Abstract
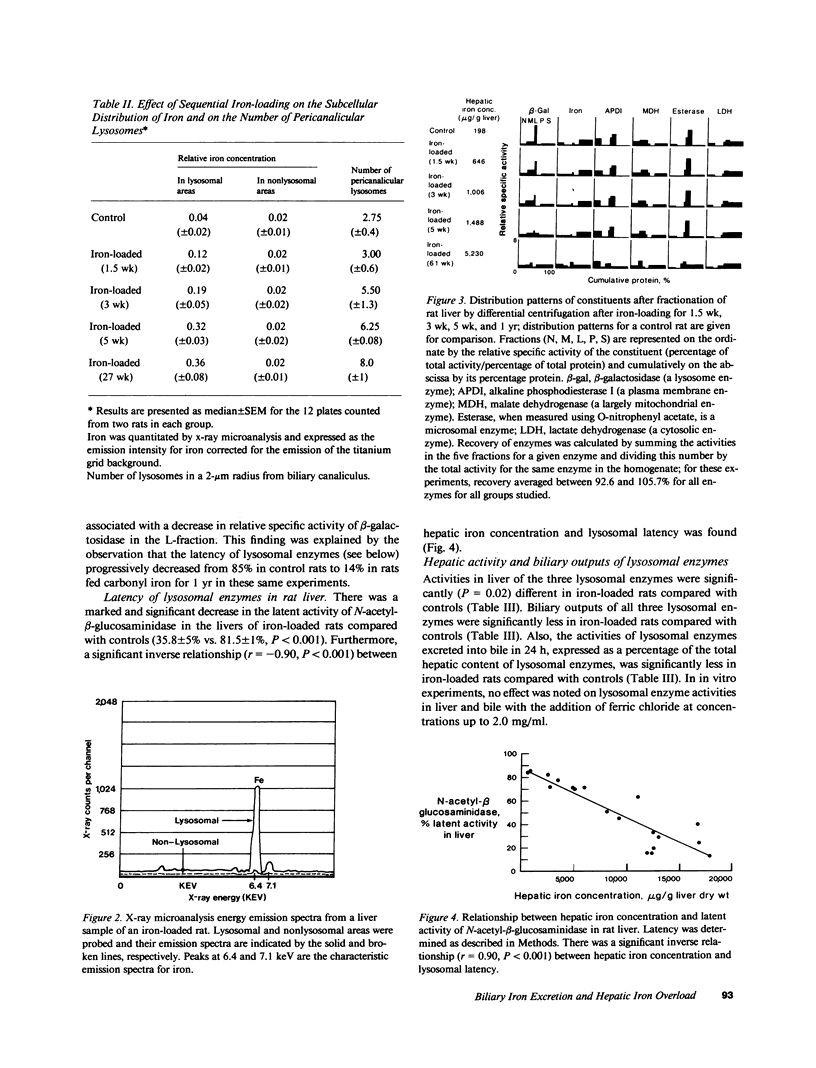
In these experiments, we assessed the role of hepatocyte lysosomes in biliary excretion of iron. We loaded rats with iron by feeding 2% carbonyl iron and collected bile for 24 h via bile fistulae from iron-loaded and control rats. In additional rats, bile was collected before and after the administration of colchicine. Rats were then killed and their livers were homogenized and fractionated for biochemical analyses or processed for electron microscopy and x-ray microanalysis. Inclusion of 2% carbonyl iron in the diet caused a 45-fold increase (P less than 0.001) in hepatic iron concentration compared with controls (1,826 +/- 159 vs. 38 +/- 6.7 micrograms/g liver, mean +/- SE). Electron microscopy with quantitative morphometry and x-ray microanalysis showed that the excess iron was sequestered in an increased number of lysosomes concentrated in the pericanalicular region of the hepatocyte. Iron loading was also associated with a twofold increase in biliary iron excretion (4.06 +/- 0.3 vs. 1.75 +/- 0.1 micrograms/g liver/24 h; P less than 0.001). In contrast, the biliary outputs of three lysosomal enzymes were significantly lower (P less than 0.0005) in iron-loaded rats compared with controls (mean +/- SE) expressed as mU/24 h/g liver: N-acetyl-beta-glucosaminidase, 26.7 +/- 4.6 vs. 66.2 +/- 13.4; beta-glucuronidase, 10.1 +/- 1.3 vs. 53.2 +/- 17.9; beta-galactosidase, 8.9 +/- 1.0 vs. 15.4 +/- 2.3. In iron-loaded rats but not in controls, biliary iron excretion was coupled to the release into bile of each of the three lysosomal hydrolases as assessed by linear regression analysis (P less than 0.001). In contrast, no relationships were found between biliary iron excretion and the biliary outputs of a plasma membrane marker enzyme (alkaline phosphodiesterase I) or total protein. After administration of colchicine, there was a parallel increase in biliary excretion of iron and lysosomal enzymes in iron-loaded rats, but not controls. We interpret these data to indicate that, in the rat, biliary iron excretion from hepatocyte lysosomes is an important excretory route for excess hepatic iron.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arborgh B. A., Glaumann H., Ericsson J. L. Studies on iron loading of rat liver lysosomes. Effects on the liver and distribution and fate of iron. Lab Invest. 1974 May;30(5):664–673. [PubMed] [Google Scholar]
- BESSIS M., CAROLI J. A comparative study of hemochromatosis by electron microscopy. Gastroenterology. 1959 Nov;37:538–549. [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M., Flynn D. M., Letsky E. A., Risdon R. A. Long-term chelation therapy in thalassaemia major: effect on liver iron concentration, liver histology, and clinical progress. Br Med J. 1974 Apr 6;2(5909):16–20. doi: 10.1136/bmj.2.5909.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell T. H., Charlton R. W., Seftel H. C. Oral iron overload. S Afr Med J. 1965 Oct 30;39(39):892–900. [PubMed] [Google Scholar]
