Abstract
The fabrication of cell-laden structures with anisotropic mechanical properties while having a precise control over the distribution of different cell types within the constructs is important for many tissue engineering applications. Automated textile technologies for making fabrics allow simultaneous control over the color pattern and directional mechanical properties. The use of textile techniques in tissue engineering, however, demands the presence of cell-laden fibers that can withstand the mechanical stresses during the assembly process. Here, the concept of composite living fibers (CLFs) in which a core of load bearing synthetic polymer is coated by a hydrogel layer containing cells or microparticles is introduced. The core thread is drawn sequentially through reservoirs containing a cell-laden prepolymer and a crosslinking reagent. The thickness of the hydrogel layer increases linearly with to the drawing speed and the prepolymer viscosity. CLFs are fabricated and assembled using regular textile processes including weaving, knitting, braiding, winding, and embroidering, to form cell-laden structures. Cellular viability and metabolic activity are preserved during CLF fabrication and assembly, demonstrating the feasibility of using these processes for engineering functional 3D tissue constructs.
1. Introduction
Textile technologies developed for making clothing and decorative fabrics can produce finely tuned 2D and 3D constructs with exquisite control over their size, shape, and porosity from natural or synthetic fibers.[1–3] These characteristics elevate the textile technologies including weaving, knitting, and braiding to an attractive platform for engineering artificial organs for both transplantation and research purposes.[4–6] Textile technologies have been exploited to mimic important mechanical characteristics of natural tissues, such as the anisotropy of cardiac muscle, tendon, and vascular walls,[5,7,8] which are known to control cellular behavior and activity.[9] In an interesting study, Moutos et al. used a customized loom to weave a 3D scaffold from poly(glycolic acid) (PGA) microfibers.[4] These scaffolds served to reinforce an agarose hydrogel construct loaded with porcine articular chondrocytes. The mechanical properties and microstructure of the fabricated scaffolds were adjusted to match the anisotropy and tension–compression nonlinearity of native articular cartilage.[4] In another study, Dai et al. created a 3D hybrid scaffold by incorporating a type I collagen within a poly(lactic-co-glycolic acid) (PLGA) knitted mesh for cartilage regeneration.[5] The knitted structure served as a reinforcing structure while collagen enhanced cell adhesion and tissue formation. Freeman et al. created poly(L-lactic acid) (PLLA) braid-twist scaffolds and showed that by adjusting the braiding and twisting angles, the mechanical properties of the created scaffolds could be tailored to simulate the biomechanical profile and mechanical properties of anterior cruciate ligament.[10] However, all these scaffolds were formed from lifeless fibers and cells had to be added after the fact. Thus, the ability to deliver cells inside of the scaffold and to control the distribution and organization of different cell types were lacking.
The incorporation of cells into the fibers prior to processing into a larger construct offers a tantalizing prospect of cell delivery both at the surface and deep within the construct with exquisite positional control while the construct is being shaped. Common methods to create cell-laden fibers include electro-spining,[11] wetspinning,[12] microfluidic spinning[6,13,14], and interfacial complexation.[15] Among these methods, microfluidic and wetspinning offer excellent control over the size, morphology, and biochemical composition of the fibers.[6,13,14] In a recent study, Oneo et al. developed a double-coaxial laminar flow microfluidic device to create meter-long functional microfibres with a mixture of extracellular matrix proteins and cells as the core and Ca-alginate as the shell.[6] The authors stated that handling of the woven structure was challenging and required a support made from another hydrogel, indicating that the mechanical strength of the fibers was not sufficient. By using great care and efforts, microfibers were assembled into cellular constructs that mimicked intrinsic morphologies and functions of living tissues by weaving and winding, highlighting the potential of living fibers in tissue engineering. For these techniques to gain traction, and be used widely and routinely, much more complex structures should be developed. Thus, living fibers that can be handled with ease and withstand the forces applied during weaving, winding, braiding, knitting, and embroidering are needed. Mechanically strong fibers will also be useful for making tissues that require high mechanical strength, such as connective tissues, vascular grafts, and bone.
Here, we introduce core-shell composite living fibers (CLFs) that comprise a mechanically rigid core and a soft shell made of a hydrogel seeded with living cells. CLFs easily withstand the mechanical constraints imposed by commonly used textile manufacturing processes including knitting, braiding, weaving, and embroidering. Indeed, CLFs possess the mechanical strength of the core thread made of single and multi-filaments, biodegradable, and non-biodegradable suturing threads, while providing a suitable environment for the proliferation of cells encapsulated within the hydrogel layer that were coated around the core thread. We assembled CLFs into 2D and 3D cell-laden constructs using the gamut of textile techniques, demonstrating the versatility of CLFs while confirming the integrity of the hydrogels and the viability of various cells. Using our approach a core material and a hydrogel comprising cells or drug microparticles can be freely combined, offering great freedom for designing scaffolds with tunable mechanical properties and programmable cell distribution.
