Summary
Memory CD8+ T cells are programmed during the primary response for robust secondary responsiveness. Here we show that CD8+ T cells responding to different epitopes of influenza virus received qualitatively different signals during the primary response that altered their secondary responsiveness. Nucleoprotein (NP)-specific CD8+ T cells encountered antigen on CD40-licensed, CD70-expressing, CD103−CD11bhi dendritic cells (DCs) at later times in the primary response. As a consequence, they maintained CD25 expression and responded to interleukin-2 (IL-2) and CD27, which together programed their robust secondary proliferative capacity and interferon-γ (IFN-γ)-producing ability. In contrast, polymerase (PA)-specific CD8+ T cells did not encounter antigen-bearing, CD40-activated DCs at later times in the primary response, did not receive CD27 and CD25 signals and were not programmed to become memory CD8+ T cells with strong proliferative and cytokine-producing ability. As a result, CD8+ T cells responding to abundant antigens, like NP, dominated the secondary response.
INTRODUCTION
The generation of memory CD8+ T cells that rapidly expand after secondary challenge is essential for sustained anti-viral immunity. Dendritic cells (DCs) prime naïve T cell responses and early studies suggest that a brief encounter between naïve T cells and antigen-bearing DCs is sufficient to trigger their differentiation into effector and memory CD8+ T cells without additional stimulation (Kaech and Ahmed, 2001; van Stipdonk et al., 2001). Later studies, however, show that repeated encounters with antigen-bearing DCs are important for optimal primary CD8+ T cell responses (McGill et al., 2008; Zammit et al., 2006) and that responding CD8+ T cells are conditioned to become functional memory cells during the contraction phase of the primary immune response, a phenomenon termed memory programming(Kaech and Wherry, 2007; Teixeiro et al., 2009; Williams et al., 2006).
The cellular and molecular basis of memory programming is not entirely understood, but is thought to require CD4+ T cell help (Shedlock and Shen, 2003; Sun and Bevan, 2003), IL-2 signaling through CD25 (Williams et al., 2006), engagement of CD27 by its ligand, CD70 (Hendriks et al., 2000) and, in some cases, interactions between CD40 and its ligand, CD154 (Borrow et al., 1996; Lee et al., 2003). In fact, the licensing of CD40- expressing DCs by CD154-expressing CD4+ T cells can be a major component of help for primary CD8+ T cell responses against some pathogens as well as non-replicating antigens due to the ability of CD40 to activate DCs (Bennett et al., 1998; Ridge et al., 1998; Schoenberger et al., 1998), and due to its ability to prevent regulatory T (Treg) cell mediated suppression (Ballesteros-Tato et al., 2013). However, primary responses to some pathogens appear to bypass the requirement for CD4 and CD40 help (Borrow et al., 1998; Hamilton et al., 2001; Whitmire et al., 1996), possibly due to direct activation of DCs through pathogen recognition receptors. Nevertheless, even when primary CD8+ T cell responses do not require CD40 signaling, memory CD8+ T cell responses are often severely impaired in Cd40−/− or Cd154−/− mice (Borrow et al., 1998), in part because of CD40-dependent expression of CD70, which engages CD27 on T cells and promotes memory CD8+ T cell programming (Feau et al., 2012; Hendriks et al., 2000).
Here we show that influenza nucleoprotein (NP)-specific and polymerase (PA)-specific memory CD8+ T cells differentially utilize the IL-2:CD25, CD70:CD27 and CD40:CD154 signaling pathways. NP-specific memory T cells have prolonged interactions with CD40-licensed, antigen-bearing DCs, maintain CD25 expression for up to 10 days after infection and utilize CD70:CD27 interactions for programming. In contrast, PA-specific CD8+ T cells concluded their interactions with antigen-bearing DCs and downregulate CD25 expression prior to day 6 after infection. As a result, PA-specific CD8+ T cells do not engage CD40-licensed, CD70-expressing DCs during the late phase of the primary response and fail to differentiate into fully programmed memory cells with robust secondary proliferative capacity. Thus, CD8+ T cells of different specificities, even during the same infection, receive qualitatively distinct sets of signals during the late phase of the primary response resulting in differential memory programming. These differences strongly impact the immunodominance hierarchy of the secondary response and may represent a mechanism to enhance the fitness of the memory T cell responses.
RESULTS
NP-specific, but not PA-specific CD8+ T cell expansion requires CD40 signaling
To determine the role of CD40 signaling in primary CD8+ T cell responses to influenza, we infected WT and Cd40−/− mice with A/PR8/34 (PR8) influenza virus and followed the kinetics of NP and PA-specific CD8+ T cell accumulation in the mediastinal lymph nodes (mLNs). We found that the initial (day 7) NP-specific CD8+ T cell response was similar WT and Cd40−/− mice (Fig 1a). However, NP-specific CD8+ T cells continued to expand through day 10 in WT mice, whereas they contracted in Cd40−/− mice (Fig. 1a). In contrast, PA-specific CD8+ T cells expanded equivalently in WT and Cd40−/− mice through day 7 and thereafter contracted equivalently in both groups (Fig. 1b). Thus, CD40-deficiency altered the kinetics of the primary CD8+ T cell response to NP, but not that of PA (Fig. 1c). Importantly, the differences in T cell accumulation did not appear to be due to altered proliferation, as NP-specific and PA-specific CD8+ T cells incorporated 5-ethenyl-2’-deoxyuridine (EdU) at similar rates in WT and Cd40−/− at all times tested (Fig. 1d).
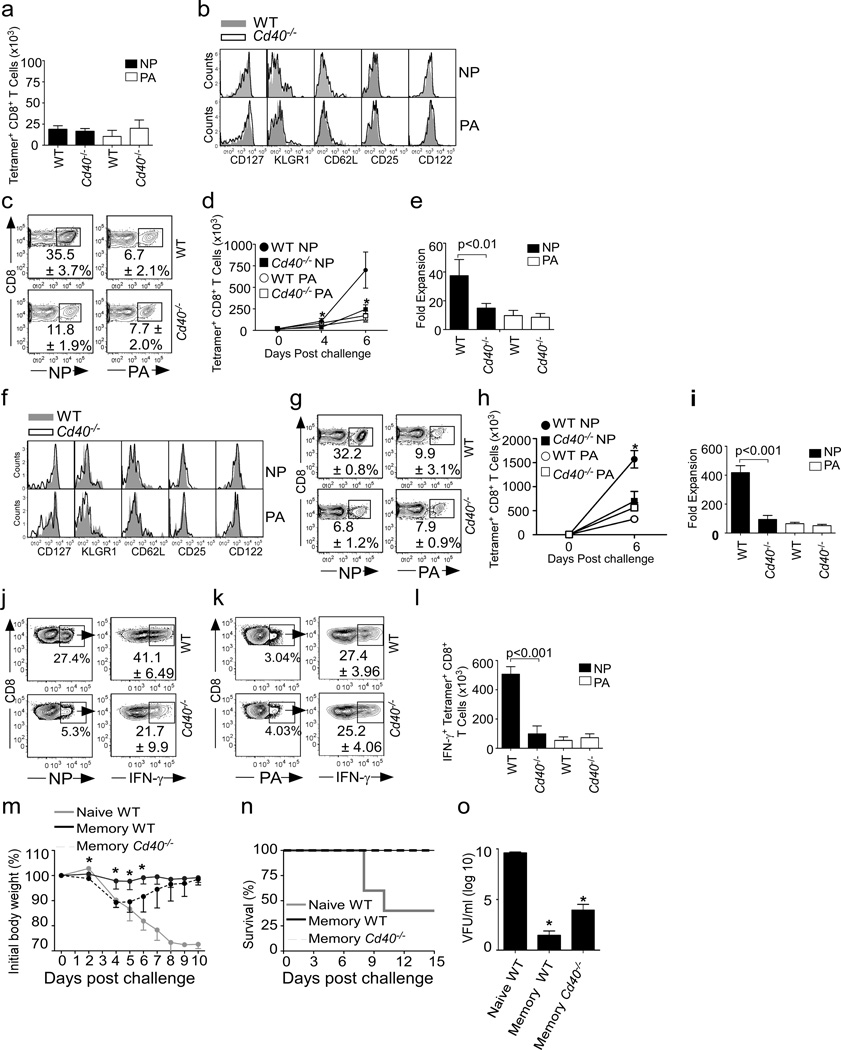
Figure 1. CD40 signaling delays the contraction of NP-specific CD8+ T cells.