- Bradford W. D., Elchlepp J. G., Arstila A. U., Trump B. F., Kinney T. D. Iron metabolism and cell membranes. I. Relation between ferritin and hemosiderin in bile and biliary excretion of lysosome contents. Am J Pathol. 1969 Aug;56(2):201–228. [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Frommer D. The binding of copper by bile and serum. Clin Sci. 1971 Dec;41(6):485–493. doi: 10.1042/cs0410485. [DOI] [PubMed] [Google Scholar]
- Grace N. D., Powell L. W. Iron storage disorders of the liver. Gastroenterology. 1974 Dec;67(6):1257–1283. [PubMed] [Google Scholar]
- Hoffstein S., Goldstein I. M., Weissmann G. Role of microtubule assembly in lysosomal enzyme secretion from human polymorphonuclear leukocytes. A reevaluation. J Cell Biol. 1977 Apr;73(1):242–256. doi: 10.1083/jcb.73.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz R., Arborgh B., Wroblewski R., Ericsson J. L. Studies on the rat liver following iron overload. Electron probe x-ray microanalysis of acid phosphatase and iron. Am J Pathol. 1979 Aug;96(2):625–640. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRusso N. F., Kost L. J., Carter J. A., Barham S. S. Triton WR-1339, a lysosomotropic compound, is excreted into bile and alters the biliary excretion of lysosomal enzymes and lipids. Hepatology. 1982 Mar-Apr;2(2):209–215. doi: 10.1002/hep.1840020204. [DOI] [PubMed] [Google Scholar]
- LeSage G. D., Baldus W. P., Fairbanks V. F., Baggenstoss A. H., McCall J. T., Moore S. B., Taswell H. F., Gordon H. Hemochromatosis: genetic or alcohol-induced? Gastroenterology. 1983 Jun;84(6):1471–1477. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez del Pino V. H., LaRusso N. F. Dissociation of bile flow and biliary lipid secretion from biliary lysosomal enzyme output in experimental cholestasis. J Lipid Res. 1981 Feb;22(2):229–235. [PubMed] [Google Scholar]
- Mak I. T., Weglicki W. B. Characterization of iron-mediated peroxidative injury in isolated hepatic lysosomes. J Clin Invest. 1985 Jan;75(1):58–63. doi: 10.1172/JCI111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R. B., Barham S. S., LaRusso N. F. Effect of chloroquine on the form and function of hepatocyte lysosomes. Morphologic modifications and physiologic alterations related to the biliary excretion of lipids and proteins. Gastroenterology. 1983 Nov;85(5):1146–1153. [PubMed] [Google Scholar]
- Sewell R. B., Mao S. J., Kawamoto T., LaRusso N. F. Apolipoproteins of high, low, and very low density lipoproteins in human bile. J Lipid Res. 1983 Apr;24(4):391–401. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Edds K. T. Microtubules: structure, chemistry, and function. Physiol Rev. 1976 Oct;56(4):709–777. doi: 10.1152/physrev.1976.56.4.709. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Valigorsky J. M., Arstila A. U., Mergner W. J., Kinney T. D. The relationship of intracellular pathways of iron metabolism to cellular iron overload and the iron storage diseases. Cell sap and cytocavitary network pathways in relation to lysosomal storage and turnover of iron macromolecules. Am J Pathol. 1973 Aug;72(2):295–336. [PMC free article] [PubMed] [Google Scholar]