2. Results and Discussion
2.1. CLF Fabrication
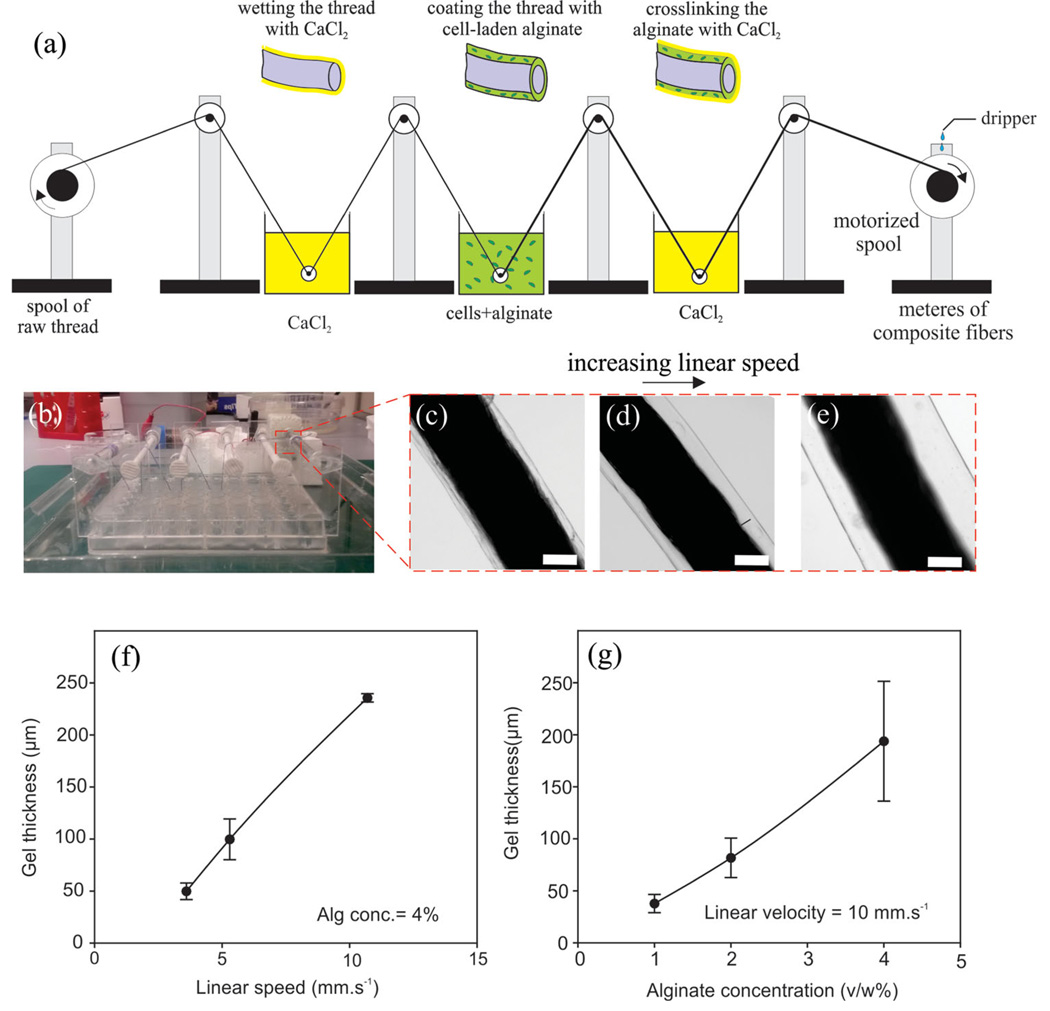
A schematic of the CLF manufacturing process and the actual fabricated device are shown in Figure 1a,b. Threads were steadily drawn through a sequence of baths containing calcium chloride (CaCl2) and Na-alginate prepolymers mixed with cells (or beads) using a motorized spool. The coating layer thickness was adjusted by changing the drawing speed (Figure 1c–e). The thickness of the hydrogel layer could be increased by increasing the drawing speed, which dragged along more solution, or by using a higher concentration (and viscosity) of Na-alginate (Figure 1f). The first CaCl2 bath wets the surface and fills the pores (for braided or twisted threads) by capillarity and promotes the gelation of Ca-alginate on the surface and the inside of the thread. The second CaCl2 bath completed the crosslinking of the Na-alginate by exchanging the Na+ with Ca+ to form a hydrogel network. A dripper loaded with tris-buffered saline (TBS) continuously rinsed the CLF at the collecting spool to rinse off excessive CaCl2 and ensure a humid environment (Figure 1a). We used alginate as an example to demonstrate our technology for engineering composite living fibers. Alginate is easy to use, gels quickly, and is approved by the FDA for certain applications. The concept of composite living fibers, however, is not limited to alginate and other types of hydrogels including photocrosslinkable materials such as methacrylated gelatin (GelMA, see further below) and thermally crosslinkable hydrogels such as collagen and gelatin can be used in this process. Moreover, alginate can be functionalized with RGD-containing cell adhesion ligands or can be blended with other biopolymers such as gelatin or collagen for applications, where cell spreading within 3D hydrogels is essential.[16,17]
Figure 1.
Fabrication of cell-laden composite living fibers (CLFs). a) Schematic of the experimental setup comprised of two baths of calcium chloride solution, a bath of cell suspension in sodium alginate, and a motorized spool. A dripper is designed to wash the excessive calcium ions from the threads and keep the threads in a wet condition. A biocompatible thread (e.g., braided suturing thread) is passed through the baths and is pulled by the motor leading to the formation of a cell-laden calcium alginate layer on the thread. More steps can be added to the setup to coat multiple layers of hydrogel containing different cell types or chemicals on a core thread. b) The coating device is in use. The size of the device is the same as a 48-well plate. c–e) Braided suturing threads coated with hydrogel at different drawing speed. Scale bars show 100 µm. f) Variation of the hydrogel layer thickness as a function of drawing speeds. g) Variation of the hydrogel layer thickness as a function of prepolymer concentration (viscosity). Error bars are standard deviation calculated from three independent measurements. The solid lines are a guide to the eye.