WT and Cd40−/− mice were infected with PR8 and the frequencies of NP-specific (a) and PA-specific (b) CD8+ T cells as well as the numbers of NP-specific and PA-specific CD8+ T cells (c) in the mLNs are shown. Data are representative of 3 experiments (mean ± s.d of 5 mice per group)(*p<0.005). (d) WT and Cd40−/− mice were injected with 0.5 mg of 5-ethynyl-2'-deoxyuridine every 6 hours starting 24 hours before sacrifice and the frequency of EdU+ cells among NP and PA-specific CD8+ T cells in the mLNs are shown. Data are representative of 3 experiments (mean ± s.d of 4–5 mice). (e–f) C57BL/6 mice were infected with PR8 and treated with 250 µg of the CD154-blocking antibody, MR1, or control antibody on day 0 or day 5 after infection. NP-specific (e) and PAspecific (f) CD8+ T cells were enumerated on day 10 in mLNs. Data are representative of 3 experiments (mean ± s.d of 5 mice per group). P values were determined using a twotailed Student´s t-test.
To directly test whether CD40 signaling was important for the initial priming of NP-specific CD8+ T cells or to delay the contraction phase, we treated WT mice with control antibody or MR1 (anti-CD154) at the time of infection or 5 days later and measured CD8+ T cell responses on day 10. We found that MR1 treatment starting on day 0 or day 5 resulted in equivalent reductions in NP-specific CD8+ T cells (Fig. 1e). In contrast, we observed no differences in the accumulation of PA-specific CD8+ T cells (Fig. 1f). These results showed that the late expansion of NP-specific CD8+ T cell response was compromised in the absence of CD40 signaling, regardless of whether initial priming occurred in a CD40 sufficient environment and further demonstrated that NP and PA-specific CD8+ T cell responses have differential requirements for CD40 signaling.
CD40 signaling programs NP-specific memory CD8+ T cells
To determine whether the altered primary response in Cd40−/− mice impacted the differentiation of influenza-specific memory CD8+ T cells, we first enumerated NP and PA-specific memory cells in WT and Cd40−/− mice 8 weeks after infection. We found that despite the differences in the primary response, the number (Fig. 2a) and phenotype (Fig. 2b) of NP-specific memory CD8+ T cells were similar in WT and Cd40−/− mice prior to secondary infection and were indistinguishable from the number and phenotype of the PA-specific memory CD8+ T cells (Fig. 2a–b).
Figure 2. NP-specific memory CD8+ T cell responses require CD40.
a–b. WT and Cd40−/− mice were infected with PR8 and the number (a) and phenotype (b) of NP-specific and PA-specific CD8+ T cells in the lungs are shown at 8 weeks. Data are representative of 3 experiments (mean ± s.d of 5 mice per group). c–e WT and Cd40−/− mice were infected with PR8 and challenged with X31 8 weeks later. The frequencies (c) and total numbers (d) of NP-specific and PA-specific CD8+ T cells in the lungs are shown. Data are representative of 3 experiments (mean ± s.d of 5 mice per group)(*p<0.005). (e) The relative expansion of NP- and PA-specific CD8+ T cells in the lungs of C57BL/6 and Cd40−/− mice was calculated on day 7. Data are representative of 3 experiments (mean ± s.d of 5 mice per group). (f) WT and Cd40−/− mice were infected with PR8 and the phenotype of NP-specific and PA-specific CD8+ T cells in the lungs was determined 100 days later. (g–i) WT and Cd40−/− mice were infected with PR8, challenged with X31 100 days later and the frequencies (g) and total numbers (h) of NPspecific and PA-specific CD8+ T cells in the lungs are shown (*p<0.005). (i) The fold expansion of NP- and PA-specific CD8+ T cells in the lungs of C57BL/6 and Cd40−/− mice was calculated on day 6. The frequency (j–k) and number (l) of IFN-γ producing cells among either WT or Cd40−/− NP and PA-specific CD8+ T cells were determined by intracellular staining and tetramer co-staining after re-stimulation with NP366–374 peptide (j and l) or PA224–233 peptides (k and l) 6 days after challenge. Data are representative of 2 experiments (mean ± s.d of 4-5 mice per group). (m–o) WT and Cd40−/− mice were infected with 500 EIU of X31 and challenged with 5000 EIU PR8 8 weeks later. As a control, naïve WT mice were infected with 5000 EIU PR8. Weight loss (m) and survival (n) are shown. Viral titers in the lungs were determined at day 6 (o). Data are representative of 2 experiments (mean ± s.d of 5-10 mice per group)(*p<0.05). P values were determined using a two-tailed Student´s t-test.
To test whether there were functional differences in the populations of memory cells, we infected WT and Cd40−/− mice with PR8, allowed memory cells to develop for 8 weeks, and challenged the memory mice with influenza A/HK-X31 (X31). Since PR8 and X31 viruses express different HA and NA subtypes (H1N1 in PR8, H3N2 in X31), antibodies generated to PR8 do not neutralize X31. However, the genome segments encoding NP and PA are identical in PR8 and X31 (Baez et al., 1980). Thus, memory T cells generated following infection with one virus will response to challenge infection with the other virus. We found that the secondary expansion of NP-specific CD8+ T cells was impaired in Cd40−/− mice, whereas the secondary expansion of PA-specific CD8+ T cells was similar in WT and Cd40−/− mice (Fig. 2c–e). Interestingly, the impaired NP-specific CD8+ T cell response in Cd40−/− mice was similar to the “normal” PA-specific CD8+ T cell response in WT mice when expressed as total numbers (Fig. 2d) or as fold-expansion from resting memory cells (Fig. 2e). Similar results were obtained when memory cells were allowed to develop for 100 days prior to X31 re-challenge (Fig. 2f–i), or when NP and PA-specific memory CD8+ T expansion was evaluated in the mLN (Supplementary Fig. 1a and b).
Given that the ability to produce interferon-γ (IFN-γ) is another hallmark of properly programmed memory CD8+ T cells (Williams et al., 2006), we next analyzed the capacity of NP-specific and PA-specific memory CD8+ T cells from WT and Cd40−/− mice to produce IFN-γ. Cells from the lungs of WT and Cd40−/− mice were stimulated with NP366–374 or PA224–233 peptides 6 days after secondary challenge and the frequency of IFN-γ- producing, NP and PA-specific CD8+ T cell populations was determined by combining tetramer and intracellular cytokine staining (Dimopoulos et al., 2009). We found that more than 40% of the NP-specific memory CD8+ T cells from WT mice made IFN-γ, but only 21% of the NP-specific CD8+ T cells from Cd40−/− mice made IFN-γ (Fig. 2j). In contrast, only 27% of the PA-specific memory CD8+ T cells from WT mice made IFN-γ, similar to the frequency in cells from Cd40−/− mice. (Fig. 2k) These differences were magnified when calculated as total numbers (Fig. 2l). Thus, the lack of CD40 signaling impaired both the secondary proliferative capacity and the IFN-γ-producing ability of NP-specific memory CD8+ T cells, whereas these characteristics were already impaired in PA-specific memory CD8+ T cells from WT mice and, as a result, the loss of CD40 had little impact.
To determine whether CD40-deficiency affected the ability of CD8+ T cells to protect against a lethal challenge, we infected WT and Cd40−/− mice with a sublethal dose of X31, allowed them to recover for 8 weeks, and then challenged the memory mice as well as naive WT mice with a normally lethal dose of PR8. As expected, naïve WT mice rapidly lost weight (Fig 2m) and 60% of the animals succumbed to infection (Fig 2n). By contrast, all WT and Cd40−/− memory mice were protected from lethal challenge (Fig 2. n). However, WT memory mice lost almost no weight after challenge infection (Fig 2m), whereas Cd40−/− memory mice lost nearly 12% body weight over the first 5 days after infection and did not recover until after day 8. Consistent with the severity of the weight loss, we found high viral titers in the lungs of all naïve WT mice, very low titers in memory WT mice and slightly increased titers in memory Cd40−/− mice on day 6 after challenge (Fig 2o).
We next determined whether CD40 signaling during the primary response was required to program functional memory NP-specific CD8+ T cells. Therefore, we sorted total CD44hiCD8+ memory T cells from WT (Fig. 3a) and Cd40−/− mice (Fig 3b) 8 weeks after primary PR8 infection and adoptively transferred equivalent numbers of NP-specific CD8+ T cells (CD45.2) into naïve CD45.1 recipient mice. We challenged recipients 24 hours after transfer with X31 and assessed the host (CD45.1+) and donor (CD45.2+) NP-specific CD8+ T cell responses in the lungs on day 6 after challenge. We found that the frequencies (Fig 3a–b), and numbers (Fig 3c), of host NP-specific CD8+ T cells were similar in the two groups. However, the frequencies (Fig 3a–b) and numbers (Fig 3d) of donor NP-specific CD8+ T cells were reduced in recipients of Cd40−/− cells compared to recipients of WT cells.
