. Annu Rev Neurosci. 2009;32:315–346. doi: 10.1146/annurev.neuro.051508.135431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93:734–747. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999a;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999b;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ BE, Ackelson AL, Baker AE, Cohen YE. Coding of auditory-stimulus identity in the auditory non-spatial processing stream. J Neurophysiol. 2008a;99:87–95. doi: 10.1152/jn.01069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ BE, Orr LE, Cohen YE. Prefrontal neurons predict choices during an auditory same-different task. Curr Biol. 2008b;18:1483–1488. doi: 10.1016/j.cub.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Nicol TG, Zecker SG, Hayes EA, Kraus N. Auditory training improves neural timing in the human brainstem. Behav Brain Res. 2005;156:95–103. doi: 10.1016/j.bbr.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Miller JM, Pfingst BE, Martin GK. Effects of reaction time performance on single-unit activity in the central auditory pathway of the rhesus macaque. J Neurosci. 1984;4:298–308. doi: 10.1523/JNEUROSCI.04-01-00298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan S, Wang X. Nonlinear spectrotemporal interactions underlying selectivity for complex sounds in auditory cortex. J Neurosci. 2009;29:11192–11202. doi: 10.1523/JNEUROSCI.1286-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Involvement of auditory cortical and hippocampal neurons in auditory working memory and reference memory in the rat. J Neurosci. 1994;14:2606–2623. doi: 10.1523/JNEUROSCI.14-05-02606.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y. Hippocampal cells have behavioral correlates during the performance of an auditory working memory task in the rat. Behav Neurosci. 1990;104:253–263. doi: 10.1037//0735-7044.104.2.253. [DOI] [PubMed] [Google Scholar]
- Samuelson LK, Smith LB, Perry LK, Spencer JP. Grounding word learning in space. PLoS One. 2011;6:e28095. doi: 10.1371/journal.pone.0028095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K, Frome Y, Scheich H. FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Hum Brain Mapp. 2007;28:1007–1022. doi: 10.1002/hbm.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Cynader MS. Basic functional organization of second auditory cortical field (AII) of the cat. J Neurophysiol. 1984;51:1284–1305. doi: 10.1152/jn.1984.51.6.1284. [DOI] [PubMed] [Google Scholar]
- Seebach BS, Intrator N, Lieberman P, Cooper LN. A model of prenatal acquisition of speech parameters. Proc Natl Acad Sci USA. 1994;91:7473–7476. doi: 10.1073/pnas.91.16.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Some features of vocal development in nonhuman primates. In: Snowdon CT, Hausberger M, editors. Social Influences on Vocal Development. Cambridge University Press; 1997. pp. 249–273. [Google Scholar]
- Shafi M, Zhou Y, Quintana J, Chow C, Fuster J, Bodner M. Variability in neuronal activity in primate cortex during working memory tasks. Neuroscience. 2007;146:1082–1108. doi: 10.1016/j.neuroscience.2006.12.072. [DOI] [PubMed] [Google Scholar]
- Simmons A, Popper AN, Fay RR. Acoustic Communication. Springer; New York: 2003. [Google Scholar]
- Smith LB, Colunga E, Yoshida H. Making an ontology: cross-linguistic evidence. In: Rakison DH, Oakes LM, editors. Early Category and Concept Development. Oxford University Press; 2003. pp. 275–302. [Google Scholar]
- Smith LB, Samuelson L. An attentional learning account of the shape bias: reply to Cimpian and Markman (2005) and Booth, Waxman, and Huang (2005) Dev Psychol. 2006;42:1339–1343. doi: 10.1037/0012-1649.42.6.1339. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Brown CH, Petersen MR. Primate Communication. Cambridge University Press; 1982. [Google Scholar]
- Song JH, Skoe E, Wong PC, Kraus N. Plasticity in the adult human auditory brainstem following short-term linguistic training. J Cogn Neurosci. 2008;20:1892–1902. doi: 10.1162/jocn.2008.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Blumberg MS, McMurray B, Robinson SR, Samuelson LK, Tomblin JB. Short arms and talking eggs: Why we should no longer abide the nativist-empiricist debate. Child Dev Perspect. 2009;3:79–87. doi: 10.1111/j.1750-8606.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait DL, Kraus N, Skoe E, Ashley R. Musical experience and neural efficiency: effects of training on subcortical processing of vocal expressions of emotion. Eur J Neurosci. 2009;29:661–668. doi: 10.1111/j.1460-9568.2009.06617.x. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Diltz MD, Averbeck BB, Romanski LM. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J Neurosci. 2006;26:11138–11147. doi: 10.1523/JNEUROSCI.3550-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Merzenich MM. Plasticity and Signal Representation in the Auditory System. Springer; New York: 2003. [Google Scholar]