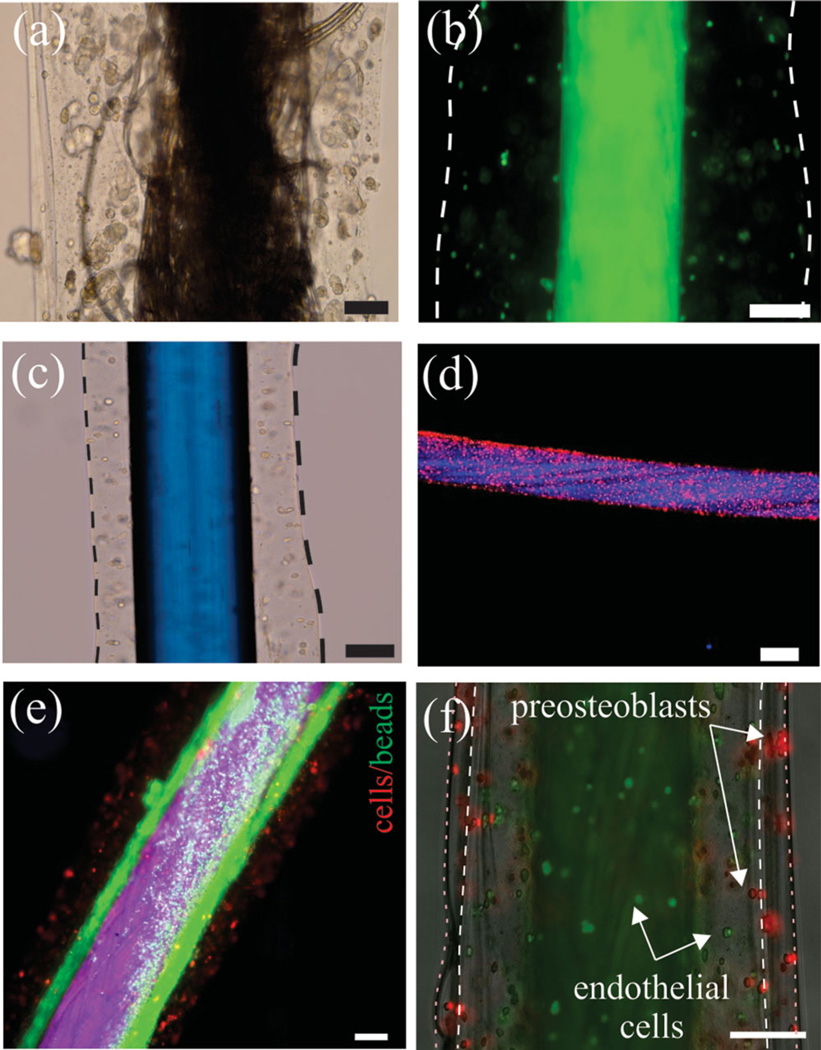
CLFs were made from multifilament twisted cotton, biodegradable suturing threads, including monofilament (polydioxanone), braided multifilament (polyglycolic acid), and gut thread (Figure 2a–d). Cells and beads were suspended in Na-alginate solutions before coating according to the protocol described in the experimental section. We noticed that the morphology, structure, and surface properties of the core thread governed the formation and uniformity of the hydrogel layer. Monofilament and braided suturing threads yielded homogeneous coatings compared to cotton threads, which comprise many loose filaments. We plasma treated non-absorbable polypropylene suturing threads to render their surface hydrophilic and to coat a uniform layer of hydrogel on their surface.
Figure 2.
Hydrogel coated threads. a) A commercially available cotton thread coated with HEK293 cells encapsulated in the Ca-alginate. b) An absorbable braided suturing thread coated with breast cancer cell-laden hydrogel. c) A non-absorbable monofilament suturing thread coated with HEK293 cell-laden hydrogel. The surface of the thread was plasma treated before the coating. d) A gut suture coated with HEK293 cell-laden hydrogel. The cells were stained with CellTracker for better visualization. e) Two layer coating on a core braided suturing thread. The inner layer containsed fluorescently labeled beads representing drug-loaded particles and the outer layer contained stained HEK293 cells. f) Two-layer coating of endothelial cells (green) and preosteoblasts (red) on a braided suturing thread. Due to lack of adhesion sites in alginate, cells did not spread and stayed rounded. Scale bars in all images show 100 µm.
Using our fabrication method, it was easy to make multilayer fibers simply by adding more reservoirs containing Na-alginate and CaCl2 to the process shown in Figure 1. We used this technique in one case to coat a braided suturing thread with a hydrogel layer comprising submicrometer beads (green) as a representative of microcapsules containing biomolecules followed by a layer containing human embryonic kidney (HEK293) (red), Figure 1e. In another example, a core thread was coated with human umbilical vein endothelial cells (HUVECs) covered by osteoblast precursor cells (MC3T3) (Figure 1f). These two cells are known to interact and promote the formation of vascularized bone tissues.[7] Using this method, it is possible to apply different cells and materials as separate layers onto a single fiber, which will be of interest for tissue engineering applications that require co-cultures of different cell types or drug releasing microcapsules.
2.2. Viability Assessment
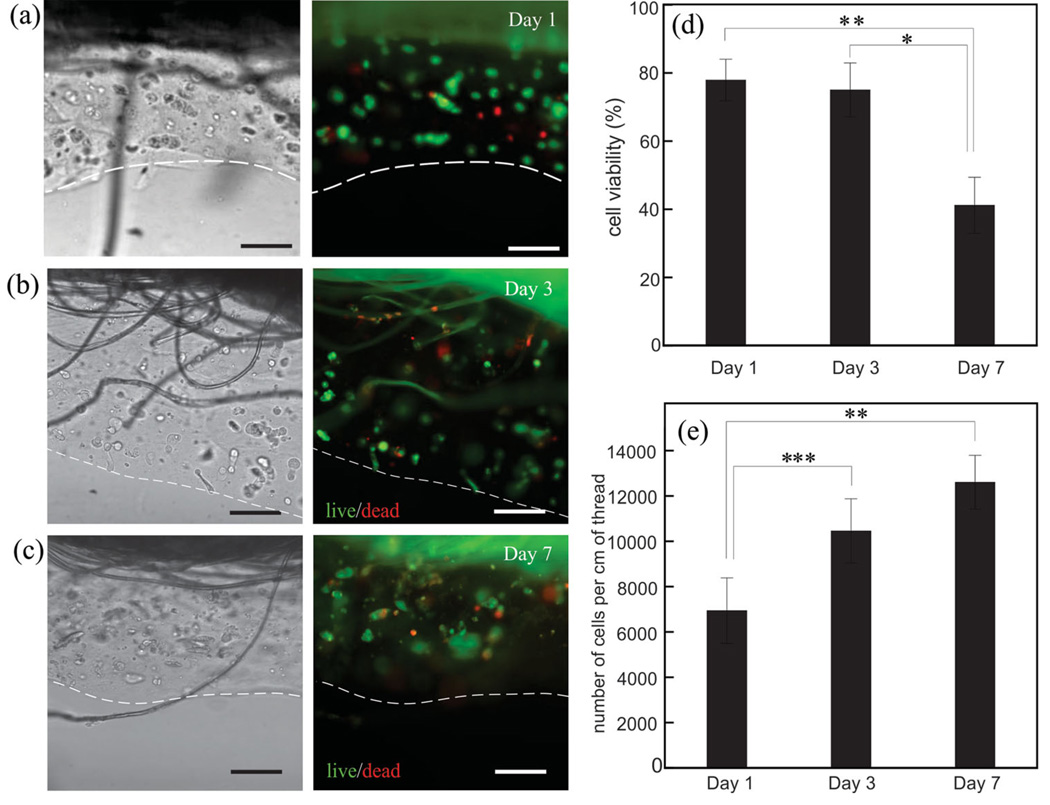
To assess the biocompatibility of our approach, we performed a live/dead cell assay and determined the cell viability using the percentage of live HEK293 cells with respect to the total cells over a period of seven days (Figure 3). Our results indicate that approximately 80% of the cells stayed alive during the first three days of incubation while the cell viability reduced to 40% at day seven (Figure 3d). The high percentage of live cells in the first three days suggests that the process of fabricating CLFs was harmless to the cells. The decrease in cell viability over time was expected as Ca-alginate hydrogel does not permit the cell anchorage. This data is consistent with other studies[16] and our results obtained from cells cultured in wetspun fibers as control group (Figure S2a, Supporting Information). To investigate cell proliferation in CLFs, we counted the total number of cells over the seven days of incubation. The linear density of cells (number of cells per cm of thread) increased from 6800 to 12 400 cells cm−1 between day 1 and day 7 of culture, demonstrating that the cells were proliferating (Figure 3e). Whereas initially cells proliferated rapidly, the lack of adhesion and the absence of migration limited the cell proliferation.