Figure 3. CD40 signaling during priming programs NP-specific memory CD8+ T cells.
a–d. WT and Cd40−/− mice (both CD45.2) were infected with PR8 and 8 weeks later, memory CD8+CD44hi T cells were sorted from the spleens and populations containing 4 × 103 WT or Cd40−/− NP-specific CD8+CD44hi T cells (a–d) or populations containing 4 × 103 WT or Cd40−/− PA-specific CD8+CD44hi T cells (e–h) were transferred into naïve CD45.1+ mice, which were infected with X31 24 hours later. The numbers of host (c) and donor (d) NP-specific CD8+ T cells as well as the number of host (g) and donor (h) PA-specific CD8+ T cells in the lungs of recipient mice are shown. Data are representative of 3 experiments (mean ± s.d of 4–5 mice per group). i–j. C57BL/6 mice were treated with 250µg of MR1 or control IgG and infected with PR8. Six weeks later, CD8+CD44hi cells were sorted from the donor mice and populations containing 4 × 103 NP-specific CD8+ T cells were transferred into CD45.2 recipient mice. The recipients were infected with X31 the next day and treated with MR1 or control IgG. The number of donor NP-specific CD8+ T cells in lungs of recipient mice were determined using flow cytometry 7 days later (i). Data are representative of 2 experiments (mean ± s.d of 4-5 mice per group). P values were calculated using a two-tailed Student's t-test.
We also transferred equal numbers of PA-specific CD8+ memory T cells from WT (Fig. 3e) and Cd40−/− mice (Fig. 3f) to CD45.1 recipients, challenged them with X31 and assayed memory T cell expansion 6 days after re-challenge. In this case, we found no differences in the expansion of host PA-specific CD8+ T cells (Fig. 3g) or the donor PA-specific CD8+ T cells from WT and Cd40−/− mice in the lungs of recipients (Fig. 3h). These data suggested that CD40 signaling during the primary response was necessary for programming NP-specific, but not PA-specific CD8+ memory T cells.
To determine whether CD40 signaling played any role in the secondary expansion of NP-specific memory CD8+ T cells, we sorted memory CD8+ T cells from WT mice that were treated with control or CD154-blocking antibody (MR1) during the primary infection (Fig. 3i). We then adoptively transferred equal numbers of NP-specific memory CD8+ T cells to naïve CD45.1 mice, treated recipient mice with control antibody or MR1 and then challenged all groups with X31. We found that the secondary expansion of donor NP-specific CD8+ T cells was not impaired by treatment with MR1 during the challenge, but that treatment with MR1 during the primary response did impair the secondary expansion of NP-specific CD8+ T cells in WT recipients (Fig. 3j). Similar results were obtained when WT donor NP-specific memory CD8+ T cells were transferred into Cd40−/− mice (data not shown). Taken together, our results confirmed that absence of CD40 signaling during primary response compromised optimal NP-specific memory T cell expansion regardless of whether re-challenge occurred in a CD40 sufficient environment.
Cd40−/− DCs poorly present influenza NP to CD8+ T cells
Given the role of CD40 signaling in DC licensing (Bennett et al., 1998; Ridge et al., 1998; Schoenberger et al., 1998), we next tested whether Cd40−/− DCs could present influenza-derived epitopes at late times during the primary immune response. To do that, we purified CD11c+ DCs from the mLNs of day 7 infected WT or Cd40−/− mice and co-cultured them for 3 days with carboxyfluorescein succinimidyl ester (CFSE)-labeled CD8+ T cells that were sorted from the mLN of influenza-infected WT mice. We found that WT DCs expanded CD8+ T cells more efficiently than did Cd40−/− DCs (Fig. 4a). We next analyzed the expansion of NP-specific and PA-specific CD8+ T cells in the cultures. We found that WT DCs expanded NP-specific CD8+ T cells much more efficiently than did Cd40−/− DCs (Fig. 4b–c). In contrast, although only a few PA-specific CD8+ T cells were expanded in either culture, they expanded to the same extent in cultures with WT or Cd40−/− DCs (Fig. 4b–c). Thus, these results suggested that lack of CD40 signaling compromised the ability of Cd40−/− DCs to expand NP-specific, but not PA-specific CD8+ T cells.
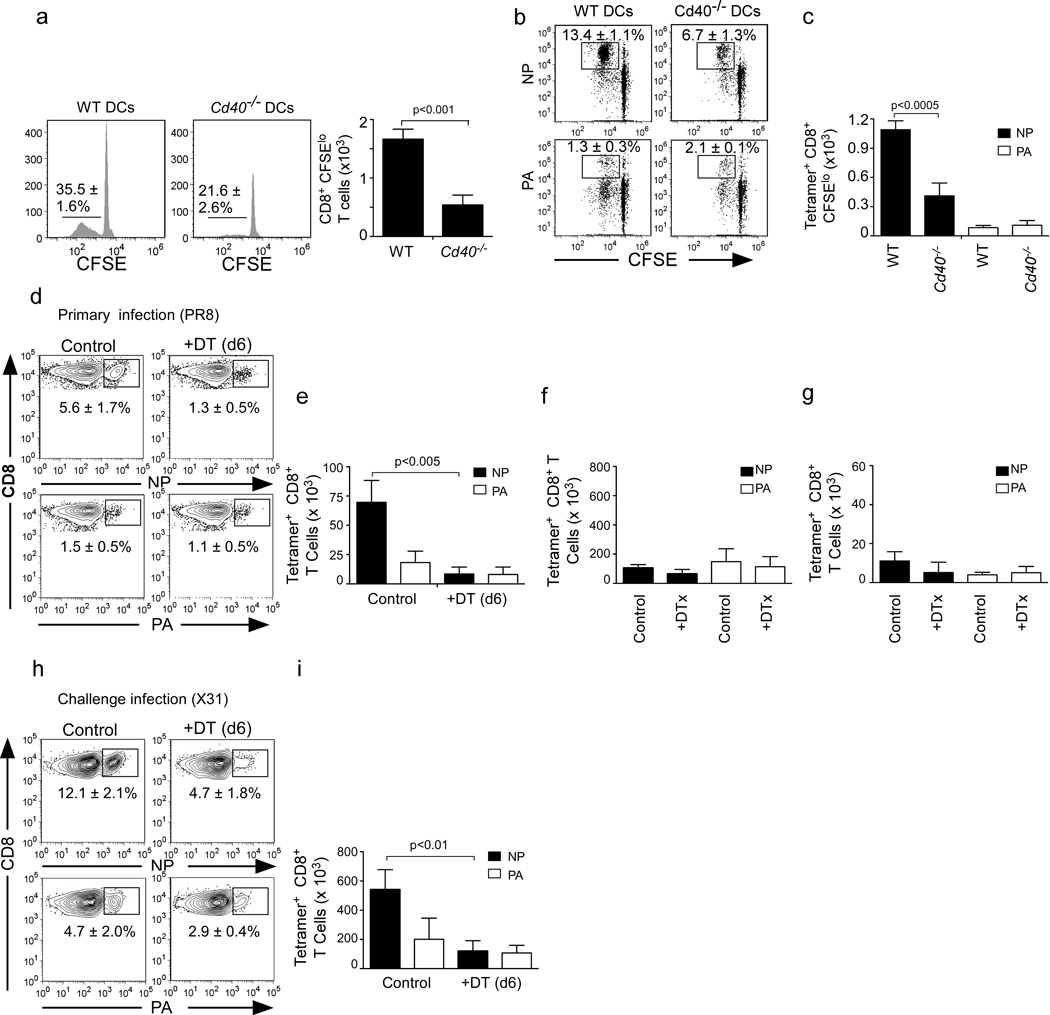
Figure 4. Limited presentation of NP by DCs in the absence of CD40.