- Taglialatela JP, Russell JL, Schaeffer JA, Hopkins WD. Visualizing vocal perception in the chimpanzee brain. Cereb Cortex. 2009;19:1151–1157. doi: 10.1093/cercor/bhn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington WJ, Rapuano KM, Hitt LA, Frum CA, Lewis JW. Humans mimicking animals: a cortical hierarchy for human vocal communication sounds. J Neurosci. 2012;32:8084–8093. doi: 10.1523/JNEUROSCI.1118-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Teufel C, Ghazanfar AA, Fischer J. On the relationship between lateralized brain function and orienting asymmetries. Behav Neurosci. 2010;124:437–445. doi: 10.1037/a0019925. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Gentner TQ. Song recognition learning and stimulus-specific weakening of neural responses in the avian auditory forebrain. J Neurophysiol. 2010;103:1785–1797. doi: 10.1152/jn.00885.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turati C. Why faces are not special to newborns: an alternative account of the face preference. Curr Dir Psychol Sci. 2004;13:5–8. [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Masfield RJW, editors. Analysis of Visual Behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- von Kriegstein K, Eger E, Kleinschmidt A, Giraud AL. Modulation of neural responses to speech by directing attention to voices or verbal content. Brain Res Cogn Brain Res. 2003;17:48–55. doi: 10.1016/s0926-6410(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Wang X. On cortical coding of vocal communication sounds in primates. Proc Natl Acad Sci USA. 2000;97:11843–11849. doi: 10.1073/pnas.97.22.11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. The unexpected consequences of a noisy environment. Trends Neurosci. 2004;27:364–366. doi: 10.1016/j.tins.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Wang X. Neural coding strategies in auditory cortex. Hear Res. 2007;229:81–93. doi: 10.1016/j.heares.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Wang X, Kadia SC. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J Neurophysiol. 2001;86:2616–2620. doi: 10.1152/jn.2001.86.5.2616. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Exp Brain Res. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- Webster W, Dunlop C, Simons L, Aitkin L. Auditory habituation: a test of a centrifugal and a peripheral theory. Science. 1965;148:654–656. doi: 10.1126/science.148.3670.654. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MW, Trehub SE, Schellenberg EG. Something in the way she sings: enhanced memory for vocal melodies. Psychol Sci. 2012;23:1074–1078. doi: 10.1177/0956797612442552. [DOI] [PubMed] [Google Scholar]
- Werker JF, Vouloumanos A. Speech and language processing in infancy: a neurocognitive approach. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 269–280. [Google Scholar]
- Wiethoff S, Wildgruber D, Grodd W, Ethofer T. Response and habituation of the amygdala during processing of emotional prosody. Neuroreport. 2009;20:1356–1360. doi: 10.1097/WNR.0b013e328330eb83. [DOI] [PubMed] [Google Scholar]
- Wollberg Z, Sela J. Frontal cortex of the awake squirrel monkey: responses of single cells to visual and auditory stimuli. Brain Res. 1980;198:216–20. doi: 10.1016/0006-8993(80)90358-3. [DOI] [PubMed] [Google Scholar]
- Wong PC, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci. 2007;10:420–422. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CD, Wang XF, Gruen E. Response to acoustic stimuli increases in the ventral cochlear nucleus after stimulus pairing. Neuroreport. 1994;5:513–515. doi: 10.1097/00001756-199401120-00037. [DOI] [PubMed] [Google Scholar]
- Woody CD, Wang XF, Gruen E, Landeira-Fernandez J. Unit activity to click CS changes in dorsal cochlear nucleus after conditioning. Neuroreport. 1992;3:385–388. doi: 10.1097/00001756-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Woolley SM. Early experience shapes vocal neural coding and perception in songbirds. Dev Psychobiol. 2012;54:612–631. doi: 10.1002/dev.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Hauber ME, Theunissen FE. Developmental experience alters information coding in auditory midbrain and forebrain neurons. Dev Neurobiol. 2010;70:235–252. doi: 10.1002/dneu.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden FG, Marsh JT. Amplitude changes of auditory potentials evoked at cochlear nucleus during acoustic habituation. Electroencephalogr Clin Neurophysiol. 1963;15:866–881. doi: 10.1016/0013-4694(63)90176-7. [DOI] [PubMed] [Google Scholar]
- Yin P, Mishkin M, Sutter M, Fritz JB. Early stages of melody processing: stimulus-sequence and task-dependent neuronal activity in monkey auditory cortical fields A1 and R. J Neurophysiol. 2008;100:3009–3029. doi: 10.1152/jn.00828.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Ziabreva I, Schnabel R, Poeggel G, Braun K. Mother’s voice “buffers” separation-induced receptor changes in the prefrontal cortex of octodon degus. Neuroscience. 2003;119:433–441. doi: 10.1016/s0306-4522(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zoloth SR, Petersen MR, Beecher MD, Green S, Marler P, Moody DB, Stebbins W. Species-specific perceptual processing of vocal sounds by monkeys. Science. 1979;204:870–873. doi: 10.1126/science.108805. [DOI] [PubMed] [Google Scholar]