Figure 3.
Cell viability assay for assessing the biocompatibility of the process. HEK293 cell-laden gels were coated on a typical cotton thread and incubated for seven days. Bright field images of the thread (left) and the corresponding fluorescent images (right) after a) one, b) three, and c) seven days of incubation. Green cells are alive and the red ones are dead. Scale bars are 200 µm and the dashed lines indicate the edges of the fibers. Initial cell density for all the results was 4 ×106 cells mL−1. d) Percentage of viable cells over time. e) Number of cells over 7 days of incubation. The error bar is the standard deviation from three independent experiments. * p < 0.05, ** p < 0.01, and *** p < 0.001.
2.3. CLFs Assembly using Textile Techniques
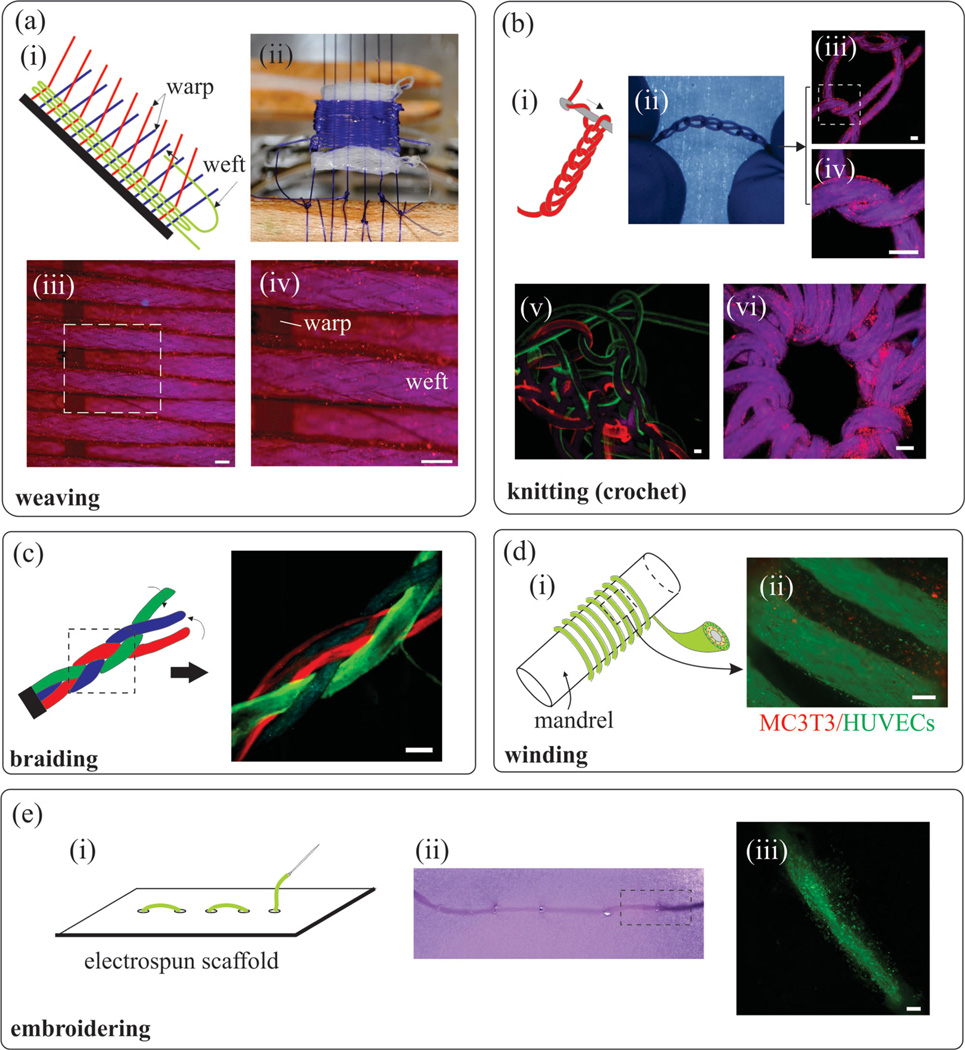
To test the compatibility of the CLFs with common textile techniques, complex 2D and 3D structures were made by weaving, braiding, knitting, winding, and embroidering under sterile condition. In weaving, warp threads were pre-stretched across the loom and the weft thread was woven across in the perpendicular direction. The diameter, strength, spacing, and the tension of both warp and weft can be used to tune the strength, porosity, morphology, and geometry of the fabric (Figure 4a–i). Woven structures are usually two-dimensional, lightweight, and flexible and have been used to make scaffolds for cartilage tissue engineering.[4,18] The microstructure of the woven construct can be tailored to meet the mechanical properties of the native tissue and enhance the transport of nutrients and oxygen to cells.[4] We modified an off-the-shelf manual weaving loom to weave CLFs (Figure S1a, Supporting Information). Two meters of HEK293 loaded CLFs (wefts) were passed through monofilament cell-free suturing threads (warps) to form a 1.5 cm × 2 cm woven structure (Figure 4a,ii–iv). The fabricated woven structure was mechanically stable and could easily be manipulated during weaving, and afterwards. Moreover, the hydrogel layer withstood all imposed mechanical forces during the weaving process and remained intact. These initial promising results suggest that it may be possible to scale up the process as well as to use automated weaving looms for a variety of applications, (Figure S1b, Supporting Information). An important point to consider is that the weaving process was conducted in a wet condition, which is not common for textile machinery.