CD8+ T cells were purified from day 7 PR8-infected WT mice, labeled with CFSE and cultured for 3 days with CD11c+ DCs purified from the mLNs of day 7 PR8-infected WT or Cd40−/− mice. The frequency and number of CFSEloCD8+ T cells are shown (a). The frequency (b) and number (c) of NP-specific and PA-specific CFSEloCD8+ T cells are shown. Data are representative of 3 experiments (mean ± s.d of 4 samples per group). d–e. C57BL/6 mice were irradiated and reconstituted with CD11c-DTR-EGFP bone marrow. Reconstituted mice were infected with PR8, injected i.p. with PBS or 60 ng DTX on day 6 after infection and then analyzed on day 12. The frequency (d) and numbers (e) of NP-specific and PA-specific CD8+ T cells in the mLNs are shown. Data are representative of 4 experiments (mean ± s.d of 4-5 mice per group). f–g. CD11c-DTREGFP BM chimeras were infected with PR8, injected i.p. with PBS or 60 ng DTX every three days between day 6 and 40. The numbers of resting NP-specific and PA-specific memory CD8+ T cells in lungs (f) and mLNs (g) are shown at 2 weeks. Data are representative of 3 experiments (mean ± s.d of 4–5 mice per group). h–i. CD11c-DTREGFP BM chimeras were infected with PR8, injected i.p. with PBS or 60 ng DTX every three days between day 6 and 40. Two weeks later, mice were challenged with X31 and the frequency (h) and numbers (i) of NP-specific and PA-specific CD8+ T cells in the lungs are shown at day 6. Data are representative of 2 experiments (mean ± s.d of 4–5 mice per group). All p values were calculated using a two-tailed Student's t-test.
Given studies showing that DCs program CD8+ T cells during the early stages of priming (Kaech and Ahmed, 2001; van Stipdonk et al., 2001), we next performed depletion studies to address whether DCs also acted later in the primary response to program NP-specific memory CD8+ T cells. In the first experiment, we reconstituted irradiated B6 recipients with bone marrow (BM) from CD11c-diptheria toxin receptor (DTR) mice, allowed them to recover for 8 weeks and infected them with PR8. We then depleted CD11c-expressing cells with DT on day 6 after infection and enumerated NP and PA-specific CD8+ T cells in the mLN on day 12. We found that the frequency (Fig. 4d) and number (Fig. 4e) of NP-specific CD8+ T cells were dramatically decreased in the mLNs of DT-treated mice, confirming that the late expansion of NP-specific CD8+ T cell required antigen presentation by DCs. In contrast, DC depletion on day 6 did not affect the accumulation of PA-specific CD8+ T cells (Fig. 4d–e).
In a second experiment, CD11c-DTR BM chimeras were treated with DT every 3 days between day 6 and day 40 after infection. Mice were then allowed to recover for two weeks, which was sufficient time for normal numbers of DCs to return (data not shown), and we enumerated NP-specific and PA-specific memory CD8+ T cells in control and DT-treated mice on day 55. We found that the numbers of NP-specific and PA-specific memory CD8+ T cells were similar in the lungs (Fig. 4f) and mLNs (Fig 4g) of both groups, suggesting that late DC depletion did not alter the number of memory NP and PA-specific CD8+ T cells generated after the primary infection. Mice were then re-challenged with X31 and the accumulation of NP and PA-specific CD8+ T cells in the lungs was assessed 6 days later. We found that the frequency (Fig. 4h) and total number (Fig. 4i) of responding NP-specific memory CD8+ T cells was compromised in the lungs of DT-treated mice. However, the frequency and number of PA-specific CD8+ T cells was not affected by DT treatment (Fig. 4h–i). Similar results were obtained in the mLN and spleen (Supplementary Fig. 2a–b). These results suggested that CD40-licensed DCs presented antigen to NP-specific, but not PA-specific CD8+ T cells at late times after infection and that sustained antigen presentation programmed NP-specific CD8+ memory T cells to optimally expand after re-challenge.
CD40 signaling controls cross-presentation by CD103−CD11b+ DCs
To better understand how CD40 signaling controls DC function in response to influenza, we next enumerated DC subsets at various times after infection in WT and Cd40−/− mice. Although WT and Cd40−/− mice contained similar numbers of most DC subsets in the mLNs, there were more CD103−CD11bhi DCs in the mLNs of WT mice than in Cd40−/− mice on day 10 (Supplementary Fig. 3a–c). However, there were no differences in the numbers of DCs in the lungs of WT and Cd40−/− mice at any time (Supplementary Fig. 3d–e). We also examined the expression of the costimulatory molecules, CD80, CD86 and CD70 and found slightly higher expression of CD80 and CD86 on CD103−CD11bhi and CD103+CD11blo tDCs in both the mLN and lungs of Cd40−/− mice (Supplementary Fig. 3f–g). In contrast, there was no difference in the expression of CD70 on these cells at any time (Supplementary Fig. 3h–i). Thus, the numbers, subset distribution and maturation of DCs in both the lungs and mLNs appeared relatively normal in Cd40−/− mice.
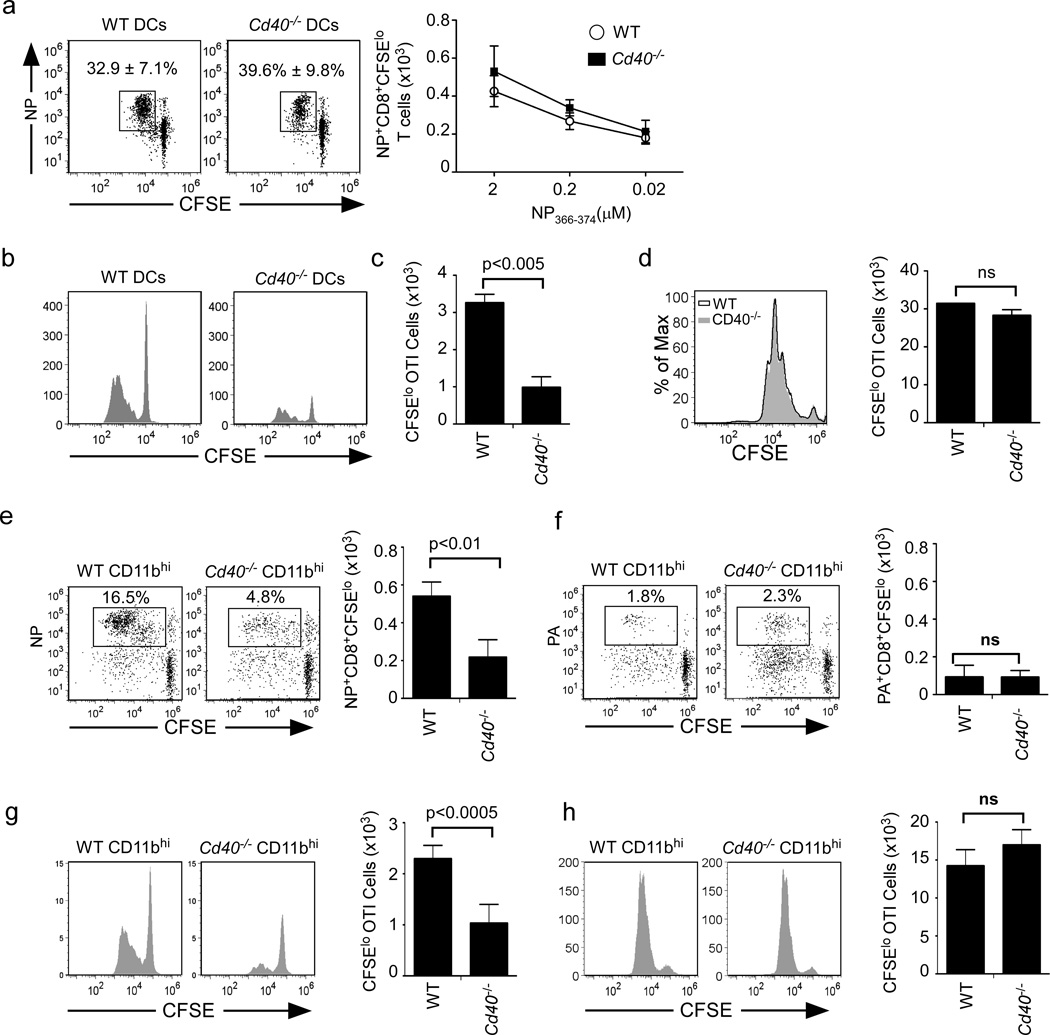
Given the apparently normal maturation of Cd40−/− DCs, we next tested whether they were functional antigen presenting cells. To do this, we purified total CD11c+ DCs from the mLNs of WT and Cd40−/− mice that had been infected with influenza 7 days earlier, pulsed them in vitro with NP366−374 peptide and cultured them with CFSE-labeled CD8+ T cells from the mLNs of day 7 influenza infected mice. We found that NP-specific CD8+ T cells proliferated similarly in response to both WT and Cd40−/− DCs pulsed with a wide range of peptide concentrations (Fig. 5a). Next, to test the capacity of Cd40−/− DCs to cross-present exogenous protein antigens, we purified total CD11c+ DCs from the mLNs of WT or Cd40−/− mice infected with influenza 7 days earlier, pulsed them with soluble OVA and cultured them with CFSE-labeled OT-I cells. We found that Cd40−/− DCs poorly cross-presented soluble OVA compared to WT DCs (Fig. 5b–c). Importantly, the failure of Cd40−/− DCs to expand OT-I T cells was reversed when we pulsed DCs with OVA257−264 peptide (Fig. 5d).
Figure 5. Cross-presentation by CD103−CD11b+ tDC is compromised in Cd40−/− mice.
a. CFSE-labeled CD8+ T cells from day 7 influenza infected mLNs were cultured for 72 hours with WT or Cd40−/− CD11c+ cells pulsed with NP366–374 peptide and the frequency (left) and number (right) of divided NP-specific CD8+ T cells is shown. Data are representative of 3 experiments with 4 samples per group. (b–d) CD11c+ cells from mLNs of day 7 influenza-infected C57BL/6 or Cd40−/− mice were pulsed with 5µg/ml OVA (b–c) or 0.5µg/ml OVA257–264 (d) and cultured for 72 hours with CFSE-labeled OTI cells. Results are representative of 3 experiments with 4 samples per group. e–f. CFSE-labeled CD8+ T cells from mLNs of day 7 infected mice were cultured with CD103− CD11b+ tDCs from the mLN of day 7 infected C57BL/6 or Cd40−/− mice and the frequency and number of divided NP-specific (e) or PA-specific CD8+ T cells (f) are shown. Data are representative of 3 experiments with 4 samples per group. (g–h) CFSElabeled OT-I cells were cultured for 72 hours with CD103−CD11b+ tDCs cells from mLNs of day 7 C57BL/6 or Cd40−/− that were pulsed with 5µg/ml OVA (g), or 0.5µg/ml OVA257–264 (h). Data are representative of 3 experiments with 4 samples per group. All p values were calculated using a two-tailed Student's t-test.
Given that CD103−CD11bhi DCs are the dominant population of DCs in the mLN and lung after influenza infection (Ballesteros-Tato et al., 2010; GeurtsvanKessel et al., 2008) and that these cells are the major subset that presents NP to CD8+ T cells at the peak of infection (Ballesteros-Tato et al., 2010), we next tested the ability of CD103−CD11bhi DCs from WT and Cd40−/− mice to induce the proliferation of effector NP-specific CD8+ T cells. We found that WT CD103−CD11bhi DCs efficiently expanded NP-specific CD8+ T cells, whereas Cd40−/− CD103−CD11bhi DCs did not (Fig. 5e). In contrast, although only a few PA-specific CD8+ T cells expanded in culture, they expanded equivalently in cultures with WT and Cd40−/− DCs (Fig. 5f). These results demonstrate that CD40 controls the ability of CD103−CD11bhi DCs to present NP, but not PA, late after infection.
To determine whether CD103−CD11bhi DCs were impaired in their ability to cross-present soluble antigens, we sorted CD103−CD11bhi DCs from infected WT or Cd40−/− mice, pulsed them with either soluble OVA protein or OVA peptide and tested their ability to prime naïve OTI cells. We found that Cd40−/− CD103−CD11bhi DCs poorly cross-presented soluble OVA protein to CD8 T cells (Fig. 5g), but that those same DCs pulsed with peptide primed naïve CD8 T cells normally (Fig. 5h). Taken together, our results suggest that CD40 signaling controls antigen processing and cross-presentation rather than the accumulation and maturation of DCs.
Control of memory programming by CD70-expressing CD103−CD11b+ DCs
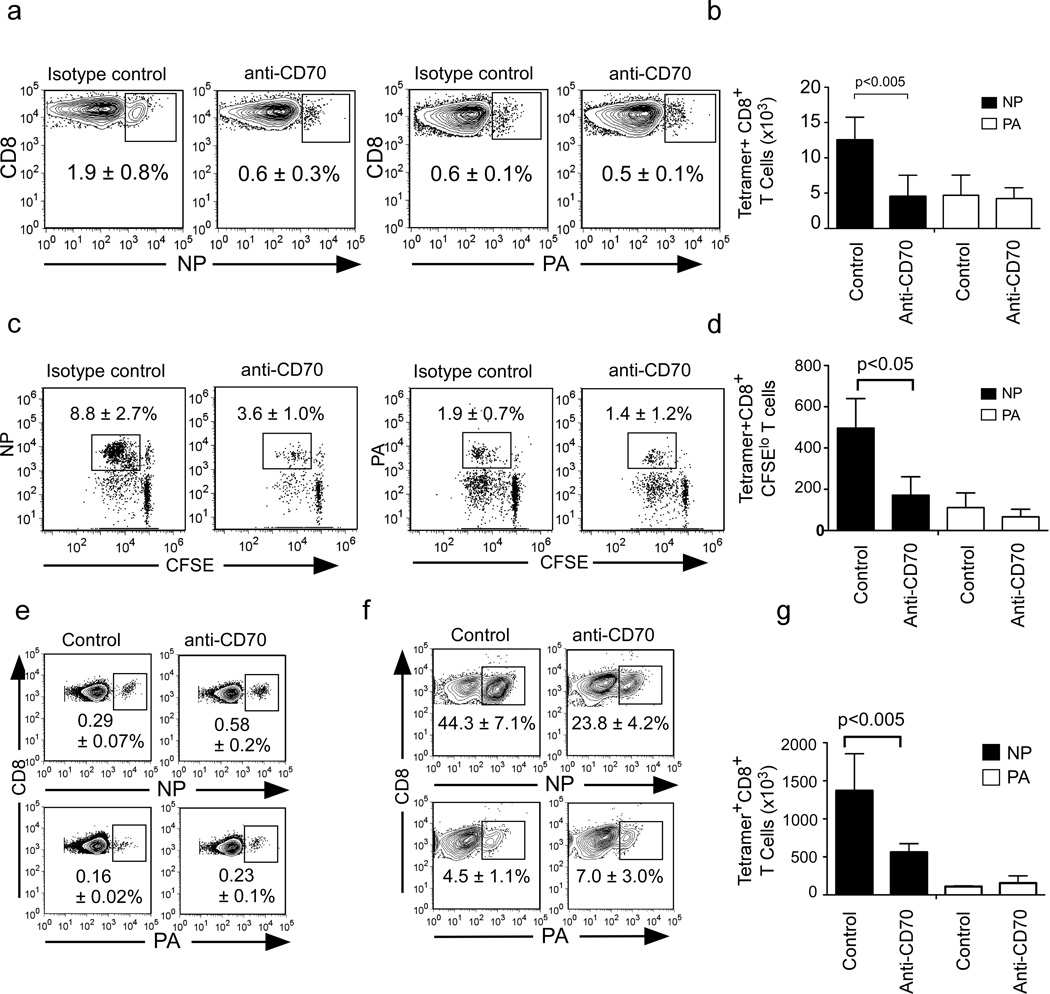
CD103−CD11bhi DCs express CD70, the ligand for CD27, which is a costimulatory molecule that facilitates the late expansion of CD8+ T cell responses and may be involved in memory programming (Ballesteros-Tato et al., 2010; Hendriks et al., 2000). To test whether CD27 engagement by CD70-expressing CD103−CD11bhi DCs was important for NP-specific or PA-specific CD8+ T cell responses, we treated WT mice with anti-CD70 blocking antibody 4 days after primary infection and enumerated NP and PA-specific CD8+ T cells on day 10. We found that anti-CD70 treatment impaired NP-specific CD8+ T cell expansion without affecting the PA-specific CD8+ T cell response (Fig. 6a–b). Similarly, we found that the in vitro expansion of NP-specific CD8+ T cells by CD103− CD11b+ DCs was also markedly inhibited by CD70 blockade, whereas the expansion of PA-specific CD8 T cells was not affected (Fig. 6c–d).
Figure 6. CD70-expressing CD103−CD11b+ DCs program NP-specific CD8+ T cells.