Figure 4.
Living fabrics made by using threads coated with cell-containing hydrogels using the most common textile manufacturing processes. a) Woven cell-laden CLFs: i) Schematic of the weaving process. The weaving process includes passing weft threads through warp threads, which are separated by raising or lowering heddles. ii) The woven structure includes monofilament suturing thread as warp and multifilament braided suturing threads coated with HEK293 cells as weft. The white section of the woven fabric is cell-free cotton thread. The size of the woven structure is 1.5 cm by 2 cm. iii) Fluorescent image of the woven structure showing the cells (red) on weft threads and iv) close up view of the dashed box in (iii). b) Knitted structures: i) Schematic of the knitting (crochet) process (ii); iii) HEK293 cell (red)-laden hydrogels were coated on suturing thread and manually knitted to form a chain-like structure; iv) Close up view of the dashed box in (iii). v) A composite knitted structure including threads coated with red and green beads; vi) A donut shape knitted structure coated with HEK293 cells. c) Braided textiles formed from intertwining three gel-coated threads. The florescent image shows a braided structure made from one uncoated and two coated (red and green) threads. d) Winded thread around a mandrel: i) Schematic of a winded double layer thread; ii) A two-layer thread including MC3T3 and HUVEC cells winded around a mandrel. e) Embroidered pattern cell-laden threads on an electrospun PGS-PCL composite scaffold. i) Schematic of the process (top) and the structure (bottom); ii) Close up view of the dashed box in (i) under fluorescent microscope. Encapsulated cells are breast cancer cells. iii) Close up view of the dashed box in (ii) showing the stitch area. All scale bars show 200 µm.
Knitting creates structures by intertwining yarns or threads in a series of connected loops in 2D or 3D (Figure 4b,i) that enables creating complex patterns. Knitted structures provide mechanical strength both in-plane and across-plane, unlike woven structures that are 2D.[1] Knitted structures have found a wide range of applications in regenerative medicine as scaffolds for engineering cartilage,[19] skin,[20] bone,[21] ligament,[22] and vascular grafts.[23] We utilized our cell- and bead-laden CLFs to fabricate a multi colored 3D knitted fabric and a donut-like construct (Figure 4b,ii,vi). The latter construct had inner and outer diameters of 5 mm and 25 mm, respectively and was made with 1.5 m of CLF (Figure 4b,vi).
Braiding is the intertwining of three or more fiber strands (Figure 4c) and allows for making complex cylindrical structures or patterns. Braided scaffolds replicate both the geometrical dimension and mechanical properties of connective tissue, blood vessels, tendons, and ligaments[2,24]. Here, we coated three strands of CLFs loaded with sub-micrometer beads on the outside, and braided them manually into a triple stranded fiber.
Winding is commonly used in textile for thread storage on mandrels, but not as the final product. However, in tissue engineering, tissue constructs can be made by winding. Here, we fabricated tubular structures by winding a thread double-coated with MC3T3 cells and HUVECs on an 18 gauge syringe needle (diameter: 1.2 mm) serving as a mandrel. Figure 4d shows that the gel layer remained intact even after the winding process indicating that CLF could withstand bending with a short radius of curvature. The winded structure can be separated from the needle forming a 3D tubular construct, which could be used to form artificial blood vessels.[6]
In embroidery, threads are woven into an existing fabric, which is widely used to add decorative imagery to textiles such as caps and t-shirts. To illustrate the possibility of using CLFs for embroidering, the fibers were secured in a needle and manually sewn through an electrospun poly(glycerol sebacate) (PGS) and poly (ε-caprolactone) (PCL) scaffold (Figure 4e). A linear pattern was formed by stitching a composite thread containing human breast cancer (MDA-MB-231/GFP) (Figure 4e,ii), while preserving the integrity of the fibers and the scaffold (Figure 4e,iii). These results indicated that it may be possible to embroider larger structures with different types of threads on preformed biocompatible scaffolds.
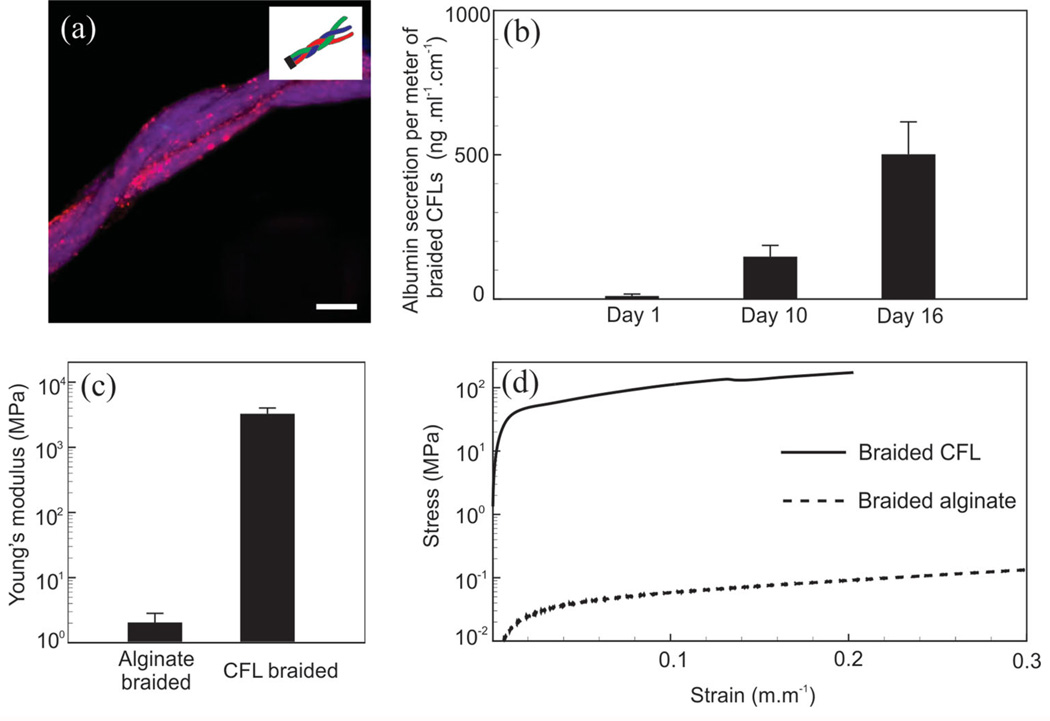
Textile techniques facilitate the co-culture of various cells in predefined patterns. However, one major concern is the cellular viability and function after the assembly process. To investigate the cellular activity after CLFs assembly, we created CLFs containing HUVECs, NIH 3T3 fibroblasts, and HepG2 hepatocytes and braided them to form a co-culture of these cells as a representative model of liver (Figure 5a). We incorporated GelMA into Ca-alginate layer to provide cells with binding sites to help with maintaining their viability and activity over the culture time. GelMA is derived from denatured collagen, which is an abundant protein found in the human body. Adding GelMA to the Ca-alginate matrix improved the adhesion of cells and enhanced their functional activities (see Figure S2b–d, Supporting Information). The fabricated braided structures were kept in culture for 16 days and cellular activity was confirmed by observing a significant increase in the measured secreted albumin (Figure 5b).