(a–b) C57BL/6 mice were infected with PR8, treated with 500µg anti-CD70 or control IgG 4 days after infection and the frequency (a) and number (b) of NP and PA-specific CD8+ T cells in the mLNs on day 10 are shown. Data are representative of 3 experiments (mean ± s.d of 4-5 mice per group). (c–d) CD8+ T cells from mLNs of day 7 infected C57BL/6 mice were cultured for 72 hours with CD103−CD11b+ tDCs and either anti- CD70 or control IgG and the frequency (c) and number (d) of divided NP and PA-specific CD8+ T cells is shown. Results are representative of 3 experiments (mean ± s.d of 4 samples). (e) C57BL/6 were infected with PR8 and treated with 500µg anti-CD70 or control IgG 4 days after infection and the frequencies of NP and PA-specific CD8+ T cells in the lungs at 8 weeks are shown. Data are representative of 2 experiments (mean ± s.d of 4-5 mice per group). (f) C57BL/6 were infected with PR8 and treated with 500µg anti-CD70 or control IgG 4 days after infection, challenged 8 weeks later with X31, and the frequencies (left) and numbers (right) of NP and PA-specific CD8+ T cells in the lungs are shown. Data are representative of 2 experiments (mean ± s.d of 4–5 mice per group). P value, two tailed Student´s t-test
To determine whether CD27 signaling played a role in memory CD8+ T cell programming, we next treated WT mice with anti-CD70 antibody 4 days after primary infection, waited 8 weeks for memory to develop and enumerated NP-specific memory CD8+ T cells. We found that the frequencies and numbers of NP-specific memory T cells were similar in mice treated with anti-CD70 or control antibodies (Fig. 6e). We next challenged memory mice with X31. We found that NP-specific memory CD8+ T cell expansion was compromised in the lungs of mice that received anti-CD70 during primary infection, but that the expansion of PA-specific memory CD8+ T cells was unaffected (Fig. 6f–g). Thus, both CD40 and CD27 are required at late times during the primary response to elicit fully functional NP-specific memory CD8+ T cells, whereas PA-specific memory CD8+ T cells develop normally in the absence of these costimulatory signals.
Costimulation through CD40 and CD27 maintain IL-2-responsive T cells
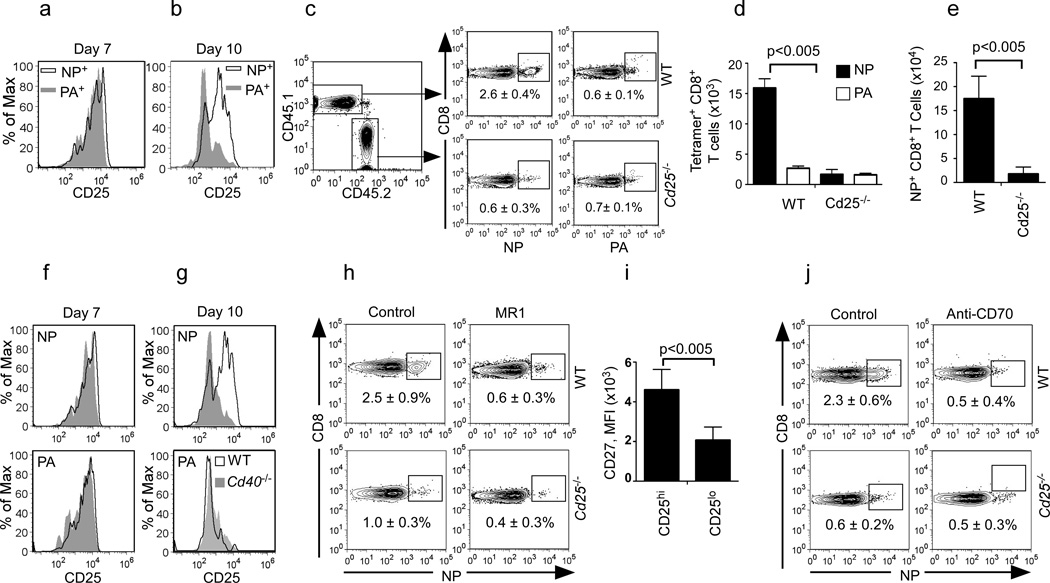
IL-2 signaling through CD25 is required for T cell expansion and memory formation (Williams et al., 2006), and may be dependent on CD40 (Wolkers et al., 2011) and CD27 co-stimulation (Huang et al., 2006). Therefore, we next determined whether NP and PA16 specific CD8+ T cells expressed CD25 after influenza infection. We found that NP and PA-specific CD8+ T cells expressed similar amounts of CD25 early after infection (Fig. 7a). However, whereas NP-specific CD8+ T cells maintained CD25 expression on day 10, PA-specific CD8+ T cells down-regulated CD25 (Fig. 7b).
Figure 7. CD40 promotes survival of CD25+ CD8+ T cells.
(a–b) Expression of CD25 on NP and PA-specific CD8+ T cells in the mLN. Data are representative of 3 experiments of 4–5 mice per group. (c–d) WT:Cd25−/− chimeras were infected with PR8 and the frequency (c) and numbers (d) of WT and Cd25−/− NP-specific and PA-specific CD8+ T cells in the mLNs on day 12 are shown. Data are representative of 3 experiments (mean ± s.d of 4-5 mice per group). (e) WT:Cd25−/− chimeras were infected with PR8, challenged with X31 8 weeks later and the numbers of WT and Cd25−/− NP-specific CD8+ T cells on day 6 are shown. Data are representative of 2 experiments (mean ± s.d of five mice per group). (f–g) Expression of CD25 on NP and PA-specific CD8+ T cells in the mLN of influenza-infected C57BL/6 and Cd40−/− mice. Data are representative of 3 experiments (4–5 mice per group). (h) WT:Cd25−/− chimeras were infected with PR8 and treated with 250 µg of MR1 or control IgG and the frequency of NP-specific CD8+ T cells from WT or Cd25−/− donors in the mLN on day 12 is shown. Data are representative of 2 experiments (mean ± s.d of 4-5 mice). (i) Expression of CD27 on CD25hi or CD25lo NP-specific CD8+ T cells in the mLN of day 10 influenzainfected mice (MFI; mean fluorescence intensity). Data are representative of 3 experiments (mean ± s.d of 4-5 mice). (j) WT:Cd25−/− chimeras were infected with PR8, treated with anti-CD70 or control antibody and the frequency of NP-specific CD8+ T cells from WT or Cd25−/− donors is shown. Data are representative of 2 experiments (mean ± s.d of 4–5 mice). P values, two tailed Student´s t-test
To test whether CD25 was important for the accumulation of NP-specific CD8+ T cells, we made mixed BM chimeras in which irradiated WT mice (CD45.1+) were reconstituted with 50% WT CD45.1+ BM and 50% CD45.2+Cd25−/− BM. Chimeric mice were infected with PR8 and the expansion of WT (CD45.1) and Cd25−/− (CD45.2) NP-specific and PA-specific CD8+ T cells was assessed. We found that although equivalent numbers of NP-specific CD8+ T cells were generated from WT and CD25-deficient CD8+ T cells 7 days after infection (data not shown), many more WT NP-specific CD8+ T cells than Cd25−/− NP-specific CD8+ T cells had accumulated by day 12 (Fig. 7c–d). In contrast, similar numbers of PA-specific CD8+ T cells were generated from WT and Cd25−/− precursors (Fig. 7c–d). Similar results were obtained in the lungs (data not shown).
We next challenged the WT:Cd25−/− chimeras with X31 8 weeks after the initial infection and measured the expansion of WT and Cd25−/− CD8+ T cells in the lung 6 days later. We found that the secondary expansion of Cd25−/− NP-specific memory CD8+ T cell was compromised compared to their WT counterparts (Fig. 7e). Thus, CD25 expression was important for both the primary and secondary expansion of NP-specific CD8+ T cells. To connect CD40 signaling and CD25 expression, we analyzed whether NP and PA-specific CD8+ T cells expressed CD25 in WT and Cd40−/− mice. We found that both NP and PA-specific CD8+ T cells expressed similar amounts of CD25 in WT and Cd40−/− mice on day 7 (Fig. 7f). In contrast, although NP-specific CD8+ T cells continued to express CD25 on day 10 in WT mice, they had decreased CD25 expression in Cd40−/− mice (Fig 7g). Unlike NP-specific CD8+ T cells, PA-specific CD8+ T cells had already down-regulated CD25 expression on day 10 after infection in WT mice and the amount of CD25 was not affected by the loss of CD40 (Fig. 7f–g). Similar results were obtained in mice treated with MR1 5 days after infection (data not shown).
To directly confirm that CD40 signaling was important for the accumulation of CD25+ NP-specific CD8+ T cells, WT:Cd25−/− chimeras were treated with MR1 to block CD40 signaling and WT and Cd25−/− NP-specific CD8+ T cells were enumerated 12 days after infection. We found that WT NP-specific CD8+ T cells accumulated to a greater extent than Cd25−/− NP-specific CD8+ T cells in isotype control-treated mice, whereas WT and Cd25−/− NP-specific CD8+ T cells accumulated similarly in MR1-treated mice (Fig. 7h). These results suggested that CD40 signaling helps to maintain CD25 expression, and thus IL-2 responsiveness, by NP-specific, but not PA-specific CD8+ T cells.