Figure 5.
Braided cell-laden structures. a) CLFs loaded with HUVECs, NIH 3T3 fibroblasts, and HepG2 hepatocytes were braided to form a liver model. Cells were stained with CellTracker (red) for clarity. b) Secreted albumin from the braided structure over 16 days of culture. c) Young’s modulus of braided CLFs and wetspun fibers. d) Stress/strain curve for braided CLFs and wetspun fibers. Error bars are standard deviation calculated from three independent samples. Scale bar shows 200 µm.
A key advantage of CLFs is the possibility of tuning the mechanical properties of the fibers and the constructs. We formed wetspun alginate fibers (4% w/v, 1 mm in diameter) and composite reinforced fibers with a core of braided suture thread (200 µm in diameter), a shell of Ca-alginate (2% v/w, 200 µm thick) and assembled them into braided structures. We measured the tensile properties of the alginate and CLFs individually. The Young’s modulus of alginate fiber was 3.72 ± 0.62 MPa in comparison with 10 495 ± 1719 MPa for CLFs. The significant difference between the mechanical properties of hydrogel and CLFs is due to the load bearing characteristics of the core layer. Thus, it is expected that the mechanical properties of the tissue construct can be tuned by selecting different core materials. The mechanical properties of braided structures fabricated using alginate and CLFs with PGA core were 1.98 ± 0.75 MPa and 3162 ± 706 MPa, respectively. These values indicate that a wide range of mechanical properties that can be achieved by assembly of fibers using braiding. The same principle is most certainly extendible to the other textile techniques discussed in this paper.
3. Conclusion
Textile technologies have been widely utilized in tissue engineering, but have been mostly limited to the fabrication of reinforcing structures and polymeric tissue scaffolds. Here, we introduced the fabrication of CLFs, which comprise of load bearing core fiber and a sheath of cell-laden hydrogel. Continuous reel-to-reel fabrication of CLFs was demonstrated by drawing a core fiber through multiple reservoirs containing cell-laden prepolymer (Na-alginate) and crosslinking reagents (CaCl2). We characterized the fabrication process and showed that by adjusting the drawing speed and the prepolymer concentration, hydrogel layers with the thickness of 50–250 µm could be made. Moreover, by passing the core fibers through multiple reservoirs, each containing a mixture of Na-alginate and a different cell, a co-culture of different cells on the same fiber was demonstrated. The biocompatibility of the CLF fabrication process was confirmed by a cellular viability assessment. Cell viability was high after the manufacturing process. Results using alginate:GelMA fibers suggest that CLFs promoting cell adhesion, proliferation, and differentiation could be fabricated.
The fibers were assembled using all major textile processes including weaving, knitting, braiding, and embroidering under sterile conditions. Our results indicate that the adhesion of the gel layer to the core thread was strong enough to withstand the mechanical loads imposed during the textile assembly processes. We braided three different CLFs containing NIH 3T3 cells, HepG2 cells, and HUVECs, respectively as a model for liver. The structures were incubated for 16 days and the concentration of albumin secreted by cells was determined as a measure for cellular activity.
The concept of CLFs is versatile and maybe applicable to a wide range of biocompatible materials including photo- (e.g., GelMA) and thermally- (e.g., collagen) crosslinkable polymers. In addition, the CLFs formation and tissue assembly processes can be completed in a matter of hours, therefore hydrolytically degradable polymers such as PGA and PLLA might also be used as core materials. We believe that CLF will serve as a building block for designing and making advanced tissue engineering constructs with complex architectures by combining multiple CLFs and using well-established textile manufacturing technologies.
4. Experimental Section
Materials
Sodium alginate, calcium chloride (CaCl2), ethylene diamine tetra acetic acid (EDTA), type-A porcine skin gelatin, and methacrylic anhydride (MA) were purchased from Sigma Aldrich (St. Louis, MO, USA). A photoinitiator (PI) was purchased from Irgacure 2959. Dulbecco's modified Eagle medium (DMEM), Alpha Minimum Essential Medium (MEM Alpha) fetal bovine serum (FBS), 0.05% trypsin-EDTA (1×), and antibiotics (Penicillin/Streptomycin) were purchased from Invitrogen (Burlington, ON, Canada). A final DMEM solution was prepared with 10% FBS and 1% antibiotics (penicillin/streptomycin). LIVE/DEAD Viability/Cytotoxicity Kit, for mammalian cells was purchased from Invitrogen (Burlington, ON, Canada). Other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) unless mentioned otherwise.