In order to connect CD25 and CD27, we next gated the NP-specific CD8+ T cells into CD25hi and CD25lo subsets and measured CD27 expression using flow cytometry. We found that CD25hi cells expressed more CD27 compared to CD25lo cells (Fig. 7i), suggesting that CD27 and CD25 expression are also functionally linked. To test this possibility, we treated WT:Cd25−/− chimeric mice with control or anti-CD70 blocking antibody 4 days after infection and enumerated WT and Cd25−/− NP -specific CD8+ T cells on day 12. We found that the accumulation of Cd25−/− NP-specific CD8+ T cells was severely impaired in chimeras treated with control antibody, whereas the accumulation of both WT and Cd25−/− NP-specific CD8+ T cells was impaired to the same extent in anti- CD70 treated mice (Fig. 7j). Thus, the loss of CD70 and CD25 appear to impact the same process of late T cell expansion, which is when memory programming occurs.
Taken together, these results suggest that CD8 T cells are programmed to become fully functional memory cells by prolonged antigen presentation and interactions between IL-2:IL2R, CD40:CD40L and CD70:CD27. In the absence of extended antigen presentation, CD8+ T cells do not receive the appropriate costimulatory signals and are not programmed to become fully functional memory cells.
DISCUSSION
Our results demonstrate that CD8+ T cells responding to different epitopes in influenza have different requirements for the CD40, CD27 and CD25 signaling pathways. Primary and secondary CD8+ T cell responses to NP require these pathways, whereas CD8+ T cell responses to PA are unchanged by their absence. This observation is contrary to the current paradigm, which suggests that CD8+ T cell responses to some types of antigens, such as purified proteins in subunit vaccines, are dependent on CD40 signaling to properly license DCs, whereas CD8 T cell responses to virulent pathogens, like influenza, may not rely on CD40:CD154 interactions because DCs are fully activated by pathogen-sensing molecules (Hamilton et al., 2001). If this paradigm is correct, then one would expect that CD8+ T cells responding to any epitope of a particular antigen or pathogen would be consistent in their requirements for CD40-mediated DC licensing. In contrast, our data demonstrate that there can be dramatic differences between CD8+ T cell responses to different epitopes from the same pathogen. Thus, factors other than initial DC activation must control the requirement for CD40 signaling for CD8+ T cell responses to some antigens.
For example, our data demonstrate that CD11c+ cells (presumably DCs) are required for the continued expansion of NP-specific CD8+ T cells beyond day 5, whereas these cells are not at all required for normal CD8+ T cell responses to PA. Thus, we conclude that the duration of antigen presentation for these two antigens is very different. Consistent with this idea, T cells responding to PA expand for the first 7 days after influenza infection and subsequently contract. In contrast, T cells responding to NP continue to accumulate through days 10–12. Given that CD103−CD11bhi DCs, which are the only cells to express CD70, the ligand for CD27, dominate the late phase of the primary response to influenza (Ballesteros-Tato et al., 2010), it makes sense that CD8+ T cells responding to epitopes that are presented during this period are exposed to qualitatively distinct DCs and utilize very different pathways of costimulation.
Each of the signaling pathways required in the late phase of the primary response (CD40, CD25, CD27) appears to control different aspects of late primary expansion and memory programming. For example, CD40 signaling appears to be important for successful cross-priming during this period. Thus, in the absence of CD40, cross priming is inefficient, NP is poorly presented and NP-specific CD8+ T cell expansion and memory programming is ineffective. Blockade of either CD70 or CD25 has no additional effect, because in the absence of antigen, costimulation is irrelevant. Antigen receptor signaling is also likely to be important for IL-2 production, which reinforces the expression of CD25. Thus, in the absence of CD40, NP is not presented and CD25 expression is not maintained.
The functions of CD25 and CD27 are also likely to be interrelated. For example, CD27 engagement by CD70-expressing DCs is probably important for preventing the apoptosis of CD8+ T cells responding to IL-2 (Dolfi et al., 2008; Peperzak et al., 2010). Thus, although IL-2 signaling is important for late primary expansion and memory programming, it does not work unless the T cells encounter CD70-expressing DCs that prevent their apoptosis and promote their survival. Importantly, none of these mechanisms apply to PA-specific CD8+ T cells, since PA is poorly presented during the late phase of the immune response. Thus the genetic ablation or pharmacological blockade of CD40, CD25 or CD27 pathways has no effect on PA-specific CD8+ T cell responses.
In light of these data, we propose an alternative model, in which CD40-licensed, CD70-expressing, CD103−CD11bhi DCs cross-present abundant antigens during the late phase of the primary response. T cells recognizing antigens on these DCs express CD25, respond to IL-2 and receive survival signals through CD27, which together program T cells to become memory T cells with robust secondary proliferative capacity and cytokine producing ability. In contrast, T cells responding to antigens like PA, which are poorly presented during the late phase of the primary response, do not receive these signals and are not programmed be become highly proliferative memory CD8+ T cells. This model is consistent with previous data showing that NP-specific memory CD8+ T cells, but not PA-specific memory CD8+ T cells, dominate the secondary response to influenza and promote beneficial outcomes (Belz et al., 2000; Crowe et al., 2003; La Gruta et al., 2010).
The differences in the presentation of NP and PA by DCs during the primary response may be explained by the nature of antigens themselves. For example, the amount of NP and PA contained in mature influenza virions is widely different - with 560 NP molecules per virion and only 8 PA molecules (one per RNA strand) per virion. Thus, one could envision a scenario in which both NP and PA are directly presented to CD8 T cells early after infection by influenza-infected DCs that are activated by pathogen recognition receptors. However, at later times after infection, when the number of virally infected cells is low and the majority of antigen is in the form of cellular debris and neutralized virions, then DCs must acquire antigens exogenously and stimulate CD8 T cells by cross-priming. Given that cross-presentation is much more efficient at high doses rather than low doses of antigen (Kurts et al., 1998), then the processing and presentation of NP would be dramatically favored over PA. This conclusion is also consistent with data showing that subdominant antigens are often poorly cross-presented (Otahal et al., 2005) and that the immunodominance hierarchy can be a function of antigen dose (Jenkins et al., 2006; La Gruta et al., 2006). Importantly, previous studies show that the recall response to PA can be enhanced by engineering the PA epitope into the influenza neuraminidase protein, which is much more abundant than polymerase (La Gruta et al., 2006). Although the previous studies did not specifically examine memory programming or a requirement for CD40, they are consistent with our model in which epitopes in more abundant proteins are preferentially cross-presented at late times in the primary response and, as a result, preferentially receive memory programming signals.
In this model, only T cells recognizing epitopes in abundant antigens would be programmed appropriately by CD70-expressing CD103−CD11bhi DCs. This model also represents a mechanism for the immune system to enhance the efficiency of memory T cell responses. Differential cross-presentation by CD103−CD11bhi DCs would lead to a selection process that favors the expansion of T cells recognizing more abundant antigens and skews memory responses towards those antigens. As a consequence, the responding memory CD8+ T cells would more likely encounter antigen on DCs as well as non-professional APCs such as lung epithelial cells and more effectively eliminate the pathogen. Thus, the fitness of the memory response would be improved. This view is consistent with studies showing that NP, but not PA derived epitopes, are strongly expressed by lung-epithelial cells (Crowe et al., 2003), the primary target of influenza virus, and that PA-specific memory CD8+ T cells are ineffective or even detrimental in controlling influenza infection when compared to NP-specific memory CD8 T cells (Crowe et al., 2003).
In summary, our data provide new insights into the mechanisms regulating memory CD8+ T cell programming as well as the role of extended antigen presentation by DCs. Collectively, this information will be useful in the rational design of vaccines and development of immunotherapies that target CD8+ T cell responses.
Experimental procedures
Mice, infections, chimeras, EdU and antibody treatment
C57BL/6 (WT), B6.129P2-Tnfrsf5tm1kik (Cd40−/−), B6.129S2-Tnfsf5tm1Imx (Cd154−/−), B6.Tgn(TcrOVA)1100Mjb (OT-I), B6.129S2-IgH-6tm1Cgn/J (µMT), B6.129S4-IL2ratm1Dw/J, (Cd25−/−) B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J (CD11c-DTR) and B6.IgHa.Thy-1a.Ptrpca (CD45.1) mice were obtained from Trudeau Institute and were bred in the University of Rochester (UR) or University of Alabama at Birmingham (UAB) animal facilities. Infections were performed intranasally in 100 µl with 500 egg infectious units (EIU) of PR8 or X31 (primary infection) and 5000 EIU of PR8 or X31 (secondary infection). Viral titers were quantified using a viral foci assay (Rangel-Moreno et al., 2008). In some experiments, mice were injected intraperitoneally with 500 µg anti-CD70 (FR70), 500 µg rat IgG2b (LTF-2), 250 µg anti-CD154 (MR-1) or 250 µg hamster IgG (all from Bioxcell). Proliferating cells were labeled by intravenously injecting 0.5 mg of EdU (Invitrogen) three times every 6 hours starting 24 hours before sacrifice. BM chimeric mice were generated by lethally irradiating recipients with 950 Rads from a 137Cs source delivered in a split dose and reconstituting them with 107 total BM cells. Mice were allowed to reconstitute for 8–12 weeks before infection. In some cases, B6 CD11c-DTR BM chimeras received an intraperitoneal injection of 60 ng DT (Sigma) on days 6 and 10 after infection. All experimental procedures involving animals were approved by the appropriate UR or UAB animal welfare committees.