Sodium alginate was dissolved in distilled water at a desired concentration. The solution was stirred overnight at 40 °C then stored at 4 °C and used for experiments for up to 2 months. Calcium chloride was dissolved in tris-buffered saline (TBS) with a 2% (w/v) concentration and its pH was adjusted to 7.0. As a chelating solution, a solution of 20 mm EDTA in 1× phosphate buffer saline (PBS) was made and stored at 4 °C. We dissolved type-A porcine skin gelatin (Sigma–Aldrich) in Dulbecco’s phosphate buffered saline (DPBS) (GIBCO) and kept it at 60 °C to make a uniform gelatin solution with desired concentration. Methacrylic anhydride was slowly added to the gelatin solution under stirring conditions. Unreacted MA and additional chemicals were removed from the solution by dialyzing the solution against deionized water using 12–14 kDa cut-off dialysis tubes (Spectrum Laboratories) for 7 days at 50 °C. The solution was freeze-dried at − 80 °C, under vacuum, and stored at room temperature. All solutions were sterilized and filtered by a 0.22 µm filter and degassed by placing them in a vacuum desiccators connected to a vacuum pump to remove bubbles.
Threads
Absorbable surgical suturing threads were purchased from SouthPointe Surgical (SoutPoint Surgical Supply, Inc., FL, USA). We used monofilament (MON-OASIS; material: polydioxanone; absorbs by hydrolysis in 180–220 days; size: 2–0 and 3–0), braided (VI-OASIS; material: polyglycolic acid; absorbs by hydrolysis in 60–90 days; size: 2–0 and 3–0), plain gut (GUT-OASIS; material: natural purified collagen; size: 2–0) suturing threads. Non absorbable suturing threads that were made from cotton threads (100% Cotton yarn Ne 30/1, Mahabad Riss Co, Iran) were used as received.
Fabrication of Electrospun Scaffolds
PGS–PCL sheets with different compositions were spun using a conventional electrospinning set-up. Fibers were collected on an aluminum foil coated with a layer of mineral oil. We dissolved PGS and PCL with the ratio of 3:1 in an anhydrous chloroform: ethanol (9:1) mixture and electrospun the fibers at 20 kV. The total polymer concentration was kept constant at 33% w/v. Other parameters, such as the distance between the needle and the collector (18 cm), flow rate (2 mL h−1) and needle diameter (21G) were kept constant during fiber fabrication. The scaffolds were spun for 20 min and dried overnight in a desiccator to remove any remaining solvent prior to further use.
Fabrication of Wetspun Living Fibers
We followed a conventional wetspinning process in which Na-alginate (4% w/v) was mixed with flourescent microbeads or cells and then injected in a bath of CaCl2 using a syringe pump. Upon injection Ca-alginate is formed in a matter of seconds which is insoluble in water. The size of the fibers can be controlled by changing the size of the injection needle as well as the viscosity of the solution. Here, we used 25G needles and an injection flow rate of 2 mL min−1, which resulted in the formation of fibers with 0.8 mm diameter.
Cell Culture
Human embryonic kidney (HEK293), human breast cancer (MDA-MB-231/GFP), green flourescent protein (GFP)-expressing human umbilical vein endothelial cells (HUVECs), mouse fibroblast cells (NIH-3T3), human hepatocytes (HepG2), and osteoblast precursor cell line derived from Musmusculus (mouse) calvaria (MC3T3) were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). All cell lines were grown in 5% CO2 at 37 °C in tissue culture polystyrene flasks. HEK293, MDA- MB-231/GFP, NIH-3T3, and HepG2 cells were cultured in DMEM containing 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin/streptomycin). HUVEC cells were cultured in endothelial cells basal medium (EBM-2; Lonza) supplemented with endothelial growth BulletKit (EGM-2; Lonza), 2% fetal bovine serum, vascular endothelial growth factor (VEGF). MC3T3 cells were cultured in MEM Alpha with ribonucleosides, deoxyribonucleosides, 2 mm L-glutamine and 1 mm sodium pyruvate. All cells were passaged every 3 days and cells passages 10–20 were used during all experiments. Cell medium was replaced every 3 days with fresh cell medium for long incubation times.
Preparation of Cell Suspension in Sodium Alginate
Cells that reached 80–90% confluency were trypsinized, resuspended in corresponding cell media, and mixed with 4% sodium alginate with a ratio of 1 to 7 to reach final cell concentrations in the range of 2–5 × 106 cells mL−1. For better visualization of structures formed by reinforced cell-laden fibers, HEK293 cells were stained with CellTracker (Invitrogen, Burlington, ON, Canada) according to the manufacturer’s protocol before their suspension in sodium alginate.
Preparation of Cell Suspension in Sodium Alginate:GelMA Mixture
To produce alginate:GelMA fibers, we dissolved the freeze-dried GelMA and Na-alginate power, and PI in DPBS at desired final concentrations. We used 0.5% (w/v) of photoinitiator for all experiments. To create CLFs hydrogels, we used alginate:GelMA (2%:10% w/v) mixture in our coating device and to coat a layer of hydrogel on the surface of the threads. We then expose the CLFs to UV for 30 s to crosslink GelMA and form an hydrogel.
Microscopy Analysis
Microscopy images were taken using a Zeiss Axiovert 40CFL (Carl Zeiss Canada Ltd., Toronto, ON, Canada) and an inverted Nikon TE2000 microscope (TE2000, Nikon, Saint-Laurent, QC, Canada). Images were recorded using a cooled CCD camera (Photometrics CoolSNAP HQ2) connected to the microscope. Black and white images were colored and merged using the software ImageJ 1.45 s (National Institutes of Health Maryland).
Tensile Test
CLFs and hydrogel fibers were fabricated and cut into pieces of 100 mm long. The samples were kept in PBS and their tensile properties were measured using an Instron 5542 mechanical tester. The fibers were sandwiched between the grips and were stretched at a constant strain rate of (0.1 mm min−1). The fibers were kept hydrated using an ultrasonic humidifier during the test, four samples for each type was tested. Similarly, CLFs and wetspun alginate fibers were manually braided into approximately 100 mm long pieces. The braid fibers were tested using the same procedure.
Supplementary Material
Acknowledgements
Financial support from NSERC, CIHR, CHRP, CFI, Genome Canada, and Genome Quebec is gratefully acknowledged. M.A. and A.T. acknowledge NSERC Postdoctoral fellowships. D.J. acknowledges support from a Canada Research Chair. A.K. acknowledges funding from the National Science Foundation CAREER Award (DMR 0847287), the office of Naval Research Young National Investigator Award, and the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
M.A. and A.T. contributed equally to this work.
The authors declare no conflict of interests in this work.