Cell preparation and flow cytometry
Cells were prepared from lungs cut into small fragments and digested for 45 min at 37°C with 0.6 mg/ml collagenase A (Sigma) and 30 µg/ml DNAse I (Sigma) in RPMI-1640 medium (GIBCO). Digested lungs were dispersed by passage through a wire mesh. Live cells were obtained by density-gradient centrifugation with 1-Step Polymorphs (Accurate Chemical). Cells were obtained from mLNs and spleens by disruption through 70 µm nylon cell strainer (BD Biosciences). Red blood cells were lysed with 150 mM NH4Cl, 10 mM KHCO3 and 0.1 mM EDTA. Fc receptors were blocked with antibody 2.4G2 (10 µg/ml; Trudeau Institute), followed by staining with MHC class I tetramers or fluorochrome-conjugated antibodies. The H-2Db class I tetramers containing NP366–374 peptide or PA224–233 peptide were generated by NIH Tetramer Core Facility. Fluorochrome-labeled anti-CD8α (53-6.7), anti-CD4 (RM4-5), anti-CD27 (L6.3A10), anti-CD40 (3/23), anti-CD44 (IM7), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD86 (GL1), anti-CD11b (MI/70), anti-Ly6C (AL-21), anti-B220 (RA3-6B2), anti-CD25 (7D4), anti-CD122 (TM-B1), anti-CD62L (MEL-14) and anti-MHC class II (AF6-120.1) were from BD Biosciences. Anti-CD11c (N418), anti-CD70 (FR70) and anti-CD103 (2E7), anti-CD80 (16-10AI), anti-CD127 (A7R34) and anti-KLGR1 (2F-1) were from eBioscience. For intracellular staining and tetramer co-staining, cells were stimulated with NP366–374 or PA224–233 peptides (2 µg/ml) and 40U of rIL-2 in the presence of Brefeldin-A (10 µg/ml) for 3 h. Cells were then surface stained, washed, fixed and intracellular staining for IFNγ (clone XMG1.2, eBioscience) was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) following the manufacturer’s instructions and adapted from (Dimopoulos et al., 2009). Flow cytometry was performed using a FACSCanto II (BD Biosciences), or a C6 Flow Cytometer (Accuri) and analyzed with Flowjo software.
Cell purification, CFSE labeling and adoptive transfer
CD8+ T cells from influenza-infected C57BL/6 mice or OT-I mice were obtained by depletion of CD11c+ cells with anti-CD11c MACS beads followed by positive selection with anti-CD8 MACS beads (Miltenyi Biotec). All T cell preparations were more than 95% pure. In some experiments, CD8+ T cells were labeled for 10 min at 37 °C with 5 µM CFSE (Molecular Probes).
CD8+CD44hi memory T cells were sorted from spleens of C57BL/6 or Cd40−/− mice using a FACSAria (BD Biosciences) after positive selection with anti-CD8 MACS beads. Cell numbers were normalized to the concentration of antigen-specific T cells and 4 × 104 CD8+CD44hi DbNP+ or CD8+CD44hi DbPA+ T cells were transferred intravenously into naïve C57BL/6, Cd40−/− or CD45.1 recipient mice. DCs were enriched from pooled mLNs of C57BL/6, Cd40−/− or Cd154−/− with anti-CD11c MACS beads. In some experiments, DC subsets were sorted with a FACSAria. All sorted DC subsets were more than 95% pure.
In vitro culture
Cells were cultured in RPMI-1640 supplemented with sodium pyruvate, HEPES, pH 7.4, nonessential amino acids, penicillin, streptomycin, 2-mercaptoethanol and 10% heatinactivated FCS (all from Gibco). Sorted DCs (1 × 103) and CFSE-labeled T cells (1 × 104) were cultured for 72 h at 37 °C in 100 µl in round-bottomed 96-well plates. In some experiments, we added soluble OVA protein at 5 µg/ml or OVA257–264, NP366–374 or PA224–233 peptides at 0.5 µg/ml. In some cases, anti-CD40 (10C8), or anti-CD70 (FR70; eBioscience) or rat IgG2b isotype-matched control antibody (KLH; Bioxcell) was added to the culture at a final concentration of 25 µg/ml.
Statistical analysis
The statistical significance of differences in mean values was analyzed with a two-tailed Student's t-test. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Highlights.
NP-specific and PA-specific CD8+ T cells differentially require CD40, CD25 and CD27
Presentation of NP is sustained for longer than 6 days, but presentation of PA is not
CD40 is necessary of DCs for cross-priming and late NP presentation
CD40-mediated late antigen-presentation maintains CD25 and CD27 on T cells
Acknowledgements
The authors would like to thank Louise Hartson for the preparation of vial stocks and personnel from the University of Rochester Flow Cytometry Core for assistance with cell sorting. This work was supported by UR, UAB and NIH grants AI061511, AI072689, HL069409 and AR048311.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baez M, Palese P, Kilbourne ED. Gene composition of high-yielding influenza vaccine strains obtained by recombination. J. Infect. Dis. 1980;141:362–365. doi: 10.1093/infdis/141.3.362. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Leon B, Lund FE, Randall TD. CD4+ T helper cells use CD154-CD40 interactions to counteract T reg cell-mediated suppression of CD8+ T cell responses to influenza. J. Exp. Med. 2013;210:1591–1601. doi: 10.1084/jem.20130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Xie W, Altman JD, Doherty PC. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge. J Virol. 2000;74:3486–3493. doi: 10.1128/jvi.74.8.3486-3493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, Flavell RA. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Tough DF, Eto D, Tishon A, Grewal IS, Sprent J, Flavell RA, Oldstone MB. CD40 ligand-mediated interactions are involved in the generation of memory CD8(+) cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J. Virol. 1998;72:7440–7449. doi: 10.1128/jvi.72.9.7440-7449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SR, Turner SJ, Miller SC, Roberts AD, Rappolo RA, Doherty PC, Ely KH, Woodland DL. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. TJ. Exp. Med. 2003;198:399–410. doi: 10.1084/jem.20022151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos N, Jackson HM, Ebert L, Guillaume P, Luescher IF, Ritter G, Chen W. Combining MHC tetramer and intracellular cytokine staining for CD8(+) T cells to reveal antigenic epitopes naturally presented on tumor cells. J. Immunol. Meth. 2009;340:90–94. doi: 10.1016/j.jim.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27-CD70 interactions. Nat. Commun. 2012;3:948. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J. Exp. Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Tvinnereim AR, Harty JT. Listeria monocytogenes infection overcomes the requirement for CD40 ligand in exogenous antigen presentation to CD8(+) T cells. J Immunol. 2001;167:5603–5609. doi: 10.4049/jimmunol.167.10.5603. [DOI] [PubMed] [Google Scholar]
- Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, Robbins PF. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MR, Webby R, Doherty PC, Turner SJ. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J. Exp. Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta NL, Kedzierska K, Pang K, Webby R, Davenport M, Chen W, Turner SJ, Doherty PC. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc. Natl. Acad. Sci. (USA) 2006;103:994–999. doi: 10.1073/pnas.0510429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, Thomas PG, Doherty PC, Turner SJ. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J. Clin. Invest. 2010;120:1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J. Exp. Med. 2003;198:1759–1764. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otahal P, Hutchinson SC, Mylin LM, Tevethia MJ, Tevethia SS, Schell TD. Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope. J Immunol. 2005;175:700–712. doi: 10.4049/jimmunol.175.2.700. [DOI] [PubMed] [Google Scholar]
- Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J. Clin. Invest. 2010;120:168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J, Carragher DM, Misra RS, Kusser K, Hartson L, Moquin A, Lund FE, Randall TD. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J Immunol. 2008;180:454–463. doi: 10.4049/jimmunol.180.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J. Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers MC, Bensinger SJ, Green DR, Schoenberger SP, Janssen EM. Interleukin-2 rescues helpless effector CD8+ T cells by diminishing the susceptibility to TRAIL mediated death. Immunol. Lett. 2011;139:25–32. doi: 10.1016/j.imlet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.