Contributor Information
Mohsen Akbari, McGill University and Genome Quebec Innovation Centre, McGill University, Montreal, Quebec, H3A 0G1, Canada; Biomedical Engineering Department, McGill University, Montreal, Quebec, H3A 2B4, Canada; Center for Biomedical Engineering, Department of Medicine, Brigham and Womenís Hospital, Harvard Medical School, Boston, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115, USA.
Ali Tamayol, McGill University and Genome Quebec Innovation Centre, McGill University, Montreal, Quebec, H3A 0G1, Canada; Biomedical Engineering Department, McGill University, Montreal, Quebec, H3A 2B4, Canada; Center for Biomedical Engineering, Department of Medicine, Brigham and Womenís Hospital, Harvard Medical School, Boston, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Veronique Laforte, McGill University and Genome Quebec Innovation Centre, McGill University, Montreal, Quebec, H3A 0G1, Canada; Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, H3A 2B4, Canada.
Nasim Annabi, Center for Biomedical Engineering, Department of Medicine, Brigham and Womenís Hospital, Harvard Medical School, Boston, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115, USA.
Alireza Hassani Najafabadi, Center for Biomedical Engineering, Department of Medicine, Brigham and Womenís Hospital, Harvard Medical School, Boston, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Ali Khademhosseini, Center for Biomedical Engineering, Department of Medicine, Brigham and Womenís Hospital, Harvard Medical School, Boston, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115, USA; Department of Physics, King Abdulaziz University, Jeddah 21569, Saudi Arabia; Department of Maxillofacial Biomedical Engineering and Institute of Oral Biology, School of Dentistry, Kyung Hee University, Seoul 130-701, Republic of Korea.
David Juncker, McGill University and Genome Quebec Innovation Centre, McGill University, Montreal, Quebec, H3A 0G1, Canada; Biomedical Engineering Department, McGill University, Montreal, Quebec, H3A 2B4, Canada; Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, H3A 2B4, Canada.
References
- 1.Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D. Biotechnol. Adv. 2013;31:669. doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Biomaterials. 2005;26:1523. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y-t, Haftel VK, Kumar S, Bellamkonda RV. Biomaterials. 2008;29:3117. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moutos FT, Freed LE, Guilak F. Nat. Mater. 2007;6:162. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 5.Dai W, Kawazoe N, Lin X, Dong J, Chen G. Biomaterials. 2010;31:2141. doi: 10.1016/j.biomaterials.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 6.Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K. Nat. Mater. 2013;12:584. doi: 10.1038/nmat3606. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, Kang Y, Yang Y, Khademhosseini A. Tissue Eng. Part B: Rev. 2012;18:363. doi: 10.1089/ten.teb.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Fan H, Wang Y, Toh SL, Goh JCH. Biomaterials. 2008;29:662. doi: 10.1016/j.biomaterials.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Annabi N, Mithieux SM, Boughton EA, Ruys AJ, Weiss AS, Dehghani F. Biomaterials. 2009;30:4550. doi: 10.1016/j.biomaterials.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Freeman JW, Woods MD, Cromer DA, Ekwueme EC, Andric T, Atiemo EA, Bijoux CH, Laurencin CT. J. Biomech. 2011;44:694. doi: 10.1016/j.jbiomech.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 11.a) Townsend-Nicholson A, Jayasinghe SN. Biomacromolecules. 2006;7:3364. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]; b) Shih Y-H, Yang J-C, Li S-H, Yang W-CV, Chen C-C. Textile Res. J. 2012;82:602. [Google Scholar]
- 12.a) Arumuganathar S, Jayasinghe SN. Biomacromolecules. 2008;9:759. doi: 10.1021/bm701322k. [DOI] [PubMed] [Google Scholar]; b) Puppi D, Dinucci D, Bartoli C, Mota C, Migone C, Dini F, Barsotti G, Carlucci F, Chiellini F. J. Bioactive Compatible Polym. 2011;26:478. [Google Scholar]
- 13.Kang E, Jeong GS, Choi YY, Lee KH, Khademhosseini A, Lee S-H. Nat. Mater. 2011;10:877. doi: 10.1038/nmat3108. [DOI] [PubMed] [Google Scholar]
- 14.Ghorbanian S, Qasaimeh MA, Akbari M, Tamayol A, Juncker D. Biomed. Microdevices. 2014 doi: 10.1007/s10544-014-9842-8. [DOI] [PubMed] [Google Scholar]
- 15.a) Leong MF, Toh JK, Du C, Narayanan K, Lu HF, Lim TC, Wan AC, Ying JY. Nat. Commun. 2013 doi: 10.1038/ncomms3353. [DOI] [PubMed] [Google Scholar]; b) Yim EKF, Wan ACA, Le Visage C, Liao IC, Leong KW. Biomaterials. 2006;27:6111. doi: 10.1016/j.biomaterials.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Rowley JA, Madlambayan G, Mooney DJ. Biomaterials. 1999;20:45. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 17.Alsberg E, Anderson K, Albeiruti A, Franceschi R, Mooney D. J. Dental Res. 2001;80:2025. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 18.Moutos FT, Guilak F. Biorheology. 2008;45:501. [PMC free article] [PubMed] [Google Scholar]
- 19.Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, Caplan AI, Guilak F, Freed LE. Biomaterials. 2010;31:2193. doi: 10.1016/j.biomaterials.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Li Q, Hu X, Ma L, You C, Zheng Y, Sun H, Han C, Gao C. J. Mech Behavior Biomed. Mater. 2012;8:204. doi: 10.1016/j.jmbbm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Rentsch B, Hofmann A, Breier A, Rentsch C, Scharnweber D. Ann. Biomed. Eng. 2009;37:2118. doi: 10.1007/s10439-009-9731-0. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo S, Cho-Hong JG, Siew-Lok T. Biomed. Mater. 2007;2:169. doi: 10.1088/1748-6041/2/3/001. [DOI] [PubMed] [Google Scholar]
- 23.Ananta M, Aulin CE, Hilborn J, Aibibu D, Houis S, Brown RA, Mudera V. Tissue Eng. Part A. 2008;15:1667. doi: 10.1089/ten.tea.2008.0194. [DOI] [PubMed] [Google Scholar]
- 24.Fang Q, Chen D, Yang Z, Li M. Mater. Sci. Eng. C. 2009;29:1527. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.